Abstract
STUDY QUESTION
What is the relationship between couple's health and fecundity in a preconception cohort?
SUMMARY ANSWER
Somatic health may impact fecundity in men and women as couples whose male partner had diabetes or whose female partner had two or more medical conditions had a longer time-to-pregnancy (TTP).
WHAT IS ALREADY KNOWN
The impact of somatic health on human fecundity is hypothesized given the reported declines in spermatogenesis and ovulation among individuals with certain medical comorbidities.
STUDY DESIGN, SIZE, DURATION
A population-based prospective cohort study recruiting couples from 16 counties in Michigan and Texas (2005–2009) using sampling frameworks allowing for identification of couples planning pregnancy in the near future. Five hundred and one couples desiring pregnancy and discontinuing contraception were followed-up for 12 months or until a human chorionic gonadotropin pregnancy was detected.
PARTICIPANTS/MATERIALS, SETTINGS, METHODS
In all, 33 (21.4%) female and 41 (26.6%) male partners had medical conditions at baseline.
MAIN RESULTS AND THE ROLE OF CHANCE
Couples’ medical comorbidity was associated with pregnancy status. Diabetes in either partner was associated with diminished fecundity, as measured by a longer TTP. Specifically, fecundability odds ratios (FORs) were below 1, indicating a longer TTP, for male partners with diabetes (0.35, 95% confidence interval (CI): 0.14–0.86) even in adjusted models (0.35, 95% CI: 0.13–0.88). Female partners with diabetes had comparable reductions in FORs; however, the analyses did not reach statistical significance (0.26, 95% CI: 0.03–1.98). Female partners with two or more medical conditions had a significantly longer TTP compared with women with no health problems (0.36, 95% CI: 0.14–0.92). Importantly, the presence of medical conditions was not associated with sexual frequency. We cannot rule out residual confounding, Type 2 errors for less prevalent medical conditions, or chance findings in light of the multiple comparisons made in the analysis.
LIMITATIONS, REASONS FOR CAUTION
The findings require cautious interpretation given that medical diagnoses are subject to possible reporting errors, although we are unaware of any potential biases that may have been introduced, as participants were unaware of how long it would take to become pregnant upon enrollment.
WIDER IMPLICATIONS OF THE FINDINGS
The current report suggests a relationship between male and female diabetes and fecundity, and possibly somatic health more globally. Moreover, while the mechanism is uncertain, if corroborated, our data suggest that early evaluation and treatment may be warranted for diabetics prior to attempting to conceive.
STUDY FUNDING/COMPETING INTEREST(S)
Intramural research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract nos. #N01-HD-3-3355, N01-HD-3-3356 and N01-HD-3-3358). The authors have no conflicts of interest to declare.
Keywords: diabetes, hypertension, hyperlipidemia, hyperthyroidism, hypothyroidism, fertility
Introduction
Approximately 15% of couples are unable to conceive after one year of unprotected intercourse and are labeled infertile which represents over 10 million Americans currently affected (Louis et al., 2013; Thoma et al., 2013). In the USA, we may consider reproductive health as separate from, and perhaps even less important than, somatic health. However, the two are intimately connected. Indeed, medical comorbidities can impact human fecundity and fertility.
In men, data suggest that obesity impairs time-to-pregnancy (TTP) and semen parameters (Nguyen et al., 2007; Sermondade et al., 2013; Eisenberg et al., 2014). Diabetes has also been associated with impaired semen quality in case–control studies (Dinulovic et al., 1990; Garcia-Diez et al., 1991). However, as some diabetic men from these studies were fathers, the clinical importance is uncertain. In addition, serum cholesterol levels in men are associated with impaired semen parameters and a longer TTP (Schisterman et al., 2014a). Moreover, investigators found increased comorbidities in infertile compared with fertile men and higher rates of comorbidities in men with poor semen quality (Salonia et al., 2009; Eisenberg et al., 2015a,b; Ventimiglia et al., 2015). Thus, current data do suggest a relationship between current health and male infertility.
In women, components of the metabolic syndrome (e.g. higher body mass index or higher serum cholesterol) relate to impaired fertility (Hassan et al., 2004; Schisterman et al., 2014a,b). Endocrine dysfunction also impacts a woman's fertility. Indeed, hyperthyroidism and hypothyroidism are both associated with menstrual irregularities and infertility (Benson et al., 1955; Joshi et al., 1993). Diabetes has also been associated with menstrual irregularities, a shorter reproductive period (delayed menarche and early menopause), hyperandrogenism and polycystic ovary syndrome (PCOS) (Liversushits et al., 2009). However, limited prospective data exist on the association between a couple's health and TTP.
Successful fertility relies on the underlying fecundity, or biological capacity for reproduction of both partners of the couple. Thus, it is important to assess both partners of a couple when evaluating reproductive health. To our knowledge, no studies have explored the health of both partners of the couple while they try for pregnancy. The goal of the current analysis was to assess the association between couples’ somatic health and couple fecundity as measured by prospectively observed TTP.
