Abstract
STUDY QUESTION
Is the length of FMR1 repeat alleles within the normal range associated with the risk of early menopause?
SUMMARY ANSWER
The length of repeat alleles within the normal range does not substantially affect risk of early menopause.
WHAT IS KNOWN ALREADY
There is a strong, well-established relationship between length of premutation FMR1 alleles and age at menopause, suggesting that this relationship could continue into the normal range. Within the normal range, there is conflicting evidence; differences in ovarian reserve have been identified with FMR1 repeat allele length, but a recent population-based study did not find any association with age at menopause as a quantitative trait.
STUDY DESIGN, SIZE, DURATION
We analysed cross-sectional baseline survey data collected at recruitment from 2004 to 2010 from a population-based, prospective epidemiological cohort study of >110 000 women to investigate whether repeat allele length was associated with early menopause.
PARTICIPANTS/MATERIALS, SETTING, METHOD
We included 4333 women from the Breakthrough Generations Study (BGS), of whom 2118 were early menopause cases (menopause under 46 years) and 2215 were controls. We analysed the relationship between length of FMR1 alleles and early menopause using logistic regression with allele length as continuous and categorical variables. We also conducted analyses with the outcome age at menopause as a quantitative trait as well as appropriate sensitivity and exploratory analyses.
MAIN RESULTS AND THE ROLE OF CHANCE
There was no association of the shorter or longer FMR1 allele or their combined genotype with the clinically relevant end point of early menopause in our main analysis. Likewise, there were no associations with age at menopause as a quantitative trait in our secondary analysis.
LIMITATIONS, REASONS FOR CAUTION
Women with homozygous alleles in the normal range may have undetected FMR1 premutation alleles, although there was no evidence to suggest this. We estimate minor dilution of risk of early menopause from the likely inclusion of some women with menopause at over 45 years in the early menopause cases due to age-rounding bias in self-reports.
WIDER IMPLICATIONS OF THE FINDINGS
There is no robust evidence in this large study that variation within the normal range of FMR1 repeat alleles influences timing of menopause in the general population, which contradicts findings from some earlier, mainly smaller studies. The FMR1 CGG repeat polymorphism in the normal range is unlikely to contribute to genetic susceptibility to early menopause.
STUDY FUNDING/COMPETING INTEREST(S)
We thank Breast Cancer Now and The Institute of Cancer Research for funding the BGS. The Institute of Cancer Research acknowledges NHS funding to the NIHR Biomedical Research Centre. The study was funded by the Wellcome Trust (grant number 085943). There are no competing interests.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: MeSH, FMR1-related primary ovarian insufficiency, Fragile X-associated primary ovarian insufficiency, FMR1 protein, human, menopause, premature menopause
Introduction
Previous studies have suggested that normal variation in number of CGG repeats in FMR1 could influence age at menopause. The 5′ untranslated region of the FMR1 gene contains a CGG repeat that varies in length, causing Fragile X syndrome at over 200 repeats, with methylation and silencing of the FMR1 gene and lack of FMRP expression. While ovarian function remains normal in women with full mutation range repeat alleles, primary ovarian insufficiency (POI) occurs in 20% of women with alleles in the premutation range of FMR1 (55–200 repeats) (Allen et al., 2014).
In the premutation range, FMRP is expressed although there are elevated levels of FMR1 mRNA, which have been found to sequester mRNA binding proteins. Although the mechanism of causation for POI remains unknown, premutation range alleles impair follicle development and induce apoptosis in mouse models (Lu et al., 2012). Also, there is a non-linear relationship between length of premutation alleles that have a dominant genetic effect and age at menopause, with earliest menopause at around 80 copies and later menopause at lower and higher copy numbers (Sullivan et al., 2005; Ennis et al., 2006; Mailick et al., 2014). It has been hypothesised that this relationship with age at menopause may continue to be observed in the range for normal length alleles (<55 repeats).
