Abstract
Introduction
Osteoporosis is a significant public health issue affecting over half of women aged over 50. With an aging population its importance is set to increase further over time. Prevention of fragility fractures avoids significant mortality and morbidity as well as saving significant direct and indirect costs to the economy. In this review, we discuss existing treatments to contextualise the treatment landscape, and demonstrate how our understanding of bone pathophysiology has led to novel therapies – in the form of combinations and altered durations of existing treatments, as well as newer drug therapies.
Sources of data
Pubmed and Embase were searched for randomised controlled trials of new therapies for osteoporosis. These searches were supplemented with material presented in abstract form at international meetings
Areas of agreement
New drugs that appear promising in the treatment of osteoporosis include the cathepsin K inhibitor, monoclonal antibodies against sclerostin, and parathyroid hormone related peptide.
Areas of controversy
Separate to the development of novel drug therapies is the issue of how best to use agents that are currently available to us; specifically which agent to choose, alone or in combination; duration of therapy; how best to identify patients at highest risk of fracture, and to ensure the highest possible adherence to medication. Many of these issues have been addressed in other excellent review papers, and will not be considered in detail here.
Growing points
As with all new treatments, we await results of long term use, and experience in ‘real life’ patient populations
Areas timely for developing research
As alluded to above, data are urgently required regarding the optimal duration of therapy; use of combination therapy; ordering of therapies for best therapeutic effect. As stratified medicine becomes more strongly considered in all areas of therapy, its merits in osteoporosis as in other musculoskeletal conditions, is timely and valuable.
Introduction
Definition of Osteoporosis
Osteoporosis is a systemic disorder characterised as the depletion of bone mass with structural deterioration of bone tissue [1]. This results in a decrease in bone mineral density (BMD) and a predisposition to fragility fractures. Dual-energy x-ray absorptiometry (DXA) is currently the criterion standard for the evaluation of BMD. DXA is used to measure BMD at the hip, neck of femur, vertebrae and wrist. DXA provides the patient’s T-score, which is the BMD value compared with that of control subjects who are young, healthy adults at the peak of their BMD. The World Health Organisation define osteoporosis in postmenopausal women as a BMD value at least 2.5 standard deviations below the average value in healthy young women (T-score) [2]. Fragility fractures are fractures which result from low energy trauma which would not usually occur in normal bone. The most common sites for fragility fractures are the vertebrae, proximal femur and distal radius. In this review, we discuss existing osteoporosis treatments to contextualise the treatment landscape, and demonstrate how our understanding of bone pathophysiology has led to novel therapies – in the form of combinations and altered durations of existing treatments, as well as newer drug therapies.
Epidemiology of Osteoporosis and Current Guidance
Osteoporosis affects approximately 30% of all postmenopausal women in the United States and in Europe [3]. With an aging population osteoporosis is becoming hugely relevant to healthcare in the UK. We know that fragility fractures carry with them significant mortality, morbidity and financial implications. Every year over 300,000 patients present with fragility fractures to hospitals in the UK [4]. In the UK those who present with hip fracture have a 30 day mortality of 8.2% [5] with permanent disability resulting in 50% of those affected [6]. Direct medical costs from fragility fractures to the UK healthcare economy were estimated at £1.8 billion in 2000, with the potential to increase to £2.2 billion by 2025, and with most of these costs relating to hip fracture care [7]. There are likely to be significant extra costs to society in days lost from the workplace by the patient and carers.
Decreasing BMD is part of the normal aging process with osteoclast activity becoming greater than osteoblast activity. The process is accelerated in females after menopause, males also tend to have a greater peak in BMD, contributing to the increased incidence in fracture presenting in older females. Risk factors are outlined in table 1.
Table 1.
Risk factors for decreased bone mineral density. Adapted with permission from Curtis et al. [9]
| Risks independent of bone mineral density | Risks dependent on bone mineral density |
|---|---|
| Age Previous history of fragility fracture Parental history of hip fracture Smoking Alcohol intake of 4 or more units per day Steroid use Rheumatoid arthritis Body mass index < 19 kg/m2 Low sunlight exposure Falls |
Drugs (glucocorticoids, aromatase inhibitors, androgen deprivation therapy, heparin therapy, proton pump inhibitors) Malabsorption Conditions resulting in prolonged immobility Untreated premature menopause, untreated hypogonadism Endocrine disease e.g. hyperthyroidism Chronic liver disease Chronic renal disease Chronic obstructive pulmonary disease |
UK guidance suggests that we consider assessing fracture risk in women over 65 and men over 75, or those over 50 with the presence of risk factors as summarised in table 1. In practice the most commonly used tool is the fracture risk assessment tool (FRAX), which can be used with or without BMD score [8]. It gives a predicted risk of major osteoporotic and hip fracture over 10 years as a percentage.
The National Osteoporosis Guideline Group (NOGG) issued updated guidance for diagnosis and management of osteoporosis in postmenopausal women and men over 50 in 2014 [10]. This guidance can advise the clinician on assessing fracture risk in different populations, use of FRAX and anti-osteoporotic drug choice and duration. Ultimately, however, treatment decision is based on individual clinician and patient choice.
Existing Pharmacological Treatments
Vitamin D and Calcium Supplementation
There has been huge debate regarding the role of vitamin D supplementation in many fields of medicine. The majority of randomised controlled trials use calcium and vitamin D supplementation, although this has been at varying doses (500mg – 1000mg calcium; 250-1200 IU vitamin D daily) therefore it is difficult to establish efficacy of treatment. The literature reviewing the benefits of calcium and vitamin D supplementation has been very conflicting. However, it is generally recommended that patients should ensure good calcium and vitamin D intake in their diet [11]. Vitamin D and calcium supplementation alone is less effective as compared to other osteoporosis treatments discussed [12].
