Abstract
Even under the most expert care, a properly constructed intestinal anastomosis can fail to heal resulting in leakage of its contents, peritonitis and sepsis. The cause of anastomotic leak remains unknown and its incidence has not changed in decades. Here, we demonstrate that the commensal bacterium Enterococcus faecalis contributes to the pathogenesis of anastomotic leak through its capacity to degrade collagen and to activate tissue matrix metalloprotease-9 (MMP9) in host intestinal tissues. We demonstrate in rats that leaking anastomotic tissues were colonized by E. faecalis strains that showed an increased collagen-degrading activity and also an increased ability to activate host MMP9, both of which contributed to anastomotic leakage. We demonstrate that the E. faecalis genes gelE and sprE were required for E. faecalis-mediated MMP9 activation. Either elimination of E. faecalis strains through direct topical antibiotics applied to rat intestinal tissues or pharmacological suppression of intestinal MMP9 activation prevented anastomotic leak in rats. In contrast, the standard recommended intravenous antibiotics used in patients undergoing colorectal surgery did not eliminate E. faecalis at anastomotic tissues nor did they prevent leak in our rat model. Finally, we show in humans undergoing colon surgery and treated with the standard recommended intravenous antibiotics, that their anastomotic tissues still contained E. faecalis and other bacterial strains with collagen-degrading/MMP9 activity. We suggest that intestinal microbes with the capacity to produce collagenases and to activate host metalloproteinase MMP9 may break down collagen in the gut tissue contributing to anastomotic leak.
INTRODUCTION
The most devastating complication following removal of an intestinal segment (resection) and its reconnection (anastomosis) is an anastomotic leak. The clinical manifestations of an anastomotic leak range from abdominal pain with fever to septic shock. In its extreme form, anastomotic leak can cause peritonitis, sepsis, and even death. Leaks are particularly prevalent in patients undergoing surgery in high risk regions of the intestine such as the rectum and esophagus (1). In the distal colon and rectal area, the anastomotic leak rate can be excessive (30–40%) forcing surgeons to routinely perform a protective diverting stoma (ileostomy, colostomy) to lessen the clinical effects of intestinal content spillage (1). This practice requires a second operation to close the diverting stoma, which itself carries significant morbidity and includes the risk of an anastomotic leak. Consequently, many patients and surgeons elect to leave the stoma as a permanent solution to avoid a second high risk surgery. Given this, there is little motivation among surgeons to eliminate the routine use of a diverting stoma in lower colorectal surgery as they have accepted that the actual causes of anastomotic leaks remain unknown and hence they are not preventable.
That intestinal microbes play a key causative role in the pathogenesis of anastomotic leak has been suggested for over 60 years. The most direct evidence was first reported in 1955 by Cohn et al. who demonstrated that repeated direct topical application of antibiotics onto anastomotic tissues accelerated healing and prevented leak in dogs undergoing colon resection and anastomosis when the supplying blood vessels were divided in a manner that resulted in gross ischemia (2). Remarkably, despite the grossly visible presence of ischemia, direct topical application of antibiotics not only prevented anastomotic leak, but it also completely reversed the ischemia. Although from 1955 to 1984 oral antibiotics were introduced as a routine part of the preparation of gastrointestinal surgery, they were soon replaced by intravenous antibiotics owing primarily to the convenience of administration and the perception that they were equally efficacious in decontaminating anastomotic tissues of potentially offending pathogens (3, 4). Despite numerous studies demonstrating the benefit of adding oral antibiotics prior to gastrointestinal surgery to prevent infection and anastomotic leak, most surgeons do not routinely administer oral antibiotics in preparation for gastrointestinal surgery (3, 4). We have recently published work that readdresses the role of bacteria in anastomotic leak in a more molecular context (5). We report that exposure of anastomotic tissues to pathogenic bacteria such as Pseudomonas aeruginosa resulted in selection of a more virulent phenotype characterized by high collagen degrading activity, which was associated with anastomotic leak (5). We hypothesized that the capacity of intestinal bacteria to degrade collagen may be an important mechanism underlying anastomotic leak. To identify additional and perhaps more common bacteria with collagen-degrading activity that might colonize anastomotic tissues following surgery, we next examined the microflora associated with anastomotic tissues using 16S rRNA and PiCRUST analyses in rats following anastomotic surgery (6). Results demonstrated a 500-fold increase in the relative abundance of the genus Enterococcus at the anastomotic site. The PiCRUST functional analysis (7) predicted the predominance of several bacterial virulence factors, one of which, coccolysin (GelE), is responsible for collagen/gelatin degradation (8, 9). These findings, coupled with the observations that bacterial-derived collagenases are known to play an important role in a variety of intestinal disorders such as those involving inflammation and necrosis, led us to explore the role of Enterococcus in anastomotic leak (10–15).
Here, we demonstrate that among commensal microbiota, Enterococcus faecalis strains with enhanced collagen-degrading activity and the capacity to activate intestinal tissue matrix metalloprotease 9 (MMP9) contribute to the pathogenesis of anastomotic leak.
RESULTS
Clinical outcome of rats following surgery
We created an anastomotic leak model in rats by performing a 1 cm colon resection (at the peritoneal reflection) and primary anastomosis, followed by devascularization of a 2 cm segment of blood supply adjacent to the anastomosis (Fig.1A). All rats that survived did well after surgery and were healthy appearing at the time of sacrifice (postoperative day 6, POD6) as judged by their feeding pattern, movement in the cage, and stool passage. There were occasional anesthesia- related deaths (<5) within 24 hours. In these cases additional rats were operated on and added to the groups to achieve equal numbers in each group. All rats were sacrificed on postoperative day 6 and underwent laparotomy for gross inspection of the anastomosis and detection of leakage. No leaks were observed in rats subjected to anastomosis alone (Anast). In contrast, 50% of rats subjected to anastomosis with devascularization (Anast + Dvasc) developed an anastomotic leak (Fig.1B). The devascularization procedure did not cause grossly visible ischemia (Fig.S1A). To confirm this, we performed endoscopy above and below the anastomotic suture line in selected rats that did not demonstrate any gross areas of ischemia (Fig. S1B). Finally, in a separate group of rats, we used confocal laser endomicroscopy following fluorophore injection to examine the microscopic blood supply of the colon just below the anastomosis and demonstrated that the segmental devascularization did not grossly interrupt the blood supply (Fig.S1C). Confocal images demonstrated normal filling of the blood vessels with the fluorophore (shown by arrows on Fig.S1C) in colon segments with or without devascularization.
Fig. 1. E. faecalis with high collagen-degrading activity is associated with anastomotic leak.
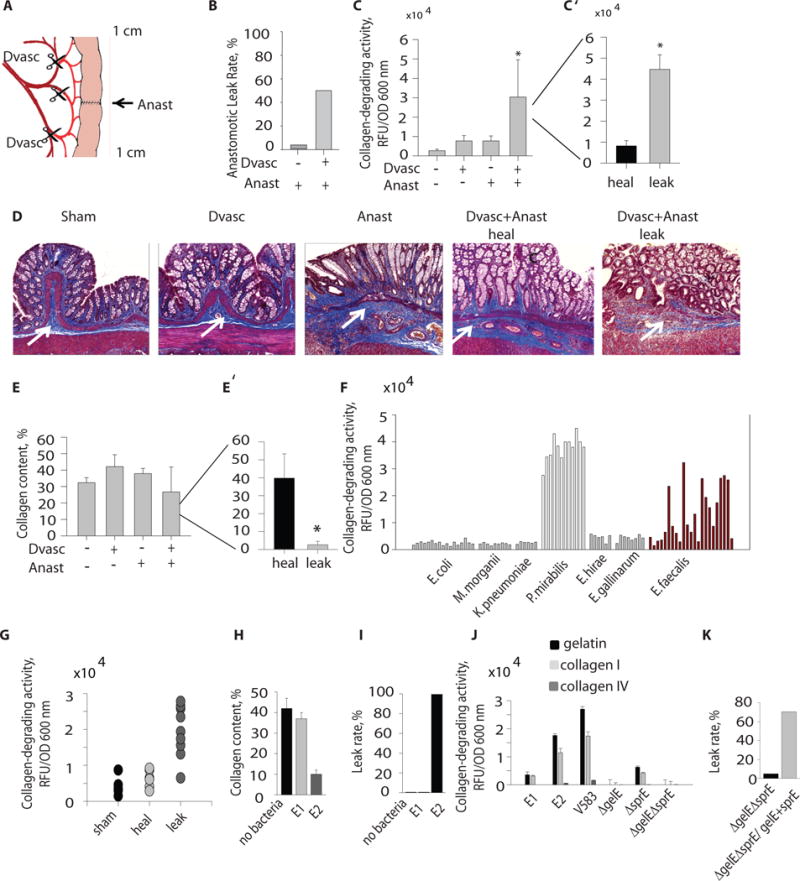
A. Rat model of anastomotic leak. B. Incidence of leak between devascularized (Dvasc) versus non- devascularized (Anast) intestinal segments of the rat model (n=15, *p<0.01). C. Collagen-degrading activity of whole microbial communities between various groups of rats showing that microbial collagen-degrading activity discriminated between leaking versus non-leaking groups (n=10/group *p<0.01, Student t-test). D. Trichrome staining of representative rat intestinal segments demonstrates collagen depletion and leakage. Collagen shown by white arrow. E. Trichrome stain density between treatment groups discriminated between rats with and without anastomotic leak (*p<0.05, Student t-test). F. Collagen-degrading activity among various cultured microorganisms shows non-uniform activity across E. faecalis strains. G. Collagen-degrading activity among various isolates of E. faecalis from sham-operated versus devascularized anastomotic tissues that either healed (heal) or leaked (leak). H. Collagen content of intestinal tissues of rats injected by E. faecalis E1 or E2 strains measured by Trichrome staining (3 slides/rat n=5/group, *p<0.01, Student t-test). I. Leak rates in rats with intestinal anastomoses that were transrectally inoculated with E1 and E2 strains of E. faecalis (n=5/group). J. Collagen-degrading capacity of E. faecalis demonstrating that the human strain V583 degraded collagen I in a GelE/SprE dependent manner (n=3/group). K. Leak rates in rats with intestinal anastomoses transrectally inoculated with the V583 derivative double mutant ΔgelEΔsprE versus the complemented double mutant ΔgelEΔsprE/gelE+sprE (n=20 in ΔgelEΔsprE group and n=17 in ΔgelEΔsprE/gelE+sprE). p<0.001, Student t-test.
