Abstract
Assessing the effects of grapes and grape powder extracted polyphenols on lipogenesis and glucose uptake in adipocytes may clarify the risk/benefit of recommending them to individuals with obesity and insulin resistance. We investigated the effect of grape powder extracted polyphenols (GPEP) on intracellular fat accumulation and glucose uptake during differentiation of 3T3-F442A preadipocytes. Total polyphenols were extracted and measured based on gallic acid equivalents (GAE). There were 2167 mg of GAE polyphenols in 100 g of grape powder. 3T3-F442A cells were incubated with GPEP, extracted from 125–500 µg GP/mL of media, until day 8 of differentiation when the cells were collected for different assays. AdipoRed™ assay and Oil Red O staining showed that GPEP induced, in a dose-dependent manner, an increase in intracellular triacylglycerol (TAG) content of adipocytes. Concomitantly, grape powder extracted polyphenols increased, in a dose-dependent manner, glucose uptake by 3T3-F442A cells, and there was a strong positive correlation between glucose uptake and the amount of TAG accumulation (r = 0.826, n = 24, P ≤ 0.001). No changes in cell viability was measured by Trypan Blue staining, suggesting that these effects were independent of cytotoxicity. Western-blot showed that GPEP upregulated protein level of glucose transport protein 4 (GLUT4), p-PKB/Akt, and p-AMPK in 3T3-F442A adipocytes. LY294002 (10 µmol/L), a phosphatidyl-inositol 3 kinase inhibitor (PI3K), reversed the effects of grape powder extracted polyphenols on cellular lipid content and glucose uptake. Furthermore, quantitative real-time polymerase chain reaction showed that GPEP increased mRNA expression of GLUT4, fatty acid synthase, lipoprotein lipase, adiponectin, and peroxisome proliferator-activated receptor γ, while it decreased mRNA expression of leptin and Insig-1. Our results indicate that GPEP may induce adipocyte differentiation via upregulation of GLUT4, PI3K and adipogenic genes. Future research may be directed toward obese individuals with insulin resistance or individuals with diabetes.
Keywords: California Table Grape Commission, grape powder, polyphenols, 3T3-F442A, adipocyte, differentiation, glucose uptake, LY294002, phosphatidyl-inositol 3 kinase, Akt, proliferator-activated receptor γ, glucose transport protein 4, adiponectin, AMP-activated protein kinase
Introduction
Obesity is a prevalent health hazard in industrialized countries and is closely associated with increased risk for a number of pathological disorders, including type 2 diabetes, hypertension, coronary heart disease, and cancer. With regard to the wide range of health implications, the need to develop new and effective strategies to prevent or treat obesity and its related health disorders has become an international emergency. Obesity results from an excess of fat tissue due to prolonged excess calorie intake along with many varied behaviors. Long-term positive energy balance results in expansion of white adipose tissue (WAT). WAT is the major energy reserve in the human body; in periods of energy excess, it stores triacylglycerol (TAG) which is mobilized during energy deprivation.
At the cellular level, obesity is characterized by increases in the number and size of adipocytes, named by hyperplasia and hypertrophy, respectively. In humans, the majority of preadipocyte differentiation occurs shortly after birth.1 This increase in adipose tissue mass enables the newborn to survive during periods of fasting.2,3 While the ability of preadipocytes to differentiate continues throughout life in response to fat storage demands, total number of adipocytes remains constant in adulthood.4 Increased number of adipocytes is, therefore, set during prenatal, childhood, and adolescence.5 However, excess accumulation of TAG in adipocytes produces large fat cells, or hypertrophic adipocytes, which may become dysfunctional and insulin resistant due to inflammation. Accordingly, investigating the effects of different food ingredients on adipocyte size and function may correct the problem caused by insulin resistance in adipocytes, thereby maintaining metabolic homeostasis.
Synthesis of TAG in adipocytes may be a defense mechanism to clear extra sources of energy, mainly glucose and fat, from blood. Thus, inhibition of this natural mechanism may increase risk of hyperglycemia and hyperlipidemia leading to glucotoxicity and lipotoxicity. Lipotoxicity plays an important role in pathogenesis of diabetes due to lipid overload in pancreatic β-cells leading to a reduction in β-cell mass.6–8
The 3T3-L1 and 3T3-F442A fibroblasts or preadipocytes are the most common in vitro models for studying adipocyte differentiation.9–11 We have selected 3T3-F442A fibroblasts because they differentiate to adipocytes spontaneously in the presence of fetal calf serum.12 Pharmacological doses of insulin accelerate the development of 3T3-F442A adipocytes. However, the 3T3-L1 cells have a low rate of spontaneous differentiation and require differentiation-inducing agents, such as dexamethasone and 1-methyl-3-isobutylxanthine, to trigger conversion into adipocytes.13 This difference can be due to increased glycerol-3-phosphate dehydrogenase activity, the lipogenic enzyme, elicited by growth hormone alone (plus serum) in 3T3-F442A cells.14 In contrast, neither of these features is expressed or stimulated by growth hormone in 3T3-L1 cells. In addition, we have previous experience with 3T3-F442A cell lines using the same methods.15
Adipocyte differentiation is a well-orchestrated multi-step process involving several genes.10 Two transcriptional factors, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα)9,10 are known as major regulators of adipogenesis and are at the core of the adipogenic cascade. PPARγ is highly adipocyte specific with its expression rapidly increasing after hormone-induced adipocyte differentiation.9–11 Sterol regulatory element binding protein (SREBP) is another key lipogenic transcription factor that is nutritionally regulated by glucose and insulin.16,17 Insulin can function upstream to PPARγ by activation of SREBP-1c.18
Insulin or insulin growth factor-1 (IGF-1) are the main hormones required for the differentiation of these established cell lines.9,19 Insulin induces translocation of glucose transport protein 4 (GLUT4) vesicles from the cytoplasm to the plasma membrane (PM) via the phosphatidyl-inositol 3 kinase-protein kinase B/Akt (PI3K-PKB/Akt) pathway in adipocytes.20–22 Increased glucose uptake, then, provides the main source of energy for TAG synthesis.