In white Europeans, the lowest observed allele length is six CGGs, and there are peaks in the distribution at 20, 23 and 30 CGGs, with 30 repeats being the most common (Murray et al., 1996). Previous studies have defined subgroups based on allele length and have reported differences in ovarian reserve between these (Gleicher et al., 2009, 2010, 2012a,b). In some studies, a greater proportion of longer normal length FMR1 alleles have been found in women with POI or diminished ovarian reserve (Bretherick et al., 2005; Bodega et al., 2006; Pastore et al., 2012). However, many of these studies were composed of fewer than 500 women. Other analyses, including two large studies in over 3000 women by Voorhuis et al., have not found an association between longer length normal FMR1 alleles and POI (Bennett et al., 2010; Voorhuis et al., 2014) and no relationship between normal length FMR1 alleles and age at menopause (Voorhuis et al., 2013). We tested the role of normal-sized FMR1 CGG repeat alleles in menopause timing in a cohort of over 2000 early menopause cases, plus over 2000 controls, drawn from a population-based study of over 110 000 women from the Breakthrough Generations Study (BGS).
Materials and Methods
Participants included
In this analysis, we included 4333 women from the BGS recruited from 2004 to 2010. The BGS is a prospective population cohort study started in 2003 to investigate the environmental, behavioural, hormonal and genetic causes of breast cancer (Swerdlow et al., 2011) (http://www.breakthroughgenerations.org.uk/). The BGS cohort includes over 110 000 women aged 16 and older at entry, recruited from the general UK population through connections to the charity Breakthrough Breast Cancer or who volunteered as a result of publicity, and female friends and family members of these participants. The study received appropriate ethical approval from the South East MREC, and informed consent was received from the participants (Swerdlow et al., 2011). Detailed menstrual histories and blood samples were collected. All women with early menopause were eligible for inclusion in our analyses. Breast cancer cases were excluded from our analyses. Of the women included, 99.5% were of white ethnicity (Table I).
Table I.
Summary statistics for individuals included in the analysis (all variables measured at recruitment unless otherwise specified).
| Early menopause cases | Controls | Post-menopausal women included in quantitative trait analysis | ||||
|---|---|---|---|---|---|---|
| Age (years) | n = 2118 | n = 2215 | n = 3805 | |||
| Mean (range) | 58.5 (22,88) | 59.4 (45,89) | 60.3 (22,89) | |||
| Median (lower quartile, upper quartile) | 58 (52,64) | 59 (53,65) | 60 (55,66) | |||
| Year of entry | n = 2118 | n = 2215 | n = 3805 | |||
| Mean (range) | 2006 (2004, 2009) | 2006 (2004, 2010) | 2006 (2004, 2010) | |||
| Median (lower quartile, upper quartile) | 2006 (2005, 2007) | 2006 (2005, 2007) | 2006 (2005, 2007) | |||
| Age at menopause | n = 2118 | n = 1687 | n = 3805 | |||
| Mean (range) | 42.5 (19,45) | 52.1 (46,62) | 46.7 (19,62) | |||
| Median (lower quartile, upper quartile) | 43 (41,45) | 52 (50,54) | 45 (43,52) | |||
| BMI at age 20 | n = 1684 | n = 1801 | n = 3053 | |||
| Mean (range) | 21.3 (12,56.5) | 21.4 (12.4,38.3) | 21.3 (12,56.5) | |||
| Median (lower quartile, upper quartile) | 21 (19.6,22.6) | 21.1 (19.8,22.7) | 21 (19.7,22.6) | |||
| BMI (kg/m2) | n = 2041 | n = 2148 | n = 3676 | |||
| Mean (range) | 25.8 (5.1,63.2) | 25.6 (14.9,54.4) | 25.7 (5.1,63.2) | |||
| Median (lower quartile, upper quartile) | 24.9 (22.7,28) | 24.9 (22.6,27.6) | 24.9 (22.7,27.8) | |||
| Height (m) | n = 2082 | n = 2192 | n = 3750 | |||
| Mean (range) | 1.63 (1.27,2.06) | 1.63 (1.37,1.83) | 1.63 (1.27,2.06) | |||
| Median (lower quartile, upper quartile) | 1.63 (1.58,1.68) | 1.63 (160,1.68) | 1.63 (1.58,1.68) | |||
| Number of births (live and still) at ≥26 weeks gestation (includes never pregnant) | n = 2111 | n = 2210 | n = 3795 | |||
| Mean (range) | 1.9 (0,10) | 2.1 (0,8) | 2.0 (0,10) | |||
| Median (lower quartile, upper quartile) | 2 (1,3) | 2 (2,3) | 2 (1,3) | |||