Drugs that Inhibit Bone Resorption
Bisphosphonates
The majority of primary and secondary osteoporosis treatment involves the use of bisphosphonate therapy, and most randomised controlled trials of osteoporosis therapy have used these agents. The nitrogen containing bisphosphonates alendronate, risedronate, ibandronate and zoledronate are analogues of inorganic pyrophosphate and inhibit farnesyl pyrophosphate synthase in the mevalonate pathway, which results in apoptosis of the osteoclasts. This leads to inhibition of bone resorption and increases in BMD. There is data to suggest inhibition can continue following cessation of the drug, with markers of bone turnover 50 percent lower 5 years after discontinuation [13].
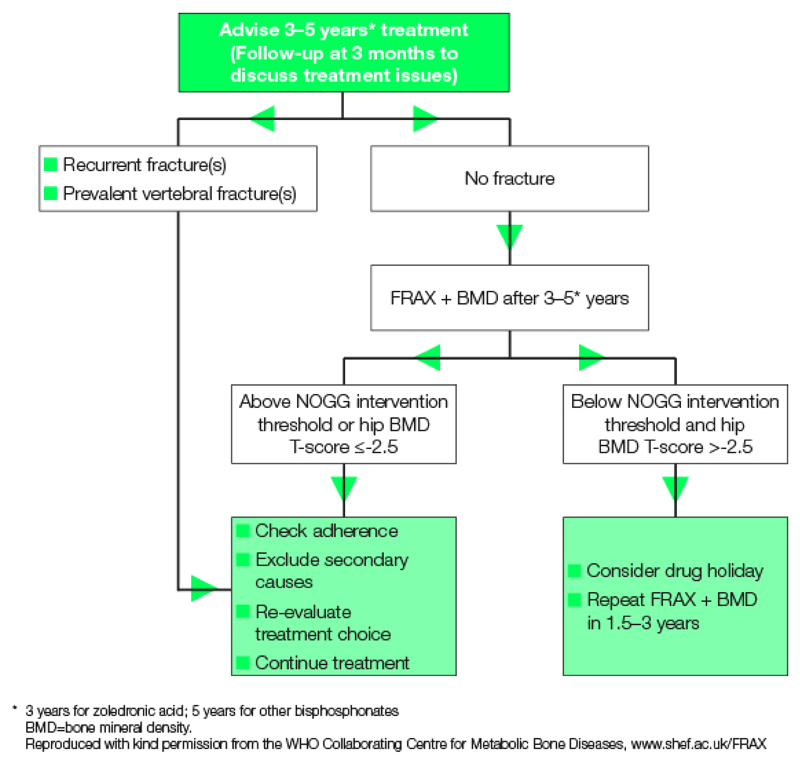
The most commonly prescribed is alendronate, given orally once weekly. The drugs must be given on an empty stomach because food and liquids interfere with gastrointestinal absorption. Suboptimal administration can result in oseophageal irritation. Poor adherence is associated with increased fracture rates [14]. If oral bisphosphates cannot be tolerated, intravenous zoledronate can be used as an alternative at a dose of 5mg yearly for 3 years. The concerning side effect of osteonecrosis of the jaw (ONJ) has been predominantly seen in larger doses of bisphosphonates given to oncology patients, and the risk of those being treated for osteroporosis is very low [15]. The American Society for Bone and Mineral Research (ASBMR) recommends that patients should be informed that there is a low risk of development of ONJ and that health providers should encourage patients to practice good oral hygiene and have regular dental visits [16]. Atypical fracture is now a recognised potential complication of prolonged use of bisphosphonates and patient reassessment after 3-5 years is now recommended, as outlined in figure 1.
Figure 1.
Algorithm for treatment monitoring with the use of bisphosphonates from the National Osteoporosis Guidelines Group (NOGG)
Strontium ranelate
Strontium ranelate is a compound made up of 2 atoms of the element strontium bound with ranelic acid. It is below calcium on the periodic table and is incorporated into bone at the same rate. Recently the use of strontium has been restricted following concern regarding cardiovascular risks. In March 2014 the European Medicines Agency concluded its review of the risks and benefits of stronium ranelate and advised that strontium ranelate should be restricted to only people with severe osteoporosis for whom there are no alternate treatments for osteoporosis [17]. Consequently there has been a large decline in its use.
Denosumab
Denosumab is a fully human RANKL (receptor activator of nuclear factor kappaB (RANK) ligand) antibody. Precursors to osteoclasts, called pre-osteoclasts, express surface receptors called RANK. RANK is activated by RANKL, which exists as cell surface molecules on osteoblasts. Activation of RANK by RANKL promotes the maturation of pre-osteoclasts into osteoclasts. Denosumab inhibits this maturation of osteoclasts by binding to and inhibiting RANKL. This mimics the natural action of osteoprotegerin, an endogenous RANKL inhibitor. This decreases bone resorption and increases BMD. It is administered via a 6 monthly subcutaneous injection. Side effects are uncommon but include transient hypocalcaemia, particularly if vitamin D deficient, and cellulitis often away from the injection site [18].