The PI3K-PKB/Akt pathway is involved in regulation of many cellular activities, including cell proliferation and apoptosis.23–25 Inhibition of PI3K with wortmannin and LY294002 blocks adipocyte differentiation in 3T3-L1 cells.26,27 Development of adipose tissue is impaired along with other abnormalities, including muscle, bone, and skin, in PKB/Akt gene knockout in mice.28 The PI3K-PKB/Akt pathway is important, not only in the regulation of adipose tissue development, but also in the development of other tissues originating from mesodermal cells.
Grapes are a favorite, available fruit that require more evaluation of their health benefits. Grape products are rich in phenolic compounds that possess antioxidant and anti-inflammatory properties. Lyophilized grape powder (GP), obtained from red, green, and blue-purple seeded and seedless California grapes, was provided by the California Table Grape Commission (CTGC). The main polyphenols in GP include Quercetin-3-glucoside (8.0%), Catechins (2.3%), Epicatechin (1.9%), Gallic acid (1.4%), Rutin (1.2%), and Resveratrol (0.53%).29 Grape powder extracted polyphenols (GPEP) modulate inflammation in human adipocytes.29 However, the effect of GPEP on lipogenesis and glucose uptake is not clear.
Assessing the effect of GP extracted polyphenols on lipogenesis and glucose uptake in adipocytes may clarify the risk/benefit of recommending it to individuals with obesity and insulin resistance. In this study, we investigated the effect of GPEP on lipogenesis and glucose uptake during differentiation of 3T3-F442A preadipocytes. Furthermore, we evaluated effect of GPEP on mRNA expression of adipogenic genes, and protein levels of GLUT4 and p-PKB/Akt. We also determined whether effect of GPEP is mediated by upregulation of PI3K and PPARγ, which induces glucose uptake via membrane translocation of GLUT4 protein. We hypothesized that GPEP would promote adipocyte differentiation to maintain healthy adipocytes by increasing glucose uptake.
Materials and methods
Cell culture and treatment preparation
Cell culture
Murine 3T3-442A preadipocytes, purchased from Dr. Howard Green (Harvard Medical School), were cultured in six-well plates (5 × cells/well/ 2 mL medium) containing maintenance medium, Dulbecco’s modified Eagle’s medium (DMEM), adjusted by American Type Culture Collection (ATCC, Manassas, VA), supplemented with 10% bovine calf serum (BCS, Fisher Scientific Company LLC, Houston, TX), and 1% penicillin/streptomycin (GIBCO, Grand Island, NY) at 37℃ in a humidified atmosphere of 10% . At ∼100% confluency, the medium was replaced by differentiating medium (DM), DMEM + 10% fetal bovine serum (FBS, Fisher Scientific Company) and 1% penicillin/streptomycin (GIBCO) supplemented by 10 µg/ml insulin (Sigma Aldrich, St. Luis, MO) for 24 h; cells were then changed to differentiation medium (DMEM with 10% FBS) supplemented with polyphenols, extracted from 125–500 µg GP/mL of media, until day 8 of differentiation when 70–80% of cells were differentiated, then cells were collected for different assays. Control cells were incubated in medium containing the same amount of ethanol. The same amount of ethanol was used for all concentrations of the grape polyphenols, as <1% ethanol. A PI3K inhibitor, LY294002 (10 µmol/L, Sigma, USA) was used as supplemental treatment to determine role of PI3K signaling pathway in mechanism.
Oil red O staining
On day 8 of differentiation, cells were rinsed with phosphate-buffered solution (PBS) twice and fixed in 1 mL of 10% formalin per well at room temperature for 1 h. Cells were then rinsed with deionized water and stained with 0.5 mL of 0.3% freshly filtered Oil Red O working solution per well at room temperature for 30 min30 to visualize cellular lipid accumulated in fat droplets. Cells were then washed with 1 mL deionized water per well three times before photomicrography of monolayer cells. Photos were taken with Nikon Eclipse TS 100 microscope (Nikon Corporation, Tokyo, Japan) equipped with a Nikon Coolpix 995 digital camera (Nikon Corporation).
Polyphenol assay
GP was provided by CTGC in aluminum bags. Total polyphenols were extracted by ethanol and measured based on gallic acid equivalents (GAE) using GA standard curve.31
Cell viability assay
Following seven days incubation of murine 3T3-F442A cells in differentiation medium including GPEP (GP 0–500 µg/mL), floaters were collected and combined with detached monolayer cells following trypsinization. Viable and dead cells were counted by Trypan Blue staining with a hemocytometer.
Intracellular triglyceride measurement
Intracellular lipid content was measured on day 7 of differentiation using AdipoRed™ Assay kit (Lonza, Walkersville, MD) according to manufacturer’s instructions. AdipoRed contains hydrophilic Nile Red stain and allows quantification of intracellular lipid droplets.32
Differentiated cells were rinsed with 2 mL PBS and then to each well, 5 mL PBS and 140 µL of AdipoRed reagent were added. After 10–15 min of incubation, the plates were positioned in a Tecan Infinite M200 microplate reader (Tecan Systems Inc., Salzburg, Austria) and fluorescence was measured with an excitation wavelength of 485 nm and an emission wavelength of 572 nm.