| n | % | n | % | n | % | |
| Ethnicity | ||||||
| White ethnicity | 2107 | 99.5 | 2204 | 99.5 | 3787 | 99.5 |
| Non-white | 11 | 0.5 | 11 | 0.5 | 18 | 0.5 |
| Smoking status | ||||||
| Never smoker | 1242 | 58.6 | 1401 | 63.3 | 2299 | 60.4 |
| Former smoker | 683 | 32.3 | 714 | 32.2 | 1146 | 32.8 |
| Current smoker | 187 | 8.8 | 99 | 4.5 | 252 | 6.7 |
| Smoking status not known | 6 | 0.3 | 1 | 0.1 | 7 | 0.2 |
| Hormone replacement therapy use | ||||||
| Never | 1036 | 48.9 | 1526 | 68.9 | 2077 | 54.6 |
| Former | 752 | 35.5 | 539 | 24.3 | 1268 | 33.3 |
| Current | 323 | 15.3 | 147 | 6.6 | 450 | 11.8 |
| Not known | 7 | 0.3 | 3 | 0.1 | 10 | 0.3 |
| Oral contraceptive use | ||||||
| Never | 526 | 24.8 | 584 | 26.4 | 1065 | 28.0 |
| Former | 1569 | 74.1 | 1591 | 71.8 | 2711 | 71.3 |
| Current | 11 | 0.5 | 35 | 1.6 | 12 | 0.3 |
| Not known | 12 | 0.6 | 5 | 0.2 | 17 | 0.4 |
| Total | 2118 | 2215 | 3805 | |||
Definition of age at natural menopause
Natural menopause was defined as cessation of menstruation for at least 6 months without known cause based on questionnaire data (‘Have your periods now stopped completely? (That is, have you now gone at least 6 months without having a period and you are not pregnant or on the contraceptive pill)’). We excluded women if periods stopped because of pregnancy, breastfeeding, surgery, hormonal contraceptive use or other types of medical treatment or if there was a medical condition or illness that could have caused amenorrhoea (e.g. polycystic ovary syndrome).
Early menopause cases and controls
A nested case–control design was used for reasons of cost-effectiveness. Early menopause cases had natural menopause at age 45 years or younger, whereas controls were women known to have had menopause (natural or surgical) at 46 years or older or, to allow sufficient suitable controls to be identified, women who were aged 46 years or older and were premenopausal. Where possible, controls were matched to cases on date of birth (within 12 months), ethnicity, year of questionnaire completion and source of recruitment. Where this was not possible, matching criteria were relaxed in the following order until a match was found: source of recruitment, year of completion and date of birth. Early menopause cases aged 45 years or younger were matched to a control aged 46 years, since all postmenopausal women aged 45 years or younger were early menopause cases. In order to exclude subjects who were genetically related to each other, we identified mother–daughter and sister–sister pairs among the selected subjects. We excluded the oldest subject when both of the individuals were cases or controls. When one was a case and the other a control, the control was replaced. Following genotyping and data cleaning, 4333 women remained in our analysis, of whom 2215 were early menopause cases (including 250 women with menopause under 40 years) and 2118 were controls.
Evaluation of FMR1 repeat length
For each subject, Asuragen Amplidex kits (http://www.asuragen.com) containing FMR1 CGG repeat region–specific primers were used to PCR amplify the FMR1 repeat region from 20 ng of genomic DNA that had been extracted from peripheral blood mononuclear cells. All PCRs were performed in 3 μl of reaction volumes in 384-well microtiter plates, using conditions recommended by the kit manufacturers. Products were size separated by capillary electrophoresis on an ABI 3730 automated sequencer (Applied Biosystems, Warrington, UK), using a ROX 1000 size standard (Asuragen, Austin, TX, USA) for estimation of product sizes. CGG repeat numbers were determined by comparison with a control individual of 52 CGG repeats. We included duplicates of ~10% of the samples on independent plates. The concordance between duplicate samples was 98.5%, excluding differences of ±1 CGG repeat. Controls included 12 no-template controls, 3 samples from females of known expansion size (largest CGG = 55, 117 and 145) and a lane containing the multiple size targets supplied by Asuragen (CGG = 20, 29, 31, 53, 117 and 196) per 384 plate.