Selective oestrogen receptor modulators (SERMs)
Raloxifene is a partial oestrogen agonist that acts as an agonist in bone, but an antagonist in other areas of the body such as the uterus and breast. It therefore has positive oestrogen effects in the bones, without other unwanted oestrogen effects elsewhere. It has been shown to reduce deteriorating BMD and vertebral fractures in post menopausal women [19]. However, there is an increased risk of venous thrombo-embolic events and patients should be assessed prior to commencing treatment; active or past history of venous thromboembolic events (VTE), including deep vein thrombosis, pulmonary embolism and retinal vein thrombosis is a contraindication to use.
Drugs that Promote Bone Formation
Teriparatide
Teriparatide is a recombinant form of parathyroid hormone (PTH)(1-34). It is currently the only widely available anabolic agent used for osteoporosis. Teriparatide increases renal re-absorption of calcium and enhances intestinal calcium absorption via its effect on one hydroxylation of 25(OH)D3. Intermittent administration of PTH increases the number of bone forming osteoblasts whereas continuous administration increases the number of bone resorbing osteoclasts. Therefore, for an anabolic effect, it is given daily as a low dose subcutaneous injection of 20μg for a period of 18-24 months. The high cost of teriparatide restricts its use to those with high fracture risk who have failed other therapies.
Combination Therapy
In recent times there has been interest in looking at combination therapies, either treatment given at the same time or following on from each other. Most trials data have assessed the combination of anabolic therapy (teriparatide) with an antiresorptive therapy (a bisphosphonate or denosumab). Although there are no trials sufficiently powered to show a difference in fracture outcome, some trials have been able to show a difference in BMD particularly at the hip: a greater BMD at the hip as compared to the spine has been a consistent finding across many trials [20]. The DATA extension study looked specifically at using two years of concomitant teriparatide and denosumab therapy and found increases in BMD more than therapy with either medication alone, and more than has been reported with any current therapy [21]. However, to date no trial looking at combination therapy has shown a reduction in fracture rate.
Bone Turnover and Pathways
Remodelling of bone is a continuous process with two phases that are normally coupled and balanced: bone resorption mediated by osteoclasts followed by bone formation mediated by osteoblasts, within a bone remodelling unit. Osteocytes, a third bone cell type, play an important role in regulating activities of osteoclasts and osteoblasts. An imbalance in this process with either an increase in bone resorption or decrease in bone formation leads to a progressive loss of bone mass and impaired bone microarchitecture. In post-menopausal osteoporosis this balance shifts to bone resorption over formation. Drugs should counteract this balance, going towards increased formation or decreased resorption (or both).
However, the challenge is that inhibiting osteoclasts may also result in decreased bone formation, as they are coupled processes. The coupling is thought to be via osteogenic factors released by osteoclasts. Inhibiting both processes may also lead to a decrease in the repair of microbreaks from normal activities and lead to defective microarchitecture [22][23]. This is likely to be an inherent problem of prolonged use of bisphosphonates and denosumab as they inhibit osteoclastogenesis, with the reduced number of osteoclasts leading to low bone turnover and hence less coupled activity of osteoblasts for bone formation. The potential consequent defective microarchitecture could increase the risk (albeit a low one) for atypical subtrochanteric fractures. Nevertheless, the association between atypical fractures and the use of bisphosphonates remains contentious, and is based on case reports and retrospective case reviews; bisphosphonates are not thought to be the sole risk factor for atypical fractures [24]. Microdamage accumulation is only one hypothesised mechanism of atypical fracture; others include reduced heterogeneity of mineralisation and cortical hardness resistant to plastic deformation [25].
With the possible link between reduced bone remodelling and atypical fractures, a drug that preserves osteoclast numbers to enable the coupled activity of bone formation by osteoblasts, but inhibits the activity of the osteoclasts could be beneficial. Recent studies suggest a hypothesis of a cell layer lining the bone marrow and forming a canopy over the whole remodelling surface, as a source of osteoblast progenitors, and that this is induced by osteoclastic factors to favour the initiation of bone formation [26].
Bone resorption is made up of the removal of the predominant constituents: inorganic bone mineral and organic bone matrix. The former is removed by acid, and the latter by cathepsin K (under acidic conditions). Therefore targeting cathepsin K rather than osteoclastogenesis should allow continued signals to osteoblasts and consequent bone formation. This has been found to be the case in ovariectomized rabbits where histology has shown that odanacatib (a cathepsin K inhibitor) induces a shorter reversal phase, higher osteoblast recruitment and an increase in osteoclast surface, whereas those treated with alendronic acid did not show these responses [27]. Accordingly odanacatib had a positive effect on bone formation rate at the same time as decreasing bone resorption, whereas alendronic acid decreased bone formation rate [28]. Whilst bone formation rate is increased at both trabecular and cortical sites in CatK knockout mice and odanacatib treated ovariectomized rabbits, in ovariectomized adult rhesus monkeys bone formation rate was site dependent (reduced at trabecular and intracortical bone but unchanged at endocortical surface and increased at the periosteal surface). However the authors suggested that odanacatib brought trabecular bone formation rate in estrogen-deficient animals to the level of intact animals [29].
Loss of function mutations in the cathepsin K gene is described in a naturally occurring disease called pycnodysostosis. It results in the loss of bone resorption and subsequent increased bone mass. It is a rare autosomal recessive syndrome of skeletal dysplasia associated with brachycephaly, wide cranial sutures, short stature, osteosclerosis, fragility fractures and high bone mineral density. Impairment of bone remodelling is reflected by a reduction in bone resorption markers (N-telopeptides of type 1 collagen (NTx) and C-telopeptides of type 1 collagen (CTx)). In cathepsin K deficient mice they show an osteopetrotic skeleton with increased trabecular and cortical bone. This histology shows a normal rate of bone mineralisation with normal or increased osteoclast numbers but a decrease in the bone matrix resorption ability of osteoclasts [30].
