Abstract
STUDY QUESTION
Is the length of the anogenital distance (AGD), a biomarker of the in-utero prenatal hormonal environment, associated with the presence of endometriomas and deep infiltrating endometriosis (DIE)?
SUMMARY ANSWER
Shorter AGD is associated with presence of endometriomas and DIE.
WHAT IS KNOWN ALREADY
It is debated whether hormonal exposure to estrogens in utero may be a risk factor for endometriosis in adulthood. AGD is a biomarker of prenatal hormonal environment and observational studies have shown an association between AGD and reproductive parameters in both sexes.
STUDY DESIGN, SIZE, DURATION
This case–control study of 114 women with endometriosis (endometriomas and/or DIE) and 105 controls was conducted between September 2014 and May 2015.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Cases were attending the Endometriosis Unit of the Hospital. Prevalent as well as incident cases, diagnosed by transvaginal ultrasound (TVUS), were included. Controls were women without endometriosis attending the gynecological outpatient clinic for routine gynecological exams. Participants completed health questionnaires, followed physical and gynecological examinations, including TVUS. Measurements from the anterior clitoral surface to the upper verge of the anus (AGDAC), and from the posterior fourchette to the upper verge of the anus (AGDAF) were obtained in all subjects. Unconditional multiple logistic regression was used to estimate the association between AGD measurements and presence of endometriomas and/or DIE while accounting for important confounders and covariates, including age, body mass index, vaginal delivery or episiotomy.
MAIN RESULTS AND THE ROLE OF CHANCE
AGDAF was related to presence of endometriomas and/or DIE. For all cases of endometriosis (endometriomas and DIE), women in the lowest tertile of the AGDAF distribution, compared with the upper tertile, were 7.6-times (95% CI 2.8–21.0; P-trend < 0.001) more likely to have endometriosis. With regard to DIE, women with AGDAF below the median, compared with those with AGDAF above the median, were 41.6-times (95% CI 3.9–438; P-value = 0.002) more likely to have endometriosis.
LIMITATIONS, REASONS FOR CAUTION
In case–control studies, information and selection bias has to be ruled out. Physicians conducting the measurement were blind to the status of the patients. Controls came from the same population as the cases. We adjusted for known and suspected confounders and covariates, but the possibility of residual confounding or chance findings should always be considered. As with all observational studies, causal inference is limited.
WIDER IMPLICATIONS OF THE FINDINGS
This study suggests that endometriosis, especially the DIE, might have a prenatal origin that may be traced back to the hormonal milieu in which the fetus develops.
STUDY FUNDING/COMPETING INTEREST
This work was supported by the Ministry of Economy and Competitiveness, ISCIII (AES), grant no. PI13/01237 and the Seneca Foundation, Murcia Regional Agency of Science and Technology, grant no. 19443/PI/14. The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: anogenital distance, deep infiltrating endometriosis, estrogens, endometriomas, endometriosis, prenatal exposures
Introduction
Endometriosis is a chronic estrogen-dependent disease characterized by endometrial glands and stroma outside the uterus, often in the peritoneal cavity (Vercellini et al., 2014), with an estimated prevalence of about 10%, and affecting 4–30% of women during their reproductive years (Leibson et al., 2004; Buck Louis et al., 2011b). Women present chronic pelvic pain, dysmenorrhea, dyspareunia or infertility (Mahmood et al., 1991; Denny and Mann, 2007), that can be incapacitating and can negatively affect their quality of life (Jones et al., 2004).
Intrauterine etiology and early-life influences are one of the etiological hypotheses being investigated for endometriosis (Missmer et al., 2004; Hediger et al., 2005; Buck Louis et al., 2007, 2013; Rizner, 2009; Somigliana et al., 2011; Wolff et al., 2013; Ferrero et al., 2014; Upson et al., 2015; Vannuccini et al., 2016).