Statistical analyses
We analysed the effect of FMR1 alleles and genotypes on odds of early menopause using logistic regression. All women had both FMR1 repeat alleles in the clinically normal range (<55 copies). All statistical analyses were performed using Stata 13.1 or 14.1 (StataCorp, College Station, TX, USA). To avoid assuming that normal length FMR1 alleles would act by the same dominant genetic mechanism as premutation alleles, we considered dominant and additive effects by investigating the effects of each individual FMR1 allele and combinations of alleles (genotypes).
In each woman, we defined ‘Allele 1’ as the shorter FMR1 repeat allele and ‘Allele 2’ as the longer of her two alleles. We tested the per repeat effect of each FMR1 allele as a continuous variable and also classified each allele into repeat categories according to size, using previously published criteria (Gleicher et al., 2014): alleles <26 repeats were considered to be ‘low’, 26–34 repeats to be ‘medium’ and >34 repeats to be ‘high’.
To test the effects of each individual FMR1 allele, statistical analyses were performed for Allele 1 and Allele 2 separately. Allele length was treated in three ways: as a continuous variable, as a nominal categorical variable (comparison of low and high to medium length as the reference category) or as an ordinal categorical variable (analysed as a continuous variable ordered from low to high).
We also analysed the effect of the FMR1 repeat allele combination, or genotype. We included each allele as a continuous variable in the same model, with/without an interaction term (Allele 1 × Allele 2). We generated six genotype categories from the combinations of repeat length category for the two alleles: low/low, low/medium, low/high, medium/medium, medium/high, high/high. The genotypes were treated as either nominal variables (comparing each genotype to medium/medium) or as ordinal variables (analysed as a continuous variable), defining the order in two ways: (i) (a) low/low, (b) low/medium, (c) low/high, (d) medium/medium, (e) medium/high, (f) high/high; or (ii) (a) low/low, (b) low/medium, (c) medium/medium, (d) low/high, (e) medium/high, (f) high/high. Exploratory analyses were performed of alternative methods of modelling combinations of the alleles: as a difference between allele length in an individual or as a mean allele length.
In a secondary analysis, we investigated the relationship between FMR1 alleles and genotypes with age at natural menopause in the 3805 postmenopausal women in our study. This was a smaller number than in the case–control study because not all controls were postmenopausal and so some were not included. The residuals of the regression on menopause age were not normally distributed due to overrepresentation of early menopause cases in the analysis; therefore, we applied a statistical correction to transform the data to a normal distribution. Briefly, this method ranked the observed values of age at menopause (randomly ordering tied values), then converted these to a percentile of a standard normal distribution.
Sensitivity analyses were carried out by conditional logistic regression analyses in 1559 fully matched case–control pairs, and by repeating the logistic regression analyses restricted to women of white ethnicity and including smoking status at time of study entry as a covariate. Smoking status and ethnicity were available for all women analysed (Table I). In the analysis of menopause as a quantitative trait, we also tested the models separately in the control group. Earlier exploratory analysis with menopause before 40 years as the outcome was consistent with the main analysis (not presented).
Statistical power was estimated using Quanto (http://biostats.usc.edu/Quanto.html). We estimated the size of genetic effect we could detect at 80% power for an additive mode of inheritance comparing the low/low genotype with low/medium and medium/medium. For case–control models, we assumed a population prevalence of 5% for early menopause.