Alternatively we may wish to target osteoblasts for its role in bone formation. Existing treatments such as oestrogens and teriparatide promote the activity of osteoblasts. The leading agents in this field relate to the Wnt signalling pathway, via an inhibitor of sclerostin (e.g. Romosozumab), and separately a PTH related peptide (PTHrp) (e.g. Abaloparatide).
In skeletal cells, activation of the canonical Wnt/β-catenin signaling pathway induces osteoblast cell differentiation [31]. It is the translocation of unphosphorylated β-catenin to the nucleus that activates these Wnt target genes. However, β-catenin is phosphorylated and degraded in the proteasome when Wnt receptor binding interactions are absent: it is the binding of Wnt to frizzled receptors and to the low-density lipoprotein receptor–related protein (LRP) 5 and 6 co-receptors that leads to the stabilization of β-catenin and its translocation to the nucleus. Through theses interactions, Wnt thereby induces osteoblastogenesis and bone formation. The activity of Wnt is modulated by extracellular antagonists that act by binding Wnt itself or by preventing its interactions with its receptor or coreceptors. Sclerostin and dickkopf 1 (Dkk-1) bind to LRP5 and LRP6. Mutations in LRP5 and LRP6, preventing the association of sclerostin or Dkk-1 with LRP5, result in increased bone mass as the Wnt pathway is thereby unimpeded. Therefore, Wnt signaling is a suitable target for the development of new anabolic therapies.
Sclerostin, encoded by the SOST gene, is an osteocyte-secreted glycoprotein that normally inhibits the Wnt signalling pathway on the cell membranes of osteoblasts, and thereby inhibits osteoblast proliferation and function. Blocking the action of sclerostin should therefore yield bone formation. Indeed patients with a genetic deficiency of sclerostin (van Buchem disease) have high bone mass and correspondingly increased bone strength that is resistant to fractures [32]. These patients tend to lead a normal life with nerve entrapment being the only problem of the high skeletal BMD.
PTHrp shares a similar structural organization to PTH that interacts with the same receptor as PTH, but it performs a different role in bone, with an important paracrine action on committed osteoblast precursors in order to enhance their differentiation and reduce osteoblast apoptosis [33]. Mice with an osteoblast-specific targeted disruption of PTHrp had marked osteoporosis characterized by impaired bone formation, even in the presence of normal levels of circulating PTH, because of the loss of the paracrine action of PTHrp [34].
Novel Therapies
Cathepsin K Inhibitor
Cathepsin K is a lysosomal cysteine proteinase expressed by osteoclasts, and is one of the enzymes that degrade type I collagen, a major component (90%) of bone matrix.
Other cathepsins B, L and S degrade collagen in other tissues such as skin and lung, so a Cathepsin K inhibitor has to be selective over these other types in order to avoid side effects such as morphea-like skin reactions and respiratory abnormalities. Cathepsin K has also been found to be expressed in other cells which may explain some of the off-target effects seen in clinical trials, including for example the macrophages and smooth muscle cells of atherosclerotic lesions [35].
Odanacatib is a highly selective cathepsin K inhibitor. In phase I clinical trials, a half life of 66-93 hours was observed, allowing for once weekly dosing [36]. A phase II randomized, multicenter, placebo-controlled trial enrolled 399 postmenopausal women with low BMD (T-score ≤-2.0 and ≥-3.5) allocated to one of the following weekly odanacatib treatments: 3, 10, 25 and 50 mg. The trial was designed as a 12-month study with a 12-month extension period [37]. In this study population of postmenopausal women with low bone density, odanacatib treatment at doses of 10, 25, and 50 mg once weekly generally resulted in dose-dependent increases, compared with placebo, in lumbar spine, total-hip, femoral neck, trochanter, and one-third radius BMD. Substantial further increases in BMD were seen in the second year. Dose dependent decreases in levels of bone resorption markers urinary CTx/creatinine ratio and serum NTx with the three higher doses were consistent with an antiresorptive effect. Nevertheless, because of the direct role of cathepsin K in the production of collagen fragments, interpretation of these bone resorption markers may be different compared to other antiresorptive drugs.
Decreases in markers of bone formation were modest and transient compared with those seen with other antiresorptive therapies (e.g., alendronate and risedronate) and were consistent with the non-significant decreases in bone-formation rate and mineralizing surface in the biopsy samples. This would be consistent with the hypothesised coupling of bone remodelling described earlier, which is enabled by the targeting of the enzyme produced by the osteoclast rather than reducing the number of osteoclasts. Indeed, when treated with odanacatib, TRAP5b (an index of osteoclast metabolic activity and cell number) was found to initially decrease but then recover to, or reach levels slightly higher than, that seen in the placebo group. This differs dramatically from the large decreases in TRAP5b seen with other antiresorptive agents [37].