Anogenital distance (AGD) is a sexually dimorphic feature, which is almost twice as long in males than in females, and a biomarker of prenatal hormonal environment (Greenham and Greenham, 1977; Kurzrock et al., 2000). In observational studies, AGD is a biomarker of prenatal exposure to endocrine disruptors (Swan et al., 2005, 2015; Bornehag et al., 2015) and androgens during the formation of the reproductive system (Dean and Sharpe, 2013; Jain and Singal, 2013). Prenatal exposure to stressful life events result in a longer (more masculine) AGD in infant girls (Barrett et al., 2013), while exposure to anti-androgens results in a shorter (more feminine) AGD in infant males (Swan et al., 2005, 2015). In adult women, AGD length is associated with female reproductive function (Mendiola et al., 2012; Mira-Escolano et al., 2014a,b; Barrett et al., 2015). We hypothesize that a prenatal estrogenic environment resulting in relatively shorter AGD will be associated with higher endometriosis risk. The aim of this study was to assess the relationship between AGD measurements, as a biomarker of intrautero hormonal milieu, and the presence of endometriomas and deep infiltrating endometriosis (DIE) in adulthood.
Materials and Methods
Study population
This case–control study conducted from September 2014 to May 2015 at the Department of Obstetrics and Gynecology of the University Hospital ‘Virgen de la Arrixaca’ in the Murcia Region (Spain). Patients were excluded from the study if they were pregnant, or having with oncological treatment or genitourinary prolapse. The age range was 18–50 years of age. Cases (n = 114) were women attending the Endometriosis Unit of the Hospital, and included prevalent and newly diagnosed cases. Diagnoses were established by medical history, and confirmed by ultrasounds [e.g. endometriotic cyst (endometriomas) or DIE] (Eskenazi et al., 2001; Abrao et al., 2007; Hsu et al., 2010; Somigliana et al., 2010; Johnson and Hummelshoj, 2013; Vercellini et al., 2014). Deep endometriosis was defined as endometriosis infiltrating the peritoneum by >5 mm. Therefore, women with endometriosis were further classified as endometriomas (n = 82) and DIE (n = 32) using symptoms, signs and ultrasound findings. When a patient presented both types of endometriosis (DIE and endometriomas), she was classified as DIE. Controls were women without endometriosis attending the gynecological outpatient clinic for routine exams (n = 105). Nine controls reported clinical symptoms compatible with endometriosis but the diagnosis was not confirmed by transvaginal ultrasound (TVUS). Written informed consent was obtained from all subjects. This study was approved by the Ethics Research Committee of the University of Murcia.
Gynecological history and physical examination
Cases and controls were interviewed in-person by gynecologists. All participants completed health questionnaires, gynecological and obstetrical histories, and underwent a gynecological examination including TVUS at a scheduled clinical visit. A visual analog scale (0 to 10) was used to measure endometriosis-associated pelvic pain (dysmenorrhea, chronic pelvic pain, dyspareunia, dysuria and dyschezia) at the time of the exam (Vincent et al., 2010). Height and weight were measured using a digital scale (Tanita SC 330-S, London, UK). Uterine and ovarian morphology were evaluated with TVUS (Voluson E-8® and 4–9 MHz transducer; General Electric Healthcare, USA). All women having disease were diagnosed by 2D, but for DIE cases, 3D was also used (30% of cases). Two gynecologists using the same methodology performed all clinical evaluations.