Results
Repeat distribution
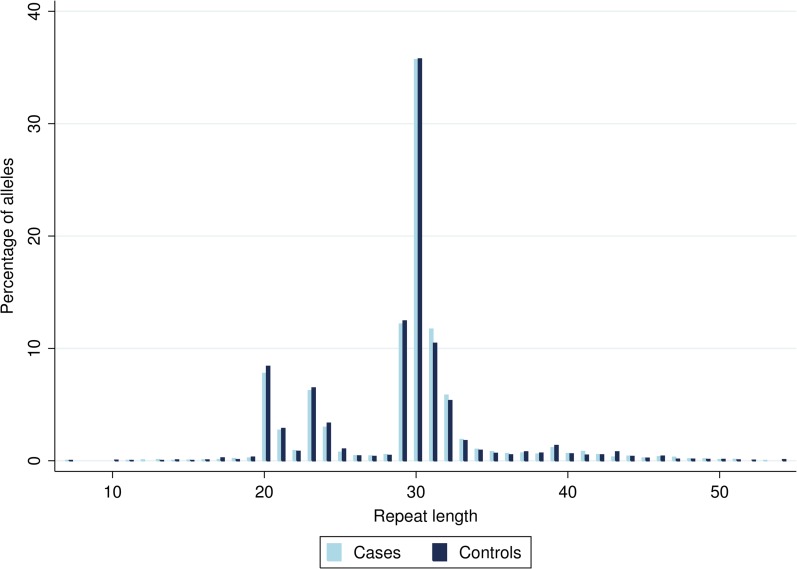
The length of the FMR1 allele repeat ranged from 7 to 54 copies (Fig. 1). The distribution of the FMR1 allele was consistent with previous studies with a mode at 30 copies, and secondary peaks at 20 and 23 copies (Fu et al., 1991; Peprah, 2012), with similar distributions in the early menopause cases and the rest of the cohort. Almost half of the women with a natural age at menopause had two medium length alleles (26–34 repeats), and almost one-third had a combination of one low allele (< 26 repeats) and one medium allele (26–34 repeats) (Table II).
Figure 1.
Percentage of FMR1 alleles of each length in early menopause cases (n = 4236) and controls (n = 4430).
Table II.
Number of women by FMR1 genotype, categorised by allele lengths.
| Genotype | Early menopause cases | Controls | Postmenopausal women included in quantitative trait analysis | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Low/low | 130 | 5.9 | 101 | 4.8 | 204 | 5.4 |
| Low/medium | 710 | 32.1 | 662 | 31.3 | 1215 | 31.9 |
| Low/high | 88 | 4.0 | 77 | 3.6 | 145 | 3.8 |
| Medium/medium | 1027 | 46.4 | 1035 | 48.9 | 1803 | 47.4 |
| Medium/high | 248 | 11.2 | 221 | 10.4 | 409 | 10.7 |
| High/high | 12 | 0.5 | 22 | 1.0 | 29 | 0.8 |
| Total | 2215 | 100.0 | 2118 | 100.0 | 3805 | 100.0 |
Note: Low < 26 repeats; medium 26–34 repeats; high 35–54 repeats.
Age of menopause
In early menopause cases (n = 2118), the median age at natural menopause was 43 years (range 19–45 years, mean 42.5 years, SD 3.1 years) compared with 52 years in the controls (n = 1687, range 46−62 years, mean 52.1 years, SD 3.1 years), although 24% (n = 528) of controls were premenopausal and therefore could not be included in the mean (Table I).
No association between length of either FMR1 repeat allele and early menopause
We found no associations for either FMR1 allele with odds of early menopause (Table III). There was no association between length of either Allele 1 or Allele 2 of FMR1 and age at menopause as a quantitative trait (P > 0.05 for all) (Table III).
Table III.
Relationship of FMR1 allele length with early menopause and age at menopause as a quantitative trait.
| Model | Variables included | Early menopause (n = 2215 cases, n = 2118 controls) | Age at natural menopause (n = 3805) | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | Effecta | 95% CI | P | ||
| Allele 1, continuous | Allele 1 (continuous) | 1.01 | 1–1.03 | 0.077 | 0.00 | −0.01–0 | 0.280 |
| Allele 1, nominal categorical | 1. Low | 0.92 | 0.81–1.04 | 0.173 | 0.05 | −0.01–0.12 | 0.125 |
| 2. Medium | ref. | ref. | |||||
| 3. High | 1.86 | 0.92–3.78 | 0.085 | −0.26 | −0.63–0.1 | 0.159 | |
| Allele 1, ordinal categorical | 1. Low | 1.11 | 0.99–1.25 | 0.075 | −0.06 | −0.12–0 | 0.063 |
| 2. Medium | |||||||
| 3. High | |||||||
| Allele 2, continuous | Allele 2 (continuous) | 1.00 | 0.99–1.01 | 0.750 | 0.00 | −0.01–0.01 | 0.672 |
| Allele 2, nominal categorical | 1. Low | 0.80 | 0.61–1.04 | 0.094 | 0.05 | −0.07–0.22 | 0.302 |
| 2. Medium | ref. | ref. | |||||
| 3. High | 0.94 | 0.8–1.11 | 0.474 | 0.01 | −0.07–0.11 | 0.679 | |
| Allele 2, ordinal categorical | 1. Low | 1.03 | 0.9–1.17 | 0.712 | −0.01 | −0.08–0.06 | 0.800 |
| 2. Medium | |||||||
| 3. High | |||||||
aEffect size is in standard deviations of inverse normally transformed age at menopause.