The phase II study was subsequently extended by a year to look at further efficacy and safety as well as the effects of discontinuation [38]. After 2 years, patients (n = 189) were re-randomized to odanacatib 50 mg weekly or placebo for an additional year. Endpoints included BMD at the lumbar spine (primary), total hip, and hip sub-regions; levels of bone turnover markers; and safety assessments. Continued treatment with 50 mg of odanacatib for 3 years produced significant increases from baseline and from year 2 in BMD at the spine (7.9% and 2.3%) and total hip (5.8% and 2.4%). The cumulative gains in BMD seen with odanacatib are similar to those seen with alendronate and zoledronic acid, although BMD increases with odanacatib did not plateau over time, as has been observed with other antiresorptive therapies. Urine cross-linked N-telopeptide of type I collagen (NTx) remained suppressed by 50% over the 3 year period compared to a lowering of 17% in the placebo group. There were no differences in skin adverse effects or upper respiratory tract infections between the odanacatib and placebo groups, except an increase in cases reported and treated as urinary tract infections in the odanacatib group (n = 12 vs. 3), that did not lead to drug discontinuation. Because odanacatib does not persist in bone, it is not surprising that after its discontinuation, much of the bone density that had been gained in the initial 2 years was lost during the following year. In those that switched from active treatment to placebo, there was an initial rapid loss in BMD over 6 months, eventually levelling off to near-baseline levels. These data are more akin to the findings with hormone-replacement therapy, denosumab, and parathyroid hormone than the bisphosphonates [38].
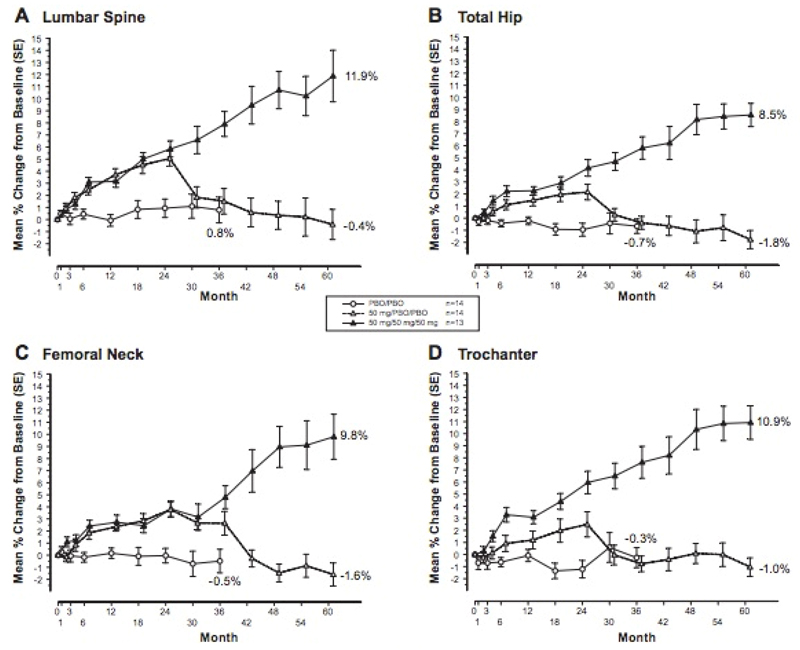
In a further extension to 5 years of this phase II trial, further monitoring of bone mineral density showed an ongoing almost linear increase from baseline at multiple sites with continuous treatment of odanacatib 50mg weekly. It also continued to show return of BMD to baseline or just below upon discontinuation of the odanacatib to placebo. Safety and adverse effects were not studied, as there was a crossover. This extension does contain small numbers (n = 13 to 14) in each arm but does show consistent results over measured sites and over time as described above (also see figure 2) [39].
Figure 2.
Percentage change from baseline in BMD over 5 years in continuous odanacatib 50mg weekly, versus discontinuation at month 24, versus placebo from months 0 to 36 [39]. – permission granted
Whilst BMD is conventionally an areal measure using DXA, volumetric information using quantitative computed tomography (QCT) scans are being used to provide further information on effects on cortical and trabecular bone. A 2-year international, randomized, double-blind, placebo-controlled phase 3 trial enrolled 214 postmenopausal women (mean age 64 years) with low areal BMD (T-score ≤-1.5 and ≥-3.5). Subjects were randomized to odanacatib 50 mg weekly or placebo, and all participants received calcium and vitamin D. Hip QCT scans at 24 months were available for 158 women (odanacatib: n=78; placebo: n=80). The cortical, subcortical, and trabecular volumetric BMD and bone mineral content of the proximal femur increased in postmenopausal women treated with odanacatib for 24 months compared to placebo [40].
The above data is promising, and an ongoing phase III fracture trial will try and translate this into the real world through reduction in fractures. LOFT is a randomized, double-blind, placebo-controlled, event-driven trial, including a pre-planned, blinded placebo-controlled extension study. The trial enrolled 16,713 women, 65 years of age or older, diagnosed with osteoporosis, who have been postmenopausal for five years or more. Patients were randomized to receive odanacatib 50 mg/week (n=8,357) or placebo (n=8,356). All patients received vitamin D (5600 IU/week) and calcium up to 1200 mg/day, if required. The three primary outcomes were radiologically determined vertebral, hip, and clinical non-vertebral fractures. Secondary end points included clinical vertebral fractures, BMD, bone turnover markers, and safety and tolerability, including bone histology [41].
The drug company producing Odanacatib, Merck, have released data from LOFT indicating that compared to patients receiving placebo, patients who received odanacatib had a: 54% relative risk reduction of new and worsening morphometric (radiographically-assessed) vertebral fractures, 47% relative risk reduction of clinical hip fractures, 23% relative risk reduction of clinical non-vertebral fractures, and 72% relative risk reduction of clinical vertebral fractures (all p<0.001). Adjudicated events of morphea-like skin lesions and atypical femoral fractures occurred more often in the odanacatib group than in the placebo group. Adjudicated major adverse cardiovascular events were generally balanced overall between the treatment groups. There were numerically more adjudicated stroke events with odanacatib than with placebo [42].