Materials and methods
Study population
We used data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study whose methodology has been described previously (Buck Louis et al., 2011). Briefly, the LIFE Study is a prospective cohort of 501 couples attempting to conceive in two geographical areas (Texas and Michigan) in 2005–2009. Couples planning pregnancy were recruited by targeting mailings from 4 counties in Michigan and 12 counties in Texas to ensure heterogeneity in couples’ baseline and lifestyle characteristics. Minimal eligibility criteria were required: females aged 18–44 years and males aged 18+ years; in a committed relationship; ability to communicate in English or Spanish; menstrual cycles between 21 and 42 days; no hormonal contraception injections during past year and no sterilization procedures or physician-diagnosed infertility. A complete description of the cohort and methods is presented elsewhere (Buck Louis et al., 2011). The most frequently cited reasons for ineligibility included: age (27%), not interested in pregnancy (19%), not in a committed relationship (19%) and moving outside study area (16%). Full human subjects’ approval was obtained from all participating institutions, and all couples gave informed consent before any data collection.
Data collection and operational definitions
All participants completed baseline interviews that were conducted by trained nurses or research assistants, usually in the couple's home. Couples were queried separately about their medical and reproductive history, lifestyle and occupational activity. For study purposes, medical history was defined by participant's responses to the following questions:
Have you ever been told by a doctor that you have any of the following health conditions? (Yes/No)
Hypothyroid (under-active thyroid), Hyperthyroid (over-active thyroid), high blood pressure, high cholesterol and diabetes
Research nurses performed the standardized anthropometric assessment using the methodology adapted from the NHANES III survey (1988). Specifically, all participants were weighed after removing shoes and excessive clothing using the digital self-calibrating Health-O-Meter scale. The nurse was instructed to take two measurements and record weight to the nearest pound. If the measurements differed by more than one pound, a third measurement was taken. Measurements were averaged for analysis consistent with the protocol. The scale is reported to be accurate up to 330 pounds. For participants with weights in excess of 330 pounds (10 (2%) male and 4 (0.8%) female partners), we relied upon self-reported weight, as it did not affect the calculation of BMI.
Height was measured using a standardized cloth tape measure. Participants were asked to remove shoes, stand erect with his back to the wall and shoulders relaxed at the sides and looking straight ahead. The nurse took two measurements rounded to the nearest one-half inch and a third if the difference was more than one-half inch. Multiple measurements were averaged and converted to kilogram and meter to calculate BMI.
Couples completed daily journals while attempting to become pregnant, until an hCG pregnancy or 12 months of trying, to capture lifestyle behaviors relevant to fecundity, sexual intercourse, medication, menstruation and pregnancy test results for female participants. To maximize all couples’ fecundity, female partners were instructed in the use of the commercially available Clearblue Easy® fertility monitors (Swiss Precision Diagnostics, formerly Unipath). Daily levels of estrone-3-glucoronide (E3G) and luteinizing hormone (LH) were tracked commencing on Day 6 of the cycle and up to 10–20 days thereafter, depending upon cycle length. Monitors indicated low, high or peak fertility, as determined by the ratio of E3G and LH, with peak fertility representing the highest LH read as indicative of impending ovulation. Women were also trained in the use of the digital Clearblue Easy® home pregnancy test for detecting hCG pregnancy, and all women's urine samples were tested prior to enrollment to ensure the absence of pregnancy. The fertility monitor is 99% accurate for detecting the LH surge and 91% accurate for peak fertility when compared with the gold standard of ultrasonography (Behre et al., 2000). Each partner of the couple was remunerated $75 for complete participation in the study.
Statistical analysis
All data were entered into a web-based data management system capable of handling the study‘s hierarchical data structure stemming from prospective longitudinal data collection at the partner and the cycle level. Data were monitored for completeness. Descriptive analysis included the inspection of missing data. Missing data for some of the covariates, namely race and education, were < 5 (~ 1%) and were replaced with most frequently observed value. Our analysis included all 501 couples.
A menstrual cycle was defined as the interval between the onset of bleeding in one cycle as reported in the daily journal with at least 2 days of bleeding with increased intensity to the onset of the next similar bleeding episode using longitudinally collected data from the daily journal and fertility monitors. This definition excluded any episodic non-cyclic bleeding. If diary data were missing on the start of menses, information was borrowed from the fertility monitor as women pressed the ‘m’ button on the day menses began resulting in a date stamp on the monitor. Pregnancy was defined as a positive home pregnancy test on the day of expected menstruation. The TTP was the number of menstrual cycles which required for a positive pregnancy test. Couples withdrawing from the study were censored at that cycle for analysis.
