Abstract
OBJECTIVES
Primary sclerosing cholangitis (PSC) often coexists with inflammatory bowel disease (IBD) and can be complicated by cholangiocarcinoma (CCA), a lethal malignancy for which reliable predictors remain unknown. We aimed to characterize the influence of colectomy and IBD duration on risk of CCA in patients with PSC-IBD.
METHODS
A retrospective review of patients with PSC-IBD seen at the Mayo Clinic, Rochester, between January 2005 and May 2013 was performed. The primary outcome was time to development of CCA and our goal was to determine whether the risk differed between patients with and without colectomy. Risk factors were assessed using univariable and multivariable Cox proportional hazard models where colectomy, IBD disease duration, and development of advanced liver disease were treated as time-dependent covariates.
RESULTS
A total of 399 patients with PSC-IBD were included in the study, of whom 137 had a colectomy and 123 patients developed CCA. Age-adjusted univariate Cox proportional hazard models demonstrated that colectomy (hazard ratio (HR) 1.53, 95% confidence interval (CI) 1.05–2.22, P =0.02) and duration of IBD (HR 1.37, 95% CI 1.15–1.63, P <0.01) were associated with an increased risk of CCA, and colonic neoplasia (HR 1.52, 95% CI 0.97–2.37, P =0.06) and colectomy for colonic neoplasia (HR 1.62, 95% CI 1.01–2.61, P =0.05) approached significance. Among patients with a history of colectomy, colonic neoplasia as the indication for surgery was associated with a particularly increased risk of CCA (HR 2.91, 95% CI 1.24–6.84, P =0.01) compared with medically refractory disease. On multivariate analysis, duration of IBD remained significantly associated with CCA (HR 1.33, 95% CI 1.11–1.60, P <0.01). The influence of IBD duration on CCA risk was not modified after colectomy (P =0.69).
CONCLUSIONS
Prolonged duration of IBD is associated with an increased risk of CCA in patients with PSC-IBD, and colectomy itself does not modify this risk. These findings identify a subset of patients who are at high risk of this lethal complication and in need of close surveillance.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease that can progress to cirrhosis and liver failure (1). PSC frequently coexists with inflammatory bowel disease (IBD), particularly ulcerative colitis (2). Compared with patients with IBD alone, individuals with PSC-IBD are at an increased risk of developing colonic dysplasia or neoplasia at a rate that approaches a lifetime risk of up to 25%, thereby mandating close surveillance with colonoscopy and biopsies annually from the time of diagnosis (3,4). Patients with IBD who develop colonic dysplasia, carcinoma, or medically refractory disease often require surgical intervention, usually with colectomy and ileal pouch anal anastomosis.
Along with an increased risk of colorectal cancer, patients with PSC with or without IBD are also at increased risk of primary hepatobiliary neoplasia and, in particular, cholangiocarcinoma (CCA). The lifetime incidence of CCA in patients with PSC ranges between 5 and 10%, with the mean age of diagnosis in the fourth decade of life (5). Although curative options are available for limited-stage disease, CCA is frequently detected in its advanced stages when prognosis is poor, and thus predictors of CCA development remain critically needed to identify at-risk patients.
The risk factors for CCA in PSC have been reported, although none are sufficiently robust or reliable enough to alter recommendations for disease surveillance (6,7). As a consequence, major international societies have not made specific recommendations for surveillance of CCA in patients with PSC, but acknowledge that, given the prognostic implications of this diagnosis, many clinicians will offer surveillance with an imaging study and carbohydrate antigen 19-9 (CA 19-9) at yearly intervals (8,9). An increased risk of CCA among patients with chronic IBD and proctocolectomy has been suggested, but this risk remains ill-defined (10,11). Identifying robust risk factors associated with CCA among patients with PSC is important to allow clinicians to risk stratify patients and guide surveillance strategies. Therefore, we sought to characterize the association between CCA, IBD disease duration, and colectomy in a large cohort of patients with PSC-IBD.