Glucose uptake
Murine 3T3-F442A preadipocytes were cultured and induced to differentiate as described above. On day 4 of differentiation, medium was changed and cells continued incubation until day 8 when an aliquot of medium was sampled for measurement of glucose concentration using a Stanbio Glucose LiquiColor kit® (Stanbio Laboratory, Boerne, TX). The difference in glucose concentration between fresh medium, added at day 4, and medium from day 8 was considered cellular glucose uptake.
RNA extraction and RT-qPCR
Following eight days incubation of murine 3T3-F442A cells with treatments, total cellular RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s protocols. Concentration and purity of isolated total RNA were determined spectrophotometrically using OD260:280 ratio. Integrity of purified total RNA was verified by detecting a 2:1 ratio for 28 S:18 S ribosomal RNA (rRNA) using gel electrophoresis. Samples were run on a 1.5% agarose gel (tris-acetate (TAE) buffer) at 80 V for 90 min and visualized by Chemi Doc XRS imaging system (Bio-Rad, Hercules, CA) following addition of 0.5 µg/ml ethidium bromide. mRNA expression levels of PPARγ, GLUT4, FAS, LPL, adiponectin, leptin, and Insig-1 were analyzed by reverse transcription (RT) followed by polymerase chain reaction (PCR); 2 µg of total RNA in a 20 μl reaction buffer was reverse transcribed into cDNA using an Oligo (dT)20 primer and SuperScript® III First-Strand kit (Invitrogen, Grand Island, NY) following manufacturer's instructions. cDNA was diluted 20-fold with RNAse free water, and 6 μl of diluted cDNA was amplified in a 25 μl PCR solution containing 250 nM of both forward and reverse primers of the gene and iQ™SYBR® Green Supermix (Bio-Rad). Primers were designed using Vector NTI Advance version 11 software (Invitrogen). Primer sequences are listed in Table 1. cDNA was denatured at 95℃ for 3 min followed by 40 cycles of PCR (94℃ for 30 s, 60℃ for 25 s, 72℃ for 25 s, and 78℃ for 9 s) by means of an iQ™5 multi-color real-time PCR detection system (Bio-Rad) with Bio-Rad iQ5 Optical System Software (version 2.1). mRNA levels of all genes were normalized using ribosomal protein L22 (RPL22) as internal control using the ΔCT method. Fold changes of gene expression were calculated by the 2−ΔΔCT method. To ensure that a single gene was amplified, the melting profile of double-stranded DNA product of PCR generated from each primer was analyzed, as described before.33
Table 1.
Primer sequences (forward and reverse) and GenBank accession numbers used in the quantitative real-time polymerase chain reaction (qRT-PCR)
| Gene | Accession # | Primer sequence |
|---|---|---|
| Pparg | NM_001127330.1 & NM_011146.2 | 5′-AGAGGGCCAAGGATTCATGACCAGG-3′ 5′-TTCAGCTTGAGCTGCAGTTCCAGGG-3′ |
| LPL, LCP1 | NM_008509.2 | 5′-TCCCTTCACCCTGCCCGAGGT-3′ 5′-CGATGACGAAGCTGGGGCTGCT-3′ |
| FAS, TNFR6 | NM_007988.3 | 5′-CCCAGGCCTTGCCGTGCAGT-3′ 5′-GCTCAGGACTGCGTGGGGCT-3′ |
| AdipoQ, Ad, adipo, Adiponectin | NM_009605.4 | 5′-CGGCAGCACTGGCAAGTTCTACTGC-3′ 5′-TTGTGGTCCCCATCCCCATACACCT-3′ |
| LEP, leptin | NM_008493.3 | 5′-TGGAGGTGAGCGGGATCAGGTTTTG-3′ 5′-TGGCACGTGGGATCTTTCAGAAGCC-3′ |
| Insig1, Insig-1 | NM_153526.5 | 5′-GCACGAGCTATTCCGGAGAAGGGTTC-3′ 5′-GGACCAACGACTGTGTCAGGAGGTCAG-3′ |
| GLUT4, Slc2a4 | NM_009204.2 | 5′-GAACCCCCTCGGCAGCGAGT-3′ 5′-ATCCGGTCCCCCAGGACCTTGC-3′ |
| RPL22 | NM_009079.3 | 5′-GCGACTTTAACTGGGCTGCTGCT-3′ 5′-GCCCACCACCCAGCCTCTCG-3′ |
pparγ: peroxisome proliferator-activated receptor gamma; LPL: lipoprotein lipase; LCP1: lymphocyte cytosolic protein 1; FAS: fatty acid synthase; TNFR6: TNF receptor superfamily member 6, AdipoQ (Ad, adipo): adiponectin; LEP: leptin; Insig1(Insig-1): insulin-induced gene 1; GLUT4: glucose transport protein 4; Slc2a4: solute carrier family 2 (facilitated glucose transporter) member 4; RPL22: ribosomal protein L22.