Cox models for discrete survival time were used for analysis of TTP, as they account for left truncation or time couples were off contraception and right censoring or couples withdrawing from the study. Fecundability odds ratio (FOR) and 95% confidence intervals (CIs) were estimated using SAS proportional odds model in SAS software (SAS version 9.2; SAS Institute, Inc). Significance was assessed for categorical data using chi-square statistics (Fisher's exact test for small sample sizes) and t-tests for continuous data using an a priori established two-sided α < 0.05. FORs estimate the odds of becoming pregnant each cycle for participants with versus without each disease conditional on not being pregnant in the previous cycle. FORs < 1 denote a reduction in fecundity or a longer TTP, and FORs > 1 denote a shorter TTP. Models were first run for female and male medical conditions modeled individually and then jointly to assess couples’ health and fecundity so that the same exact adjustments were made for all models. This later step is in keeping with the couple-dependent nature of pregnancy where both partners’ medical histories can be considered. All regression models were adjusted for age (continuous, years), race (white (reference), others), BMI (continuous, kg/m2), education (college educated (reference), others), smoking (yes (reference), no), alcohol (12 or more drinks in the past 12 months, yes (reference), no) and vigorous physical activity (> 1× per week, yes (reference), no). We further assessed the presence of medical conditions in one or both partners and modeled it.
Results
In all, the LIFE Study comprised 501 couples of whom 347 (69%) achieved an observed pregnancy, whereas 54 (11%) did not and 100 (20%) withdrew at some point from the study. The mean ages of male and female partners were 31.8 ± 4.9 and 30.0 ± 4.1 years, respectively (Table I). Most couples were white and college educated with slightly less than half having previously been pregnant or fathered a pregnancy. Female partners had higher rates of thyroid disorders than male partners (9.0% versus 0.8%), whereas the latter had higher rates of hypercholesterolemia (8.2% versus 15.6%) and hypertension (4.0% versus 10.4%) than the former.
Table I.
Baseline characteristics of cohort by partner, LIFE Study, 2005–2009 (n = 501). Data are n (%) unless stated otherwise.
| Females (n = 501) | Males (n = 501) | P-value | |
|---|---|---|---|
| Age (mean, SD) | 29.98 (4.13) | 31.77 (4.92) | <0.01 |
| 20–30 | 256 (51.1) | 176 (35.1) | <0.01 |
| 30–40 | 245 (48.9) | 303 (60.5) | |
| 40+ | 0 (0.0) | 22 (4.4) | |
| BMI (mean, SD) | 27.60 (7.31) | 29.82 (5.55) | <0.01 |
| <25 | 229 (45.8) | 84 (16.9) | <0.01 |
| 25–30 | 136 (27.2) | 206 (41.5) | |
| 30–35 | 66 (13.2) | 131 (26.4) | |
| 35+ | 69 (13.8) | 75 (15.1) | |
| White | 393 (78.9) | 394 (79.1) | 0.94 |
| College educated | 470 (94.6) | 452 (91.1) | 0.04 |
| Prior maternity/paternity | 235 (47.2) | 239 (48.0) | 0.80 |
| Smoker | 56 (11.2) | 74 (14.8) | 0.37 |
| At least 12 drinks of any kind of alcoholic beverage in the past 12 months | 374 (74.7) | 428 (85.4) | <0.01 |
| Vigorous physical activity > 1/week | 200 (39.9) | 211 (42.1) | 0.48 |
| Hyperthyroidism | 6 (1.2) | 0 (0.0) | 0.01 |
| Hypothyroidism | 39 (7.8) | 4 (0.8) | <0.01 |
| High cholesterol | 41 (8.2) | 78 (15.6) | <0.01 |
| Diabetes | 6 (1.2) | 14 (2.8) | <0.01 |
| High blood pressure | 20 (4.0) | 52 (10.4) | <0.01 |
| Comorbidities | 0.36 | ||
| 0 | 406 (81.0) | 389 (77.6) | |
| 1 | 80 (16.0) | 91 (18.2) | |
| 2+ | 15 (3.0) | 21 (4.2) |
Couples’ medical conditions and habits were associated with pregnancy status. In both partners, diabetes was associated with a lower likelihood of pregnancy. In addition, the overall number of medical conditions was positively associated with TTP in women (Table II).
Table II.
Partners health, lifestyle and pregnancy status, LIFE Study, 2005–2009. Data are n (%).