METHODS
Study design
Patients diagnosed with PSC-IBD and seen at the Mayo Clinic in Rochester, Minnesota, between January 2005 and May 2013 were identified for this retrospective study. Eligible subjects were identified through a search of the electronic medical record using the terms “primary sclerosing cholangitis” or “PSC.” The diagnosis of PSC was based on accepted criteria, including biochemical and cholangiographic features (12), and IBD was diagnosed based on characteristic endoscopic and/or histopathologic findings. Patients met inclusion criteria for the study if they had a confirmed diagnosis of both PSC and IBD, were >18 years of age, and who underwent cross-sectional imaging of the abdomen and endoscopic retrograde cholangiopancreatography (ERCP).
Study variables
Medical records including clinical notes, laboratory tests, and radiographic, endoscopic, and pathologic studies were reviewed for confirmation of diagnosis and to characterize disease course. Demographic data including age and gender were recorded. Dates of PSC and IBD diagnosis and complications of each disease were documented. Need for colectomy as well as date, type, and indication for surgery were noted. Indication for colectomy was recorded as refractory disease, neoplasia, or unknown reasons. Neoplasia included low-grade dysplasia, high-grade dysplasia, or confirmed adenocarcinoma. Those patients who required a colectomy for both active disease and neoplasia were considered to have neoplasia as their primary surgical indication.
Complications of PSC including CCA, advanced liver disease (defined as presence of gastroesophageal varices, ascites, or encephalopathy), and need, date, and indication for liver transplantation (either end-stage liver disease or CCA) were also recorded. CCA was diagnosed based on definitive histopathology from resection, biopsy, or cytology specimens or based on characteristic cross-sectional imaging features (13). Smoking history and use of ursodeoxycholic acid as noted in the medical record were also documented. The study was approved by the Mayo Clinic Institutional Review Board.
Statistical analysis
Descriptive statistics were used to define the baseline characteristics of the cohort. Qualitative variables were summarized as proportions and continuous variables as means with s.d. The primary analysis assessed whether time to development of CCA differed between PSC-IBD patients who had or had not undergone colectomy. Risk factors for CCA were assessed using univariable and multivariable Cox proportional hazard models where colectomy, IBD disease duration, and development of advanced liver disease were treated as time-dependent covariates. Models were constructed using age as the timescale that adjusts for an age effect. All PSC-IBD patients with a clinic visit during the January 2005 to May 2013 observation period were initially included in the study. To minimize immortal time bias, risk assessment started in 2005 or following diagnosis of both PSC and IBD if after 2005; thus, only patients who were alive and without an outcome by this date were included in the analysis. The observation period for included subjects was censored at development of an end point (i.e., death, liver transplantation, or CCA) or at date of last clinical follow-up. IBD duration was defined as date of diagnosis to date of end point or last clinical follow-up and was calculated from date of diagnosis to the point of entry into the study with the analysis timescale and IBD duration measured in years and IBD duration considered as a linear term. A linear relationship between IBD duration and CCA risk was confirmed. IBD duration was not censored at colectomy. To further investigate the influence of colectomy on CCA risk, we considered a Cox model that included IBD duration, colectomy, and the interaction between the IBD duration and colectomy. This model allowed an estimate of whether the influence of IBD duration on time to development of CCA changed before and after colectomy. A significant interaction term would suggest that the influence of IBD duration on CCA was modified after colectomy. A sensitivity analysis for the primary end point was performed in a combined cohort including the PSC-IBD patients with ERCP and a random sample of two-thirds of all PSC-IBD patients without ERCP seen during the same study period. Case weights were used allowing the sampled patients without ERCP to represent all patients without ERCP seen during the study period. CCA rates were calculated by colectomy, age, and follow-up time-specific person-years. The relationship of CCA rate vs. age and time since diagnosis was modeled using a smoothing spline within a generalized additive model assuming a Poisson error distribution and displayed graphically using the predicted values.
RESULTS
Patient characteristics
During the study period, 407 patients with PSC-IBD were evaluated at the Mayo Clinic, Rochester, and were eligible for the study. After exclusion of patients with missing diagnosis dates or follow-up information and those who had reached an end point before 2005, 399 patients with PSC-IBD were included in the final analysis. Of this cohort, 67% (n =268) were male and 33% (n =131) were female. The mean follow-up was 9.4±7.9 years after PSC-IBD diagnosis. IBD was diagnosed a mean of 10.5±12.0 years before PSC. Most patients (86%, n =343) had ulcerative colitis as the phenotypic manifestation of IBD, 13% (n =50) had Crohn’s disease, and 2% (n =6) had indeterminate colitis. Of the 399 patients, 34% (n =137) had a colectomy, of whom a majority (70%, n =94) had an ileal pouch anal anastomosis. The indication for colectomy was colonic neoplasia in 45% (n =61), medically refractory disease in 37% (n =51), and not available in the medical record in 18% (n =25) of patients.