Anogenital measurements
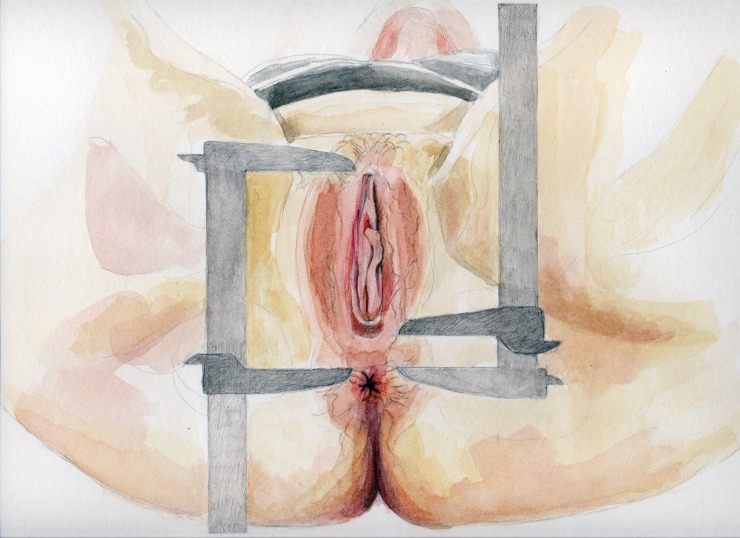
Women lay in the lithotomy position with thighs at 45° to the examination table. Using a digital caliper (Stainless Steel Digital Caliper, VWR® International, LLC, West Chester, PA, USA), AGDAC was measured from the anterior clitoral surface to the upper verge of the anus, and AGDAF from the posterior fourchette to the upper verge of the anus (Fig. 1). Two gynecologists unaware of the patient’ gynecological status measured each distance three times. Average values of the six measurements were used in the analyses.
Figure 1.
Landmarks for two measurements of anogenital distance (AGD): AGDAC, from the anterior clitoral surface to the upper verge of the anus (left) and AGDAF, from the posterior fourchette to the upper verge of the anus (right).
Statistical analyses
Descriptive statistics are presented using raw data. Continuous variables were compared using unpaired Student T tests, and categorical variables were compared with χ2 tests. Unconditional multiple logistic regression was used to explore the association between presence of endometriosis (endometriomas and/or DEI) (yes/no) and AGD (in tertiles from the whole distribution of cases and controls, with the highest tertile as reference category) using odds ratios (OR). Age (years) and BMI (kg/m2) were included as potential confounders in the model, and vaginal delivery and episiotomy were included as covariates as they may affect AGD. Tests for trend in the ORs across exposure strata for AGD measures were calculated using logistic models that included categorical terms as continuous variables. Coefficients of variation (%CV) were used to assess intra- and inter-examiner variability in AGD measurements. All tests were two-tailed at 0.05 significance level. Analyses were conducted with IBM SPSS 20.0 (IBM Corporation, Armonk, New York, USA).
Results
For the whole population, cases were older and had had more birth deliveries than controls, but were similar for other demographic and lifestyle factors (Table I). AGDAC and AGDAF had normal distributions, were correlated [Pearson correlation (r) = 0.52, P < 0.001] and had a positive association with BMI (P-values < 0.01). Intra- and inter-examiner CV were 5% and 10% for AGDAF and AGDAC, respectively. Intraclass correlation coefficients were above 0.95 for both AGD measurements. Cases showed significantly shorter AGDAF compared with controls.
Table I.
Comparison of the general characteristics of controls and cases of endometriomas and deep infiltrating endometriosis (DIE).