Notes: OR, odds ratio; ref., reference category.
No association between FMR1 repeat genotype and early menopause
FMR1 repeat genotype was not associated with early menopause or age at menopause as a quantitative trait (P > 0.05 for all) (Table IV).
Table IV.
Relationship of FMR1 genotype with early menopause and age at menopause as a quantitative trait.
| Model | Variables included | Early menopause (n = 2215 cases, n = 2118 controls) | Age at natural menopause (n = 3805) | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | Effecta | 95% CI | P | ||
| Allele 1, Allele 2, continuous | Allele 1 (continuous) | 1.01 | 1–1.03 | 0.079 | 0.00 | −0.01–0 | 0.328 |
| Allele 2 (continuous) | 1.00 | 0.98–1.01 | 0.805 | 0.00 | −0.01–0.01 | 0.808 | |
| Allele 1, Allele 2 and interaction, continuous | Allele 1 (continuous) | 0.98 | 0.9–1.06 | 0.586 | 0.00 | −0.04–0.04 | 0.934 |
| Allele 2 (continuous) | 0.97 | 0.91–1.04 | 0.367 | 0.00 | −0.03–0.04 | 0.836 | |
| Allele 1 × Allele 2 (interaction) | 1.00 | 1–1 | 0.384 | 0.00 | 0–0 | 0.793 | |
| Genotype, nominal categorical | 1. Low/low | 0.77 | 0.59–1.01 | 0.063 | 0.09 | −0.05–0.24 | 0.202 |
| 2. Low/medium | 0.93 | 0.81–1.06 | 0.265 | 0.06 | −0.02–0.13 | 0.127 | |
| 3. Low/high | 0.87 | 0.63–1.19 | 0.384 | 0.04 | −0.13–0.21 | 0.619 | |
| 4. Medium/medium | ref. | ref. | |||||
| 5. Medium/high | 0.88 | 0.72–1.08 | 0.230 | 0.05 | −0.06–0.16 | 0.349 | |
| 6. High/high | 1.82 | 0.9–3.7 | 0.098 | −0.25 | −0.61–0.12 | 0.190 | |
| Genotype, ordinal categorical (order 1) | 1. Low/low | 1.04 | 0.99–1.09 | 0.132 | −0.02 | −0.05–0 | 0.110 |
| 2. Low/medium | |||||||
| 3. Low/high | |||||||
| 4. Medium/medium | |||||||
| 5. Medium/high | |||||||
| 6. High/high | |||||||
| Genotype, ordinal categorical (order 2) | 1. Low/low | 1.03 | 0.97–1.09 | 0.399 | −0.02 | −0.05–0.01 | 0.309 |
| 2. Low/medium | |||||||
| 3 .Medium/medium | |||||||
| 4. Low/high | |||||||
| 5. Medium/high | |||||||
| 6. High/high | |||||||
OR, odds ratio; ref., reference category.
aEffect size is in standard deviations of inverse normally transformed age at menopause.
Notes: The genotypes were treated as either nominal variables (comparing each genotype to medium/medium) or as ordinal variables, defining the order in two ways: (i) 1. low/low, 2. low/medium, 3. low/high, 4. medium/medium, 5. medium/high, 6. high/high; (ii) 1. low/low, 2. low/medium, 3. medium/medium, 4. low/high, 5. medium/high, 6. high/high.
Sensitivity analyses
In the fully matched case–control analysis, we found no robust associations of either FMR1 allele or genotype with odds of early menopause, although ‘low’ lengths of Allele 2 (OR = 0.72, 95% CI 0.52−0.99, P = 0.044) and the ‘low/low’ genotype (OR = 0.69; 95% CI 0.50–0.96; P = 0.028) were associated with decreased odds of early menopause (P > 0.05 for all other results) (Supplementary Table 1 and 2).