CCA occurred in 31% (n =123) of patients in the cohort. Diagnosis was based on definitive histology alone in 24% (n =29), consistent radiographic findings in 23% (n =28), and both histology and imaging in 51% (n =63). Advanced liver disease was present in 39% (n =156) of patients overall and 35% (n =43) of patients who developed CCA. Varices were the most common manifestation and were found in 86% (n =134). One-quarter of patients (n =99) underwent liver transplantation, with 54% (n =53) of them having a primary indication of CCA. Additional clinical characteristics of the cohort are summarized in Tables 1 and 2.
Table 1.
Clinical characteristics of 399 patients diagnosed with both PSC and IBD
| Characteristic | N | Included patients |
|---|---|---|
| Gender (males) | 399 | 268 (67%) |
| Age at PSC diagnosis | 391 | 40.2±14.4 |
| Age at IBD diagnosis | 357 | 29.2±14.7 |
| Age at CCA diagnosis | 123 | 49.7±12.4 |
| Duration of IBD at inclusion | 357 | 10.5±11.6 |
| IBD phenotype | 399 | |
| Ulcerative colitis | 343 (86%) | |
| Crohn’s disease | 50 (13%) | |
| Indeterminate colitis | 6 (2%) | |
| Colectomy before PSC diagnosis | 399 | 52 (13%) |
| UDCA use | 309 | 147 (48%) |
| Smoking | 309 | 26 (8%) |
| CCA | 399 | 123 (31%) |
| Liver transplantation | 399 | 99 (25%) |
| Liver transplant for CCA | 53 (54%) | |
| Time-dependent covariates | ||
| Colectomy (% ever) | 399 | 137 (34%) |
| Varices (% ever) | 398 | 134 (34%) |
| Ascites (% ever) | 399 | 104 (26%) |
| Encephalopathy (% ever) | 398 | 43 (11%) |
| Any advanced liver disease (% ever) | 398 | 156 (39%) |
CCA, cholangiocarcinoma; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid.
Table 2.
Characteristics of the 137 patients who had a colectomy
| Characteristic | N | Included patients |
|---|---|---|
| Time from IBD onset to colectomy (years) | 95 | 12.5±9.9 |
| Age at colectomy | 137 | 37.4±13.6 |
| Reason for colectomy | 137 | |
| Refractory disease | 51 (37%) | |
| Neoplasia | 61 (45%) | |
| Dysplasia (LGD/HGD) | 45 (33%) | |
| Adenocarcinoma | 16 (12%) | |
| Unknown indication | 25 (18%) | |
| Type of colectomy | 134 | |
| IPAA | 94 (70%) | |
| Total proctocolectomy/end ileostomy | 20 (15%) | |
| Subtotal colectomy | 9 (7%) | |
| Ileorectal anastomosis | 8 (6%) | |
| Other | 3 (2%) |
HGD, high-grade dysplasia; IBD, inflammatory bowel disease; IPAA, ileal pouch anal anastomosis; LGD, low-grade dysplasia.
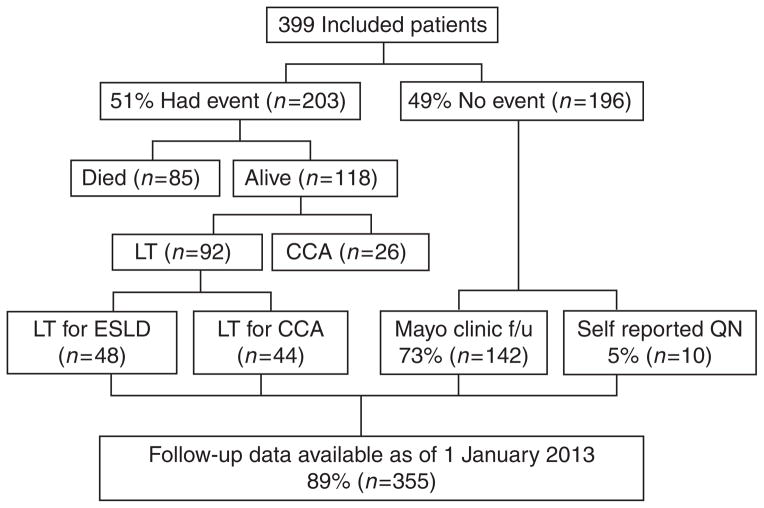
Of the 399 included patients, 51% (n =203) had an event (i.e., CCA, liver transplantation, and/or death) and 49% (n =196) did not. We were able to ascertain clinical status on or after 1 January 2013 in 89% (n =355) of the entire cohort and 78% (n =152) of those who were free of an event (Figure 1).