Collagen-degrading activity of E. faecalis is associated with anastomotic leak
We measured collagen-degrading activity in whole bacterial communities recovered by swabbing anastomotic tissues at the time of sacrifice and performing assays for collagen degradation capabilities on the entire recovered microbiota. Results demonstrated that the collagen-degrading activity of microbes enabled discrimination between leaking versus non-leaking anastomotic sites; microbes with increased collagen-degrading activities predominated in leaking anastomotic tissues (Fig.1C′). Histologic examination demonstrated visible collagen depletion in rats subjected to anastomosis and devascularization that had anastomotic leakage compared to rats without anastomotic leakage (Fig.1D). Similarly, collagen content was significantly different between rats with leaking versus non-leaking anastomoses (Fig.1E′, p< 0.05, student t-test), whereas there were no overall differences in collagen content between non-devascularized and devascularized anastomoses (Fig.1E). There was a clear association between enhanced collagen-degrading activity (Fig.1C′) and attenuated collagen content within the anastomotic leak group (Anast+Dvasc) (Fig.1E′). In addition, measurements of collagen-degrading activity of whole tissue extracts demonstrated a significant difference between leaking vs healed anastomotic tissues within the group of rats undergoing anastomosis and devascularization (Fig.S2).
In order to identify the bacterial strains with high collagen-degrading activity, we cultured bacteria recovered from anastomotic tissues by swabbing. E. faecalis and Proteus mirabilis, members of the commensal microbiota, had the highest collagen-degrading activity among all isolates. P. mirabilis strains uniformly had high activity, whereas isolates of E. faecalis showed both high and low collagen-degrading activity (Fig.1F). Notably, the mean collagen-degrading activity of E. faecalis strains was significantly increased (p<0.01) in rats with anastomotic leakage compared to those without (Fig.1G). No such correlation was observed with P. mirabilis as all isolates were high collagenase producers. Therefore, we decided to focus on E. faecalis as its collagen-degrading activity might be a potential discriminator between anastomotic leakage and non-leakage. We called low collagenase producing E. faecalis strains E1 and high collagenase producers E2. Time-dependent measurements of collagen-degrading activity revealed that E2 strains started to degrade collagen significantly earlier (p<0.01) and at a higher level compared to E1 strains (Fig.S3). To determine other phenotypic differences between E1 and E2 strains, we screened these strains in Caenorhabditis elegans killing assays and observed that the E2 strain was more virulent than E1 (Fig.S4). This finding was in agreement with previous work showing that collagenase plays a key role in the virulence and lethality of E. faecalis (16–18). Next, to define the role of collagen-degrading activity among E. faecalis strains in the pathogenesis of anastomotic leak, we selected a pair of strains from our isolates from rat anastomoses that were low collagenase producers (E1) and high collagenase producers (E2). We introduced live cultures of E1 and E2 (~106 cfu in 0.1 ml solution) via enema into rats following colon resection and anastomosis without devascularization and monitored the development of anastomotic leak after sacrifice on POD6 as in previous experiments. Rats were administered systemic cefoxitin and mucolytics (systemic atropine, topical n-acetyl cysteine) to mimic the clinical preparation of the colon for surgery that involves elimination of the normal flora with antibiotics and depletion of mucosal mucus with a purgative bowel preparation (19). Results demonstrated that E2, but not E1, caused anastomotic leak associated with depletion of intestinal collagen (Fig. 1H, I). Further characterization of E1 and E2 demonstrated that E2 had a greater capacity to degrade gelatin and collagen I (but not collagen IV) (Fig.1J). We also observed this capacity to degrade collagen I but not collagen IV with the well-characterized human isolate E. faecalis V583 (Fig.1J). Purified GelE from E. faecalis, a zinc metalloproteinase, has been shown to cleave numerous substrates including collagen fragments (20). GelE encoded by the gelE gene is co-transcribed in a quorum sensing-dependent manner with sprE that encodes the serine protease SprE (21). We therefore used mutants derived from E. faecalis V583 including ΔgelE, ΔsprE, and ΔgelEΔsprE and observed that both GelE and SprE are involved in the degradation of collagen I (Fig.1J). Complementation of ΔgelE with gelE and ΔgelEΔsprE with gelE+sprE, led to higher collagenase activity compared to the wild-type strain. However complementation of ΔsprE with sprE did not lead to increased collagen-degrading activity as compared to the non-complemented mutant (Fig.S5). As others have suggested, it is possible that a critical balance between GelE and SprE is required for their proper function (22). The collagen-degrading activity of E2 and the E. faecalis V583 mutant was significantly suppressed by the zinc chelator 1,10-phenanthroline when tested using either gelatin or collagen I as a substrate (Fig.S6A) confirming the involvement of a zinc metalloproteinase. However, suppression of gelatin and collagen I degradation by 1,10-phenanthroline was not complete. We observed residual collagen-degrading activity in both strains (Fig.S6B), which suggests involvement of non-metalloprotease activity thereby supporting the role of SprE in collagen degradation.
In order to define the role of GelE and SprE in anastomotic leak in our rat model, we introduced the V583 derivative double mutant ΔgelEΔsprE or ΔgelEΔsprE complemented with gelE plus sprE via enema similar to experiments with E1 and E2. Rats were sacrificed on POD6 and anastomotic leak rates were determined by gross inspection. Results demonstrated a significant difference in leak rates between the two groups (p<0.0001) with ΔgelEΔsprE causing a low rate of leakage of 5% (1/20 rats) and ΔgelEΔsprE complemented with gelE+spreE causing a high rate of leakage of 70% (12/17 rats) (Fig.1K).
Activation and cleavage of intestinal MMP9 is associated with anastomotic leak and can be induced by E. faecalis in a GelE/SprE-dependent manner
In addition to bacterial collagen-degrading proteases, host extracellular matrix degrading enzymes can also contribute to anastomotic leakage. Among them, intestinal MMP9 has been shown to play an important role in the pathogenesis of anastomotic leak (23). Normally, as part of the response to tissue injury, zinc-dependent MMP9 degrades components of the extracellular matrix including collagen for remodeling and wound healing. Excessive activation of MMP9 could tip the balance during anastomotic healing such that collagen degradation results in leakage instead of healing. Other inflammatory signals could be also triggered in addition to, or as a result of, MMP9 activation (24) and could participate in excessive inflammation at the site of the anastomosis. Zymography analysis of tissue extracts demonstrated higher MMP9 activity in leaking vs healed anastomoses among groups of rats subjected to anastomosis plus devascularization (Fig.S7). Based on our observation that leaking anastomotic tissues were colonized by high collagenase producing E faecalis, we next hypothesized that E. faecalis may activate MMP9 in anastomotic tissues. It has been previously demonstrated that certain bacteria can activate tissue MMP9 (25, 26), however, this does not seem to have been demonstrated for E. faecalis to our knowledge. To test this, we incubated rat colon explants isolated from normal healthy non-treated rats with live bacterial cells and cell-free supernatants of E1 and E2 strains. Zymography, an electrophoretic method for measuring proteolytic activity, was performed on tissue extracts and demonstrated that the E. faecalis E2 strain induced cleavage of host proteases resulting in the appearance of an 86 kDa band (Fig. 2A). In order to confirm that this band corresponded to MMP9, we performed Western blot analysis using anti-MMP9 antibodies. Results confirmed that the band was MMP9 (Fig. 2B). To examine direct cleavage of MMP9 by the E. faecalis isolates, recombinant pro-MMP9 (r-MMP9) was used as a substrate. Results demonstrated that only the E2 strain was able to activate pro-MMP9 (Fig. 2C). We hypothesized that either GelE or SprE is involved in the proteolytic cleavage of pro-MMP9. To test this, we incubated r-MMP9 with the human E. faecalis isolate V583 and its derivative mutants ΔgelE and ΔsprE (8). Zymography demonstrated that the GelE-deficient mutant failed to cleave MMP9, whereas the SprE-deficient mutant cleaved MMP9 leading us to conclude that GelE was responsible for the activation of MMP9 (Fig. 2D). To verify the involvement of GelE in the activation of MMP9, we performed ex vivo experiments using the mouse peritoneal macrophage cell line J774. J774 macrophages were incubated with V583 and its derivative mutants ΔgelE, ΔsprE and ΔgelEΔsprE and MMP9 measured by both zymography (Fig. 2E) and Western blot (Fig. 2E′). Surprisingly, we observed the opposite effect with the mutants whereby SprE, not GelE, appeared to be required for MMP9 cleavage (Fig. 2E, 2E′). We speculated that the discrepancy between human recombinant MMP9 versus naturally occurring MMP9 from macrophages may be due to protein folding differences influencing cleavage activity. GelE is a metalloproteinase with broad substrate specificity and is known to cleave unfolded proteins (27), whereas SprE is a serine protease with narrow substrate specificity (22) and thus may be incapable of cleaving proteins that are not properly folded. To verify the exclusive role of SprE, we next examined the complemented mutants ΔgelE/gelE, ΔsprE/sprE, and ΔgelEΔsprE/gelE+sprE for their ability to activate macrophage MMP9. Results indicated that complementation of ΔsprE with sprE restored MMP9 activation and increased the formation of the truncated form of MMP9 (Fig.S8). Complementation of the double mutant ΔgelEΔsprE with gelE+sprE also restored MMP9 activation (Fig. S8). Surprisingly, complementation of ΔgelE with gelE enhanced MMP9 activation suggesting that GelE is important for E. faecalis activation of MMP9 (Fig. S8). However, we cannot invoke a specific and independent role for either GelE or SprE in MMP9 activation.