Fractionation of membrane and non-membrane proteins
Pooled cell pellets were obtained from two six-well plates per each group by scraping cells into medium and centrifuging at 1258 × g for 5 min at 4℃. Cell pellets were washed in PBS and resuspended in 0.5 mL of buffer (10 mM HEPES-KOH, pH 7.4), mixed with protease inhibitor cocktail. Cell suspension was homogenized by passing it through a 22G1 needle 30 times followed by short time sonication on ice (3 × 5 s) and centrifugation at 1000 × g for 10 min at 4℃. Supernatant was used to obtain the membrane fraction, and the pellet, by centrifugation at 20,000 × g for 20 min. Acetone was added to the supernatant to precipitate the non-membrane proteins, kept for 30 min at −20℃, and centrifuged at 20,000 × g for 15 min at 4℃. Both the membrane and non-membrane pellets were resuspended in SDS lysis buffer (10 mM Tris-HCl [pH 6.8], 1% (w/v) SDS, 100 mM NaCl, and 1 mM EGTA). Protein concentration of each extract was measured using the BCA™ Protein Assay Kit (Pierce, USA). All extracts were then mixed with an equal volume of buffer (62.5 mM Tris-HCl, pH 6.8, 15% [w/v] SDS, 8 mol/L urea, 10% [v/v] glycerol, and 100 mmol/L dithiothreitol) and 1/6 volume of the 4 × g SDS loading buffer (150 mmol/L Tris-HCL, pH 6.8, 12% [w/v] SDS, 30% glycerol, 6% β-mercaptoethanol, and bromphenol blue) and stored at −80℃. All fractions were incubated at 37℃ for 20 min prior to loading an 8% SDS polyacrylamide gel.
Statistics
Experiments were repeated three times. One-way analysis of variance (ANOVA) was performed to assess the differences in means between groups using Prism® 4.0 software (GraphPad Software Inc., SanDiego, CA) and SPSS-19. A Pearson product-moment correlation coefficient was computed to assess the relationship between the amount of TAG accumulation and glucose uptake. Levels of significance were considered as P ≤ 0.05.
Results
Total polyphenols were extracted from GP by ethanol and measured using based on GAE. There was about 2167 mg GAE of polyphenols in 100 g of GP (∼2%).
Next, effect of GPEP on differentiation of murine 3T3-F442A cells was determined following eight days incubation of cells with GPEP (GP 0–500 µg /mL) during differentiation period. Figure 1(a) to (d) shows that GPEP induced a concentration-dependent increased in number of lipid droplets stained by Oil Red O in each cell and the total amount of visible lipids.
Figure 1.
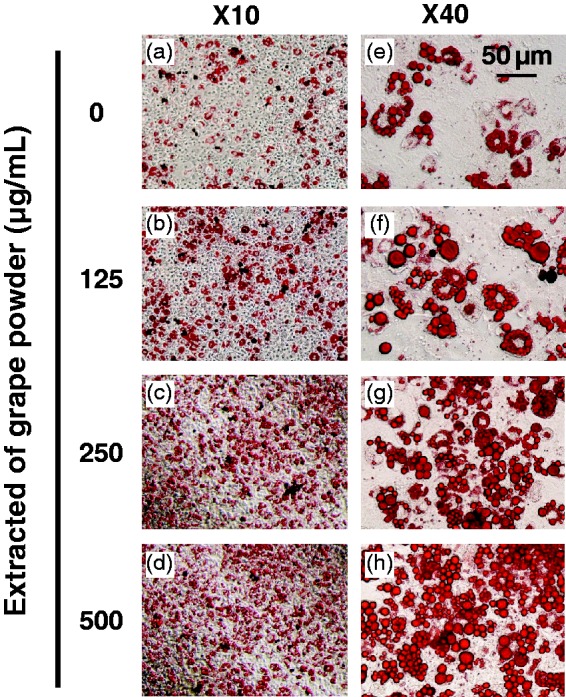
Grape powder extracted polyphenols (GPEP)-induced differentiation of 3T3-F442A preadipocytes. Oil Red O staining and photomicrography of 3T3-F442A adipocytes showing GPEP (GP 0–500 µg/mL) of medium increase the amount of intracellular fat droplets in a dose-dependent manner. Pictures are shown with two magnification power, (× 10 and × 40)
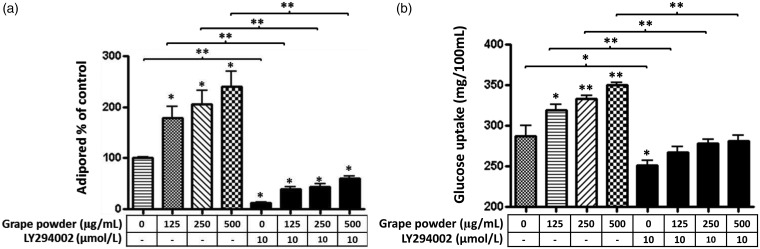
These visual effects were confirmed by measuring the amount of intracellular TAG accumulation using AdipoRed™ (Figure 2(a)). GPEP dose-dependently increased lipogenesis during differentiation of adipocytes (P ≤ 0.05). As expected, LY294002, a PI3K inhibitor, decreased cellular TAG content and rescued the effect of GPEP on intracellular TAG accumulation, significantly (P ≤ 0.01) (Figure 2(a)).
Figure 2.