Figure 1.
Flow diagram demonstrating outcomes and follow-up for patients included in the cohort. CCA, cholangiocarcinoma; ESLD, end-stage liver disease; f/u, follow-up; LT, liver transplant; QN, questionnaire.
Overall rate of CCA diagnosis
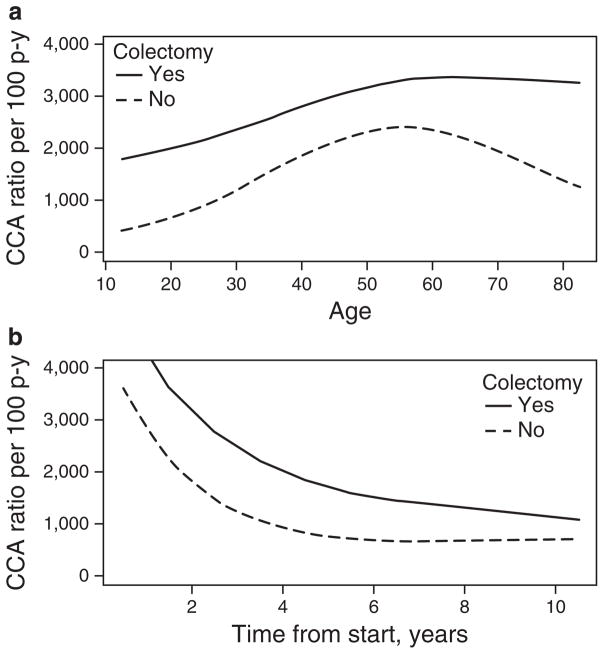
The absolute risk of CCA increased with age and was higher in those patients who had a colectomy compared with those who did not (Figure 2a). In patients 0–29 years, 30–39 years, 40–49 years, 50–64 years, and 65+ years of age who did not have a colectomy, the rate of CCA was 9.9, 48.9, 64.2, 67.6, and 37.3 per 1,000 person- years, respectively, compared with 61.5, 67.4, 84.0, 89.3, and 95.8 per 1,000 person-years, respectively, among those who did have a colectomy. The rate of CCA from time of PSC-IBD diagnosis was also higher in those who had a colectomy compared with those who did not (Figure 2b) and rates were highest in the first 5 years after diagnosis of PSC-IBD.
Figure 2.
Rates of cholangiocarcinoma (CCA) stratified by time of follow-up with and without colectomy among 399 patients diagnosed with both primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD). The rates of CCA by (a) age and (b) time of observation are shown.
Impact of colectomy on CCA risk
CCA was diagnosed in 39% (n =53) of patients who had a colectomy compared with 27% (n =70) of patients who did not have a colectomy. Considering the entire cohort of 399 patients, univariate age-adjusted Cox proportional hazard models revealed that colectomy (P =0.02) and duration of IBD (P <0.01) were significantly associated with an increased risk of CCA. In addition, the presence of colonic dysplasia or neoplasia (P =0.06) and colectomy performed for an indication of colonic neoplasia (P =0.05) were suggestive of increased CCA risk (Table 3). After adjusting for IBD duration, none of the other risk factors remained statistically associated with CCA (Supplementary Table S1 online). Moreover, on multivariable analysis, duration of IBD remained significantly associated with an increased risk of CCA with a hazard ratio (HR) of 1.33 (95% confidence interval (CI) 1.11–1.60, P <0.01). Not surprisingly, IBD duration was also associated with an increased risk of colectomy (HR 1.41, 95% CI 1.10–1.82, P <0.01) in the cohort. A random sample of an additional 186 PSC-IBD patients without ERCP who were evaluated during the study period were also assessed, and after exclusion of patients with missing data a sensitivity analysis including 157 patients supported the association between duration of IBD and increased risk of CCA (HR 1.53, 95% CI 1.30–1.80, P <0.001). Analysis of the interaction between IBD duration and colectomy on risk of CCA demonstrated that there was no evidence to suggest that the influence of IBD duration on CCA changed after colectomy (P =0.69), suggesting that the increased risk persists despite surgical “cure” of colonic disease.