We identified smoking as a potential confounder, since smoking is associated with earlier menopause (Gold, 2011;Morris et al., 2012). Consistent with this, there was an association between being a current smoker and earlier menopause in our analyses (OR = 2.13; 95% CI 1.65–2.75; P = 6.4 × 10–9); however, length of FMR1 allele or genotype was not associated with smoking (P > 0.05 for all) (data available on request). When the analyses were adjusted for smoking, the results were consistent with the main analysis (data available on request). The results remained consistent with the main analysis when analyses were restricted to women of white ethnicity (data available on request) or when the secondary analysis of age at menopause was carried out in only the control group (Supplementary Tables 3 and 4). In exploratory analyses, neither mean repeat length nor difference in repeat length was associated with early menopause or age at menopause (P > 0.05 for all).
Discussion
We found no robust association between normal length FMR1 alleles and early menopause. This is unlikely to be due to a lack of power since we estimated that we were powered to detect an odds ratio <0.85 or >1.18 per low allele (<26 repeats) in the analysis of all cases and controls (with similar values for matched analysis). This is similar in size to estimated odds ratios for early menopause (≤45 years) of 1.13–1.85 per allele for common single-nucleotide polymorphisms (SNPs) in the same study cohort (Murray et al., 2011). For the analysis of age at menopause as a quantitative trait, we estimated that we were powered to detect a change of about 0.5 years per low allele, similar to the 0.1–0.9 years per allele effect sizes for common SNPs (Day et al., 2015). Indeed, we were sufficiently powered to detect a strong effect of smoking on reducing age of menopause, which has been previously observed in this same study cohort (Morris et al., 2012).
As well as demonstrating no association between normal FMR1 allele length and risk of early menopause, our results corroborate a null association between FMR1 normal length alleles and quantitative age at menopause from a population-based study not focussed on early menopause (Voorhuis et al., 2013). Accelerated loss of ovarian reserve in women with low alleles and better ovarian reserve with high alleles has been reported in studies including up to 521 women (Gleicher et al., 2010, 2012a,b, 2014). Our study is not consistent with these findings since lower AMH levels, and hence ovarian reserve, predict earlier menopause (Aydogan and Mirkin, 2015); however, reduced ovarian reserve does not necessarily result in POI and this may explain the discrepancy (Gleicher et al., 2015).
Although we were well-powered to detect an association, our calculations do not take into account factors affecting the accuracy of the data collected or that would have reduced our power to detect an association. Long FMR1 alleles are harder to detect; therefore, women with homozygous alleles may actually be heterozygotes with an undetected premutation repeat. Of the 4333 women, 21% were homozygotes, but there was high concordance between duplicated samples, and the proportion of homozygotes in early menopause cases and controls was not statistically different. We would expect such genotyping errors to result in a higher proportion of homozygotes in early menopause cases, since premutation repeats are a known aetiological risk factor for early menopause (Sherman, 2000).
Another factor that might have contributed to the lack of an observed association is the potential dilution of risk of early menopause from misidentification of early menopause cases. Previous studies have observed rounding bias towards reporting values ending in 0 or 5 when women are asked to recall their age at menopause (Hahn et al., 1997); therefore, some women may have rounded down their menopause age to 45. We estimate, based on the distribution in our data by reported age in single years, that this may have occurred in 7% of early menopause cases. Hence, the consequent dilution of risk would have been minor and does not account for the lack of an observed association. We controlled for two potential confounders in our analysis: ethnicity and smoking. Ethnicity is known to affect FMR1 allele length (Peprah, 2012), and we found no association with smoking.
In summary, in a large population-based study, we found no association between normal length FMR1 repeats and risk of the clinically relevant outcome early menopause, and replicated a null association with age at menopause as a quantitative trait. The FMR1 CGG repeat polymorphism in the normal range does not influence risk of early menopause and is therefore unlikely to contribute to genetic susceptibility to early menopause.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Acknowledgements
We thank Breast Cancer Now and the Institute of Cancer Research for support, and the BGS participants and study staff, and the doctors, nurses and other health care staff and data providers who have contributed to the study.