The effect of grape powder extracted polyphenols (GPEP) with and without LY294002, a phosphatidyl-inositol 3 kinase (PI3K) inhibitor, on intracellular triglyceride accumulation (a) and glucose uptake (b) by 3T3-F442A preadipocytes. The cells were incubated with GPEP (GP 0–500 µg/mL) in the presence or absence of LY294002 (10 µmol/L) until day 8 of differentiation (n = 8). Intracellular triglyceride amount was measured by AdipoRed assay. The difference of glucose concentration in medium between day 8 and fresh medium was used for glucose uptake report (values are mean ± SD; *P < 0.05; **P < 0.01)
To clarify effect of GPEP on glucose uptake and correlation between lipogenesis and glucose uptake, the amount of glucose consumption by cells during differentiation was measured. The difference of glucose concentration in media between day 8 of differentiation and fresh media was measured via Stanbio Glucose LiquiColor kit® and used as glucose uptake. GPEP increased glucose uptake (Figure 2(b)) during differentiation of adipocytes, dose-dependently (P ≤ 0.05), and this uptake in glucose was positively correlated with intracellular TAG content (r = 0.826, n =24, P =0.001). About 86% of the variation in TAG accumulation between adipocytes treated by GP 0–500 µg/mL can be explained by the variation in glucose uptake (R2= 0.856). Overall, there was a strong positive correlation between the amount of TAG accumulation and glucose uptake. In addition, to see whether the inducing effect of GPEP on glucose uptake could be mediated by PI3K signaling pathway, we examined if LY294002 reversed the effect of GPEP on glucose uptake. As shown in Figure 2(b), LY294002 (10 µmol/L) reversed the effect of GPEP on glucose uptake significantly (P ≤ 0.05), concomitant with its reversal effect on lipogenesis.
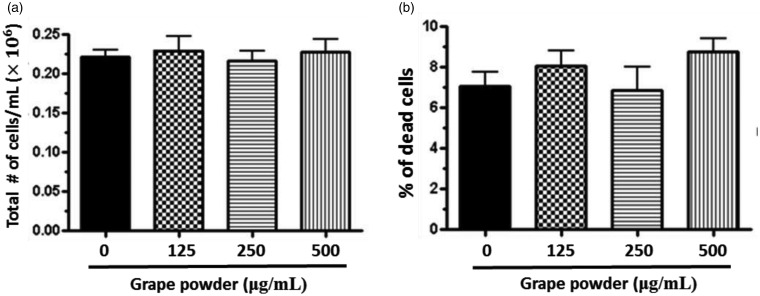
There was no effect of cytotoxicity following eight days incubation of 3T3-F442A cells with GPEP (GP 0–500 µg/mL) when the total number of cells (Figure 3(a)) and the % of dead cells were assessed with Trypan Blue staining (Figure 3(b)).
Figure 3.
The effect of grape powder extracted polyphenols (GPEP) on viability of 3T3-F442A adipocytes. The cells were incubated with GPEP (GP 0–500 µg/mL) until day 8 of differentiation. Total cell count (A) and the % of dead cells (B) were measured by Trypan Blue staining and hemocytometer
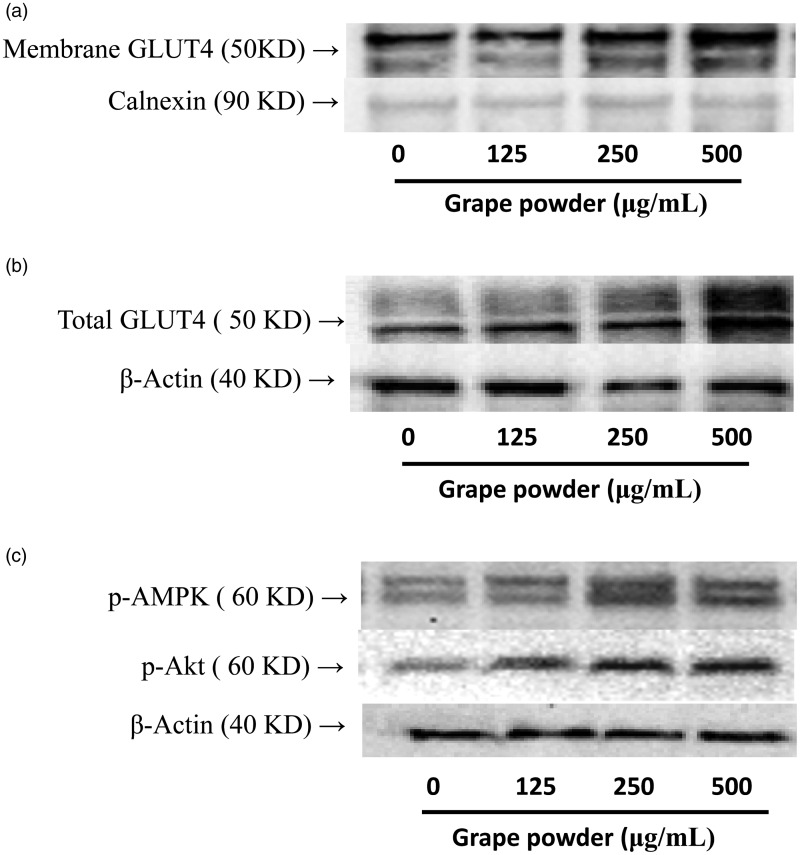
Furthermore, to investigate if the effect of GPEP on glucose uptake can be explained by the protein expression of membrane-associated GLUT4, the amount of GLUT4 protein was measured in the membrane fraction of adipocytes. GPEP induced (Figure 4(a)) the expression of GLUT4 protein. We also measured the protein expression of GLUT4 in total protein extraction. GPEP increased the total protein content of GLUT4 protein (Figure 4(b)). The protein expression of phosphorylated protein kinase B/Akt (p-PKB/Akt) and p-AMPK was measured in non-membrane fraction of 3T3-F442A adipocytes. GPEP upregulated the protein expression of p-PKB/Akt and p-AMPK (Figure 4(c)).
Figure 4.