| Pregnant (n = 347) | Not pregnant (n = 54) | Withdrawn (n = 100) | P-value | |
|---|---|---|---|---|
| Females | ||||
| White | 287 (83.0) | 40 (74.0) | 66 (6.06) | <0.01 |
| College educated | 328 (95.6) | 52 (96.3) | 90 (90.0) | 0.08 |
| Prior maternity/paternity | 182 (52.9) | 10 (18.5) | 43 (43.0) | <0.01 |
| Smoker | 23 (6.6) | 10 (18.5) | 23 (23.0) | <0.01 |
| Alcohol use | 261 (75.2) | 40 (74.1) | 73 (73.0) | 0.90 |
| Vigorous physical activity > 1/week | 147 (42.4) | 17 (31.5) | 36 (36.0) | 0.21 |
| Hyperthyroidism | 3 (0.9) | 3 (1.9) | 2 (2.0) | 0.59 |
| Hypothyroidism | 26 (7.5) | 13 (8.4) | 8 (8.0) | 0.90 |
| High cholesterol | 27 (7.8) | 14 (9.1) | 8 (8.0) | 0.71 |
| Diabetes | 1 (0.3) | 5 (3.3) | 4 (4.0) | <0.01 |
| High blood pressure | 11 (3.2) | 9 (5.8) | 6 (60) | 0.37 |
| Comorbidities | <0.01 | |||
| 0 | 285 (82.1) | 40 (74.0) | 81 (81.0) | |
| 1 | 57 (16.4) | 12 (22.2) | 11 (11.0) | |
| 2+ | 5 (1.4) | 2 (0.4) | 8 (8.0) | |
| Males | ||||
| White | 286 (83.0) | 42 (78.0) | 66 (66.0) | <0.01 |
| College educated | 326 (94.8) | 49 (90.7) | 77 (78.6) | <0.01 |
| Prior maternity/paternity | 183 (53.0) | 15 (28.3) | 41 (41.0) | <0.01 |
| Smoker | 37 (10.7) | 10 (18.5) | 27 (27.0) | <0.01 |
| Alcohol use | 301 (86.7) | 43 (79.6) | 84 (84.0) | 0.35 |
| Vigorous physical activity > 1/week | 150 (43.2) | 24 (44.4) | 37 (37.0) | 0.50 |
| Hyperthyroidism | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Hypothyroidism | 1 (0.3) | 3 (1.9) | 2 (2.0) | 0.16 |
| High cholesterol | 53 (15.3) | 25 (16.3) | 16 (16.0) | 0.94 |
| Diabetes | 5 (1.4) | 9 (5.8) | 5 (5.0) | 0.02 |
| High blood pressure | 31 (9.0) | 21 (13.6) | 18 (18.0) | 0.02 |
| Comorbidities | ||||
| 0 | 276 (79.5) | 43 (79.6) | 70 (70.0) | 0.30 |
| 1 | 57 (16.4) | 10 (18.5) | 24 (24.0) | |
| 2+ | 14 (4.0) | 1 (0.22) | 6 (6.0) |
NA, not applicable.
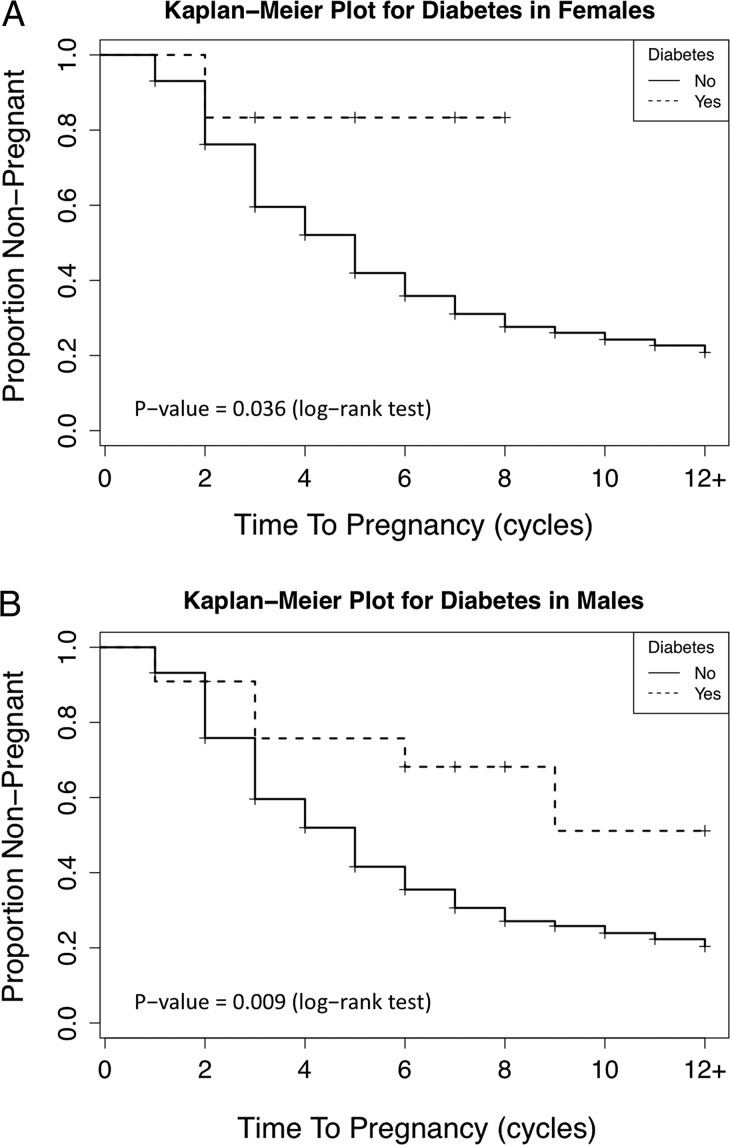
A notable observation was the consistent reduction in fecundability for all medical conditions irrespective of partner as reflected by FORs < 1.0 in Table III. However, the CIs for most FORs did not reach statistical significance. Diabetes in either partner was associated with diminished fecundity, as measured by a longer TTP (Fig. 1). Specifically, a 65% reduction in fecundity was observed for men with diabetes (0.35, 95% CI: 0.14–0.86) reflecting a longer TTP and remained so in adjusted models (0.35, 95% CI: 0.13–0.88). When both partner's medical conditions were jointly modeled, the point estimates for the FORs remained similar. However, the statistical significance was lost in fully adjusted models, possibly a reflection of more covariates in the model (Table III). Women with diabetes showed comparable reductions in FORs as did men, although the CI included one (0.26, 95% CI: 0.03–1.98), possibly reflecting lower prevalence in female (1.2%) than male (2.8%) partners. Importantly, of the six women reporting diabetes, four did not complete the study. It is important to note that women with diabetes were more likely to self-report PCOS (33%) compared with women without diabetes (5% P = 0.04). In addition, we did not find that couples were composed of men and women with similar medical conditions.