| Characteristics | Controls (n = 105) |
All endometriosis (n = 114) |
P-valuec | DIE (n = 32) |
P-valuec | Endometriomas (n = 82) |
P-valuec | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 5–95 | Mean | SD | Median | 5–95 | Mean | SD | Median | 5–95 | Mean | SD | Median | 5–95 | ||||
| Age (years) | 29.7 | 6.0 | 31.0 | 20.0–39.0 | 36.2 | 7.5 | 37.0 | 22.9–48.0 | <0.001 | 37.6 | 6.1 | 37.5 | 25.3–47.7 | <0.001 | 35.8 | 7.8 | 36.5 | 21.9–48.5 | <0.001 |
| Body mass index (BMI) (kg/m2) | 23.8 | 5.4 | 22.2 | 17.6–36.2 | 23.5 | 3.8 | 22.5 | 18.5–30.8 | 0.47 | 23.2 | 3.1 | 22.6 | 18.5–29.7 | 0.58 | 23.6 | 4.0 | 22.5 | 18.4–31.2 | 0.53 |
| Age at menarche (years) | 11.9 | 1.3 | 12.0 | 9.2–14.0 | 12.2 | 1.4 | 12.0 | 10.0–14.0 | 0.16 | 12.1 | 1.9 | 12.0 | 9.0–14.0 | 0.16 | 12.2 | 1.3 | 12.0 | 10.6–14.0 | 0.16 |
| Anogenital distance (AGDAF) (mm) | 27.3 | 5.7 | 26.2 | 18.9–38.9 | 23.5 | 5.8 | 22.3 | 15.3–33.8 | <0.001 | 19.1 | 3.5 | 18.3 | 14.4–27.9 | <0.001 | 25.3 | 5.6 | 24.6 | 18.2–35.2 | 0.02 |
| Anogenital distance (AGDAC) (mm) | 75.7 | 11.7 | 73.6 | 58.1–99.4 | 73.8 | 12.1 | 72.9 | 55.6–95.6 | 0.24 | 68.9 | 12.8 | 71.3 | 44.1–90.8 | <0.01 | 75.7 | 11.4 | 75.6 | 58.3–97.5 | 0.99 |
| Percentage (%) | |||||||||||||||||||
| Alcohol consumptiona | 74.5 | 75.3 | 0.90 | 75.0 | 0.96 | 75.3 | 0.90 | ||||||||||||
| Tobacco consumptionb | 39.2 | 51.5 | 0.09 | 57.1 | 0.13 | 50.0 | 0.15 | ||||||||||||
| Have had: | |||||||||||||||||||
| Endometriosis surgery | – | 36.0 | – | 40.6 | – | 34.1 | – | ||||||||||||
| Vaginal delivery | 21.0 | 41.2 | 0.001 | 28.1 | 0.47 | 46.3 | 0.001 | ||||||||||||
| Episiotomy | 16.2 | 10.5 | 0.22 | 3.1 | 0.07 | 13.4 | 0.60 | ||||||||||||
| Parity | |||||||||||||||||||
| 0 | 77.1 | 62.3 | 76.7 | 57.3 | |||||||||||||||
| 1 | 16.9 | 17.9 | 6.7 | 22.7 | |||||||||||||||
| 2 | 3.6 | 18.9 | 16.7 | 18.7 | |||||||||||||||
| 3+ | 2.4 | 0.9 | – | 1.3 | |||||||||||||||
AGDAF: Anogenital distance from the upper verge of the anus to the posterior fourchette.
AGDAC: Anogenital distance from the upper verge of the anus to the anterior clitoral surface.
SD: standard deviation; 5–95: 5th–95th percentile.
aDid you ever drink alcoholic beverages with a frequency of at least one a month?
bHave you ever smoked?
cT-student/Mann–Whitney U-test or χ2 test, compared with control subjects.
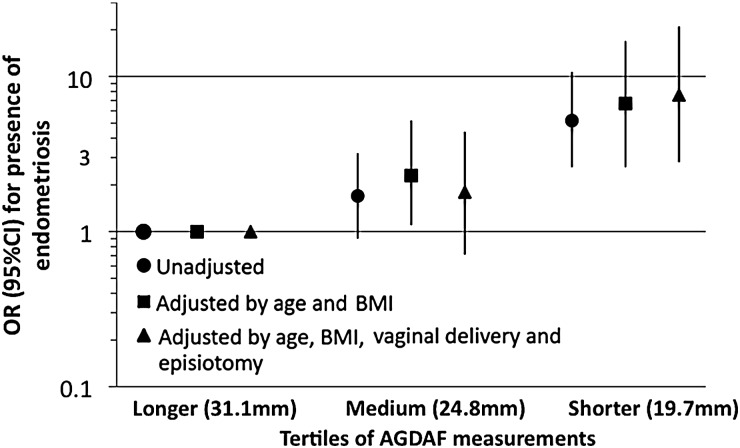
Table II presents AGD measurements in tertiles (or by median for DIE) and ORs. AGDAF, but not AGDAC, was related to presence of endometriomas and/or DIE (P-values, <0.001–0.04) in the adjusted models. For all cases of endometriosis (endometriomas and DIE), women with AGDAF in the lowest compared with the upper tertile were 7.6-times (95% CI 2.8–21.0; P-trend < 0.001) more likely to have endometriosis (Fig. 2). The strength of the association decreased when the analysis was restricted to women with endometriomas (OR 3.5, 95% CI 1.3–9.4; P-trend < 0.05). Women with DEI and AGDAF below the median were 41.6 times (95% CI 3.9–438; P-value = 0.002) more likely to have endometriosis than those above the median.