The effect of grape powder extracted polyphenols (GPEP) on the expression of membrane GLUT4 (a), total GLUT4 (b), p-Akt and p-AMPK (c) proteins in 3T3-F442A adipocytes following eight days incubation with GPEP (GP 0–500 µg/L) (n = 4)
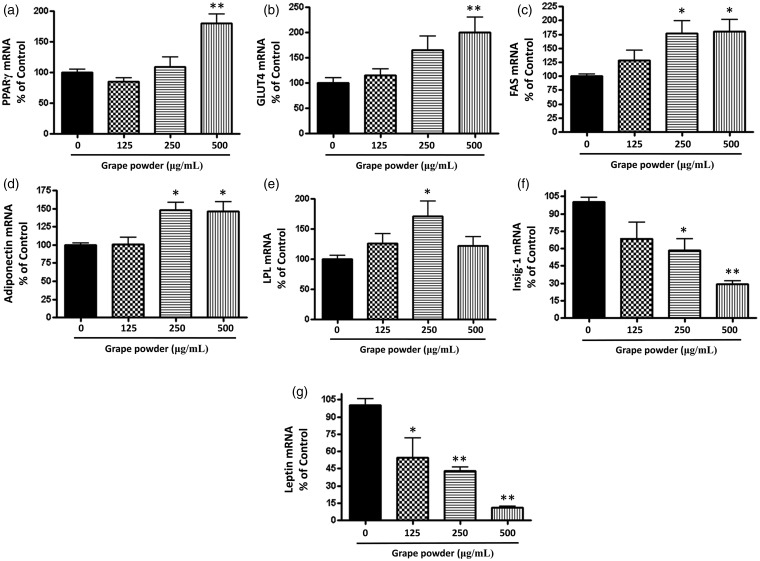
The effect of GPEP on the expression of PPARγ and its target genes, GLUT4, adiponectin, and lipoprotein lipase (LPL), and the expression of fatty acid synthase (FAS), leptin, and Insig-1 were detected in adipocytes following eight days incubation with GPEP (GP 0–500 µg/mL). The results from quantitative real-time polymerase chain reaction (qRT-PCR) showed that mRNA expression of PPARγ, GLUT4, FAS, LPL, and adiponectin was upregulated, while that of leptin, and Insig-1 was downregulated (Figure 5(a) to (g)) significantly (P ≤ 0.05).
Figure 5.
The effect of grape powder extracted polyphenols (GPEP) on expression of adipogenic genes in 3T3-F442A adipocytes. Following eight days incubation with GPEP (GP 0–500 µg/mL), mRNA expression of PPARγ (a), GLUT4 (b), FAS (c), Adiponectin (d), LPL (e), Insig-1 (f), and Leptin (g) in 3T3-F442A adipocytes were quantified with qRT-PCR (n = 5; values are mean ± SD; *P < 0.05; **P < 0.01)
Discussion
Previous studies have been shown that polyphenols extracted from GP provided by CTGC attenuate inflammation in human macrophages and in human adipocytes when exposed to macrophage-conditioned media.29 Quercetin and resveratrol, two main polyphenols in GP, attenuate tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes.34,35 Moreover, high fat-fed obese mice supplemented with GP had better glucose tolerance and lower systemic inflammation.36 In this study, we investigated the effects of GPEP on lipogenesis, glucose uptake, and underlying mechanism.
Our results show that GPEP induce the differentiation of 3T3-F442A adipocytes and intracellular TAG accumulation. Consistent with the increased intracellular TAG accumulation, the expression of FAS mRNA was significantly upregulated. Concomitantly, GPEP increase glucose uptake by 3T3-F442A cells dose-dependently. There was a strong significant positive correlation between intracellular TAG accumulation and glucose uptake, suggesting that grape polyphenols may induce adipocyte differentiation by increasing glucose uptake. Furthermore, GPEP increased the expression of GLUT4 mRNA significantly. The expression of GLUT4 protein in both adipocyte membrane and total fraction were also upregulated, indicating that both translocation and synthesis of GLUT4 protein, the main glucose transport protein in adipocytes which responds to insulin,37 were increased by GPEP in 3T3-F442A adipocytes.
The possibility of cytotoxicity was investigated by measuring the total number of adipocytes and the percentage of dead cells. There was no significant change in cell viability, suggesting that these effects were independent of cytotoxicity.
Activation of PPARγ promotes terminal differentiation of adipocytes through upregulation of adipogenic target genes.38 Adipocyte differentiation is induced by increased glucose uptake and expression of GLUT4, a PPARγ regulated gene.39 Our results show that GPEP unregulated the expression of PPARγ and other PPARγ-regulated adipogenic genes including LPL and adiponectin, a biomarker for insulin sensitivity.40
There are several reports indicating a relation between the activation of PPARγ pathway and insulin sensitivity that results in increased glucose uptake. Thiazolidodiones exert their biological effects on insulin sensitivity through binding to PPARγ.41 Mutations in PPARγ in both rodents and humans are associated with insulin resistance.42–44 The production of bioactive molecules, named adipokines, derived from adipose tissue, is another potential mechanism which explains the cross talk between the PPARγ pathway and insulin sensitivity. The plasma levels of adiponectin, an adipokine selectively released by adipocytes,45 are correlated with adipose tissue mass and insulin sensitivity.46
Leptin and adiponectin are the most important hormones produced by adipose tissue which have a significant role in energy homeostasis. Leptin is a cytokine produced in proportion to fat stores and acts on hypothalamic nuclei to reduce food intake and promote energy expenditure in rodents.47,48 Conversely, lack of leptin signaling because of a mutation in leptin or leptin receptor gene results in increased food intake with reduced energy expenditure in rodents and humans in spite of obesity.47–49 In this study, GPEP decreased the mRNA expression of leptin in 3T3-F442A adipocytes, while upregulating the expression of adipogenic genes. At this point, we do not have enough evidence to explain this result.