Table III.
Medical comorbidities and fecundability odds ratios (FORs), LIFE Study, 2005–2009.
| Males and females separately modeled (n = 501) | Couple based models (n = 501) | |||
|---|---|---|---|---|
| Unadjusted FOR (95% CI) | Adjusted FOR (95% CI)a | Unadjusted FOR (95% CI) | Adjusted FOR (95% CI)a | |
| Females | ||||
| Hyperthyroidism | 0.529 (0.16, 1.76) | 0.617 (0.183, 2.084) | 0.529 (0.16, 1.76) | 0.617 (0.180, 2.106) |
| Hypothyroidism | 0.720 (0.47, 1.11) | 0.774 (0.499, 1.203) | 0.743 (0.48, 1.14) | 0.816 (0.523, 1.272) |
| High cholesterol | 0.806 (0.53, 1.23) | 0.824 (0.533, 1.273) | 0.803 (0.52, 1.23) | 0.829 (0.532, 1.290) |
| Diabetes | 0.187 (0.03, 1.39) | 0.259 (0.034, 1.975) | 0.220 (0.03, 1.65) | 0.295 (0.038, 2.318) |
| High blood pressure | 0.635 (0.33, 1.20) | 0.763 (0.392, 1.486) | 0.629 (0.33, 1.19) | 0.789 (0.397, 1.568) |
| Comorbidities | ||||
| 0 | Referent | Referent | Referent | Referent |
| 1 | 0.858 (0.63, 1.17) | 0.906 (0.656, 1.251) | 0.852 (0.62, 1.16) | 0.946 (0.679, 1.318) |
| 2+ | 0.305 (0.12, 0.76)* | 0.363 (0.144, 0.915)* | 0.298 (0.12, 0.74)* | 0.345 (0.135, 0.879) |
| Hypothyroidism | 0.269 (0.04, 2.01) | 0.225 (0.030, 1.685) | 0.299 (0.04, 2.25) | 0.254 (0.033, 1.928) |
| High cholesterol | 0.936 (0.68, 1.29) | 0.942 (0.679, 1.307) | 0.930 (0.67, 1.28) | 0.916 (0.658, 1.277) |
| Diabetes | 0.347 (0.14, 0.86)* | 0.348 (0.1337, 0.884)* | 0.373 (0.15, 0.93)* | 0.383 (0.149, 0.986) |
| High blood pressure | 0.794 (0.53, 1.19) | 0.853 (0.565, 1.288) | 0.788 (0.53, 1.18) | 0.861 (0.567, 1.308) |
| Comorbidities | ||||
| 0 | Referent | Referent | Referent | Referent |
| 1 | 0.853 (0.62, 1.16) | 0.908 (0.662, 1.247) | 0.827 (0.61, 1.13) | 0.886 (0.643, 1.222) |
| 2+ | 0.854 (0.48, 1.53) | 0.835 (0.457, 1.523) | 0.849 (0.47, 1.53) | 0.821 (0.444, 1.517) |
*P < 0.05.
aModels adjusted for age, race, BMI, education, smoking, alcohol and vigorous physical activities.
Insufficient number of men with hyperthyroidism avoided.
FOR, fecundability odds ratio.
Figure 1.
Kaplan Meier plot of the proportion of couples not pregnant by time-to-pregnancy stratified by diabetes status for women (A) and men (B), LIFE Study.