Anti Sclerostin Antibody
Romosozumab is a humanized monoclonal antibody that blocks sclerostin from inhibiting osteoblast maturation and function. A phase I multicenter, randomized, double-blind, placebo-controlled, ascending-dose study looked at the safety and tolerability of multiples doses of romosozumab as its primary study endpoints. For the 16 healthy men and 32 healthy postmenopausal women with low bone mass studied, multiple doses of romosozumab were well tolerated and associated with significant improvements in BMD of the lumbar spine in every dose cohort, with maximum increases from baseline that ranged from 4% to 7%. These improvements persisted from the end of treatment at 12 week, through to the end of follow up at 24 weeks after initiation of study treatment. Interestingly, in the pharmacodynamic analyses, multiple doses of romosozumab increased bone formation markers such as PINP, but decreased the bone resorption marker serum CTx, suggesting an uncoupling of remodeling [43]. Wnt activation resulting from the inhibition of sclerostin has been associated with decreased bone resorption both in humans and in animal models, probably owing to direct or indirect actions on osteoclasts through the Wnt pathway. The mechanism by which the sclerostin pathway interacts with bone resorption may involve the OPG-RANKL axis because the osteoclast inhibitor OPG is considered a downstream target of Wnt/-catenin signalling. However, in vivo data demonstrating an effect of Scl-Ab on serum OPG levels is limited [44].
A phase II, multicenter, international, randomised, placebo-controlled, parallel-group, eight-group study, in which the primary end point was the percentage change from baseline in BMD at the lumbar spine after 12 months treatment has reported. The eight groups were split into five varying doses of romosozumab, oral alendronate, subcutaneous teriparatide, or placebo injections. The 419 participants were postmenopausal females with a low BMD (T-score ≤-2.0 and ≥-3.5) and no prior fragility or vertebral fracture. Romosozumab was associated with a significant mean change in lumbar BMD at month 12 of +11.3% in the 210 mg monthly dose compared with a decrease of 0.1% in the placebo group and increases of 4.1% with alendronate and 7.1% with teriparatide. Assessment of bone turnover markers again showed the uncoupling of remodelling. Circulating markers of bone formation increased rapidly with romosozumab but returned to baseline values despite continued administration: increases were noted 1 week after the initial dose was administered and were greatest at month 1. Levels of bone formation marker returned to baseline values or fell below baseline values between months 2 and 9, depending on the dose and the marker. By comparison, a decrease in a circulating marker of bone resorption was maintained over the 12-month dosing period. The reason for the transitory nature of the effect on bone formation is unclear. It may be due to changes in counter-regulatory signaling pathways in the control of bone remodeling. The overall incidence of adverse events was balanced between groups, with the exception of the increased frequency of injection-site reactions in the romosozumab groups as compared with the other groups [45]. Phase III studies are currently underway.
Blosozumab is another anti-sclerostin antibody but remains in phase II development and has not advanced to phase III studies.
Parathyroid Hormone Related Protein (PTHrp)
PTHrP has been shown to be well tolerated, even at larger doses compared to that for teriparatide, the latter having issues with adverse effects and mild hypercalcemia at higher doses. Initial studies also showed that there were rapid increases in spine BMD of 6-8% in 3 months, and that it appeared to be a pure skeletal anabolic agent with no associated increased in bone resorption markers seen [46].
Abaloparatide is a novel synthetic peptide analog of PTHrP. There has been a multi-center, multi-national, double-blind placebo controlled trial in which postmenopausal women were randomly assigned to receive 24 weeks of treatment with daily subcutaneous injections of placebo, abaloparatide, 20, 40, or 80 μg, or teriparatide, 20 μg. At 24 weeks, lumbar spine BMD increased by 2.9, 5.2, and 6.7% in the abaloparatide, 20-, 40-, and 80-μg groups, respectively, and 5.5% in the teriparatide group. The increases in the 40- and 80-μg abaloparatide groups and the teriparatide group were significantly greater than placebo (1.6%). Femoral neck BMD increased by 2.7, 2.2, and 3.1% in abaloparatide, 20-, 40-, and 80-μg groups, respectively, and 1.1% in the teriparatide group. The increase in femoral neck BMD with abaloparatide, 80 μg was significantly greater than placebo (0.8%). Total hip BMD increased by 1.4, 2.0, and 2.6% in the abaloparatide, 20-, 40-, and 80-μg groups, respectively. The total hip increases in the 40- and 80-μg abaloparatide groups were greater than both placebo (0.4%) and teriparatide (0.5%) [47]. Phase III trials are underway but not yet published in a peer-reviewed journal. The Phase III ACTIVE study has been reported to show a similar 80% reduction in vertebral fracture rate with abaloparatide compared to teriparatide, but a statistically significant reduction in non-vertebral fractures with abaloparatide compared with placebo, and this was not seen with teriparatide compared with placebo [48].
Conclusions
The range of treatments available to us to manage osteoporosis is expanding as we gain a better understanding of the pathophysiology of bone remodelling and bone fragility. Interestingly an uncoupling of bone formation and resorption has been found in the newer drugs in development that favours increased bone mineral density. As yet the long-term effects of our new drugs are obviously uncertain, just as the atypical fractures with the use of bisphosphonates over long periods have become apparent with time. Although the newer drugs all show significant increases in lumbar bone mineral density, we do not yet have evidence that these newer treatments are any better than existing treatments at reducing the risk of non-vertebral fractures. Newer treatments (odanacatib, romosozumab, and abaloparatide) are likely to be used as second or third line treatments after an initial period of 3 to 5 years on a bisphosphonate, to try and increase BMD and counter concerns about continuing suppression of bone remodelling and possible implications for atypical fracture. Guidance in many countries including the UK dictates the first choice of agent used in osteoporosis; there is a current evidence gap around effectiveness of agents used in different sequence, although, for example, observational data have suggested that the effectiveness of teriparatide therapy is not removed by prior treatment with bisphosphonate treatment. A well-tolerated drug with minimal long-term adverse effects, that equally reduces risk of non-vertebral fractures as well as vertebral fractures, has not yet been found but remains the holy grail. No drug is effective if the patient fails to take it; a particular challenge is ensuring good compliance.