Authors’ roles
A.M. designed the study. K.S.R. carried out statistical analysis and drafted the manuscript. M.J.S. and A.J.S. selected the study cohort and provided the BGS data. C.E.B. conducted the genotyping. M.N.W. advised on statistical analysis. All authors were involved in revising and approving the manuscript.
Funding
We thank Breast Cancer Now and The Institute of Cancer Research for funding the BGS. The Institute of Cancer Research acknowledges NHS funding to the NIHR Biomedical Research Centre. The study was funded by the Wellcome Trust (grant number 085943).
Conflict of interest
No conflicts of interest are declared.
References
- Allen EG, Grus WE, Narayan S, Espinel W, Sherman SL. Approaches to identify genetic variants that influence the risk for onset of fragile X-associated primary ovarian insufficiency (FXPOI): a preliminary study. Front Genet 2014;5:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogan B, Mirkin S. The utility of measuring anti-Müllerian hormone in predicting menopause. Climacteric 2015;18:777–789. [DOI] [PubMed] [Google Scholar]
- Bennett C, Conway G, Macpherson J, Jacobs P, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Human Reprod 2010;25:1335–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodega B, Bione S, Dalpra L, Toniolo D, Ornaghi F, Vegetti W, Ginelli E, Marozzi A. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod 2006;21:952–957. [DOI] [PubMed] [Google Scholar]
- Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet 2005;117:376–382. [DOI] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet 2006;14:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG Jr, Warren ST et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991;67:1047–1058. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Barad DH. Prospectively assessing risk for premature ovarian senescence in young females: a new paradigm. Reprod Biol Endocrinol 2015;13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Weghofer A, Barad DH. How the FMR1 gene became relevant to female fertility and reproductive medicine. Front Genet 2014;5:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Mullerian hormone. Fertil Steril 2009;91:1700–1706. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online 2010;20:768–775. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Kim A, Barad DH. Comparison of ovarian FMR1 genotypes and sub-genotypes in oocyte donors and infertile women. J Assist Reprod Genet 2012. a;29:529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Kim A, Barad DH. The impact in older women of ovarian FMR1 genotypes and sub-genotypes on ovarian reserve. PLoS One 2012. b;7:e33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol 1997;146:771–775. [DOI] [PubMed] [Google Scholar]
- Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet 2012;21:5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Hong J, Greenberg J, Smith L, Sherman S. Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. Am J Med Genet B Neuropsychiatr Genet 2014;165b:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DH, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the Breakthrough Generations Study. Am J Epidemiol 2012;175:998–1005. [DOI] [PubMed] [Google Scholar]
- Murray A, Bennett CE, Perry JR, Weedon MN, Jacobs PA, Morris DH, Orr N, Schoemaker MJ, Jones M, Ashworth A et al. Common genetic variants are significant risk factors for early menopause: results from the Breakthrough Generations Study. Hum Mol Genet 2011;20:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Youings S, Dennis N, Latsky L, Linehan P, McKechnie N, Macpherson J, Pound M, Jacobs P. Population screening at the FRAXA and FRAXE loci: molecular analyses of boys with learning difficulties and their mothers. Hum Mol Genet 1996;5:727–735. [DOI] [PubMed] [Google Scholar]
- Pastore LM, Young SL, Baker VL, Karns LB, Williams CD, Silverman LM. Elevated prevalence of 35-44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci 2012;19:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peprah E. Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet 2012;76:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000;97:189–194. [DOI] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2005;20:402–412. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Jones ME, Schoemaker MJ, Hemming J, Thomas D, Williamson J, Ashworth A. The Breakthrough Generations Study: design of a long-term UK cohort study to investigate breast cancer aetiology. Br J Cancer 2011;105:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, Fauser BC, Ploos van Amstel HK, van der Schouw YT, Broekmans FJ. The association of CGG repeats in the FMR1 gene and timing of natural menopause. Hum Reprod 2013;28:496–501. [DOI] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, Janse F, Ploos van Amstel HK, Goverde AJ, Lambalk CB, Laven JS, van der Schouw YT, Broekmans FJ, Fauser BC. The significance of fragile X mental retardation gene 1 CGG repeat sizes in the normal and intermediate range in women with primary ovarian insufficiency. Hum Reprod 2014;29:1585–1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.