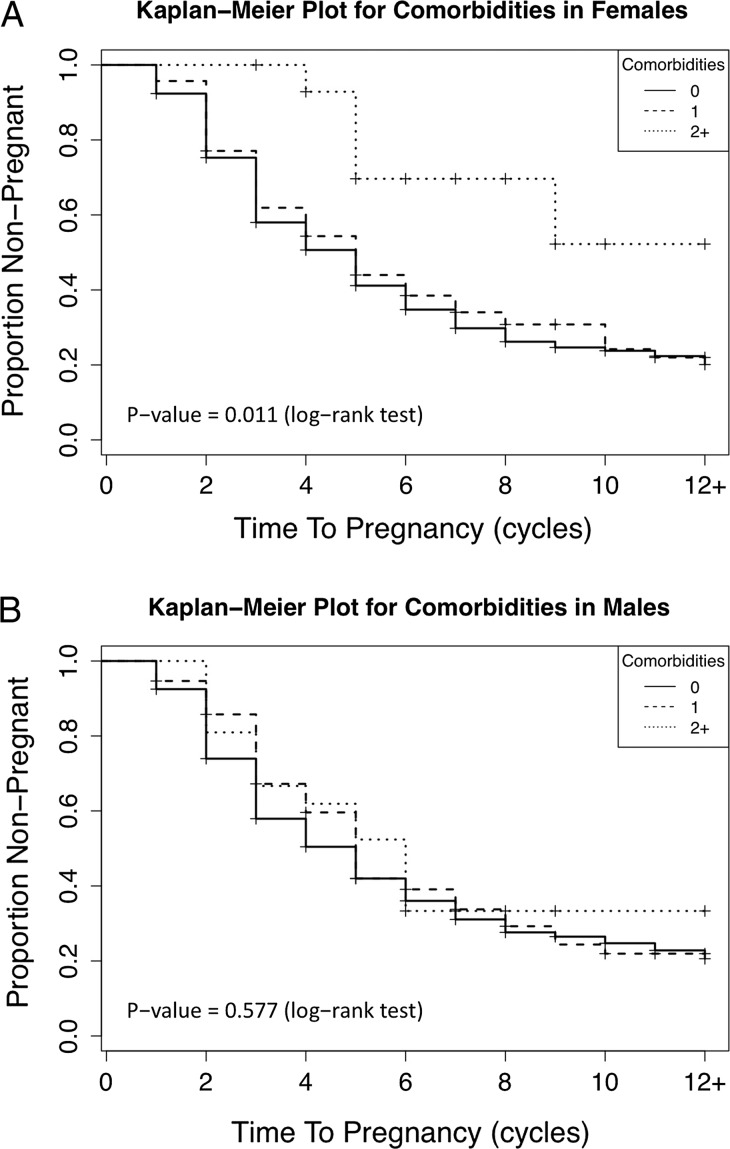
The composite exposure of interest (i.e. number of medical conditions) was positively associated with TTP for women (Fig. 2A). This corresponded to a 64% reduction in fecundity for the females only (0.36, 95% CI: 0.14–0.92) and a 65% reduction in fecundity for the couple-based models (0.35, 95% CI: 0.14–0.88). No significant relation between composite medical problems in men and in TTP was identified (Fig. 2B).
Figure 2.
Kaplan Meier plot of the proportion of couples not pregnant stratified by the number of comorbidities (0—solid line, 1—dashed line, 2+—dotted line) for women (A) and men (B), LIFE Study.
As sexual frequency may be linked to health and affect TTP, we examined the monthly sexual frequency by couples’ medical histories and observed no clear evidence of an association. However, women with high blood pressure reported a lower frequency of sexual intercourse than those without (6.02 and 7.88, P = 0.057).
Discussion
To our knowledge, the LIFE Study is the first prospective cohort study with preconception enrollment of couples to examine the relationship between somatic health of both partners of the couple and fecundity. We identified a longer TTP in couples with diabetes, especially with a diabetic male partner.
Moreover, a positive association was observed between the number of medical comorbidities in female partners and couples’ TTP. The link between somatic and reproductive health is particularly important given the increasing prevalence of comorbidities in couples attempting to conceive as couples delay pregnancy attempts until older ages along and the prevalence of overweight and obesity increases in adults (Aguilar et al., 2015). Moreover, as over 4 million reproductive aged men and women have diabetes, with over 25% of Americans with diabetes remaining undiagnosed, our findings may have an important implication of reproduction in the USA (Prevention, 2014).
The relation between diabetes and male reproductive function has been studied with mixed findings, but most studies suggest a mild impact on sperm production (Jangir et al., 2014). While some studies have identified impairments in semen parameters in diabetic men in comparison with unaffected men (Padron et al., 1984; Garcia-Diez et al., 1991; Delfino et al., 2007), other authors have reported normal semen production (Dinulovic et al., 1990; Eisenberg et al., 2015a–c). Still others reported improvement in sperm counts especially in men with diabetic neuropathy (Ali et al., 1993). Adding to the uncertainty, one study which reported reduced semen quality noted that several men with diabetes were fathers suggesting that the clinical impact of semen findings is unclear (Garcia-Diez et al., 1991). Other studies have reported a higher prevalence of primary and secondary infertility or usage of assisted reproductive technology in diabetic men compared with the general population (Bener et al., 2009; Mulholland et al., 2011). In addition, impaired IVF outcomes have also been reported among male partners with diabetes (Mulholland et al., 2011). Authors have attributed impaired fertility among men with diabetes to alterations in the hypothalamic–pituitary–gonadal axis or increased reactive oxygen specimens in the ejaculates of diabetic men as biological explanations (Lopez-Alvarenga et al., 2002; Dhindsa et al., 2004; Agbaje et al., 2007).
Diabetes has been associated with sexual dysfunction which could lead to reproductive difficulties (Kasturi et al., 2008). While overall health is associated with sexual function, we did not find a relationship between sexual frequency and medical conditions in this cohort suggesting that sexual dysfunction does not explain the longer TTP in reproductive aged diabetic men. It is important to note, however, that our study may have limited statistical power in light of our relatively small number of affected partners or couples. As such, we cannot rule out possible type II errors.