Table 3.
Age-adjusted predictors of CCA among 399 patients diagnosed with PSC-IBD
| Variable | Age-adjusted HR (95% CI) | P value |
|---|---|---|
| Gender (male) | 1.31 (0.88–1.96) | 0.18 |
| Duration of IBD (per 10 years) | 1.37 (1.15–1.63) | <0.001 |
| IBD phenotype | ||
| UC | 1 | |
| Crohn’s disease | 0.72 (0.37–1.39) | 0.33 |
| Indeterminate colitis | 0.59 (0.08–4.30) | 0.60 |
| UDCA use | 1.19 (0.67–2.12) | 0.55 |
| Smoking | 1.42 (0.53–3.85) | 0.49 |
| Colectomy | 1.53 (1.05–2.22) | 0.02 |
| Varices | 0.92 (0.55–1.52) | 0.74 |
| Ascites | 1.26 (0.68–2.30) | 0.46 |
| Encephalopathy | 0.26 (0.04–1.90) | 0.19 |
| Any advanced liver disease | 0.87 (0.53–1.43) | 0.59 |
| Dysplasia/adenocarcinoma | 1.52 (0.97–2.37) | 0.06 |
| Adenocarcinoma | 1.21 (0.55–2.66) | 0.63 |
| Reason for colectomy | ||
| Colectomy for refractory disease | 0.67 (0.32–1.41) | 0.29 |
| Colectomy for neoplasia | 1.62 (1.01–2.61) | 0.05 |
| Unknown reason | 3.84 (2.15–6.87) | <0.01 |
CCA, cholangiocarcinoma; CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; UC, ulcerative colitis; UDCA, ursodeoxycholic acid.
Impact of indication for colectomy
The risk of CCA differed between those who had a colectomy for neoplasia compared with those whose primary indication was medically refractory disease. Compared with those without colectomy, the HR for CCA in patients with colectomy for colonic neoplasia was 1.62 (95% CI 1.01–2.61, P =0.05). In contrast, the risk of CCA was not significantly increased among patients who had colectomy for medically refractory disease compared with those with an intact colon (HR 0.67, 95% CI 0.32–1.41, P =0.29). When the age-adjusted analysis was limited to just those patients with colectomy, a significantly increased risk of CCA was seen among those with a colectomy for colonic neoplasia compared with those with colectomy for medically refractory disease (HR 2.91, 95% CI 1.24–6.84, P =0.01). Repeating the analysis starting at 1 year after colectomy to exclude patients with potentially prevalent CCA (i.e., subclinical CCA already existing at the time of colectomy), a significantly increased risk of CCA remained among patients with colectomy for neoplasia (HR 2.94, 95% CI 1.25–6.94, P =0.01). After adjusting for IBD duration in the subset of patients who had surgery, colectomy for colonic neoplasia approached significance (HR 2.68, 95% CI 1.01–7.07, P =0.05). The type of colectomy did not seem to influence the risk of CCA. Patients with ileal pouch anal anastomosis did not have a significantly different risk of CCA compared with patients with any other surgical procedure (HR 0.68, 95% CI 0.34–1.35, P =0.27).
DISCUSSION
To our knowledge, this is the largest study to date examining risk factors for CCA in patients with PSC and the only study to specifically address this risk in patients with PSC-IBD. Our results demonstrate that prolonged duration of IBD is associated with an increased risk of CCA in patients with PSC-IBD. We also demonstrate that colectomy, and in particular colectomy performed for colonic neoplasia or dysplasia rather than medically refractory disease, is associated with an increased risk of CCA, in keeping with the known association between IBD duration, colectomy, and colonic neoplasia. An additional novel observation was that the risk of CCA was not modified after colectomy, suggesting that colonic resection itself does not ameliorate the risk of CCA associated with prolonged IBD disease duration.