Over the next five years we should have more long term phase III results to place these newer treatments amongst our existing options. Current efforts have been geared towards bone but neglect the link between muscle and bone, and an integrated approach may be more effective in treating musculoskeletal aging to reduce falls and fractures. For this reason we may expect new treatments to embrace both osteoporosis and sarcopenia. Sarcopenia is the progressive and generalized loss of muscle mass that can lead to a loss of strength or performance. Whilst it is more established that muscle and bone interact anatomically and through biomechanical signals through “mechanostat theory”, the paracrine and endocrine signals that co-ordinate their development is a more recent research point. Studies suggest a bone-muscle unit where the skeletal muscle secretome releases molecules that affect bone, which in turn produce osteokines from osteoblasts and osteocytes that impact muscle cells. These molecular factors are not yet fully identified. Newly emerging pathways involving these molecular factors includes activin-signaling inhibitors such as myostatin-neutralising antibodies-propeptides, recombinant follistatin, follistatin derivatives and soluble activin receptors or myokines. More central regulation of both bone and muscle could also be targeted and includes possible targets of growth hormone and growth hormone secretagogues, androgens, and selective androgen receptor modulators (SARMs) [49].
However, there have been safety concerns with these agents related to off-target effects of manipulating widespread signalling pathways. For instance, activin pathway inhibitors have been reported to cause telangiectasia, bleeding and gonadotropin suppression. SARMs have been specifically targeted to muscle and bone to try and avoid the undesirable effects of androgens. One example of a SARM is ostarine and phase I-III clinical trials have shown an increase in lean mass and physical function in elderly men, post-menopausal women and cancer patients. However, effects on BMD have not been demonstrated, possibly due to short study periods of up to 3 months [50]. Beyond five years, in the long term, these agents may supplant existing treatments for patients either over post-menopausal age with low BMD or an equivalent to be defined state of sarcopenia.
Genomics may also potentially help identify potential novel osteoporosis drug targets. Sclerostin has already been identified as a Wnt signalling target, but other potential targets include LRP4, LRP5/LRP6, SFRP4, WNT16 and NOTUM. NOTUM is a lipase that inactivates WNTs by removing the palmitoleic acid group needed to bind to frizzled receptors. Notum KO mice have been found to have elevated cortical bone thickness and strength [51].
In conclusion, a greater understanding of bone pathophysiology is offering us newer treatments in the forms of cathepsin K inhibitor, monoclonal antibodies against sclerostin, and parathyroid hormone related peptide, all with promising data on BMD, and positive preliminary reports on reduced fracture rates for abaloparatide and odanacatib.
References
- 1.Friedman AW. Important determinants of bone strength: beyond bone mineral density. J Clin Rheumatol. 2006;12:70–77. doi: 10.1097/01.rhu.0000208612.33819.8c. [DOI] [PubMed] [Google Scholar]
- 2.Czerwiński E, Badurski JE, Marcinowska-Suchowierska E, Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil. 2007;9(4):337–56. [PubMed] [Google Scholar]
- 3.Melton LJ, III, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective: How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–10. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 4.British Orthopaedic Association. The care of patients with fragility fracture guidelines. 2007 [Google Scholar]
- 5.National Hip Fracture Database National report – Summary. 2013 http://www.nhfd.co.uk/20/hipfractureR.nsf/0/CA920122A244F2ED802579C900553993/$file/NHFD%20Report%202013.pdf.