Fig. 2. E. faecalis strains with high collagen-degrading activity also activate host intestinal MMP9.
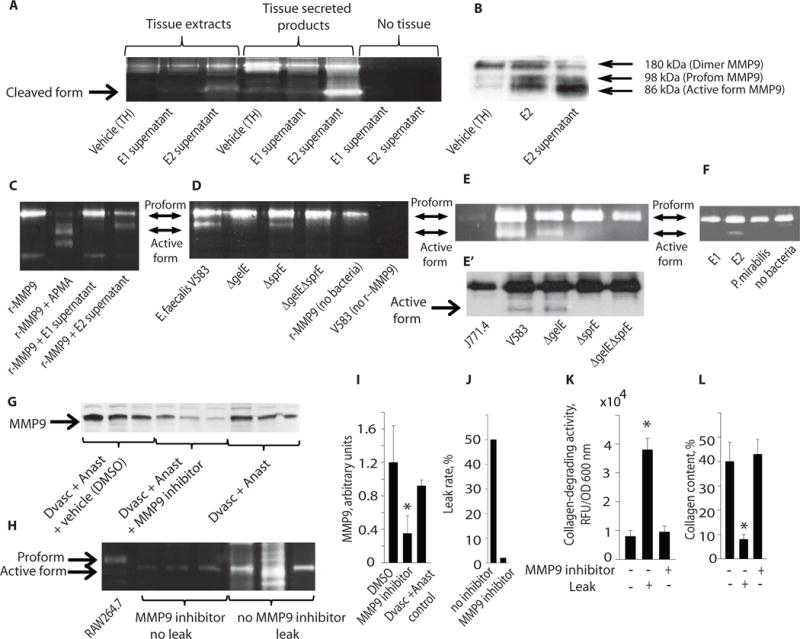
A. Zymography, a eletrophorectic technique to determine proteolytic activity that is indicated by Coomassie staining, of normal rat intestinal tissue exposed to supernatants obtained from high collagenase producing E2 strains of E. faecalis reveals host protease cleavage with the appearance of an 86 kDa band. The three bands on the left side (tissue extracts) represent proteins extracted from tissues; the three bands in the middle (tissue secreted products) represent proteins secreted by tissues; the two bands on the right (no tissue) show negative results for E. faecalis supernatants not exposed to tissues. Visualization of the proteolytic activity appears as clear bands over a deep blue background after Coomassie staining. Vehicle consisted of TH- (Todd-Hewitt broth) in which E. faecalis is usually grown. B. Western blots demonstrate that E2 supernatant cleaves MMP9 to its active form. C. Zymography demonstrates the ability of E2 to cleave human recombinant pro-MMP9 (r-MMP9). APMA, an agent known to induce MMP9 cleavage, was used as a positive control. D. The effect of the human laboratory strain of E. faecalis (V583) and its gelE and sprE mutants on human r-MMP9 cleavage is shown. E,E′. Zymography (E) and Western blot (E′) analyses of MMP9 activation in a murine macrophage cell line J774.1 incubated with E. faecalis V583 and its derivative mutants ΔGelE, ΔSprE, and ΔGelEΔSprE. F. Zymography analysis of MMP9 activation in the murine macrophage cell line J774.1 incubated with E. faecalis strains E1 and E2, and Proteus mirabilis is shown. G–L. Shown are effects of pharmacologic inhibition of MMP9 in rat tissues following colon resection, anastomosis, and devascularization: intestinal tissue MMP9 by Western blot (G), zymography (H), band densities of Western blot (I), anastomotic leak rate (J), collagen-degrading activity (K), and collagen content (L) (n=3, p<0.01, Student t-test).
Finally, given that P. mirabilis expressed high collagen-degrading activity, we measured its MMP9 cleavage activity using the macrophage MMP9 assay. As seen in Fig. 2F, P. mirabilis did not cleave MMP9 consistent with its lack of association with anastomotic leak. Thus, pathogens with the dual capacity to degrade collagen and cleave MMP9, such as E. faecalis, appear to be associated with anastomotic leak in this rat model.
To define the role of MMP9, we performed reiterative studies in separate groups of rats using a specific MMP9 inhibitor(MMP9 Inhibitor I, Calbiochem, Cat. # 444278-500UG). All rats underwent colon resection + anastomosis + devascularization with and without the MMP9 inhibitor. Results demonstrated that pharmacologic inhibition of MMP9 suppressed MMP9 production in anastomotic tissues (Fig. 2G–I) and prevented anastomotic leak (Fig. 2J) in association with suppression of the collagen-degrading activity of whole colonizing microbiota (Fig. 2K) and preservation of intestinal collagen content (Fig. 2L).
We next determined the influence of the intestinal microbiota on key inflammatory mediators present in intestinal tissues in rats 6 days following resection, anastomosis and segmental devascularization, as performed in previous experiments. These mediators included HIF1-α (hypoxia inducible factor), iNOS (inducible nitric oxide) and MPO (myeloperoxidase) in addition to MMP9 activation (Fig. 3). We directly applied a triple antibiotic solution (ciprofloxacin, metronidazole, and neomycin) to anastomotic tissues via enema both immediately after surgical anastomotic construction and again on postoperative day one. Rats were then treated with and without antibiotic enemas and assigned to the following groups: 1. sham operation, 2. devascularization alone (Dvasc) – i.e., no resection or anastomosis, 3. resection + anastomosis without devascularization (Anast), 4. resection + anastomosis + devascularization (Anast + Dvasc). All rats were sacrificed on POD6 and proteins were extracted from colon or anastomotic segments and analyzed by Western blot for MMP9, HIF1-α, iNOS, and MPO. Data demonstrated that the MMP9 expression in tissues exposed to both anastomosis and devascularization was attenuated when rats were exposed to antibiotics (Fig. 3A,B, Fig. S9). Zymography confirmed that the highest level of MMP9 activation was in the Anast+Dvasc group and that this increase was attenuated by topical antibiotics (Fig. 3C). HIF-1α expression was similarly induced by devascularization alone or anastomosis alone compared to the untreated tissues. A clear synergistic effect on inflammatory mediators was seen when anastomotic construction was combined with devascularization, an effect that could be markedly attenuated when anastomotic tissues were exposed to the triple topical antibiotics. This synergism was also observed for iNOS and MPO, where topical antibiotic treatment attenuated expression of both inflammatory markers. Taken together, these results indicate that the intestinal microbiota contributes to the amplification of MMP9, HIF1-α, and inflammation during anastomotic surgery.
Fig. 3. Antibiotic treatment of rats with anastomosis plus devascularization.