Table II.
Odds ratio (OR) for cases of endometriomas and deep infiltrating endometriosis (DIE) versus controls according to tertiles of AGD measures, taking the third tertile as a reference or divided by the median (for DIE).
| All endometriosis (n = 114) versus controls (n = 105) |
||||||||
|---|---|---|---|---|---|---|---|---|
| AGD in tertiles (median for each tertile) | Cases | Controls | Odds ratioa (95% CI) | P-trend | Odds ratiob (95% CI) | P-trend | Odds ratioc (95% CI) | P-trend |
| AGDAF | ||||||||
| 3rd (31.1 mm) | 26 | 48 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 2nd (24.8 mm) | 34 | 38 | 1.7 (0.9–3.2) | 2.3 (1.1–5.2) | 1.8 (0.71–4.4) | |||
| 1st (19.7 mm) | 54 | 19 | 5.2 (2.6–10.7) | <0.001 | 6.7 (2.6–16.9) | <0.001 | 7.6 (2.8–21.0) | <0.001 |
| AGDAC | ||||||||
| 3rd (86.1 mm) | 37 | 36 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 2nd (73.6 mm) | 36 | 37 | 0.95 (0.50–1.8) | 1.3 (0.57–2.9) | 1.9 (0.75–4.6) | |||
| 1st (63.3 mm) | 41 | 32 | 1.2 (0.65–2.4) | 0.68 | 1.8 (0.77–4.0) | 0.40 | 2.1 (0.82–5.2) | 0.26 |
| Endometriomas (n = 82) versus controls (n = 105) |
||||||||
| AGD in tertiles (median for each tertile) | Cases | Controls | Odds ratioa (95% CI) | P-trend | Odds ratiob (95% CI) | P-trend | Odds ratioc (95% CI) | P-trend |
| AGDAF | ||||||||
| 3rd (31.8 mm) | 21 | 43 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 2nd (25.5 mm) | 26 | 35 | 1.5 (0.74–3.2) | 2.1 (0.87–5.1) | 1.7 (0.64–4.4) | |||
| 1st (21.1 mm) | 35 | 27 | 2.7 (1.3–5.5) | 0.03 | 3.7 (1.5–9.3) | 0.02 | 3.5 (1.3–9.4) | 0.04 |
| AGDAC | ||||||||
| 3rd (86.6 mm) | 27 | 35 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 2nd (74.3 mm) | 27 | 36 | 0.97 (0.48–1.9) | 1.6 (0.69–3.8) | 2.0 (0.76–5.1) | |||
| 1st (65.1 mm) | 28 | 34 | 1.1 (0.53–2.2) | 0.97 | 2.1 (0.88–5.2) | 0.24 | 2.6 (0.96–7.2) | 0.16 |
| DIE (n = 32) versus controls (n = 105) |
||||||||
| AGD divided by median (median for each group) | Cases | Controls | Odds ratioa (95% CI) | P-value | Odds ratiob (95% CI) | P-value | Odds ratioc (95% CI) | P-value |
| AGDAF | ||||||||
| 2nd (30.1 mm) | 3 | 65 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 1st (20.6 mm) | 29 | 40 | 15.7 (4.5–54.9) | <0.001 | 24.4 (4.1–146) | <0.001 | 41.6 (3.9–438) | 0.002 |
| AGDAC | ||||||||
| 2nd (81.2 mm) | 12 | 56 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 1st (67.5 mm) | 20 | 49 | 1.9 (0.85–4.3) | 0.12 | 2.9 (0.85–10.4) | 0.09 | 2.6 (0.69–9.4) | 0.15 |
AGDAF: Anogenital distance from the upper verge of the anus to the posterior fourchette.