Previous authors reported the impact of diabetes on female reproductive function. Abnormal ovulatory cycles and early menopause have been identified in women with diabetes relative to unaffected women (Kjaer et al., 1992; Roumain et al., 1998; Codner et al., 2011; Schweiger et al., 2011). Indeed, women with Type I diabetes have been shown to have lower fertility rates compared with the general population, although fertility may improve with better glycemic control (Jonasson et al., 2007). The association between type 2 diabetes and fertility may be explained for some women by underlying PCOS, which is a leading cause of infertility (Nandi et al., 2013). It has been shown that insulin resistance, obesity and diabetes mellitus are strongly associated with PCOS. Moreover, PCOS and Type 2 diabetes have many of the same risk factors (Liversushits et al., 2009). Indeed, the current study found a strong coexistence between the two. It is well-established that insulin resistance impairs conception. However, with most current studies, it can be difficult to separate the impact of endocrinopathy also from the present obesity.
While individual medical problems have been explored in relation to female fecundity, the current report represents the first study to look at overall health and its impact on fecundity. In men, it is known that increased medical comorbidities are associated with male infertility and impaired semen production (Salonia et al., 2009; Eisenberg et al., 2015a–c; Ventimiglia et al., 2015). Thus, it is not surprising that a woman's overall health will impact her fecundity. Indeed obesity, which increases the risk of concurrent health problems (e.g. hypertension, diabetes and hyperlipidemia), is known to impair a woman's fertility (Bolumar et al., 2000; Wise et al., 2010,2013). It is important to note that BMI was adjusted for in the current analysis suggesting an effect of somatic health conditions independent of obesity. As diabetes, thyroid disorders and dyslipidemia have been shown to alter ovulatory and uterine function, the combination of such disorders appears to measurably impair fecundity (Benson et al., 1955; Joshi et al., 1993; Liversushits et al., 2009; Schisterman et al., 2014a,b). While our numbers were inadequate to determine whether the medical conditions or treatment may explain this relationship, the findings merit further research.
Given the link between a man's somatic health and fertility, we were surprised that male composite medical conditions were not associated with TTP (Salonia et al., 2009; Eisenberg et al., 2015a–c; Ventimiglia et al., 2015). It is important to note that although the only model for male health that significantly impacted fecundity was diabetes, the point estimates for all individual and composite comorbidities were < 1 implying a positive relation between male somatic and reproductive health. However, as fertility is a joint enterprise, impairments in one partner may be compensated for by the potential of the other. For example, a relative lower fecundability in a male partner with diabetes may be overcome with a young healthy partner, although we were not able to assess possible combinations. In addition, our study is unable to determine the direction of causality. In a similar manner, some cases of male infertility have been associated with both incident and prevalent health conditions (e.g. heart disease) due to uncertain mechanisms (Eisenberg et al., 2015a–c).
Our study findings are strengthened by our population-based sampling framework rather than reliance on a convenient or clinically based sample. This study is the first prospective population-based TTP study with preconception enrollment of both partners. Both male and female comorbidities were collected, enabling a couple-based approach for studying fecundity. Still, the findings require cautious interpretation given that self-reported medical history may be subjected to possible reporting errors, although we are unaware of any potential biases that may have been introduced, as participants were unaware of their reproductive outcome or TTP upon enrollment. We were not able to corroborate self-reported medical history in any way, and we do not have information on the clinical management or severity of diseases. Our findings need to be cautiously interpreted within these important limitations and the potential for residual confounding or chance findings in light of the number of comparisons made in the analysis. In addition, given the modest number of participants with certain comorbidities, our estimates are imprecise and we cannot rule out the possibility of type II error for null findings. Finally, the extent to which our findings may be generalizable to other populations remains to be established, particularly for populations who differ in composition from ours.
Nevertheless, our study suggests a relationship between male and female diabetes, somatic health and fecundity. As these are potentially modifiable factors, future research should determine if clinical treatment or lifestyle changes will improve couple fecundity. Moreover, while the mechanism is uncertain, if corroborated, our data suggest, early evaluation and treatment may be warranted for diabetics attempting to conceive.
Acknowledgements
We thank the Reproductive Health Assessment Team, Biomonitoring and Health Assessment Branch, National Institute of Occupational Safety and Health for conducting the semen analyses through a Memo of Understanding with the NICHD.
Authors’ roles
M.E. and G.B.L. conceived the study. G.B.L. obtained the data. R.S. and J.M. analyzed the data. All authors interpreted the analyses. M.E. drafted the paper. All authors critically revised the manuscript.
Funding
Intramural research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract nos. #N01-HD-3-3355, N01-HD-3-3356 and N01-HD-3-3358).
Conflict of interest
The authors have no relevant conflicts.