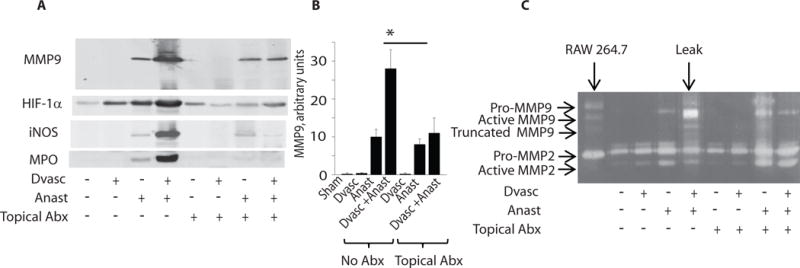
Antibiotic treatment (Abx) of rats with anastomosis plus devascularization (Anast + Dvasc) attenuated MMP9 activation, and expression of HIF1-α (hypoxia inducible factor), iNOS (inducible nitric oxide) and MPO (myeloperoxidase). (A) Western blot analyses of rat tissues subjected to devascularization (Dvasc), anastomosis (Anast) or Anast + Dvasc, in the presence or absence of direct topical application of ciprofloxacin, metronidazole, and neomycin (topical Abx) via enema. Experiments were performed on 3 rats per group; displayed immunoblot is representative of all results. (B) The evaluation of band intensities using Image J software demonstrated the abundance of the active form of MMP9 in the rat group Anast + Dvasc, and loss of active MMP9 after topical application of antibiotics. n=3, p<0.01, Student t-test. (C) Zymography analysis confirmed the abundance of the active form of MMP9 in the Anast + Dvasc rat group, and loss of the active form of MMP9 by topical application of antibiotics.
We next compared the topical antibiotic regimen with parenteral cefoxitin, the most common antibiotic used as a single agent for prophylaxis in colon surgery as recommended by the Center for Medical Services (CMS) Surgical Quality Improvement Project (SCIP) (28). It has been well documented that cefoxitin and related cephalosporins do not eliminate E. faecalis. In fact E. faecalis has been shown to “bloom” in the intestine following a single parenteral dose of cefoxitin (29). As might be predicted, only the topical antibiotic regimen prevented anastomotic leak (Fig. 4A) in association with suppression of bacterial collagenase (Fig. 4B), preservation of intestinal collagen (Fig. 4C), and lower expression of intestinal MMP9 (Fig. 4D,D’ and Fig. S10). High collagenase producing strains of E. faecalis were recovered from anastomotic tissues in rats treated with cefoxitin (Fig. S11). No E. faecalis strains were recovered from rats treated with the topical antibiotic regimen administered via enema (Fig. S11).
Fig. 4. Topical antibiotic treatment applied to rat anastomotic tissues prevents leak.
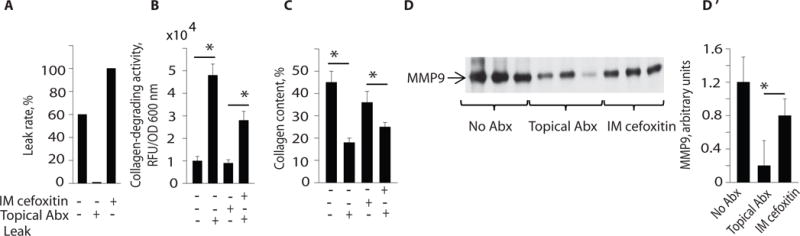
Effect of intramuscular (IM) cefoxitin (systemic antibiotic treatment) versus direct topical antibiotic treatment with ciprofloxacin, flagyl and neomycin via enema on post-operative day (POD) 0 and 6. (A–C) Shown are the effects of systemic and topical antibiotic treatment on leak rate, collagen-degrading activity of whole microbial communities, and intestinal collagen content in rats that underwent surgical resection, anastomosis, and devascularization (n=10, *P<0.01, Student t-test). (D, D′). Effect of IM cefoxitin versus topical antibiotic treatment on intestinal tissue MMP9 activity (n=3, *P<0.05, Student t-test).
Bacterial strains present on human colon tissues at the time of anastomotic construction are capable of degrading collagen and activating MMP9
We next determined whether human colon anastomotic segments harbored microbial organisms that expressed the “leak phenotype” defined by their ability to degrade collagen and activate MMP9. Eleven consecutive patients undergoing colon surgery were studied under IRB protocol approved by the University of Chicago. During surgery, once the colon segment was resected, the distal and proximal ends were immediately swabbed for microbial analysis. Swabs were used to isolate DNA for 16S rRNA analysis and in addition were cultured to test individual growing organisms for collagen-degrading activity and their ability to cleave human r-MMP9. All eleven patients received intravenous (IV) cefoxitin prophylaxis according to the SCIP recommendations and all recovered from surgery and were discharged home (28). One patient (patient #10) was readmitted due to complications. Of 64 cultured strains, only two species, P. aeruginosa and E. faecalis, showed significant collagenase activity (Fig. 5A) and cleaved MMP9 (Fig. 5B,C). 16S rRNA analysis demonstrated a significant disruption of the normal microbial community structure and membership distribution in anastomotic tissues among the 11 patients. This is not surprising given that patients received purgative intestinal cleansing solutions and antibiotics prior to surgery. Normally the dominant phyla in the colon are Bacteroidetes and Firmicutes which are known to protect the underlying intestinal epithelium from invasion by pathogenic bacteria and as well are known to induce a health promoting immune response. Under normal conditions, Proteobacteria represent less than 1% of the indigenous microbiota in the colon, however when they predominate they are often associated with intestinal pathology. Several of our patient displayed a reversal in the ratio of health-related Bacteroidetes to disease-related Proteobacteria (Fig. 6A,B)- opposite of what would normally be expected. Of particular note was the ratio of Proteobacteria to Bacteroidetes in the swab from the distal end of the colon of patient #10 that showed a ratio of Proteobacteria to Bacteroidetes of 3:1, indicative of a highly imbalanced microbiota (Fig. 6A,C). In this patient, the relative abundance of the phylum Proteobacteria (50.12%) which consists mainly of the class Gammaproteobacteria (44.47%) (Fig. 6B,D) and the family Enterobacteriaceae (43.1%), which encompasses the greatest number of human pathogens. Thus among all 11 patients, this patient had the most pronounced reversal of the ratio of Bacteroidetes to Proteobacteria (3:1) and was culture positive with a collagenolytic P. aeruginosa strain that cleaved MMP9. This patient was discharged after surgery and then readmitted for an intestinal obstruction and a suspected abdominal abscess. Subsequently the patient developed a clinical course characteristic of an anastomotic leak including small bowel obstruction up to the point of the ileocolic anastomosis, perianastomotic inflammation and free air, and a fluid collection. The patient was treated with percutaneous drainage, nasogastric tube decompression and broad spectrum antibiotics. An anastomotic leak, however, was not confirmed with a contrast enema as this was a right hemicolectomy and a full contrast enema or colonoscopy would have been risky and difficult. The patient’s course gradually resolved with conservative management alone. Finally, while one of the eleven patients harbored a collagenolytic E. faecalis strain, this patient did not develop a clinical leak perhaps owing to less disruption in this patient’s indigenous microbiota.
Fig. 5. Isolation of bacterial species from resected intestinal samples from surgical patients.
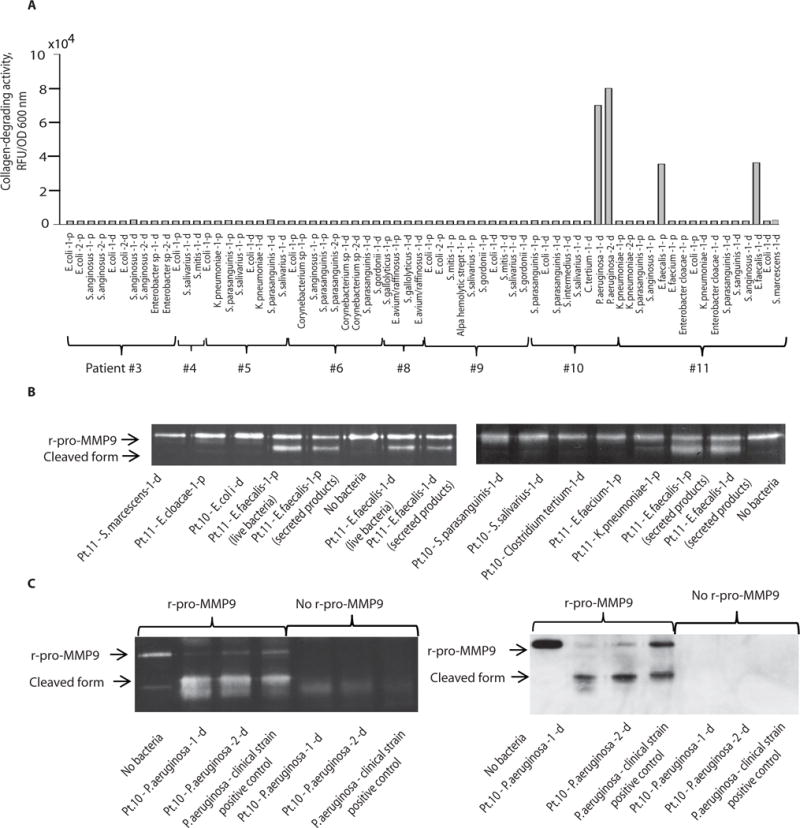
Shown are species identification, collagen-degrading activity, and MMP9 cleavage capacity among 68 bacterial isolates cultured from the proximal (p) and distal (d) ends of resected intestinal specimens from 11 patients undergoing colon surgery. A. Culture results of bacterial species and their collagen-degrading activity from patients #3 to #11; patients #1 and #2 were culture negative. B. Zymography demonstrating the ability of isolated strains of E. faecalis to activate cleavage of human recombinant pro-MMP9 (r-pro-MMP9) to its active form. C. Zymography (left panel) and Western blot (right panel) demonstrating the ability of P. aeruginosa human isolates to activate cleavage of human r-pro-MMP9 to its active form. P. aeruginosa positive control is a human stool isolate maintained in our laboratory that is known to have high collagen-degrading activity as well as the ability to cleave MMP9.