AGDAC: Anogenital distance from the upper verge of the anus to the anterior clitoral surface.
aUnadjusted OR.
bOR adjusted by age and BMI.
cOR adjusted by age, BMI, vaginal delivery and episiotomy.
Figure 2.
Unadjusted and adjusted odds ratio (OR) (95% CI) for the associations of tertiles [Longer (tertile 3), Medium (tertile 2) and Shorter (tertile 1)] of AGDAF measurements in relation to presence of all endometriosis (cases; n = 114 versus controls; n = 105).
Discussion
We found a strong association between AGDAF and presence of endometriomas and DIE, suggesting that the intrauterine hormonal milieu during prenatal life may play an important role in the development of endometriosis.
An increased rate of endometriosis was described in women prenatally exposed to diethylstilbestrol (Missmer et al., 2004), and women who were regularly fed soy formula as infants were shown to have more than twice the risk of endometriosis compared with unexposed women (Upson et al., 2015).
It has been long argued that endometriosis, an estrogen-dependent disease, may have an intrauterine etiology (Missmer et al., 2004; Hediger et al., 2005; Buck Louis et al., 2007, 2013; Rizner, 2009). The development of the uterine endometrial gland begins in utero and is completed in puberty (Gray et al., 2001). Early hormonal signaling disruptions may result in altered morphology and function from very early on. Evidence of endometriosis has been found in the autopsies of female human fetuses at various gestational ages (Signorile et al., 2010, 2012). In our study, we were not able to measure specific exposures in utero but prenatal exposure to monobutyl phthalate in amniotic fluid has been inversely correlated with the anogenital index in female infants (Huang et al., 2009).
Few studies have explored AGD in women. A longer AGD has been related to higher ovarian follicular number (Mendiola et al., 2012) and to higher testosterone levels (Mira-Escolano et al., 2014b). Moreover, longer AGDAF in young women is associated with irregularities in their mother's menstrual cycle before pregnancy (Mira-Escolano et al., 2014a), suggesting that the prenatal environment may exert a long-lasting influence in the reproductive tract of female offspring. An altered female AGD may also be an important biomarker of the human ovarian dysgenesis syndrome (Buck Louis et al., 2011a).
The current study showed significant associations with the presence of endometriomas and DIE for AGDAF, but not for AGDAC. There is not a clear interpretation for this difference at the moment. Similarly, almost all studies have reported associations for the short (equivalent) measurement (anus-scrotum) in males (Eisenberg et al., 2011; Mendiola et al., 2011) and females (Mira-Escolano et al., 2014b). However, it might be interesting and useful to take both measures, until a significant body of normative data has been accumulated in adults.
Selection and measurement bias has to be considered. Controls were patients attending the public hospital in the same period, and they stem from the same population from which cases emerged. Although TVUS cannot replace surgery for the diagnosis of noninvasive endometriosis (Nisenblat et al., 2016), TVUS has a relatively high values for sensitivity (93%) and specificity (96%) for endometriomas. Nonetheless, misclassification of disease status may have occurred, but, if present, it would contribute to underestimating the true magnitude of the association. AGD measures were performed by two gynecologists that were not members of the endometriosis unit and were unaware of the patient's status.
AGD is an anthropometric measurement that for what is known (Thankamony et al., 2009) may be stable across the lifespan of an individual. Therefore, the status of the case, prevalent versus incident, should not affect the relationship between AGD and presence of endometriomas and/or DIE. Recently, it has been reported that AGD in adult rats displays a certain degree of plasticity, which may be mediated by modulation of local androgen/estrogen activities (Mitchell et al., 2015). However, it has also been shown that AGD is stable across the women's menstrual cycle (Barrett et al., 2015).