The findings of this study are consistent with previous reports from smaller cohorts. For example, in their study of 161 patients with PSC, Burak et al. (10) found an increased risk of CCA in patients undergoing colectomy on univariate but not multivariate analysis. Furthermore, in their cohort of 58 patients, Broome et al. (14) identified an increased risk of CCA among patients with PSC and associated colonic dysplasia or carcinoma. In contrast, in a larger Scandinavian cohort, Boberg et al. (11) did not find an increased risk of CCA among patients with colectomy (P =0.48) or colon cancer (P =0.25), although prolonged IBD duration was indeed found to be associated with increased CCA risk. These differences may be explained by the relatively small number of patients with colectomy (n =83) and CCA (n =48) in the said study as compared with our cohort.
The clinical implications of these findings are significant given the age of onset of IBD, the lifetime risk of colectomy, the poor prognosis associated with CCA, and the paucity of reliable predictors available to identify patients at increased risk of this complication. Despite medical advances, ~30–40% of patients with ulcerative colitis ultimately require surgery at some time during their disease course, and ~10% of patients with Crohn’s colitis eventually require large bowel resection (15). Indications for surgery in both ulcerative colitis and Crohn’s disease include severe acute disease, chronic disease resistant to medical therapy, or complications such as dysplasia or cancer. Particularly in the setting of PSC, where a prolonged subclinical phase of IBD may go unrecognized (16), colectomy may reflect a chronically diseased colon that places patients at increased risk of CCA.
The pathobiological mechanisms underlying our findings remain unclear but may be related to the altered bile acid milieu or altered microbiome (17,18) and metabolome (19–21) that exist in a chronically inflamed colon and can persist after colectomy. Altered bile acid profiles in the setting of chronic IBD and after colectomy have been reported (22–24), and whether these changes could injure the biliary epithelia via the enterohepatic circulation or modify the biliary microenvironment to promote malignant transformation remains plausible, although unconfirmed. Changes to the gut microbiome in the setting of colonic inflammation (25,26) and with colectomy (18) have also been reported; but whether these changes could result in downstream effects on biliary epithelia leading to carcinogenesis remains to be determined. Recently, Duboc et al. (27) observed altered fecal bile acid profiles and dysbiosis of the microbiome in patients with active IBD compared with those with quiescent disease and healthy controls. How these changes could affect PSC and its complications remain a ripe area for future study. Finally, given the association between colonic neoplasia and CCA in our study, it is important to consider the possibility of a shared genetic predisposition toward developing cancer in these patients who seem to be particularly susceptible to developing malignant disease. Future efforts should investigate whether genes regulating tumor development and growth are somehow defective at the genomic level or altered by other nongenomic mechanisms in these patients.
At our center, we do not have a standardized approach for CCA surveillance specific to patients with PSC-IBD; although, in general, all patients with PSC with and without IBD undergo surveillance for hepatobiliary neoplasia with yearly cross-sectional imaging (usually magnetic resonance cholangiopancreatography) and CA 19-9. Although this approach is mentioned by international societies as being employed by many clinicians, it is not formally recommended because of the lack of a robust evidence base. It is unlikely that that this monitoring strategy alone influenced our results to a significant degree as this surveillance approach is not specific to the subset of PSC-IBD patients, nor specific to our center. Rather, the large number of events in our cohort likely reflects the nature of our referral practice; though consequently, it also allowed for adequate statistical power to identify this subset of PSC patients who are at a particularly high risk of CCA. In our multivariable model, duration of IBD was the only independent predictor of increased risk of CCA with a 33% increased risk per 10 years of IBD. Furthermore, in the subset of PSC-IBD patients with colectomy, those having surgery with an indication of colonic neoplasia were at a significantly higher risk of CCA relative to those who had colectomy for refractory disease (HR 2.68, 95% CI 1.01–7.07), even after adjustment for IBD disease duration. Thus, targeted structured surveillance strategies in these high-risk subgroups (namely, those with prolonged IBD and those with colectomy done for colonic neoplasia) may be an appropriate strategy.
The strength of our study includes the large well-defined patient cohort with clearly defined outcomes. Our findings are consistent with previous reports that have suggested an association between chronic IBD, colectomy, and CCA and refine the existing body of literature by focusing on patients with PSC and coexisting IBD.