Fig. 6. Microbial composition of resected intestinal specimens from 11 patients undergoing elective colon surgery.
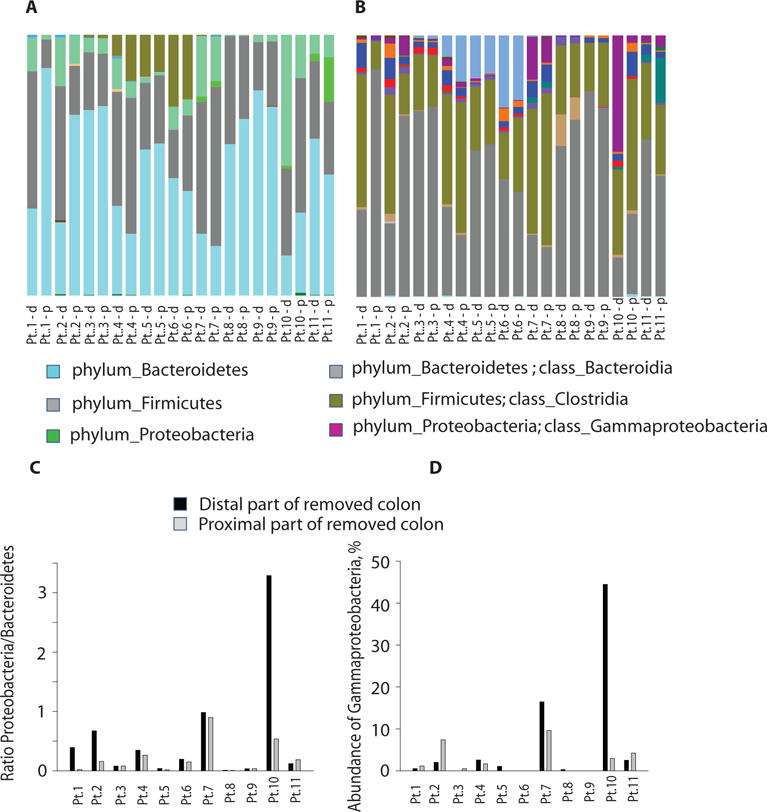
A, B. 16S rRNA analysis at the phylum (A) and class (B) levels of the microbial community composition of the proximal (p) and distal (d) ends of resected colon samples from 11 patients. C. Ratio of Proteobacteria (disease-related) to Bacteroidetes (health promoting) in the 11 patients (Pt) who underwent elective colon surgery. D. Gammaproteobacteria was the most abundant class in patient #10.
DISCUSSION
Data from the present study offer the possibility that members of intestinal commensal microflora, such as E. faecalis, may contribute to anastomotic leak. Here we show in rats, that the current method of antibiotic prophylaxis used in humans (i.e., systemic administration of cefoxitin) fails to eliminate E. faecalis in association with anastomotic leak. A recent study examining the fecal microbiota of patients undergoing colorectal surgery for cancer before and after surgery demonstrated that, despite the use of both intravenous (i.e., a second generation cephalosporin) and oral antibiotics (kanamycin, metronidazole), E. faecalis and P. aeruginosa were among the most dominant pathogens remaining in feces after surgery with a several log-fold increase in their concentration and prevalence over the course of recovery and hospitalization (30). Others have also demonstrated the high prevalence of Enterococcus following colon surgery despite adequate antibiotic prophylaxis (31). Here, we found that E. faecalis contributes to anastomotic leak through its collagenolytic and MMP9-activating functions. Our rat model suggests that there may exist a “leak phenotype” among intestinal microbes that colonize anastomotic tissues. This phenotype appears to be a function of a given bacterial strain’s collagen-degrading activity and its ability to cleave host intestinal MMP9 to its active, extracellular matrix-degrading form (32). MMPs, and in particular MMP9, are known to be associated with inflammation (33, 34) in general, and anastomotic leak (35–38) specifically. We show that E. faecalis can activate MMP9 in a manner that is dependent on GelE and SprE, both of which appear to play a key role in anastomotic leak. In these experiments we used V583, a vancomycin-resistant strain of E. faecalis (39). It should be noted that vancomycin-resistant E. faecalis strains represent only a minority of all vancomycin resistant Enterococci infections (40–46). However, risk factors for vancomycin-resistant E. faecalis acquisition include previous antibiotic exposure and invasive procedures (44, 45), factors that are present in patients undergoing colorectal surgery. Although the prevalence of vancomycin-resistant E. faecalis among patients undergoing colorectal surgery is unknown, perhaps it should be examined in light of our results.
Notably, E. faecalis degrades collagen I and activates MMP9, which in turn degrades collagen IV (47). Both collagen I and IV play an important role in maintenance and repair of the extracellular matrix (48, 49). The observation that E. faecalis degrades collagen I and activates MMP9 to its active form suggests a dual mechanism by which E. faecalis might complicate anastomotic healing. Some evidence for this was provided by the observation that either bacterial elimination with topical antibiotic treatment or MMP9 inhibition prevented anastomotic leak. It is important to recognize, however, that parenteral cefoxitin did not kill E. faecalis in our rat model nor did it prevent leakage. It is noteworthy that the most commonly used antibiotics in colon surgery (i.e., second and third generation cephalosporins) do not eliminate E. faecalis in the gut, but in fact allow it to proliferate and predominate (29, 50, 51). Although many have advocated the use of non-absorbable oral antibiotics in colon surgery including the use of a combination of oral kanamycin and erythromycin (52), whether they actually eliminate collagen-degrading E. faecalis and other such organisms from anastomotic tissues remains unknown.
Our results from patients undergoing colon surgery were not powered to determine the positive predictive value of the collagenase/MMP9 cleaving phenotype of bacterial strains on anastomotic leak. However, our rat data suggest that it may be worth testing the predictive value of the collagenase/MMP9 cleaving bacterial phenotype on anastomotic leak in a clinical trial of patients undergoing colorectal surgery. How the disruption of normal microbiota community structure due to prolonged illness and antibiotic treatment affects proliferation of bacteria expressing the “leak phenotype” remains to be defined. Our preliminary survey of 64 bacterial strains isolated from human anastomotic tissues demonstrated that only P. aeruginosa and E. faecalis expressed the collagen degrading/MMP9 cleaving phenotype. However, the background microbial composition in the patient harboring P. aeruginosa was highly disrupted and this was the only patient with a suspected anastomotic leak. Given these findings, we are currently planning a clinical trial in which we will use serial endoscopic surveillance of anastomotic tissues following lower colon surgery over the entire course of healing (21 days). We plan to directly swab anastomotic tissues during the serial endoscopic exam and identify tissue-associated microbial composition and phenotype; the microbial community structure, phenotype, and species that develop over time will be investigated.
There are some limitations to our study. First, we did not culture obligate anaerobes at anastomotic sites, which may play a contributory role in anastomotic leak. It is known that anaerobic bacteria such as Porphyromonas (Bacteroides) gingivalis (53), Bacteroides fragilis (54) and Clostridium histolyticum (55) produce collagenases. Application of metagenomics/metatranscriptomics complemented with proteomics and metabolomics would be an ideal approach to more completely define the roles that microbial community structure, membership, and function play in the pathogenesis of anastomotic leak. Although we attempted to do this in our human samples using 16S rRNA analysis, our clinical study was not set up nor powered to fully employ this approach. We are planning a clinical trial in which the microbes present on anastomotic tissues can be comprehensively analyzed over the full course of healing and their role in leak determined. Second, our rat model has certain limitations in terms of recapitulating the anastomotic leak that occurs in humans. Most anastomotic leaks are discovered on Computed Tomography (CT) scans and show up as inflammation or fluid collections adjacent to the anastomotic site. Leaks are not directly visualized during surgery as most patients do not undergo reoperation. The precise definition of anastomotic is thus debated and both contrast enema and CT scanning are known to carry significant false negative rates (56). Our approach was to directly examine all anastomoses in rats for evidence of dense adhesion, dehiscence, inflammation and purulence that clearly contrasted with a healthy non-inflamed anastomosis without adhesions. We believe this assessment is in line with what might be seen clinically in patients.
In summary, we present evidence that microbial pathogenesis may underlie anastomotic leak and may involve pathogens, such as E. faecalis, that break down collagen and cleave host tissue MMP9.