In conclusion, our results provide the first evidence of a strong association between a biomarker of hormonal prenatal environment in women and the presence of endometriomas and DIE. Nevertheless, we are cautious in interpreting the results of the associations between AGDAF and DIE, due to the unstable estimates of these relationships. Our results, if confirmed, have important implications for endometriosis in terms of prevention, clinical practice and future research.
Authors' roles
A.M.T.-C., L.C.-L., J.M., M.L.S.-F. and A.N. were involved in the study conception and design. L.C.-L., R.J.-V., A.I.H.-P., S.C.-B., A.C.-B., M.T.P.-S. and M.L.S.-F. were involved in study execution and acquisition of data. L.C.-L., M.L.S.-F., M.T.P.-S., J.M., A.N. and A.M.T.-C. contributed to data analysis and interpretation. J.M., M.L.S.-F. and A.M.T.-C. drafted the manuscript. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
Funding
This work was supported by the Ministry of Economy and Competitiveness, ISCIII (AES), grants no PI13/01237 and the Seneca Foundation, Murcia Regional Agency of Science and Technology, grant no 19443/PI/14. Funding to pay the Open Access publication charges for this article was provided by the Ministry of Economy and Competitiveness, ISCIII (AES), grant no PI13/01237.
Conflict of interest
The authors have no competing interests to declare.
Acknowledgements
We thank Mr Antonio Martínez-Mendoza (M.D.) and Mrs M. Carmen Llanos (M.D.) for performing TVUS, and the patients for their participation. We thank Prof. Elena Vicente-Herranz very much for her expertise in medical illustration.
References
- Abrao MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod 2007;22:3092–3097. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C, Swan SH. Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiol Behav 2013;114–115:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Swan SH. Stability of proposed biomarkers of prenatal androgen exposure over the menstrual cycle. J Dev Orig Health Dis 2015;6:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jönsson BA, Lindh CH, Jensen TK, Bodin A, Jonsson C, Janson S, Swan SH. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect 2015;123:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peña JB. Intrauterine exposures and risk of endometriosis. Hum Reprod 2007;22:3232–3236. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. J Dev Orig Health Dis 2011. a;2:25–35. [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A et al. . Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011. b;96:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, Varner MW, Kennedy A, Giudice L, Fujimoto VY et al. . Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril 2013;100:162–169.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab 2013;98:2230–2238. [DOI] [PubMed] [Google Scholar]
- Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl 2012;35:330–339. [DOI] [PubMed] [Google Scholar]
- Denny E, Mann CH. Endometriosis-associated dyspareunia: the impact on women's lives. J Fam Plann Reprod Health Care 2007;33:189–193. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One 2011;6:e18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Bonsignore L, Olive D, Samuels S, Vercellini P. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril 2001;76:929–935. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Remorgida V, Maganza C, Venturini PL, Salvatore S, Papaleo E, Candiani M, Leone Roberti Maggiore U. Aromatase and endometriosis: estrogens play a role. Ann N Y Acad Sci 2014;1317:17–23. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001;65:1311–1323. [DOI] [PubMed] [Google Scholar]
- Greenham LW, Greenham V. Sexing mouse pups. Lab Anim 1977;11:181–184. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril 2005;84:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LE Jr. A mixture of the ‘anti-androgens’ linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod 2004;71:1852–1861. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Khachikyan I, Stratton P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin Obstet Gynecol 2010;53:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int 2009;35:14–20. [DOI] [PubMed] [Google Scholar]
- Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Hum Reprod 2013;28:2343–2349. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod 2013;28:1552–1568. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynaecol 2004;25:123–133. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Jegatheesan P, Cunha GR, Baskin LS. Urethral development in the fetal rabbit and induction of hypospadias: a model for human development. J Urol 2000;164:1786–1792. [PubMed] [Google Scholar]