References
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod 2007;22:1871–1877. [DOI] [PubMed] [Google Scholar]
- Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- Ali ST, Shaikh RN, Siddiqi NA, Siddiqi PQ. Semen analysis in insulin-dependent/non-insulin-dependent diabetic men with/without neuropathy. Arch Androl 1993;30:47–54. [DOI] [PubMed] [Google Scholar]
- Timothy GL, Alex FR, Reynaldo M. Anthropometric Standardization Reference Manual. Champaign: Human Kinetic Books, 1988. [Google Scholar]
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus?. Int Urol Nephrol 2009;41:777–784. [DOI] [PubMed] [Google Scholar]
- Benson RC, Dailey ME. The menstrual pattern in hyperthyroidism and subsequent posttherapy hypothyroidism. Surg Gynecol Obstet 1955;100:19–26. [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol 2000;151:1072–1079. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codner E, Eyzaguirre FC, Iniguez G, Lopez P, Perez-Bravo F, Torrealba IM, Cassorla F. Ovulation rate in adolescents with type 1 diabetes mellitus. Fertil Steril 2011;95:197–202, 202 e191. [DOI] [PubMed] [Google Scholar]
- Delfino M, Imbrogno N, Elia J, Capogreco F, Mazzilli F. Prevalence of diabetes mellitus in male partners of infertile couples. Minerva Urol Nefrol 2007;59:131–135. [PubMed] [Google Scholar]
- Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–5468. [DOI] [PubMed] [Google Scholar]
- Dinulovic D, Radonjic G. Diabetes mellitus/male infertility. Arch Androl 1990;25:277–293. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014;29:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Chen Z, Ye A, Buck Louis GM. Relationship between physical occupational exposures and health on semen quality: data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril 2015. a;103:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril 2015. b;103:66–71. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril 2015. c;105:629–636. [DOI] [PubMed] [Google Scholar]
- Garcia-Diez LC, Corrales Hernandez JJ, Hernandez-Diaz J, Pedraz MJ, Miralles JM. Semen characteristics and diabetes mellitus: significance of insulin in male infertility. Arch Androl 1991;26:119–128. [DOI] [PubMed] [Google Scholar]
- Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril 2004;81:384–392. [DOI] [PubMed] [Google Scholar]
- Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev 2014;10:147–157. [DOI] [PubMed] [Google Scholar]
- Jonasson JM, Brismar K, Sparen P, Lambe M, Nyren O, Ostenson CG, Ye W. Fertility in women with type 1 diabetes: a population-based cohort study in Sweden. Diabetes care 2007;30:2271–2276. [DOI] [PubMed] [Google Scholar]
- Joshi JV, Bhandarkar SD, Chadha M, Balaiah D, Shah R. Menstrual irregularities and lactation failure may precede thyroid dysfunction or goitre. J Postgrad Med 1993;39:137–141. [PubMed] [Google Scholar]
- Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl 2008;29:251–259. [DOI] [PubMed] [Google Scholar]
- Kjaer K, Hagen C, Sando SH, Eshoj O. Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J Clin Endocrinol Metab 1992;75:524–529. [DOI] [PubMed] [Google Scholar]
- Liversushits A, Seidman DS. Fertility issues in women with diabetes. Women‘s Health 2009;5:701–707. [DOI] [PubMed] [Google Scholar]
- Lopez-Alvarenga JC, Zarinan T, Olivares A, Gonzalez-Barranco J, Veldhuis JD, Ulloa-Aguirre A. Poorly controlled type I diabetes mellitus in young men selectively suppresses luteinizing hormone secretory burst mass. J Clin Endocrinol Metab 2002;87:5507–5515. [DOI] [PubMed] [Google Scholar]
- Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Mallidis C, Agbaje I, McClure N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online 2011;22:215–219. [DOI] [PubMed] [Google Scholar]
- Nandi A, Poretsky L. Diabetes and the female reproductive system. Endocrinol Metab Clin North Am 2013;42:915–946. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men‘s body mass index and infertility. Hum Reprod 2007;22:2488–2493. [DOI] [PubMed] [Google Scholar]
- Padron RS, Dambay A, Suarez R, Mas J. Semen analyses in adolescent diabetic patients. Acta Diabetol Lat 1984;21:115–121. [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta: U.S. Department of Health and Human Services, 2014. [Google Scholar]
- Roumain J, Charles MA, de Courten MP, Hanson RL, Brodie TD, Pettitt DJ, Knowler WC. The relationship of menstrual irregularity to type 2 diabetes in Pima Indian women. Diabetes care 1998;21:346–349. [DOI] [PubMed] [Google Scholar]
- Salonia A, Matloob R, Gallina A, Abdollah F, Sacca A, Briganti A, Suardi N, Colombo R, Rocchini L, Guazzoni G et al. Are infertile men less healthy than fertile men? Results of a prospective case–control survey. Eur Urol 2009;56:1025–1031. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, Browne RW, Barr DB, Chen Z, Louis GM. Lipid concentrations and couple fecundity: the LIFE study. J Clin Endocrinol Metab 2014. a;99:2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, Chen Z, Browne RW, Boyd Barr D, Kim S, Buck Louis GM. Lipid concentrations and semen quality: the LIFE study. Andrology 2014. b;2:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger BM, Snell-Bergeon JK, Roman R, McFann K, Klingensmith GJ. Menarche delay and menstrual irregularities persist in adolescents with type 1 diabetes. Reprod Biol Endocrinol RB&E 2011;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015;104:48–55. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod 2013;28:2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod 2010;25:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]