Our observation that LY294002, an inhibitor of PI3K, reversed the effect of GPEP on cellular lipid content and glucose uptake supports the hypothesis that differentiation of adipocytes is coordinated by PI3K signaling pathways and that they are promoted by grape polyphenols. Consistently, the protein level of p-PKB/AKT, the protein phosphorylated and activated by PI3K, was upregulated by GPEP in adipocytes. This evidence supports the hypothesis that grape polyphenols activate insulin sensitivity and PI3K signaling pathways in adipocytes. As a result, grape polyphenols may benefit individuals with insulin resistance.
We have also examined the effect of GPEP on mRNA expression of insulin-induced gene 1 (Insig-1), an inhibitory factor of lipogenesis during differentiation of adipocytes50 to support inducing effect of GPEP on insulin pathway. Real-time-qPCR showed downregulation of Insig-1 expression in 3T3-F442A adipocytes treated by GPEP (GP 0–500 µg/mL) for eight days during differentiation period. SREBP1c presents as a membrane-bound precursor bound to SREBP-cleavage-activating protein (SCAP) and is tethered in the endoplasmic reticulum (ER). SCAP is a regulatory protein required for the proteolytic cleavage of the SREBP, which is located in the ER as an integral membrane protein. Insig-1 binds SCAP in the ER and blocks proteolytic processing required for SREBP activation. Upon its release from the ER (stimulated by insulin), SCAP-SREBP1c moves to the Golgi apparatus, where proteolytic cleavage frees its basic helix-loop-helix component for translocation to the nucleus. Because SCAP escorts SREBP from the ER to Golgi for proteolytic processing into an active transcription factor, the binding of SCAP by Insig-1 effectively prevents SREBP activation and thus blocks its action on gene transcription.51 Once in the nucleus, SREBP1c induces transcription of genes encoding lipogenic enzymes16 and adipocyte differentiation.52 In the Insig-1 – overexpressing cells fatty acid binding protein (FABP or αP2), PPARγ2, carbohydrate response element binding protein (ChREBP), and SREBP1c mRNA were significantly decreased.50 Consistently, our results indicating the reverse correlation between Insig-1 mRNA expression and that of PPARγ, GLUT4, LPL, FAS, and Adiponectin which are lipogenic genes during differentiation of adipocytes.
As a key physiological energy sensor, AMP-activated protein kinase (AMPK) is a major regulator of cellular and organismal energy homeostasis that coordinates multiple metabolic pathways to balance energy supply and demand and ultimately modulate cellular and organ growth.53 AMPK is able to maintain cellular energy homeostasis at a constant level. AMPK-dependent phosphorylation of ACC inhibits its enzyme activity to suppress malonyl-CoA synthesis, thereby relieving inhibition of fatty acid uptake into mitochondria and enhancing fatty acid oxidation. Thus, AMPK allows cells to utilize an alternative source of energy such as lipids when the cells do not have access to carbohydrates, the preferred energy source. In addition to this metabolic switch, AMPK stimulates gene expression of GLUT4 as well as glucose uptake by inducing GLUT4 translocation through inhibition of TBC1D1 and AS160, two Rab-GTPase-activating protein (Rab-GAP) proteins.54,55 AMPK phosphorylates and inhibits AS160, leading to Rab activation and increased PM localization of GLUT4 and glucose uptake.55 AS160 is a Rab-GAP protein which functions as a brake on translocation of GLUT4 to PM by inactivating cognate Rab proteins.56–58 Insulin causes phosphorylation of AS160 at the Akt phosphorylation sites and its dissociation from GLUT4 vesicles.58 Translocation of GLUT4 and glucose uptake are regulated by both PI3K and AMPK.59–62Consistent with these information, our results show that GPEP upregulated the protein level of p-AMPK and p-PKB/AKT.
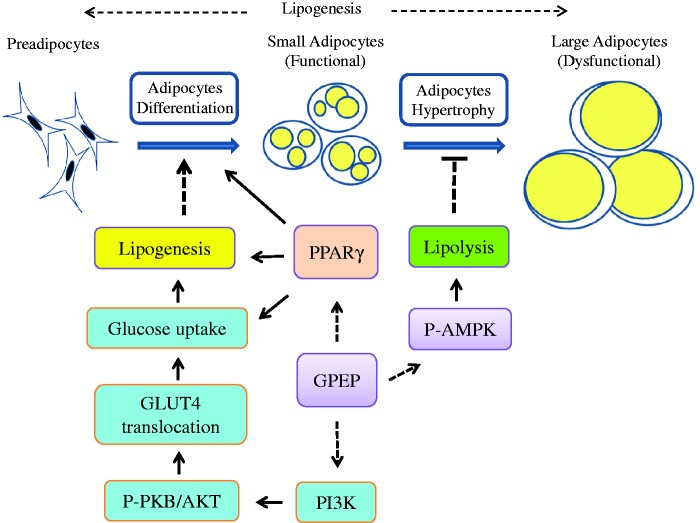
We, therefore, put forth a new model (Figure 6) and hypothesize that grape polyphenols activate lipolysis pathways in adipocytes through upregulation of p-AMPK. Therefore, it may prevent the progression of small differentiated adipocytes to large hypertrophic and possibly dysfunctional adipocytes. Having more small functional adipocytes as a result of inducing adipocyte differentiation may maintain metabolic homeostasis and energy balance in the whole system. By maintaining adipocytes in their optimum functional state, grape polyphenols may help the metabolic homeostasis in the body which is the result of fat turnover in adipocytes. However, we need to determine the activation of lipolytic pathways by grape polyphenols, and because GP contains different polyphenols, future studies of individual polyphenols on adipocyte differentiation and glucose uptake are also needed. Another point is that regulation of lipogenesis in murine cell lines, such as 3T3-F442A cells, used in the present study, may be different from primary human adipocytes due to different genetic, hormonal, and growth factors.63 Thereby, future studies on human adipocytes are required to confirm these results.
