Abstract
NK cells are the first line of defense against infected and transformed cells. Defective NK cell activity has been shown to increase susceptibility for viral infections and reduce tumor immune-surveillance. With age, the incidence of both infectious diseases and malignancy rises dramatically suggesting that impaired NK cell function might contribute to disease in these individuals. We found an increased frequency of NK cells with high expression of the inhibitory Killer Cell Lectin-like Receptor G1 (KLRG1) in individuals >70 years. The role of KLRG1 in ageing is not known and the mechanism of KLRG1-induced inhibition of NK cell function is not fully understood. Here we report that NK cells with high KLRG1 expression spontaneously activate the metabolic sensor AMP-activated protein kinase (AMPK) and that activation of AMPK negatively regulates NK cell function. Pre-existing AMPK activity is further amplified by ligation of KLRG1 in these cells, which leads to internalization of the receptor and allows interaction with AMPK. We show that KLRG1 activates AMPK by preventing its inhibitory de-phosphorylation by protein phosphatase PP2C rather than inducing de novo kinase activation. Finally, inhibition of either KLRG1 or AMPK prevented KLRG1-induced activation of AMPK and reduction in NK cell cytotoxicity, cytokine secretion, proliferation and telomerase expression. This novel signaling pathway links metabolic sensing, effector function and cell differentiation with inhibitory receptor signaling that may be exploited to enhance NK cell activity during ageing.
Introduction
The increase of human life expectancy is associated with a greater incidence and severity of many infections and malignancy in older subjects (1–4). The identification of mechanisms that are responsible for the immune decline is therefore essential for the rationalization of ways to improve health during later life. The expansion of T lymphocytes after immune activation takes a finite time resulting in a lag phase before sufficient numbers of T cells are generated (5). During this period, the host is vulnerable to infections that spread rapidly and/or cause severe pathology and this is prevented by NK cells (6). NK cells are a first line of defense against viral infections through their cytotoxic activity and ability to secrete cytokines without prior activation (7, 8). Individuals with rare genetic NK cell defects are susceptible to lethal herpes virus infections early after infection but recover when optimal T cell responses are mobilized 1-2 weeks later (9). Therefore, the efficient function of both T and NK cells are required to combat infection at different phases of the immune response. Although NK cell numbers generally increase during ageing with a shift from an undifferentiated CD56bright to a differentiated CD56dim phenotype (10–13) these cells have reduced cytotoxicity and also decreased capacity to secrete cytokines such as IFN-γ, MIP-1α and IL-8 (14). This reduction in NK cell function may lead to decreased immunity especially during the early stages of infection in older subjects (6) and lead to an increased susceptibility and greater severity of viral infections in this population.
NK cell function is determined by a balance of activating and inhibitory signals delivered by activating receptors which recognize stress-induced ligands and inhibitory receptors which engage MHC class I or MHC class-like molecules on healthy cells, respectively (15, 16). KLRG1 is a C-type lectin-like inhibitory receptor with an immune receptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain (17). It binds to the ubiquitously expressed cell adhesion molecules E-, N- and R-Cadherins (18) and binding of E-Cadherin to KLRG1 prevents lysis of E-Cadherin-expressing target cells (18). In humans, KLRG1 is expressed by 50-80% of NK and 20-40% of T cells (19). Expression of the receptor is found on mature (CD56dim) NK cells (19, 20) and terminally differentiated T cells (18, 21, 22). While KLRG1 expression on T cells was shown to dramatically increase with age (21–25), data on KLRG1 expression on NK cells in elderly individuals is scarce and controversial (26, 27). In a previous study by Hayhoe et al (27), KLRG1 expression was found to be decreased instead of increased in old subjects. However unlike in the present investigation where all the samples were freshly isolated, the study by Hayhoe et al used a mixture of fresh and frozen samples for the analysis. Recent data from our group suggest that KLRG1 expression is reduced considerably during cryopreservation which would explain the discrepancy between the data (unpublished observations). High KLRG1 expression correlates with low proliferative capacity (28, 29) impaired IFN-γ secretion (29, 30) and increased apoptosis (29) in NK cells. Moreover, in patients chronically infected with hepatitis C, blockade of KLRG1 signaling restored defective protein kinase B (PKB/Akt) phosphorylation and IFN-γ secretion (29) suggesting that KLRG1 actively contributes to functional defects observed in KLRG1-positive cells.
AMP-responsive protein kinase (AMPK) is a protein sensor that integrates intracellular cues such as low ATP levels and DNA damage signals to regulate cell function (31, 32). We previously identified a novel AMPK-dependent pathway in highly differentiated CD4+ T cells that is activated by senescence and low nutrient sensing signals to inhibit proliferation and telomerase activity (32). Once active, AMPK triggers p38 MAP-Kinase activation and the inhibition of either molecule restores proliferation and telomerase activity (32). Since terminally-differentiated NK cells with impaired immune function accumulate during ageing (14) we hypothesized that the decline in NK cell function during ageing may also be regulated by an analogous AMPK-dependent mechanism. We found that independently of its canonical inhibitory target Akt (22, 29) KLRG1 stimulated AMPK activity in NK cells with high KLRG1 expression (KLRG1bright) and signaling through this pathway inhibited NK cell cytotoxicity, IFN-γ production, proliferation and telomerase. Furthermore, KLRG1 was internalized upon ligation, bound to AMPK directly and prevented its inhibitory de-phosphorylation by PP2C phosphatases. This is a hitherto unrecognized mechanism of cell surface inhibitory receptor function in lymphocytes where AMPK activation is amplified through protection from de-phosphorylation, rather than de novo kinase activation. The central implication of these observations is that the inhibition of the KLRG1 / AMPK signaling may restore NK cell function that is relevant for immune enhancement during ageing and in patients with malignancy.
Materials and Methods
Study population and blood sample collection
After written informed consent was obtained, heparinized peripheral blood samples were taken from healthy volunteers without previous record of an autoimmune or malignant disease. Where donors are stratified by age, young is defined as ≤35 years old (age range 20-34 years, median 27 years, mean 25.8 years) and old as ≥70 years of age (age range 70-86 years, median 75 years, mean 76.9 years).
Cell Isolation
PBMC were isolated from heparinized peripheral blood using Ficoll hypaque (Amersham Biosciences). NK cells were isolated from PBMC with the NK Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. To obtain NK cell subsets with differential KLRG1 expression, NK cells were first labeled with a biotinylated anti-KLRG1 antibody (BioLegend), washed and then labeled with Anti-Biotin beads (Miltenyi Biotec) according to the manufacturer’s instructions. KLRG1bright NK cells were obtained by passing KLRG1-labeled NK cells through a magnetic column (Miltenyi Biotech) where KLRG1bright NK cells were retained (positive fraction). Flow-through was run over two additional magnetic columns sequentially to obtain KLRG1dim NK cells (positive fraction). All remaining cells were considered KLRG1neg (negative fraction). Cell surface KLRG1 expression after cell isolation was determined with Streptavidin-Cy5 (BioLegend) or a directly labeled anti-KLRG1 antibody (BioLegend) (Fig. S1B).