MATERIALS AND METHODS
Study Design
The rationale for the study was based on performing a low colon anastomosis at the junction of the rectum and colon where the highest incidence of anastomotic leak occurs clinically. Based on preliminary data, power analyses were carried out to determine the number of animals in each group required for statistical significance. Rules for stopping the experiment were to be enacted if rats appeared moribund or in any distress whereby they were sacrificed immediately and excluded from results. However this was not the case for any animals in the study. There were 5 anesthetic deaths that occurred shortly after the initial surgery (within 6 hours). These animals were excluded from the study analysis. Devascularization was performed to divide the blood vessels supplying the removed segment of colon to mimic the clinical performance of intestinal surgery. The overall objectives of the study were to develop a model in which a significant (i.e. 50%) leak rate was observed in order to analyze tissues and determine the molecular mechanisms of anastomotic leak. Standard measurements (i.e histology, gross observation) were used to determine leak, and western blotting, microbial analyses and zymography were employed to determine molecular markers of leak. Randomization of rats to the various groups was performed by randomly picking rats housed in groups of 5 to one group or another. All analyses, including the gross determination of leak were performed in a blinded fashion where the analysis was performed by a person blinded to treatment. Sample size and replicates are included in the figure legends. Endpoint selection was based on using gross inspection of the anastomotic sites on postoperative day 6 as the hard endpoint of the study given that it is a common time at which an intestinal anastomosis leaks clinically.
Rat model of colorectal anastomosis
Adult, male Wistar rats 250–300 g (Charles River Laboratory, Chicago, IL) were used for all experiments. Rats underwent general anesthesia and laparotomy with segmental colon resection at the peritoneal reflect and primary colorectal anastomosis. Devascularization of the anastomotic segment was carried out by dividing the feeding blood vessel 1 cm above and below the anastomotic suture line (57). Full details of the anastomotic surgery and other related procedures such as involving treatment with antibiotics and MMP9 inhibitors, anastomotic leak evaluation, blood vessels visualization, and intestinal collagen content are outlined in the supplemental materials.
Bacterial strains
Bacterial strains isolated in this study were used in defined experiments for collagen degrading activity and MMP9 activation assays. Among them, E. faecalis E1 and E. faecalis E2 were additionally used in rat model. E. faecalis V583 and its derivative mutants ΔgelE, ΔsprE, ΔgelEΔsprE and complemented mutants ΔgelE/gelE, ΔsprE/sprE, and ΔgelEΔsprE/gelE+sprE were provided by Lynn Hancock (58). All strains were stored in 10% glycerol stock at −80°C. Only cells freshly plated from stock were used in experiments. Cells from stock were plated onto tryptic soy broth (TSB) plates, grown overnight at 37°C and were further used as designed. For complemented mutants, spectinomycin, 500 μl/ml was added to TSB.
Microbial culture, anastomotic tissue analysis
In a separate experimental run, four groups of 10 rats each were randomly assigned to normal unoperated controls, devascularization alone (Dvasc), resection + anastomosis only (Anast) and resection, anastomosis, and devascularization (Dvasc + Anast). Colorectal anastomotic tissues were then inspected for leak, opened, and swabbed for microbial analysis. Swabs were cultured on media specific for gram-negative and gram-positive bacteria, and bacterial species were identified as previously described (59, 60). Tissues were collected and analyzed for MMP9 and inflammatory markers.
Enterococcal inoculation of colorectal anastomosis
To test the hypothesis that E. faecalis strains can cause anastomotic leak, we altered the animal model described above. As previously described, 1 hour prior to laparotomy rats were given two 5 ml rectal enemas of atropine (0.2 mg / ml; Sigma-Aldrich) and N-acetyl-L-cysteine (20 mg / ml; Sigma-Aldrich) followed by two 5 ml rectal enemas of 0.9% NaCl to remove the protective mucus layer (61). Additionally, to rid the intestine of normal flora, cefoxitin (50 mg/kg) was given via IM injection 30 minutes prior to incision. After completion of the anastomosis, E. faecalis strains were intestinally inoculated via a 5 ml rectal enema of 1:100 diluted overnight culture in Todd-Hewitt broth (THB). As described above, the animals were sacrificed on POD6 to evaluate for anastomosis integrity.Human Studies. Under approval from the Institutional Review Board at the University of Chicago and Northshore University Hospital IRB11-0481, eleven consecutive patients undergoing colon surgery were consented to participate in the study. When the colon sample was removed by the operating surgeon, the distal and proximal ends were immediately swabbed for 16S rRNA analysis and aerobic culture. In each case whether the patient recovered and was discharged uneventfully or whether the patient developed an anastomotic leak was determined by communicating with the operating surgery.
Collagen degrading activity was assessed as previously described (5) using an EnzChek Gelatinase/Collagenase Assay Kits (Molecular Probes, Eugene, OR). Details are outlined in supplemental materials. MMP9 activation assays. We have used human recombinant MMP9, murine macrophage MMP9, and intestinal tissue MMP9 to test for the MMP9 activation. Detailed methods are displayed below.
Recombinant MMP9
Recombinant human pro-enzyme MMP9 (r-MMP9) (Calbiochem, Cat# PF038) was used as a substrate. The r-MMP9 was diluted to a final concentration of 1 μg/ml in the assay buffer (50 mM Tris-HCl pH 7.5, 10 mM CaCl2, 0.05% Triton X-100). Detection of MMP9 cleaving activity was performed as previously described (62). Briefly, 30 μl of bacterial culture grown in THB for 6 h (~ OD600nm=1.0 which corresponded approximately 5×108 cells/ml as measured by plating of 10-fold dilutions) was incubated with 20 µl of 1 μg/ml r-MMP9 for 2 h. After centrifugation, 5 μl of the supernatant was mixed with an equal volume of 2X SDS-loading buffers, and subjected to zymography. The positive control included 4-amino-phenyl-mercuric acetate (Sigma) known to induce auto cleavage of MMP9 (63). Fresh 100 mM stock of APMA in DMSO was prepared, and MMP9 was activated by adding APMA to a final concentration of 1 mM followed by incubation at 37°C for 3h.
Murine macrophage MMP9
The murine macrophage cell line (J774) was cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum. The macrophage cells were harvested and resuspended in DMEM to a concentration 2×106 cells/ml. The cells were seeded on 12 well plate, 2 ml for each well, and incubated for 1h allowed to adhere to plate surface. E. faecalis strains were grown in THB to log phase and density of cells was adjusted to OD600 =1.0. 30 μl bacterial cell suspensions were added to each well. After 4h co-incubation, supernatants were collected, clarified by centrifugation for 10 min at 10, 000 g and analyzed by zymography and Western blot.
Intestinal tissue MMP9
E. faecalis strains E1 and E2 were grown overnight in THB followed by centrifugation (5,000 rpm, 6 min) to separate bacterial cells and secreted fraction (supernatant). Bacterial pellet was then diluted in fresh THB to OD600nm=0.2, and 500 μl of culture suspension was added to 100 μg of colon tissues. Similarly, 500 μl of filter-sterilized supernatant was added to 100 μg colon tissues. After 4 h of incubation at 37ºC, colon tissues and their conditioned media were collected and subjected to Zymography and Western blot.
Western blot
The resected from colon tissues were weighted, immediately placed in the liquid nitrogen, and kept at −80C. The ice cold lysis buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl. 0.5% Triton X-100 and PMSF) was added to ~ 80 mg of grinded tissues (1:10; 80 mg tissues /800 μl buffer). After vortexing and 15 min incubation on ice, the lysates were centrifuged at 10000 rpm for 15 min to remove the debris. The supernatants (15 μl that contained ~40 μg of protein) were boiled for 5 min with Laemmli sample buffer, electrophoresed through 10% SDS-polyacrylamide gels and then transferred onto PVDF membranes (Immobilon-P, Millipore). Ponceau staining was used as a loading control. The membranes were blocked in Tris-buffered saline containing Tween 20 (TBST) (pH 7.4) added with 4% non-fat dry milk (Labscientific) for 1 h and Incubated with primary anti-MMP-9, iNOS (Abcam), HIF1- α, and MPO (Novus Biological) antibodies followed by the correspond HRP coupled secondary antibody. The dilution of antibodies was performed as recommended by the vendors. The membranes were developed using ECL Western Blotting Detection Reagents (G&E Helthcare). Densitometry analyses were performed using ImageJ software.
Collagen degrading activity in anastomotic tissues
Tissue extracts from above described preparation were used to measure total collagen degrading activity in anastomotic tissues. Tissue extracts containing 60 μg of total protein were suspended in buffer containing 50 mM Tris-HCl, 0.15 M NaCl, 5 mM CaCl2, 1 μM ZnCl2, (pH 7.6) supplemented with 10 μl of 1 mg/ml fluorescent gelatin in total volume of 200 μl in 96 well plates Incubation was performed at 37ºC under static conditions, and fluorescence was measured at 480/520 nm. All experiments were performed in triplicate.
Zymography assay is described in details in supplemental materials
Caenorhabditis elegans killing assay
E. faecalis E1 and E2 strains were tested for virulence/killing capacity by assessing mortality of C. elegans fed on E. faecalis lawns (64). Full details are outlined in the supplemental materials.