- Leibson CL, Good AE, Hass SL, Ransom J, Yawn BP, O'Fallon WM, Melton LJ III. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil Steril 2004;82:314–321. [DOI] [PubMed] [Google Scholar]
- Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, van den Driesche S. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl 2010;33:279–287. [DOI] [PubMed] [Google Scholar]
- Mahmood TA, Templeton AA, Thomson L, Fraser C. Menstrual symptoms in women with pelvic endometriosis. Br J Obstet Gynaecol 1991;98:558–563. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect 2011;119:958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Roca M, Mínguez-Alarcón L, Mira-Escolano MP, López-Espín JJ, Barrett ES, Swan SH, Torres-Cantero AM. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional. Environ Health 2012;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira-Escolano MP, Mendiola J, Mínguez-Alarcón L, Roca M, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Anogenital distance of women in relation to their mother's gynaecological characteristics before or during pregnancy. Reprod Biomed Online 2014. a;28:209–215. [DOI] [PubMed] [Google Scholar]
- Mira-Escolano MP, Mendiola J, Mínguez-Alarcón L, Melgarejo M, Cutillas-Tolín A, Roca M, López-Espín JJ, Noguera-Velasco JA, Torres-Cantero AM. Longer anogenital distance is associated with higher testosterone levels in women: a cross-sectional study. BJOG 2014. b;121:1359–1364. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril 2004;82:1501–1508. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Mungall W, McKinnell C, Sharpe RM, Cruickshanks L, Milne L, Smith LB. Anogenital distance plasticity in adulthood: implications for its use as a biomarker of fetal androgen action. Endocrinology 2015;156:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2:CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizner TL. Estrogen metabolism and action in endometriosis. Mol Cell Endocrinol 2009;307:8–18. [DOI] [PubMed] [Google Scholar]
- Signorile PG, Baldi F, Bussani R, D'Armiento M, De Falco M, Boccellino M, Quagliuolo L, Baldi A. New evidence of the presence of endometriosis in the human fetus. Reprod Biomed Online 2010;21:142–147. [DOI] [PubMed] [Google Scholar]
- Signorile PG, Baldi F, Bussani R, Viceconte R, Bulzomi P, D'Armiento M, D'Avino A, Baldi A. Embryologic origin of endometriosis: analysis of 101 human female fetuses. J Cell Physiol 2012;227:1653–1656. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vercellini P, Vigano’ P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal. Hum Reprod 2010;25:1863–1868. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Abbiati A, Paffoni A, Benaglia L, Vercellini P, Fedele L. Perinatal environment and endometriosis. Gynecol Obstet Invest 2011;72:135–140. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S et al. . Study for future families research team: decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005;113:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, Redmon JB, TIDES Study Team. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 2015;30:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Ong KK, Dunger DB, Acerini CL, Hughes IA. Anogenital distance from birth to 2 years: a population study. Environ Health Perspect 2009;117:1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, Scholes D, Holt VL. Early-life factors and endometriosis risk. Fertil Steril 2015;104:964–971.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannuccini S, Lazzeri L, Orlandini C, Tosti C, Clifton VL, Petraglia F. The potential influence of in utero and early neonatal exposures on the later development of endometriosis. Fertil Steril 2016, pii: S0015-0282(15)02307-9. doi:10.1016/j.fertnstert.2015.12.127. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- Vincent K, Kennedy S, Stratton P. Pain scoring in endometriosis: entry criteria and outcome measures for clinical trials. Report from the Art and Science of Endometriosis meeting. Fertil Steril 2010;93:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray LE Jr. Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose–response study. Toxicol Sci 2002;65:71–86. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Sun L, Hediger ML, Sundaram R, Peterson CM, Chen Z, Buck Louis GM. In utero exposures and endometriosis: the Endometriosis, Natural History, Disease, Outcome (ENDO) Study. Fertil Steril 2013;99:790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Li ZL, Wu CY, Liu YM, Lin H, Wang SH, Xiao WF. Endocrine traits of polycystic ovary syndrome in prenatally androgenized female Sprague–Dawley rats. Endocr J 2010;57:201–209. [DOI] [PubMed] [Google Scholar]