Cell culture
NK cells were cultured in complete medium (RPMI-1640 supplemented with 10% heat-inactivated FCS, 100 U/ml Penicillin, 100 mg/ml Streptomycin, and 2 mM L-glutamine; all from Invitrogen) unless otherwise stated. For glucose deprivation experiments (6h or over night) glucose-free RPMI-1640 (Life Technologies) was used. MCF-10A cells were cultured in DMEM/F12 medium (Invitrogen) containing 5% horse serum (Life Technologies), 20 ng/ml Epidermal Growth Factor (EGF, PeproTech), 0.5 mg/ml Hydrocortisone (Sigma-Aldrich), 100 ng/ml Cholera Toxin (Sigma-Aldrich), 10 μg/ml Insulin (Sigma-Aldrich), 100 U/ml Penicillin, and 100 mg/ml Streptomycin (both from Invitrogen).
Flow Cytometry
The following antibodies (all from BioLegend unless otherwise indicated) were used: anti-KLRG1-biotin (2F1), anti-KLRG1-PE (2F1), anti-CD3 (UCHT1, BD BioScience), anti-CD56 (HCD56), anti-CD16 (3G8, BD BioScience), and anti-CD7 (M-T701). Biotin-conjugated antibodies were detected using Cy5- or Cy3-conjugated Streptavidin (BioLegend). Annexin V staining was performed using Annexin V Binding Buffer and Annexin V (both from BioLegend). Ki67 staining was performed (B56, BD BioScience) with the Foxp3 Staining Set (Miltenyi Biotec) according to the manufacturer’s instructions. When using total PBMC, NK cells were identified as CD3-CD7+ lymphocytes (33) (Fig. S1A). All samples were acquired on a LSRII flow cytometer (BD Biosciences). Data were analyzed using FlowJo®_V10 software (Tree Star, Ashland, OR).
Phosphoflow Cytometry
After cell surface staining on ice, cells were fixed with Cytofix Buffer (BD Biosciences) for 10 min at 37°C and permeabilized with ice-cold Perm Buffer III (BD Biosciences) followed by staining with the following antibodies for 30 min at room temperature: anti-p-AMPKα (Thr172) rabbit mAb (40H9), anti-p-ATM (Ser1981) rabbit mAb (D6H9), anti-γH2Ax-PE (Ser139) mouse mAb (20E3) (all from Cell Signaling Technology), and anti-p-Akt (Ser473) mouse mAb (M89-61, BD Bioscience). Primary antibodies were detected with goat-anti-rabbit or goat-anti-mouse or donkey anti-rabbit IgG (all from Life Technologies) for 30 min at room temperature. All samples were acquired immediately after the staining on a LSRII flow cytometer (BD Biosciences). Data were analyzed using FlowJo®_V10 software (Tree Star, Ashland, OR).
Calcein release cytotoxicity assay
The cytotoxic capacity of NK cells was assessed using the E-Cadherin expressing breast cancer epithelial cell line MCF-10A as target. Briefly, 20,000 MCF-10A cells were plated/well of a 96 flat-bottom plate 24 hours prior to the cytotoxicity assay. On the day of the assay, MCF-10A cells were labeled with Calcein-AM (Sigma-Aldrich) at 10μM for 1 hour before co-culture with NK cells in complete medium containing 500 IU/ml rhIL-2 (Miltenyi Biotech) and as described elsewhere (34). NK cells transfected with siRNA specific for KLRG1 or scrambled control siRNA for 36 hours were pre-treated with the AMPK agonist A769662 (Tocris Bioscience) at 150 μM or equivalent DMSO as negative control for 2 hours before being added to the target cells. Effector and target cells were combined at a ratio of 40:1 in triplicate and cultured in complete medium containing 500 IU/ml rhIL-2 (Miltenyi Biotech) for 4 hours. After 4 hours of co-culture fluorescence was measured in 75 μl of cell culture supernatant using a Spectramax Gemini spectrofluorimeter. Specific lysis was calculated as % killing = (test release–spontaneous release) / (max release–spontaneous release) x 100.
Granzyme B and IFN-γ analysis
For quantification of granzyme B and IFN-γ in cell culture supernatants a Cytometric Bead Array Assay was used (Human Granzyme B and Human IFN- γ Flex Set from BD Biosciences). Measurements were performed according to the manufacturer’s instructions. The lower limit of detection was 4 pg/ml for Granzyme B and 0.8 pg/ml for IFN- γ.
Introduction of siRNA into primary human NK cells
siRNA was introduced into freshly isolated KLRG1bright NK cells by electroporation using the Amaxa® Human NK Cell Nucleofector® Kit and Nucleofector® technology (Lonza) according to the manufacturer’s instructions. Briefly, 3x106 freshly isolated KLRG1bright NK cells were resolved in Nucleofector® solution provided by the manufacturer and 200 nmol PP2C (PP2Cα siRNA, sc-42937, Santa Cruz Biotechnology) or 300 nmol KLRG1 siRNA (KLRG1 siRNA, sc-42937, Santa Cruz Biotechnology). Nucleofection was performed with the Nucleofector™ I Device Program U-01 (Lonza). Cells were then transferred into 12-well cell culture plates containing pre-equilibrated complete medium and incubated for 36 hours. NK cells transfected with KLRG1 siRNA were cultured in complete medium containing 500 IU/ml rhIL-2 (Miltenyi Biotech). Knock-down efficiency of KLRG1 siRNA was monitored by measuring cell surface expression of KLRG1 by flow cytometry (Fig. S3A). Knock-down of PP2Cα was confirmed by immunoblot analysis (rabbit-anti-PP2Cα mAb, clone D18C10, XP, from Cell Signaling Technology) (Fig. S3B). In all experiments a scrambled control siRNA (sc-37007, Santa Cruz Biotechnology) was used.
Lentiviral transduction of primary human NK cells
Freshly isolated KLRG1bright NK cells were pre-activated with K562 target cells (effector : target ratio = 10:1) and 200 IU rhIL-2 (Miltenyi Biotech) for 48 hours. On day 2, cells were transduced with pHIV1-Siren lentiviral particles as previously described (32). On day 6 of culture, cells were transferred to a 96-well plate coated with an anti-KLRG1 mAb (BioLegend) or a IgG control Ab (BioLegend) both at 10 µg/ml and K562 target cells were added at an effector : target ratio = 10:1 and fresh IL-2 at 200 IU/ml. On day 9 of culture, apoptosis was assessed by Annexin V staining and proliferation by Ki67 staining. Telomerase reverse transcriptase (hTERT) expression was measured by intranuclear staining with the anti-TERT mAb (ab68781, Abcam). On day 9, NK cells were re-stimulated with K562 target cells at an effector : target ratio = 10:1 and fresh IL-2 at 200 IU/ml and cultured for a total of 12 days (for experimental design see Fig. S4A).