We expand on current knowledge by demonstrating that neoplasia as an indication for colectomy confers a particularly increased risk of CCA; and importantly, colectomy itself does not appear to modify the increased risk of CCA associated with prolonged IBD disease duration.
The limitations of the study include the possibility of referral bias given the increased likelihood of patients with severe and complicated disease being seen in specialized centers; our center in particular manages a high volume of patients with CCA. In addition, this cohort included PSC-IBD patients who underwent ERCP, thus representing a selected population of PSC patients. The ERCP criterion was originally included as a means of identifying all histologically confirmed events. ERCP is generally reserved for PSC patients with symptoms, rising levels of CA-19-9, and/or imaging evidence of dominant strictures (10). At our center, ~60% of patients fulfill these criteria, thus still representing a very significant subset of PSC patients as a whole. This is consistent with the literature that suggests that 36–57% of patients with PSC develop a dominant stricture during their disease course (1). To address this, we performed a sensitivity analysis that also included PSC patients without ERCP, and this also demonstrated a statistically higher risk of CCA with increasing duration of IBD, supporting the conclusion that IBD duration is an independent predictor of CCA risk in the full spectrum of patients with PSCIBD. Despite the high prevalence of CCA in our cohort, we are reassured by other studies that have previously suggested similar associations. By analyzing all variables together in one large cohort, our study clarifies that duration of IBD is a risk factor that independently increases CCA risk and this variable may influence the previously identified associations between CCA, colectomy, and colonic neoplasia. Finally, data were also limited to what was available in the medical record, and this could affect results through inadequately reported variables and confounders. For example, the indication for colectomy was unavailable in 18% (n =25) of patients, and whether knowledge of the reason for surgery in these patients would affect the strength of identified associations is unknown. These patients may reflect the referral bias of the cohort as 88% (n =22) had colectomies performed at other centers and were referred to Mayo Clinic with either concern for CCA or complications of PSC such as biliary obstruction with challenging ERCP. Furthermore, of these patients, 86% had their colonic surgery before 1998 (range 1957–1998); thus suggesting prolonged IBD disease duration. In fact, the median duration of IBD was significantly longer in patients with an unknown indication for colectomy compared with those with colonic neoplasia and refractory disease (23.0 years vs. 12.9 years vs. 3.5 years, respectively, P <0.01). These factors together likely contribute to the increased risk of CCA in patients with unknown indications for colectomy in our unadjusted and adjusted models (Supplementary Table S1).
Based on our findings, prolonged IBD disease duration is associated with an increased risk of CCA in patients with PSC-IBD and this risk persists after colectomy. This defines a patient population who may benefit from close interval surveillance for hepatobiliary neoplasia with cross-sectional imaging and serologic testing (e.g., CA 19-9). Patients who have colectomy, particularly with the indication of colorectal neoplasia or dysplasia, are also at an increased risk and warrant close surveillance. Identifying patients with early CCA has significant prognostic implications as curative treatment options such as liver transplantation are only available for early-stage disease. Future studies identifying the mechanisms underlying the association between IBD duration, colectomy, colonic dysplasia, and CCA will be valuable in further understanding the pathobiology of this devastating complication.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
The lifetime incidence of cholangiocarcinoma (CCA) in patients with primary sclerosing cholangitis (PSC) is 5–10%.
CCA often presents in an advanced stage and is associated with high morbidity and mortality.
Curative options for management of CCA are only available for early-stage and localized disease.
WHAT IS NEW HERE
Prolonged inflammatory bowel disease (IBD) duration is an independent predictor of increased risk of CCA in patients with PSC-IBD and this risk persists after colectomy.
Colectomy performed for colonic dysplasia or neoplasia but not for medically refractory disease is associated with an increased risk of CCA.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: Konstantinos N. Lazaridis, MD.
Specific author contributions: Aliya F. Gulamhusein was involved in planning and conducting the study, collecting and interpreting data, and drafting and revising the manuscript. John E. Eaton was involved in planning and conducting the study, collecting and interpreting data, and drafting the manuscript. James H. Tabibian was involved in planning the study, interpreting data, and drafting the manuscript. Elizabeth J. Atkinson was involved in conducting the study, interpreting data, and drafting the manuscript. Brian D. Juran was involved in planning and conducting the study, interpreting data, and drafting and revising the manuscript. Konstantinos N. Lazaridis was involved in planning and conducting the study, collecting and interpreting data, and drafting and revising the manuscript. All authors have approved the final draft submitted.