16S rRNA analysis of bacterial community structure
Composition of the bacterial microflora was analyzed by 16S rRNA V4 iTAG amplicon sequencing analysis (65, 66) using DNA recovered from tissue swabs. Full details are outlined in the supplemental materials.
Statistical analysis
Statistical analysis was performed using SigmaPlot software. Student t-tests were used when analyzing the differences between two means, whereas ANOVA with Bonferroni correction was used when >2 means were compared. Kaplan-Maier survival plot was analyzed using SPSS software. Significance was determined as a p value <0.05.
Supplementary Material
Fig. S1. Images of selected rat colon segments following colon resection with and without segmental devascularization.
Fig. S2. Collagenolytic activity of tissue extracts from leaking anastomosis compared to non-leaking anastomosis.
Fig. S3. Time course-dependent degradation of gelatin by E. faecalis isolates E1 and E2.
Fig. S4. Kaplan-Meier survival curves of C. elegans feeding on E1 and E2 strains of E. faecalis.
Fig. S5. Gelatin degradation by complemented mutants.
Fig. S6. Effect of Zinc-chelating compound 1,10-phenanthroline on collagen-degrading activity in E. faecalis E2 and V583 strains.
Fig. S7. Zymography of tissue extracts in leaking tissues.
Fig. S8. Zymography analysis of macrophage activation of MMP9 by wildtype V583, its mutants lacking GelE and SprE, and its complemented mutants ΔgelE/gelE, ΔsprE/sprE, and ΔgelEΔsprE/gelE+sprE.
Fig. S9. Western blot analyses to identify MMP9 in tissues of rats subjected to devascularization (Dvasc), anastomosis (Anast) or Anast + Dvasc, in the presence or absence of direct topical application of ciprofloxacin, metronidazole, and neomycin via enema.
Fig. S10. Effect of topical antibiotics on the activation of MMP9.
Fig. S11. Gelatin degradation by individual strains recovered from anastomotic tissues after intramuscular injection of cefoxitin or topical application of ciprofloxacin, metronidazole, and neomycin.
Accessible Summary: Can our own intestinal microbes prevent adequate healing following colon surgery?
We examined the effect of a bacterium Enterococcus faecalis, normally present in the intestine, for its capacity to contribute to anastomotic leak, the most feared complication following intestinal surgery. We demonstrated that intestinal Enterococcus faecalis can produce a tissue destroying enzyme that affects the normal healing process by breaking down collagen, a protein that is critical to fully seal the intestine following its removal and reconnection. Enterococcus faecalis also activates an intestinal enzyme, MMP9, further contributing to anastomotic leak. Finally, we demonstrated that the most common antibiotic used in intestinal surgery does not eliminate Enterococcus faecalis and thus does not prevent leak.
Acknowledgments
We thank the Core Facility Human Tissue Resource Center, University of Chicago for tissue sectioning, staining, and examination of slides.
Funding. This study was funded by a pilot and feasibility grant (JCA) from the NIH Digestive Disease Research Center Core (DDRCC) P30 DK42086.
Footnotes
Author contributions. BDS- study design participation, performed animal experiments, collagenase assays, histochemistry analysis, collected samples, analyzed data, performed statistics, wrote portions of the manuscript., NB- performed western blots, zymography, macrophage assays, study design participation, wrote portions of the methods PML- performed collagenase assays, participated in study design, AZ- performed C elegans experiments, participated in study design, intellectual direction BS- performed animal experiments, mutational analysis, wrote portions of the methods, JL- performed animal experiments, mutational analysis, wrote portions of the methods, RK- performed zymography and western blots with NB, performed animal experiments, mutational analysis, wrote portions of the methods, MW-intellectual participation, performed animal experiments, participated in study design, VK- performed endoscopy and confocal laser endomicroscopy, CB- performed bacterial identifications, SL, GA, LEH, JG, KU analyzed the data; JPM and MS collected the human samples; OZ and JCA designed all the experiments, analyzed the data, and wrote the paper.
Competing interests. The authors declare no competing interests.
SUPPLEMENTARY MATERIALS
References
- 1.Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Annals of surgery. 2007 Aug;246:207. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn I, Jr, Rives JD. Antibiotic protection of colon anastomoses. Annals of surgery. 1955 May;141:707. doi: 10.1097/00000658-195505000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deierhoi RJ, Dawes LG, Vick C, Itani KM, Hawn MT. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. Journal of the American College of Surgeons. 2013 Nov;217:763. doi: 10.1016/j.jamcollsurg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Englesbe MJ, et al. A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Annals of surgery. 2010 Sep;252:514. doi: 10.1097/SLA.0b013e3181f244f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivas AD, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PloS one. 2012;7:e44326. doi: 10.1371/journal.pone.0044326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shogan BD, et al. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014;2:35. doi: 10.1186/2049-2618-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langille MG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013 Sep;31:814. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurlow LR, et al. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infection and immunity. 2010 Nov;78:4936. doi: 10.1128/IAI.01118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng J, Teng F, Murray BE. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infection and immunity. 2005 Mar;73:1606. doi: 10.1128/IAI.73.3.1606-1612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington DJ. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infection and immunity. 1996 Jun;64:1885. doi: 10.1128/iai.64.6.1885-1891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnerlich JL, Ritter JH, Kirby JP, Mazuski JE. Simultaneous necrotizing soft tissue infection and colonic necrosis caused by Clostridium septicum. Surgical infections. 2011 Dec;12:501. doi: 10.1089/sur.2010.054. [DOI] [PubMed] [Google Scholar]
- 12.Schlapbach LJ, Ahrens O, Klimek P, Berger S, Kessler U. Clostridium perfringens and necrotizing enterocolitis. The Journal of pediatrics. 2010 Jul;157:175. doi: 10.1016/j.jpeds.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Bos J, et al. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 May 15;40:e78. doi: 10.1086/429829. [DOI] [PubMed] [Google Scholar]
- 14.Rein JM, Cosman B. Bacteroides necrotizing fasciitis of the upper extremity. Case report. Plastic and reconstructive surgery. 1971 Dec;48:592. doi: 10.1097/00006534-197112000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Engelbert M, Mylonakis E, Ausubel FM, Calderwood SB, Gilmore MS. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infection and immunity. 2004 Jun;72:3628. doi: 10.1128/IAI.72.6.3628-3633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayaoglu G, Orstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2004;15:308. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 17.Jha AK, Bais HP, Vivanco JM. Enterococcus faecalis mammalian virulence-related factors exhibit potent pathogenicity in the Arabidopsis thaliana plant model. Infection and immunity. 2005 Jan;73:464. doi: 10.1128/IAI.73.1.464-475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergis EN, et al. Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due to Enterococcus faecalis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Sep 1;35:570. doi: 10.1086/341977. [DOI] [PubMed] [Google Scholar]
- 19.Bucher P, Gervaz P, Egger JF, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Diseases of the colon and rectum. 2006 Jan;49:109. doi: 10.1007/s10350-005-0215-5. [DOI] [PubMed] [Google Scholar]
- 20.Makinen PL, Clewell DB, An F, Makinen KK. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain 0G1-10) The Journal of biological chemistry. 1989 Feb 25;264:3325. [PubMed] [Google Scholar]
- 21.Qin X, Singh KV, Weinstock GM, Murray BE. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. Journal of bacteriology. 2001 Jun;183:3372. doi: 10.1128/JB.183.11.3372-3382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawalec M, Potempa J, Moon JL, Travis J, Murray BE. Molecular diversity of a putative virulence factor: purification and characterization of isoforms of an extracellular serine glutamyl endopeptidase of Enterococcus faecalis with different enzymatic activities. Journal of bacteriology. 2005 Jan;187:266. doi: 10.1128/JB.187.1.266-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumpf M, et al. Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery. 2005 Feb;137:229. doi: 10.1016/j.surg.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. The Journal of pharmacology and experimental therapeutics. 2006 Sep;318:933. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- 25.de Bentzmann S, et al. Pseudomonas aeruginosa virulence factors delay airway epithelial wound repair by altering the actin cytoskeleton and inducing overactivation of epithelial matrix metalloproteinase-2. Laboratory investigation; a journal of technical methods and pathology. 2000 Feb;80:209. doi: 10.1038/labinvest.3780024. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto T, et al. Activation of human matrix metalloproteinases by various bacterial proteinases. The Journal of biological chemistry. 1997 Feb 28;272:6059. doi: 10.1074/jbc.272.9.6059. [DOI] [PubMed] [Google Scholar]