Immunoprecipitation
For co-immunoprecipitation of AMPK, PP2C and KLRG1 cell lysate from 10x106 isolated KLRG1bright NK cells was prepared as previously described (32). Cell lysate was split into two and incubated either with a polyclonal anti-AMPKα antibody or an isotype control antibody (both from Cell Signaling Technology). Immunoprecipitated proteins were then detected by immunoblot analysis with an anti-KLRG1 (ab170959; Abcam), PP2C and AMPK antibody (both from Cell Signaling Technology) and confirmation-specific mouse anti-rabbit IgG antibody (L27A9; Cell Signaling Technology) or a mouse antibody to rabbit IgG light chain (L57A3; Cell Signaling Technology), followed by a secondary anti-mouse IgG antibody (7076; Cell Signaling) and ECL Prime Western detection kit (GE Healthcare).
In vitro Phosphatase assay
AMPK immunoprecipitates were obtained as described above. After extensive washing, PP2C-like (PP2ase) activity was measured using the ProFluor® Ser/Thr PPase Assay according to the manufacturer’s instructions (Promega), and normalized against total levels of immunoprecipitated AMPK. Immunoprecipitated AMPK was detected by ELISA-based assays according to the manufacturer’s instructions (ab181422; Abcam).
Image Stream flow cytometry
Purified KLRG1dim and KLRG1bright NK cells were incubated with either AMPK agonist A-769662 (150 μM) or DMSO as a negative control for 12 hours followed by KLRG1 ligation or an isotype control antibody (both from BioLegend) for 2 h at 37°C. Subsequently, cells were fixed with 2% paraformaldehyde at 37°C for 10 min and permeabilized with ice-cold Perm Buffer III (BD Biosciences) for 30 min on ice. KLRG1 was stained with a biotinylated anti-KLRG1 mAb followed by Streptavidin-Cy5 staining (both from BioLegend). p-AMPKα was stained with an anti-p-AMPKα (Thr172) rabbit mAb and goat-anti-rabbit IgG (both from Cell Signaling Technology). Samples were run on an Amnis® Image Stream cytometer using INSPIRE® software, magnification 60x. Data were compensated and analyzed using IDEAS® v.6.1 software (Amnis). Co-localization of KLRG1 and p-AMPK was determined on a single cell basis using Bright Detail Similarity (BDS) Score analysis. Co-localization was considered as a BDS of ≥ 2.0.
Statistics
GraphPad Prism software was used to perform all statistical analyses. For parametric data Student’s t-test or repeated measures ANOVA test with Greenhouse-Geisser correction were used. For non-parametric data Wilcoxon matched-pairs signed rank test or Friedman test were used. P values <0.05 were considered as significant.
Ethical Approval
The present study was approved by the Ethical Committee of the Royal Free and University College London Medical School. Written informed consent was obtained from all study participants.
Results
KLRG1bright NK cells accumulate with age
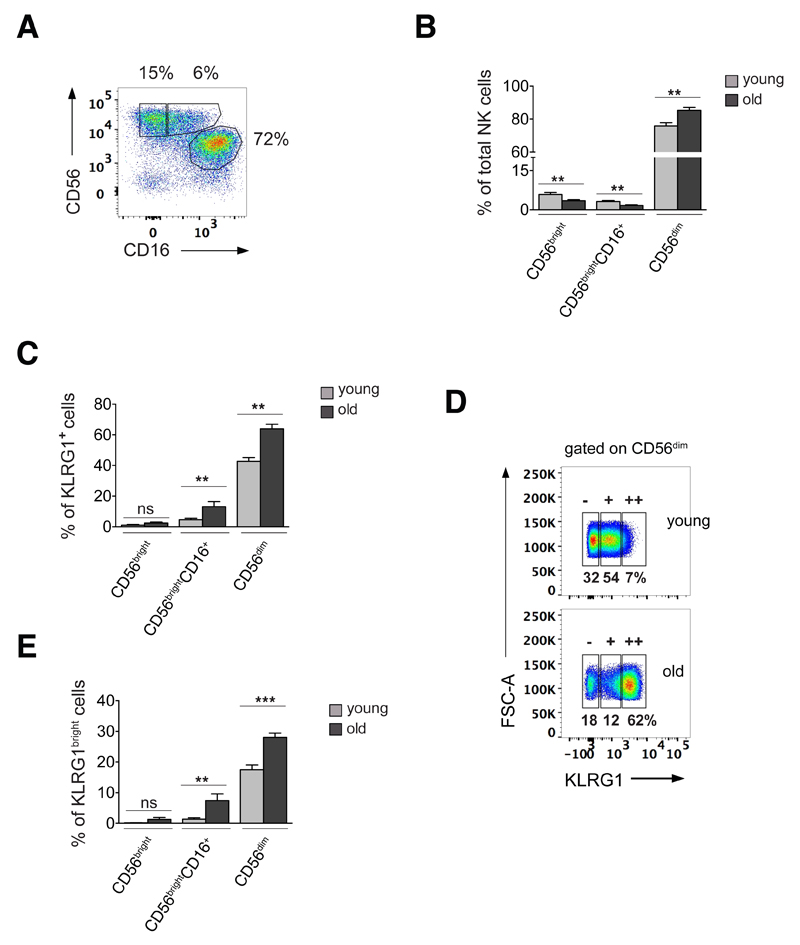
On the basis of their relative expression of CD56 and CD16, we identified three subsets of human NK cells in peripheral blood: CD56dim NK cells (CD56+CD16++, 80.5 ± 7.2%), CD56bright (CD56++CD16-, 4.6 ± 2.8%) and CD56brightCD16+ (CD56++CD16+, 2.39 ± 1.2%) NK cells (Fig. 1A, Fig. S1A). When comparing relative NK cell numbers between young (≤ 35 years) and old (≥ 70 years) donors, we found, consistent with reports from other groups (13, 35, 36), that the percentage of CD56dim NK cells was significantly increased in older individuals while the percentage of the more immature CD56bright and CD56brightCD16+ NK cell subsets was reduced compared to young donors (Fig. 1B). When comparing expression of KLRG1 on NK cell subsets in young and old donors, we found that irrespective of age, KLRG1 expression was predominant within the more differentiated CD56dim NK cell subset (Fig. 1C). Moreover, old donors had an increase in the frequency of KLRG1-expressing NK cells compared to young donors, and this was true for the CD56brightCD16+ and CD56dim NK cell subsets (Fig. 1C). In addition to an increase in KLRG1-positive NK cells, we found a higher expression level of KLRG1 in NK cells of old compared to young donors (Fig. 1D). Since the inhibitory capacity of KLRG1 is known to be directly proportional to the cell surface expression level of the receptor (37), we stratified CD56dim NK cells, the main NK cell population expressing KLRG1, based on their relative expression of the receptor into cells with no KLRG1 (KLRG1neg), intermediate (KLRG1dim) or high (KLRG1bright) expression levels (Fig. 1D). Using this approach, we found a significant increase in the percentage of KLRG1bright NK cells in old donors compared to young (Fig. 1E). This was most pronounced within the CD56dim NK cell compartment, while KLRG1 expression was low in CD56bright NK cells of both age groups (Fig. 1C and 1E).