- 6.Sernbo I, Johnell O. Consequences of a hip fracture: a prospective study over 1 year. Osteoporos Int. 1993;3(3):148–53. doi: 10.1007/BF01623276. [DOI] [PubMed] [Google Scholar]
- 7.Burge RT, Worley D, Johansen A, et al. The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ. 2001;4:51–52. [Google Scholar]
- 8.Fracture risk assessment. c2011 Https://wwwshefacuk/FRAX/ Retrieved 22 June, 2016, from https://www.shef.ac.uk/FRAX/
- 9.Curtis EM, Moon RJ, Dennison EM, Harvey NC, Cooper C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin Med (Lond) 2015 Dec;15(Suppl 6):s92–6. doi: 10.7861/clinmedicine.15-6-s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compston J, et al. National Osteoporosis Guideline Group (NOGG) Mar, 2014. Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. [Google Scholar]
- 11.Tang BMP, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet (London, England) 2007;370(9588):657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 12.Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. doi: 10.1136/bmj.b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bone HG, Hosking D, Devogelaer J-P, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–99. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 14.Patrick AR, Brookhart MA, Losina E, et al. The complex relation between bisphosphonate adherence and fracture reduction. J Clin Endocrinol Metab. 2010;95(7):3251–9. doi: 10.1210/jc.2009-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan A. Bisphosphonate-associated osteonecrosis of the jaw. Can Fam physician Médecin Fam Can. 2008;54(7):1019–21. [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicine Agency: Strontium ranelate: cardiovascular risk. 2014 [Google Scholar]
- 17.Khosla S, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral, Research,American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 18.Bone HG, Chapurlat R, Brandi M-L, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98(11):4483–92. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 20.Cosman F. Combination therapy for osteoporosis: a reappraisal. Bonekey Rep. 2014;3:518. doi: 10.1038/bonekey.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res. 2007;22(10):1502–9. doi: 10.1359/jbmr.070609. [DOI] [PubMed] [Google Scholar]
- 23.Stepan JJ, Burr DB, Pavo I, et al. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone. 2007;41(3):378–85. doi: 10.1016/j.bone.2007.04.198. [DOI] [PubMed] [Google Scholar]
- 24.Rizzoli R, Åkesson K, Bouxsein M, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: A European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011;22(2):373–390. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai PA, Vyas PA, Lane JM. Atypical femoral fractures: a review of the literature. Curr Osteoporos Rep. 2013;11(3):179–87. doi: 10.1007/s11914-013-0167-y. [DOI] [PubMed] [Google Scholar]
- 26.Jensen PR, Andersen TL, Pennypacker BL, Duong LT, Engelholm LH, Delaissé JM. A supra-cellular model for coupling of bone resorption to formation during remodeling: Lessons from two bone resorption inhibitors affecting bone formation differently. Biochem Biophys Res Commun. 2014;443(2):694–699. doi: 10.1016/j.bbrc.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Jensen PR, Andersen TL, Pennypacker BL, Duong LT, Delaissé J-M. The bone resorption inhibitors odanacatib and alendronate affect post-osteoclastic events differently in ovariectomized rabbits. Calcif Tissue Int. 2014;94(2):212–22. doi: 10.1007/s00223-013-9800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennypacker BL, Duong LT, Cusick TE, et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011;26(2):252–62. doi: 10.1002/jbmr.223. [DOI] [PubMed] [Google Scholar]
- 29.Cusick T, Chen CM, Pennypacker BL, et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res. 2012;27(3):524–537. doi: 10.1002/jbmr.1477. [DOI] [PubMed] [Google Scholar]
- 30.Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14(10):1654–63. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 31.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 33.Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest. 2005;115(9):2322–4. doi: 10.1172/JCI26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao D, He B, Jiang Y, et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. 2005;115(9):2402–11. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou WS, Li Z, Gordon RE, et al. Cathepsin k is a critical protease in synovial fibroblast-mediated collagen degradation. Am J Pathol. 2001;159(6):2167–77. doi: 10.1016/S0002-9440(10)63068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoch SA, Zajic S, Stone J, et al. Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther. 2009;86(2):175–82. doi: 10.1038/clpt.2009.60. [DOI] [PubMed] [Google Scholar]
- 37.Bone HG, McClung MR, Roux C, et al. Odanacatib, a Cathepsin-K Inhibitor for Osteoporosis: A Two-Year Study in Postmenopausal Women With Low Bone Density. J Bone Miner Res. 2009;25(5) doi: 10.1359/jbmr.091035. 091029141139034–41. [DOI] [PubMed] [Google Scholar]
- 38.Eisman JA, Bone HG, Hosking DJ, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26(2):242–251. doi: 10.1002/jbmr.212. [DOI] [PubMed] [Google Scholar]
- 39.Langdahl B, Binkley N, Bone H, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Five years of continued therapy in a phase 2 study. J Bone Miner Res. 2012;27(11):2251–2258. doi: 10.1002/jbmr.1695. [DOI] [PubMed] [Google Scholar]
- 40.Engelke K, Fuerst T, Dardzinski B, et al. Odanacatib treatment affects trabecular and cortical bone in the femur of postmenopausal women: results of a two-year placebo-controlled trial. J Bone Miner Res. 2015;30(1):30–8. doi: 10.1002/jbmr.2292. [DOI] [PubMed] [Google Scholar]
- 41.Bone HG, Dempster DW, Eisman JA, et al. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the Long-Term Odanacatib Fracture Trial. Osteoporos Int. 2015;26(2):699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merck Announces Data from Pivotal Phase 3 Fracture Outcomes Study for Odanacatib, an Investigational Oral, Once-Weekly Treatment for Osteoporosis [Internet] 2014 Available from: http://www.mercknewsroom.com/news-release/research-and-development-news/merck-announces-data-pivotal-phase-3-fracture-outcomes-st.
- 43.Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014;54(2):168–78. doi: 10.1002/jcph.239. [DOI] [PubMed] [Google Scholar]
- 44.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33(5):747–83. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 45.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz MJ, Tedesco MB, Sereika SM, et al. Safety and tolerability of subcutaneous PTHrP(1-36) in healthy human volunteers: a dose escalation study. Osteoporos Int. 2006;17(2):225–30. doi: 10.1007/s00198-005-1976-3. [DOI] [PubMed] [Google Scholar]
- 47.Leder BZ, O’Dea LSL, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 48.Tucker M. Novel Agent Abaloparatide Reduces Fractures, Including Wrist [Internet] 2015 Available from: http://www.medscape.com/viewarticle/841015.
- 49.Reginster J-Y, Beaudart C, Buckinx F, Bruyère O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19(1):31–6. doi: 10.1097/MCO.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girgis CM. Integrated therapies for osteoporosis and sarcopenia: from signaling pathways to clinical trials. Calcif Tissue Int. 2015;96(3):243–55. doi: 10.1007/s00223-015-9956-x. [DOI] [PubMed] [Google Scholar]
- 51.Brommage R. Genetic Approaches To Identifying Novel Osteoporosis Drug Targets. J Cell Biochem. 2015;116(10):2139–2145. doi: 10.1002/jcb.25179. [DOI] [PubMed] [Google Scholar]