- 27.Waters CM, Antiporta MH, Murray BE, Dunny GM. Role of the Enterococcus faecalis GelE protease in determination of cellular chain length, supernatant pheromone levels, and degradation of fibrin and misfolded surface proteins. Journal of bacteriology. 2003 Jun;185:3613. doi: 10.1128/JB.185.12.3613-3623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deierhoi RJ, Dawes LG, Vick C, Itani KM, Hawn MT. Choice of Intravenous Antibiotic Prophylaxis for Colorectal Surgery Does Matter. Journal of the American College of Surgeons. 2013 Sep 14; doi: 10.1016/j.jamcollsurg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Kager L, Malmborg AS, Nord CE, Pieper R. The effect of short-term cefoxitin prophylaxis on the colonic microflora in patients undergoing colorectal surgery. Infection. 1982 Nov-Dec;10:338. doi: 10.1007/BF01642294. [DOI] [PubMed] [Google Scholar]
- 30.Ohigashi S, et al. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013 Sep;17:1657. doi: 10.1007/s11605-013-2270-x. [DOI] [PubMed] [Google Scholar]
- 31.Komen N, et al. Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: analysis of parameters predictive for evident anastomotic leakage. International journal of colorectal disease. 2014 Jan;29:15. doi: 10.1007/s00384-013-1776-8. [DOI] [PubMed] [Google Scholar]
- 32.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. The FEBS journal. 2011 Jan;278:28. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- 33.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. The Journal of pathology. 2003 Jul;200:448. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. Journal of leukocyte biology. 2005 Jul;78:279. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 35.Pasternak B, Matthiessen P, Jansson K, Andersson M, Aspenberg P. Elevated intraperitoneal matrix metalloproteinases-8 and -9 in patients who develop anastomotic leakage after rectal cancer surgery: a pilot study. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2010 Jul;12:e93. doi: 10.1111/j.1463-1318.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- 36.Agren MS, et al. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006 Jul;140:72. doi: 10.1016/j.surg.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Krarup PM, et al. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. International journal of colorectal disease. 2013 Aug;28:1151. doi: 10.1007/s00384-013-1697-6. [DOI] [PubMed] [Google Scholar]
- 38.Agren MS, Jorgensen LN, Delaisse JM. Matrix metalloproteinases and colon anastomosis repair: a new indication for pharmacological inhibition? Mini reviews in medicinal chemistry. 2004 Sep;4:769. [PubMed] [Google Scholar]
- 39.Paulsen IT, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003 Mar 28;299:2071. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 40.Merquior VL, et al. Emergence and characterisation of vanB vancomycin-resistant Enterococcus faecalis in Rio de Janeiro, Brazil. Memorias do Instituto Oswaldo Cruz. 2012 Jun;107:557. doi: 10.1590/s0074-02762012000400020. [DOI] [PubMed] [Google Scholar]
- 41.Zheng B, Tomita H, Inoue T, Ike Y. Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections: first report of the isolation and identification of the pheromone-responsive plasmids pMG2200, Encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac) Antimicrobial agents and chemotherapy. 2009 Feb;53:735. doi: 10.1128/AAC.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilela MA, et al. Identification and molecular characterization of Van A-type vancomycin-resistant Enterococcus faecalis in Northeast of Brazil. Memorias do Instituto Oswaldo Cruz. 2006 Nov;101:715. doi: 10.1590/s0074-02762006000700002. [DOI] [PubMed] [Google Scholar]
- 43.Lopez M, Cercenado E, Tenorio C, Ruiz-Larrea F, Torres C. Diversity of clones and genotypes among vancomycin-resistant clinical Enterococcus isolates recovered in a Spanish hospital. Microb Drug Resist. 2012 Oct;18:484. doi: 10.1089/mdr.2011.0203. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa K, et al. Comparison of the clinical characteristics and outcomes associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant E. faecium bacteremia. Antimicrobial agents and chemotherapy. 2012 May;56:2452. doi: 10.1128/AAC.06299-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omotola AM, et al. Risk factors for and epidemiology of community-onset vancomycin-resistant Enterococcus faecalis in southeast Michigan. American journal of infection control. 2013 Dec;41:1244. doi: 10.1016/j.ajic.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 46.Gangurde N, Mane M, Fatale S. Prevalence of Multidrug Resistant Enterococci in a Tertiary Care Hospital in India: A Growing Threat. Open Journal of Medical Microbiology. 2014 Jan;4:11–15. [Google Scholar]
- 47.Gioia M, et al. The collagen binding domain of gelatinase A modulates degradation of collagen IV by gelatinase B. Journal of molecular biology. 2009 Feb 20;386:419. doi: 10.1016/j.jmb.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Brasken P, Lehto M, Renvall S. Fibronectin, laminin, and collagen types I, III, IV and V in the healing rat colon anastomosis. Annales chirurgiae et gynaecologiae. 1990;79:65. [PubMed] [Google Scholar]
- 49.Brasken P, Renvall S, Sandberg M. Fibronectin and collagen gene expression in healing experimental colonic anastomoses. The British journal of surgery. 1991 Sep;78:1048. doi: 10.1002/bjs.1800780908. [DOI] [PubMed] [Google Scholar]
- 50.Kager L, et al. Antibiotic prophylaxis with cefoxitin in colorectal surgery: effect on the colon microflora and septic complications–a clinical model for prediction of the benefit and risks in using a new antibiotic in prophylaxis. Annals of surgery. 1981 Mar;193:277. doi: 10.1097/00000658-198103000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komen N, et al. Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: Analysis of Parameters Predictive for Evident Anastomotic Leakage. International journal of colorectal disease. 2013 Oct 11; doi: 10.1007/s00384-013-1776-8. [DOI] [PubMed] [Google Scholar]
- 52.LeVeen HH, et al. Effects of prophylactic antibiotics on colonic healing. American journal of surgery. 1976 Jan;131:47. doi: 10.1016/0002-9610(76)90419-0. [DOI] [PubMed] [Google Scholar]
- 53.Bodinka A, et al. Polymerase chain reaction for the identification of Porphyromonas gingivalis collagenase genes. Oral microbiology and immunology. 1994 Jun;9:161. doi: 10.1111/j.1399-302x.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 54.Moncrief JS, et al. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infection and immunity. 1995 Jan;63:175. doi: 10.1128/iai.63.1.175-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bond MD, Van Wart HE. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984 Jun 19;23:3077. doi: 10.1021/bi00308a035. [DOI] [PubMed] [Google Scholar]
- 56.Kornmann VN, van Ramshorst B, Smits AB, Bollen TL, Boerma D. Beware of false-negative CT scan for anastomotic leakage after colonic surgery. International journal of colorectal disease. 2014 Apr;29:445. doi: 10.1007/s00384-013-1815-5. [DOI] [PubMed] [Google Scholar]
- 57.Daams F, et al. Local ischaemia does not influence anastomotic healing: an experimental study. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 2013;50:24. doi: 10.1159/000348411. [DOI] [PubMed] [Google Scholar]
- 58.Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. Journal of bacteriology. 2008 Aug;190:5690. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaborin A, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio. 2014;5:e01361. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romanowski K, et al. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PloS one. 2012;7:e30119. doi: 10.1371/journal.pone.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kistler EB, Alsaigh T, Chang M, Schmid-Schonbein GW. Impaired small-bowel barrier integrity in the presence of lumenal pancreatic digestive enzymes leads to circulatory shock. Shock. 2012 Aug;38:262. doi: 10.1097/SHK.0b013e31825b1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oggioni MR, et al. Pneumococcal zinc metalloproteinase ZmpC cleaves human matrix metalloproteinase 9 and is a virulence factor in experimental pneumonia. Molecular microbiology. 2003 Aug;49:795. doi: 10.1046/j.1365-2958.2003.03596.x. [DOI] [PubMed] [Google Scholar]
- 63.Sorsa T, et al. Activation of type IV procollagenases by human tumor-associated trypsin-2. The Journal of biological chemistry. 1997 Aug 22;272:21067. doi: 10.1074/jbc.272.34.21067. [DOI] [PubMed] [Google Scholar]
- 64.Zaborin A, et al. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proceedings of the National Academy of Sciences of the United States of America. 2009 Apr 14;106:6327. doi: 10.1073/pnas.0813199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007 Aug;73:5261. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME journal. 2012 Aug;6:1621. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Images of selected rat colon segments following colon resection with and without segmental devascularization.
Fig. S2. Collagenolytic activity of tissue extracts from leaking anastomosis compared to non-leaking anastomosis.
Fig. S3. Time course-dependent degradation of gelatin by E. faecalis isolates E1 and E2.
Fig. S4. Kaplan-Meier survival curves of C. elegans feeding on E1 and E2 strains of E. faecalis.
Fig. S5. Gelatin degradation by complemented mutants.
Fig. S6. Effect of Zinc-chelating compound 1,10-phenanthroline on collagen-degrading activity in E. faecalis E2 and V583 strains.
Fig. S7. Zymography of tissue extracts in leaking tissues.
Fig. S8. Zymography analysis of macrophage activation of MMP9 by wildtype V583, its mutants lacking GelE and SprE, and its complemented mutants ΔgelE/gelE, ΔsprE/sprE, and ΔgelEΔsprE/gelE+sprE.
Fig. S9. Western blot analyses to identify MMP9 in tissues of rats subjected to devascularization (Dvasc), anastomosis (Anast) or Anast + Dvasc, in the presence or absence of direct topical application of ciprofloxacin, metronidazole, and neomycin via enema.
Fig. S10. Effect of topical antibiotics on the activation of MMP9.
Fig. S11. Gelatin degradation by individual strains recovered from anastomotic tissues after intramuscular injection of cefoxitin or topical application of ciprofloxacin, metronidazole, and neomycin.