Figure 1. KLRG1bright NK cells accumulate with age.
(A) NK cell subsets in peripheral blood stratified by CD56 and CD16 expression into CD56bright (CD56++CD16-), CD56brightCD16+, and CD56dim (CD56+CD16++) NK cells from one representative young donor (≤35 years) and (B) cumulative data from 15 young (≤35 years, gray bars) and 15 old donors (≥70 years; black bars) analysed as in Fig. 1A. (C) Percentages of KLRG1-positive NK cells within each NK cell subset defined as in Fig. 1A and as assessed in 15 young (gray bars) and 15 old donors (black bars). (D) CD56dim NK cells were further divided into cells expressing no KLRG1 (KLRG1neg), intermediate (KLRG1dim) and high levels of the receptor (KLRG1bright) indicated by -, +, and ++ respectively. One representative young and one representative old donor are shown. Numbers indicate percentages of KLRG1neg, KLRG1pos and KLRG1bright NK cells in total CD56dim NK cells. (E) Percentage of KLRG1bright NK cells in each NK cell subset as defined in Fig. 1A in 15 young (gray bars) and 15 old donors (black bars). All experiments were performed on total PBMC applying the gating strategy illustrated in Fig. S1A. All bars represent mean values ± SEM, p-values were calculated using unpaired Student’s t-Test.
KLRG1 activates AMPK signaling in KLRG1bright NK cells
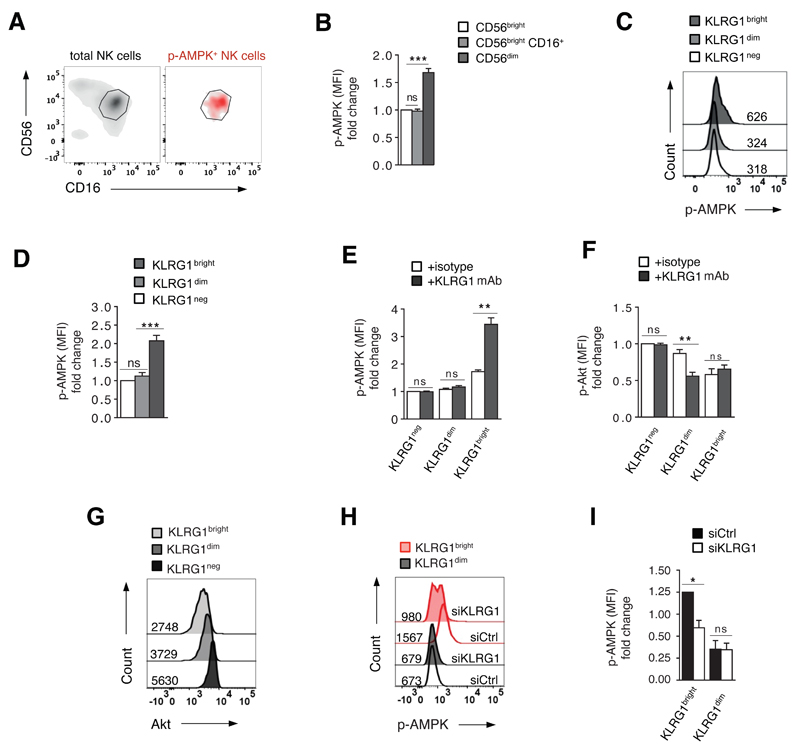
We have previously shown that AMPK is spontaneously active (Thr172 phosphorylation in the AMPK activation loop) in highly differentiated / senescent T cells (32), and we questioned whether this also occurred in CD56dim NK cells that accumulate with age. We gated on the three NK cell populations as defined in Fig. 1A, and found that AMPK was only active in the CD56dim NK cell subset (Fig. 2A and 2B). Since these cells also express high levels of KLRG1 (Fig. 1C-E), we investigated whether both molecules were detectable within the same cell (Fig. 2C). When we isolated NK cells on the basis of negative, dim or bright KLRG1 expression by magnetic bead isolation (Fig. S1B), we found that only KLRG1bright NK cells exhibited spontaneous AMPK activation (Fig. 2C and 2D). Functional analysis of KLRG1bright NK cells revealed low proliferative activity (Fig. S2A), telomere shortening (Fig. S2B) and low expression of the catalytic component of telomerase (hTERT; Fig. S2C), as well as endogenous phosphorylation of both the DNA damage response protein H2AX (γH2Ax; Fig. S2D) and its upstream activator, Ataxia telangiectasia mutated protein (p-ATM; Fig. S2E), compared to KLRG1dim and KLRG1neg NK cells. This data indicate that KLRG1bright cells express multiple characteristics of cellular senescence (38, 39) and suggest that activation of AMPK in KLRG1bright NK cells may be derived from cell senescence signals (32).
Figure 2. KLRG1 activates AMPK signaling in KLRG1bright NK cells.
(A) CD56dim NK cells spontaneously activate AMPK (Thr172). Gray density plot shows total NK cells isolated directly ex vivo from one representative old donor, stratified to CD56 and CD16 expression. Red density plot shows only NK cells that spontaneously activate AMPK (AMPK phosphorylation at Thr172 assessed directly ex vivo) within total NK cells isolated directly ex vivo from the same donor stratified to CD56 and CD16. The gate indicates CD56dim NK cells. (B) Cumulative data from 10 donors showing spontaneous AMPK-phosphorylation (Thr172) in NK cell subsets within total PBMC as described in Fig. S1A. Data are shown as fold change in MFI of p-AMPK normalized to the CD56bright population. (C) Phosphorylation of AMPK (Thr172) as analysed directly ex vivo in purified KLRG1neg, KLRG1dim and KLRG1bright NK cells from the same representative donor. Numbers next to histograms indicate MFI of p-AMPK (Thr172). (D) Cumulative data from 10 donors showing AMPK-phosphorylation in KLRG1bright, KLRG1dim and KLRG1neg NK cells as assessed directly ex vivo in total PBMC. Data are shown as fold change in MFI of p-AMPK (Thr172) normalized to KLRG1neg NK cells. (E) KLRG1 ligation in freshly purified KLRG1neg, KLRG1dim and KLRG1bright NK cell subsets enhances AMPK activity in KLRG1bright NK cells only, whereas Akt inhibition is only seen in KLRG1dim cells (F); pooled data from 5 independent experiments all performed on freshly isolated KLRG1 subsets. (G) Akt expression in freshly purified KLRG1neg, KLRG1dim and KLRG1bright NK cell subsets assessed by intracellular staining, representative histogram from 1 out of 3 donors tested. (H) Knock-down of KLRG1 by siRNA reduces AMPK phosphorylation in KLRG1bright but not in KLRG1dim NK cells as compared to control siRNA. Data from one representative donor as assessed on freshly purified KLRG1bright and KLRG1dim NK cells. (I) Cumulative data from 4 independent experiments analysed as in (H). Bars represent mean values ± SEM (Fig. 2B, 2E, 2F) and median values ± IQR. p-values were calculated using Student’s t-Test for parametric data and Friedman test for non-parametric data.