Financial support: This work was supported by a grant to Konstantinos N. Lazaridis from the National Institutes of Health R01 DK84960, the A.J. and Sigismunda Palumbo Charitable Trust, the Chris M. and Nicole Jockisch Carlos, and the Thalia N. and Chris M. Foundation.
Potential competing interests: None.
References
- 1.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet. 2013;382:1587–99. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra K, van Erpecum KJ, van Nieuwkerk KM, et al. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270–6. doi: 10.1002/ibd.22938. [DOI] [PubMed] [Google Scholar]
- 3.Torres J, Pineton de Chambrun G, Itzkowitz S, et al. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497–508. doi: 10.1111/j.1365-2036.2011.04753.x. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen KK, Lindstrom L, Cvancarova M, et al. Colorectal neoplasia in patients with primary sclerosing cholangitis undergoing liver transplantation: a Nordic multicenter study. Scand J Gastroenterol. 2012;47:1021–9. doi: 10.3109/00365521.2012.685754. [DOI] [PubMed] [Google Scholar]
- 5.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabibian JH, Enders F, Imam MH, et al. Association between serum IgE level and adverse clinical endpoints in primary sclerosing cholangitis. Ann Hepatol. 2014;13:384–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 9.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 11.Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–11. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 12.MacCarty RL, LaRusso NF, Wiesner RH, et al. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. doi: 10.1148/radiology.149.1.6412283. [DOI] [PubMed] [Google Scholar]
- 13.Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 1. Peripheral cholangiocarcinoma. AJR Am J Roentgenol. 1995;165:1427–31. doi: 10.2214/ajr.165.6.7484579. [DOI] [PubMed] [Google Scholar]
- 14.Broome U, Lofberg R, Veress B, et al. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–8. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 15.Hancock L, Mortensen NJ. How often do IBD patients require resection of their intestine? Inflamm Bowel Dis. 2008;14(Suppl 2):S68–S69. doi: 10.1002/ibd.20600. [DOI] [PubMed] [Google Scholar]
- 16.Broome U, Lofberg R, Lundqvist K, et al. Subclinical time span of inflammatory bowel disease in patients with primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:1301–5. doi: 10.1007/BF02049156. [DOI] [PubMed] [Google Scholar]
- 17.Martinez C, Antolin M, Santos J, et al. Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol. 2008;103:643–8. doi: 10.1111/j.1572-0241.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohyama A, Ogawa H, Funayama Y, et al. Bacterial population moves toward a colon-like community in the pouch after total proctocolectomy. Surgery. 2009;145:435–47. doi: 10.1016/j.surg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Bjerrum JT, Wang Y, Hao F, et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics. 2015;11:122–33. doi: 10.1007/s11306-014-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansson J, Willing B, Lucio M, et al. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS One. 2009;4:e6386. doi: 10.1371/journal.pone.0006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramanian K, Kumar S, Singh RR, et al. Metabolism of the colonic mucosa in patients with inflammatory bowel diseases: an in vitro proton magnetic resonance spectroscopy study. Magn Reson Imaging. 2009;27:79–86. doi: 10.1016/j.mri.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Jahnel J, Fickert P, Hauer AC, et al. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42:1423–31. doi: 10.1124/dmd.114.058065. [DOI] [PubMed] [Google Scholar]
- 23.Natori H, Utsunomiya J, Yamamura T, et al. Fecal and stomal bile acid composition after ileostomy or ileoanal anastomosis in patients with chronic ulcerative colitis and adenomatosis coli. Gastroenterology. 1992;102:1278–88. [PubMed] [Google Scholar]
- 24.Hylander E, Ladefoged K, Nielsen ML, et al. Excretion, deconjugation, and absorption of bile acids after colectomy for ulcerative colitis. Comparative studies in patients with conventional ileostomy and patients with Kock’s reservoir. Scand J Gastroenterol. 1986;21:1137–43. doi: 10.3109/00365528608996434. [DOI] [PubMed] [Google Scholar]
- 25.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–9. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 27.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–9. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.