We next examined effects of KLRG1 ligation on AMPK activity in purified KLRG1neg, KLRG1dim and KLRG1bright NK cells. We used phosphorylation of Akt (Ser473), an established KLRG1 inhibitory target (22, 29), as a readout control. The addition of an agonistic KLRG1 antibody to freshly isolated KLRG1neg, KLRG1dim or KLRG1bright NK cells robustly enhanced AMPK activity in KLRG1bright NK cells but had no effect in either the KLRG1dim or KLRG1neg population (Fig. 2E). In contrast, Akt phosphorylation was not altered in response to KLRG1 ligation in KLRG1bright or KLRG1neg NK cells but was inhibited in KLRG1dim cells (Fig. 2F). No change in either AMPK or Akt activity could be detected when an irrelevant isotype control antibody was used (Fig. 2E, 2F). Correspondingly, there was relatively low Akt expression in freshly purified KLRG1bright NK cells that exhibit endogenous AMPK activity (Fig. 2G vs 2C) compared to KLRG1dim and KLRG1neg NK cells.
To corroborate these findings, we silenced KLRG1 by siRNA in ex vivo isolated KLRG1bright and KLRG1dim NK cells (Fig. S3A). Knock-down of KLRG1 reduced AMPK phosphorylation in KLRG1bright NK cells but had no significant effect on KLRG1dim cells that lack endogenous AMPK phosphorylation (Fig. 2H, 2I). Together, these findings show that KLRG1 activates AMPK signaling in highly differentiated primary human NK cells. Furthermore, Akt inhibition and AMPK activation constitute two independent pathways of KLRG1 function that may operate at different stages of NK cell differentiation.
KLRG1 activates AMPK by preventing its de-phosphorylation
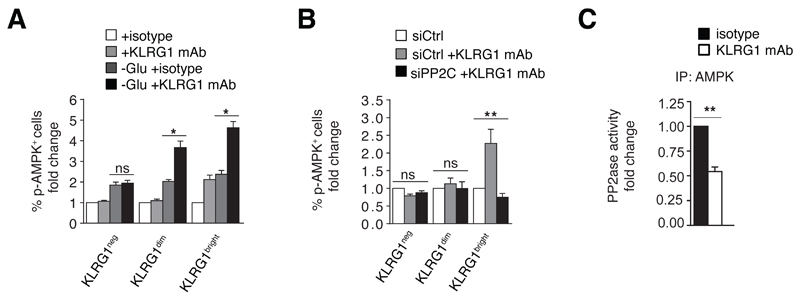
As KLRG1 ligation only enhanced AMPK phosphorylation in NK cells with pre-existing (endogenous) AMPK activity, we reasoned that KLRG1 engagement may activate AMPK through inhibition of de-phosphorylation. In ex vivo isolated KLRG1 subsets, ligation of KLRG1 induced AMPK activation only in KLRG1bright NK cells (Fig. 3A). When we cultured purified KLRG1neg, KLRG1dim and KLRG1bright NK cells in glucose-free medium (40) AMPK phosphorylation was detected in all three cell subsets (Fig. 3A). In glucose-starved NK cells, KLRG1 engagement with the activating KLRG1 antibody enhanced AMPK activity not only in KLRG1bright but also in KLRG1dim cells by 2-3 fold (Fig. 3A). There were no KLRG1-related changes in AMPK activation in KLRG1neg NK cells (Fig. 3A). This indicates that KLRG1 ligation enhances pre-existing AMPK activity. We therefore reasoned that KLRG1 might regulate AMPK function by preventing its inhibitory de-phosphorylation by upstream phosphatases rather than directly inducing de novo AMPK activation.
Figure 3. KLRG1 activates AMPK by preventing its de-phosphorylation.
(A) Pre-existing AMPK activity is required for KLRG1 regulation of AMPK. Purified KLRG1neg, KLRG1dim and KLRG1bright NK cells were left untreated or cultured in glucose-free medium for 6 hours followed by KLRG1 ligation; n=5 independent experiments. An isotype antibody was used as negative control. (B) Phosphorylation of AMPK in purified KLRG1neg, KLRG1dim and KLRG1bright NK cells transfected with siRNA to PP2Cα (siPP2C) or control siRNA (siCtrl) for 36 hours followed by a 2 hour stimulation with the activating KLRG1 antibody or medium alone; n=3 independent experiments. (C) Activity of PP2C-like phosphatases in AMPK immune-precipitates from KLRG1bright NK cells stimulated with an activating KLRG1 mAb or an isotype control antibody (2h, 37°C). Phosphatase activity was normalized to immunoprecipitated AMPK by ELISA (n=3). Bars represent median values ± IQR. p-values were calculated using a Friedman test for non-parametric data.
De-phosphorylation of AMPK by upstream phosphatases, mainly protein phosphatase-2C (PP2C), represents an important mechanism of AMPK regulation (41, 42). We tested if KLRG1 enhanced AMPK activity through inhibition of PP2C. Immunoblot analysis revealed comparable PP2C expression levels in all three KLRG1 subsets (data not shown). We then silenced PP2C by siRNA (Fig. S3B) and found that KLRG1 ligation failed to enhance AMPK activity in KLRG1bright NK cells in the absence of PP2C (Fig. 3B). Of note, PP2C knock-down did not alter KLRG1 expression or cell viability in these cells (data not shown), indicating that un-responsiveness of PP2C-silenced NK cells to KLRG1 ligation was not due to a reduction in KLRG1 cell surface expression. Thus, KLRG1 activates AMPK through a PP2C-dependent mechanism.
To assess if KLRG1 signaling modulates activity of PP2C-like AMPK-associated phosphatases, we ligated KLRG1 on KLRG1bright NK cells, immunoprecipitated AMPK and did ELISA-based in vitro phosphatase assays. We found that KLRG1 ligation significantly reduced phosphatase activity in AMPK immunoprecipitates compared to IgG control ligation (Fig. 3C). These data show that KLRG1 signaling impedes de-phosphorylation of AMPK, a process mediated by the phosphatase PP2C.
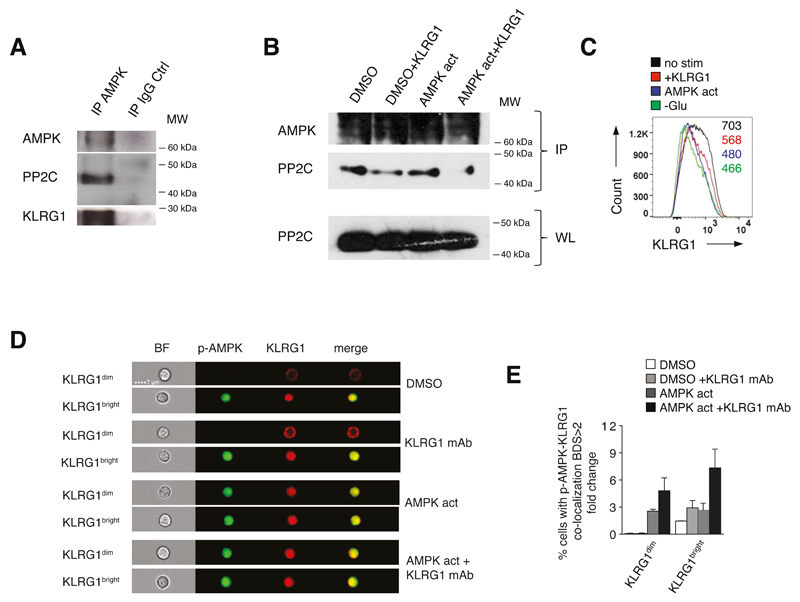
KLRG1 interacts with AMPK and undergoes internalization
We immunoprecipitated AMPK from freshly isolated KLRG1bright NK cells, and found that KLRG1, AMPK and PP2C interacted endogenously (Fig. 4A). As KLRG1 signaling inhibits phosphatase activity in the AMPK complex (Fig. 3C), we reasoned that KLRG1 ligation may disrupt the AMPK-PP2C interaction. When we immunoprecipitated AMPK from KLRG1dim NK cells treated with the AMPK agonist A-769662 or DMSO vehicle control, we found that KLRG1 ligation dissociated PP2C from AMPK, a process that was most evident on an AMPK activation background (Fig. 4B, lane 3 and 4).
Figure 4. KLRG1 interacts with AMPK and undergoes internalization.
(A) AMPK, PP2C and KLRG1 interact endogenously. KLRG1bright NK cells were lysed, immunoprecipitated with AMPK or IgG control antibody, followed by immunoblotting. Representative of two separate experiments. (B) KLRG1 signaling disrupts AMPK-PP2C interaction. Isolated KLRG1dim NK cells were cultured with the AMPK agonist A-769662 (150 μM) or DMSO as vehicle control overnight, with or without KLRG1 ligation for additional 2 hours the following day. AMPK-bound PP2C was detected in AMPK immunoprecipitates by immunoblotting. Whole-cell lysates (WL) of PP2C protein served as loading control throughout. Representative of two separate experiments. (C) KLRG1 down-regulation in purified KLRG1bright NK cells cultured over night in complete medium then stimulated with an activating KLRG1 antibody (2h, 37°C; red histogram), the AMPK agonist A-769662 (150 μM overnight; blue histogram) or cultured in glucose-free medium (overnight; green histogram) compared to KLRG1bright NK cells cultured over night in complete medium only (black histogram). Mean fluorescence intensity values of cell surface KLRG1 are shown throughout. (D) Image Stream analysis of phosphorylated AMPK (p-AMPK) and KLRG1 co-localization in purified KLRG1dim and KLRG1bright NK cells (magnification 60x). Co-localization occurs endogenously in KLRG1bright NK cells that spontaneously activate AMPK (1C, top quarter) but requires exogenous AMPK activity in the KLRG1dim population (1C, third quarter: AMPK act). AMPK was activated by A-769662 (150 μM, overnight) with or without KLRG1 ligation. A DMSO vehicle solution was used as control. Cumulative data from 2 independent experiments are shown for KLRG1dim and KLRG1bright NK cells indicating percentages of NK cells positive for p-AMPK-KLRG1 co-localization (BDS>2) shown as fold change compared to untreated NK cells (E).
When we activated AMPK in purified KLRG1bright NK cells by KLRG1 ligation, glucose-deprivation or selective AMPK agonist stimulation (43), we observed a consistent reduction in cell surface KLRG1 expression (Fig. 4C). We reasoned that KLRG1 might have been internalized to facilitate interaction with AMPK. To test for internalization of KLRG1, we compared KLRG1dim NK cells that lack endogenous AMPK activation and KLRG1bright NK cells that spontaneously activate AMPK. To this end, we cultured isolated KLRG1dim and KLRG1bright NK cells over night in the presence of the AMPK agonist A-769662 or DMSO vehicle control followed by ligation of KLRG1 for 2 hours and Image-Stream analysis.
On a DMSO background, we found that KLRG1bright but not KLRG1dim NK cells showed endogenous AMPK activation and co-localization of KLRG1 and p-AMPK (Fig. 4D top panel and Fig. 4E). When KLRG1 was ligated, we found enhanced p-AMPK/KLRG1 co-localization in KLRG1bright cells only (Fig. 4D second panel and Fig. 4E). In contrast, in KLRG1dim NK cells AMPK was refractory to triggering of the receptor per se (Fig. 4D second panel and Fig. 4E). Because pre-existing AMPK activity is essential for KLRG1 regulation of AMPK (see above), we reasoned that enforcing AMPK activation in KLRG1dim cells would reconstitute p-AMPK/KLRG1 co-localization even in this less differentiated population. On an AMPK activation background, we indeed observed KLRG1 and p-AMPK co-localization not only in KLRG1bright but also in KLRG1dim NK cells (Fig. 4D third panel and Fig. 4E). KLRG1 ligation now enhanced p-AMPK/KLRG1 co-localization further in both cell-types (Fig. 4D fourth panel and Fig. 4E). Quantification of co-localization is shown in Fig. 4E for all four conditions and quantification of KLRG1 and p-AMPK signals is shown in Fig. S3C. We obtained comparable results when probing co-localization of KLRG1 and total AMPK by Image-Stream (data not shown). Together, these data support a model whereby KLRG1 internalizes upon ligation, an event that requires pre-existing AMPK activation, and further enhances AMPK signaling.
AMPK dependence of KLRG1-induced inhibition of NK cell function
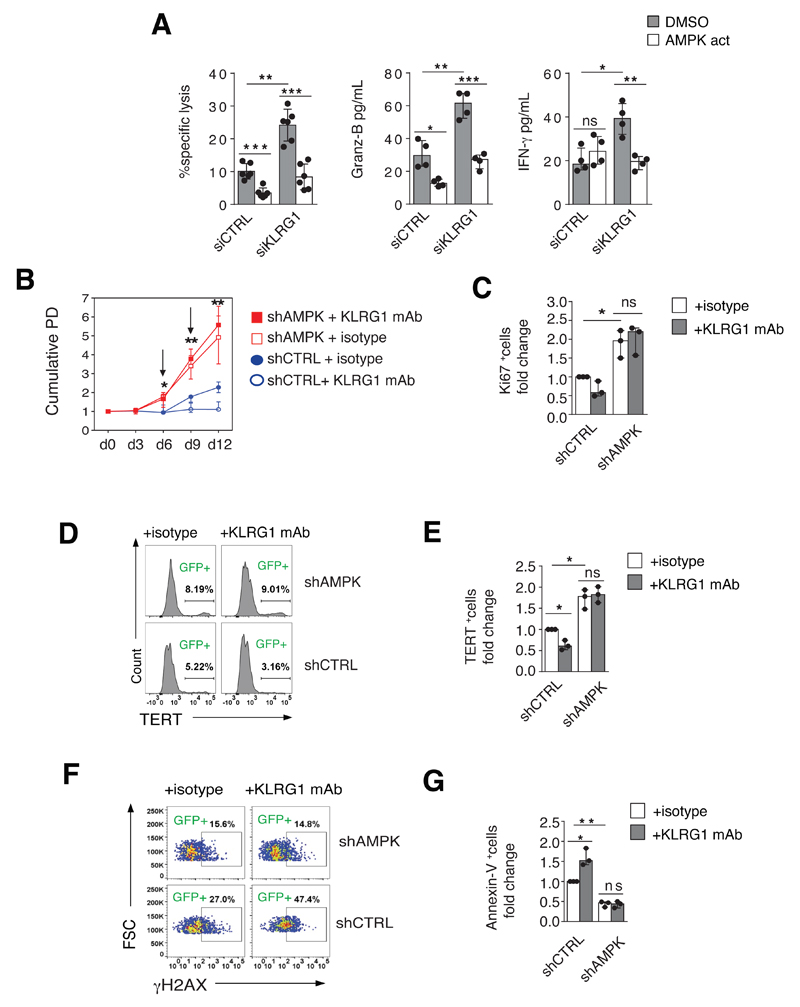
We next investigated the functional implications of inhibiting NK cells via the KLRG1 / AMPK axis. We probed KLRG1 function by co-culturing KLRG1bright NK cells with the breast-cancer cell line MCF-10A, which expresses abundant levels of the KLRG1 ligand E-cadherin (44). Knock-down of KLRG1 by siRNA increased killing capacity (measured by direct tumor cell lysis), as well as release of granzyme B and IFN-γ secretion of KLRG1bright NK cells toward the MCF-10A cell line (Fig. 5A). Importantly, this process was AMPK-dependent as all three functions examined were abolished by addition of the AMPK agonist A-769662 to the KLRG1 silenced NK cells (Fig. 5A). These data identify an inhibitory pathway of KLRG1 function in primary human NK cells that requires AMPK signaling.
Figure 5. AMPK dependence of KLRG1-induced inhibition of NK cell function.
(A) Cytotoxic activity assessed by calcein-release lysis assay in purified KLRG1bright NK cells transfected with siRNA specific for KLRG1 (siKLRG1) or control siRNA (siCtrl) for 36 hours and tested vis-à-vis the E-Cadherin-expressing tumor cell line MCF-10A (4 hours co-culture). Throughout cell culture NK cells were kept in complete medium supplemented with 500 IU/ml rhIL-2. Release of granzyme B and IFN-γ under the same conditions are also shown. Reliance of KLRG1 on AMPK activity was probed by addition of the AMPK agonist A-769662 (150 μM) to the transfected cells 2 hours prior to the cytotoxicity assay. A DMSO solution was used as vehicle control. Data are pooled from 6 donors (specific lysis) or 5 donors (granzyme B and IFN-γ). (B-G) KLRG1 ligation negatively regulates NK cell activity in AMPK-proficient cells only. Purified KLRG1bright NK cells were transduced with lentiviral vectors containing silencing shRNA to AMPK (shAMPK) or scrambled control RNA (shCtrl) as described. On days 6 and 9 NK cells were re-stimulated with feeder cells (K562, effector to target ratio 10:1) and rhIL-2 200 IU/ml. On day 6, NK cells were transferred onto plates coated with the activating KLRG1 antibody or an isotype control antibody, respectively. Throughout, cumulative data is shown as fold change compared to shCtrl-transduced cells stimulated with an isotype control antibody. Events were gated on GFP+ (effectively transduced) cells only, and data are pooled from three independent experiments. Bars represent median ± IQR. P-values were calculated using a Student’s t-Test. (B) Population doubling time (PD) of shAMPK- (red curves) and shCtrl-transduced NK cells (blue curves) either stimulated with an activating KLRG1 antibody or an isotype control antibody. Arrows represent time point of stimulation with feeder cells and fresh rhIL-2. (C) Ki-67 expression on day 9 of cell culture. (D) Histogram analysis of telomerase reverse transcriptase (TERT) expression on day 9 from one representative experiment and (E) pooled data from 3 independent experiments. (F) DNA damage as assessed by H2Ax-phosphorylation (representative data from 2 independent experiments) and (H) apoptosis assessed by Annexin V staining on day 9 of cell culture as described above.
To further dissect the link between AMPK and KLRG1 function, we silenced AMPK in primary human NK cells followed by KLRG1 ligation. We activated purified KLRG1bright NK cells with the MHC class I-deficient NK target cell line K562 and rhIL-2 for 48 hours, then transduced cells with lentiviral vectors encoding shRNAs to either AMPK (shAMPK) or an irrelevant scrambled control RNA (shCtrl). Four days post-transduction, we transferred NK cells to plates coated with either KLRG1 or an isotype control antibody and re-activated them up to 6 days as above (for experimental design and transfection efficiency see Fig. S4). The knock-down of AMPK restored NK cell expansion (Fig. 5B), proliferation (Fig. 5C), hTERT expression (Fig. 5D, E) but reduced DNA damage (Fig. 5F) and apoptosis (Fig. 5G) compared to KLRG1bright NK cells transduced with shCtrl. For each function examined, the inhibitory effect induced by KLRG1 ligation was robust in NK cells transduced with shCtrl but was totally abolished in AMPK-silenced cells (Fig. 5B-G). Of note, AMPK knock-down did not alter KLRG1 cell surface expression in NK cells (data not shown). Thus, AMPK activation is a new inhibitory pathway of KLRG1 function in primary human NK cells, and blocking this pathway restores NK cell responsiveness.
Discussion
We have identified a previously unrecognized function of the inhibitory receptor KLRG1 in linking cell surface inhibitory receptor signaling to AMPK activation in human NK cells. NK cells integrate signals from inhibitory and activating receptors to detect and eliminate infected and transformed cells (45, 46). While activating NK cell receptors recognize stress-induced ligands on target cells, inhibitory NK cell receptors recognize MHC class I and MHC class I-like molecules on healthy cells (8, 46) as a means of protecting healthy tissue from NK cell attack. It is conceivable, that an imbalance toward inhibitory immune-receptor signaling, as we now demonstrate for KLRG1 in individuals >70 years, increases the activation threshold of NK cells and leads to attenuation of effector function. While this mechanism potentially protects from auto-reactivity and collateral damage during infection, it might render hosts more susceptible to infection and/or malignancy by attenuating NK cell function. Therefore, we suggest that blocking of the KLRG1 / AMPK signaling axis may boost NK cell activity in the older population.
Our data support a model whereby persistent (endogenous) AMPK activity is regulated through protection from de-phosphorylation rather than by upstream kinase activation. The phosphatase PP2C is known to de-phosphorylate (and thus inactivate) AMPK both in vitro and in vivo (41, 42) but the precise physiological mechanism by which PP2C itself is regulated in humans was not known. We now show that KLRG1 internalizes upon ligation, binds directly to AMPK and stimulates its function by inhibiting PP2C-like phosphatase activity. Correspondingly, KLRG1 ligation strongly diminished PP2C binding to AMPK in human NK cells. This links AMPK activation with surface inhibitory receptor signaling, suggesting that immune-inhibitory receptors, that increase with age, chronic viral infections and cancer (14, 47, 48) may orchestrate AMPK-dependent metabolic pathways to inhibit NK cell function.
We further demonstrate that KLRG1 requires pre-existing AMPK activity to stimulate the AMPK pathway. KLRG1 thus seems to function as a natural ‘AMPK enhancer’ in NK cells, incapable of triggering de novo kinase activation but inducing potent signal amplification. This occurs endogenously in highly differentiated NK cells that exhibit senescence characteristics such as pre-existing DNA damage and spontaneous AMPK activity (32). We showed recently that endogenous DDR signaling activates AMPK in senescent T cells, and we now extend this observation to highly differentiated human NK cells. This raises the paradox of why constitutive activation of a low-energy sensor molecule would require continuous energy-consumption to fuel its own activation in senescent cells. As KLRG1 impedes AMPK de-phosphorylation, we propose a model in which endogenous AMPK activity is maintained by active surface inhibitory receptor signaling rather than continuous de novo upstream activation. Beyond biochemical considerations, the central implication for this is that endogenous AMPK activity may be controlled by means of surface inhibitory-receptors in human NK cells that was previously unknown.
It remains to be determined whether KLRG1 has a similar role in highly differentiated human T cells that also express high levels of KLRG1 (19, 49) and spontaneously activate AMPK (32). Furthermore, it is not known if this pathway may be shared with other T cell inhibitory receptors such as CTLA-4 and PD-1, for which blocking antibodies have been approved or are in clinical development.
In summary, we demonstrate that there is an intimate relationship between senescence and energy sensing pathways in NK cells that can be further modulated by inhibitory receptor engagement. Intervention in this signaling axis may enhance NK cell activity.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the Swiss National Foundation (P300PB_161092 and P2BSP3_151877 to B.M.D.), the Wellcome Trust (AZR00630 to A.L.), the Ministry of Education of Brazil (BEX9414/14-2 to L.P.C.), the Biotechnology and Biological Science Research Council (BB/L005328/1 to A.N.A.). A.L. is a Sir Henry Wellcome Trust Fellow sponsored by Prof. Michael L Dustin (University Of Oxford). S.M.H. is funded by the William Harvey Research Foundation.
References
- 1.Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nature reviews Immunology. 2004;4:737–743. doi: 10.1038/nri1440. [DOI] [PubMed] [Google Scholar]
- 2.Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967-1989. International journal of epidemiology. 1993;22:334–340. doi: 10.1093/ije/22.2.334. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann RJ, Monroe PW. Bacteremic urinary tract infection in older people. J Am Geriatr Soc. 1996;44:927–933. doi: 10.1111/j.1532-5415.1996.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 4.Berger NA, Savvides P, Koroukian SM, Kahana EF, Deimling GT, Rose JH, Bowman KF, Miller RH. Cancer in the elderly. Trans Am Clin Climatol Assoc. 2006;117:147–155. discussion 155-146. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature immunology. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French AR, Yokoyama WM. Natural killer cells and viral infections. Current opinion in immunology. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 7.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. Journal of immunology. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nature reviews Immunology. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orange JS. Natural killer cell deficiency. The Journal of allergy and clinical immunology. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. quiz 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz CT, Moore MB, Bradley S, Shelton BJ, Lutgendorf SK. Reciprocal age related change in natural killer cell receptors for MHC class I. Mechanisms of ageing and development. 2005;126:722–731. doi: 10.1016/j.mad.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Lorenzo G, Balistreri CR, Candore G, Cigna D, Colombo A, Romano GC, Colucci AT, Gervasi F, Listi F, Potestio M, Caruso C. Granulocyte and natural killer activity in the elderly. Mechanisms of ageing and development. 1999;108:25–38. doi: 10.1016/s0047-6374(98)00156-0. [DOI] [PubMed] [Google Scholar]
- 12.Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Lutz CT, Karapetyan A, Al-Attar A, Shelton BJ, Holt KJ, Tucker JH, Presnell SR. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. Journal of immunology. 2011;186:4590–4598. doi: 10.4049/jimmunol.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing research reviews. 2013;12:1069–1078. doi: 10.1016/j.arr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature immunology. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parham P. Killer cell immunoglobulin-like receptor diversity: balancing signals in the natural killer cell response. Immunology letters. 2004;92:11–13. doi: 10.1016/j.imlet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Guthmann MD, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc Natl Acad Sci U S A. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. The Journal of experimental medicine. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Experimental gerontology. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 22.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 23.Henson SM, Akbar AN. KLRG1--more than a marker for T cell senescence. Age. 2009;31:285–291. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bigley AB, Spielmann G, Agha N, Simpson RJ. The Effects of Age and Latent Cytomegalovirus Infection on NK-Cell Phenotype and Exercise Responsiveness in Man. Oxid Med Cell Longev. 2015;2015:979645. doi: 10.1155/2015/979645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Human immunology. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. Journal of immunology. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 29.Wang JM, Cheng YQ, Shi L, Ying RS, Wu XY, Li GY, Moorman JP, Yao ZQ. KLRG1 negatively regulates natural killer cell functions through the Akt pathway in individuals with chronic hepatitis C virus infection. Journal of virology. 2013;87:11626–11636. doi: 10.1128/JVI.01515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. Journal of immunology. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 31.Evans AM, Peers C, Wyatt CN, Kumar P, Hardie DG. Ion channel regulation by the LKB1-AMPK signalling pathway: the key to carotid body activation by hypoxia and metabolic homeostasis at the whole body level. Advances in experimental medicine and biology. 2012;758:81–90. doi: 10.1007/978-94-007-4584-1_11. [DOI] [PubMed] [Google Scholar]
- 32.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nature immunology. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milush JM, Long BR, Snyder-Cappione JE, Cappione AJ, 3rd, York VA, Ndhlovu LC, Lanier LL, Michaelsson J, Nixon DF. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood. 2009;114:4823–4831. doi: 10.1182/blood-2009-04-216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clinical and diagnostic laboratory immunology. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 36.Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann M, Schweier O, Pircher H. Different inhibitory capacities of human and mouse KLRG1 are linked to distinct disulfide-mediated oligomerizations. Eur J Immunol. 2012;42:2484–2490. doi: 10.1002/eji.201142357. [DOI] [PubMed] [Google Scholar]
- 38.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature reviews Molecular cell biology. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 39.Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. Journal of immunology. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 40.Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. European journal of immunology. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. The Biochemical journal. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. The Journal of biological chemistry. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 43.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. The Journal of biological chemistry. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer research. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 45.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JS. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92:245–255. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nature reviews Immunology. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 47.Crawford A, Wherry EJ. Inhibitory receptors: whose side are they on? Nature immunology. 2007;8:1201–1203. doi: 10.1038/ni1107-1201. [DOI] [PubMed] [Google Scholar]
- 48.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaser C, Kaufmann M, Pircher H. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. Journal of immunology. 1998;161:6451–6454. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.