Figure 6.
Hypothesized model showing the effect of GPEP on lipogenesis in adipocytes. GPEP induces differentiation of preadipocytes by activating both PPARγ and PI3K signaling pathways. Activation of PPARγ induces both glucose uptake and lipogenesis. Activation of PI3K signaling pathway by GPEP results in phosphorylation and activation of PKB/AKT protein that causes translocation of GLUT4 protein to plasma membrane. Then, GLUT4 facilitates glucose uptake in adipocytes. On the other hand, GPEP, by activating AMPK through phosphorylation, may help prevent adipocyte hypertrophy. As the main energy sensor inside the cell, AMPK induces lipolysis, thereby, preventing progression of small functional adipocytes to dysfunctional large adipocytes. Optimum health of adipocytes can be achieved by inducing adipocyte differentiation while preventing adipocyte hypertrophy. Functional small adipocytes can maintain energy balance by fat turnover
AMPK: AMP-activated protein kinase; GPEP: grape powder extracted polyphenols; p-: phosphorylated; PI3K: phosphatidyl-inositol 3 kinase; PKB: protein kinase B; PPARγ: peroxisome proliferator-activated receptor γ; PKB/Akt: protein kinase B/Akt. → based on cited references,  hypothesized
hypothesized
In summary, we propose that grape and grape polyphenols induce adipocyte differentiation by increasing glucose uptake. The induced effect of GPEP on adipocyte differentiation and glucose uptake is mediated via upregulation of PI3K and PPARγ signaling pathways, which are related to increased synthesis and membrane translocation of GLUT4 protein. By upregulation of p-AMPK, GPEP may upregulate energy expenditure and lipolysis which prevents the progression of adipocytes to hypertrophic insulin-resistant adipocytes. GP may be beneficial in obesity-related insulin resistance and diabetes.
Acknowledgements
We thank Dr. Nathaniel Mills for technical assistance, and Dr. Brian W. Beck for BLAST search. This research was supported by Texas Woman’s University Research Enhancement Program.
Authors’ contributions
Both authors participated in the design, execution, and interpretation of the studies, analysis of the data, and writing and review of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Burdi AR, Poissonnet CM, Garn SM, Lavelle M, Sabet MD, Bridges P. Adipose tissue growth patterns during human gestation: a histometric comparison of buccal and gluteal fat depots. Int J Obes 1985; 9: 247–56. [PubMed] [Google Scholar]
- 2.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 1995; 64: 345–73. [DOI] [PubMed] [Google Scholar]
- 3.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell 1983; 35: 657–66. [DOI] [PubMed] [Google Scholar]
- 4.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature 2008; 453: 783–7. [DOI] [PubMed] [Google Scholar]
- 5.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci 1997; 92: 3–11. [DOI] [PubMed] [Google Scholar]
- 6.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes 1995; 44: 863–8. [DOI] [PubMed] [Google Scholar]
- 7.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001; 50: S118–21. [DOI] [PubMed] [Google Scholar]
- 8.Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J 2001; 15: 312–21. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78: 783–809. [DOI] [PubMed] [Google Scholar]
- 10.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 2012; 81: 715–36. [DOI] [PubMed] [Google Scholar]
- 11.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 1975; 5: 19–27. [DOI] [PubMed] [Google Scholar]
- 12.Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A 1978; 75: 6107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen OM, Smith CJ, Fung C, Rubin CS. Development of hormone receptors and hormone responsiveness in vitro, Effect of prolonged insulin treatment on hexose uptake in 3T3-L1 adipocytes. J Biol Chem 1978; 253: 7579–83. [PubMed] [Google Scholar]
- 14.Zezulak KM, Green H. The generation of insulin-like growth factor-1 – sensitive cells by growth hormone action. Science 1986; 233: 551–3. [DOI] [PubMed] [Google Scholar]
- 15.Torabi S, Mo H. Trans, trans-farnesol as a mevalonate-derived inducer of murine 3T3-F442A pre-adipocyte differentiation. Exp Biol Med (Maywood). Epub ahead of print 8 December 2015. DOI: 1535370215620855. [DOI] [PMC free article] [PubMed]
- 16.Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans 2002; 30: 1091–5. [DOI] [PubMed] [Google Scholar]
- 17.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab 2008; 8: 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne VA, Au WS, Lowe CE, Rahman SM, Friedman JE, O'Rahilly S, Rochford JJ. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem J 2009; 425: 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell 1974; 1: 113–6. [Google Scholar]
- 20.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol 1997; 17: 1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 1999; 19: 4008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishiki M, Randhawa VK, Poon V, Jebailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem 2005; 280: 28792–802. [DOI] [PubMed] [Google Scholar]
- 23.Blair LA, Bence-Hanulec KK, Mehta S, Franke T, Kaplan D, Marshall J. Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival. J Neurosci 1999; 19: 1940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem 2001; 276: 32814–21. [DOI] [PubMed] [Google Scholar]
- 25.Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 1999; 19: 7203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomiyama K, Nakata H, Sasa H, Arimura S, Nishio E, Watanabe Y. Wortmannin, a specific phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic differentiation of 3T3-L1 cells. Biochem Biophys Res Commun 1995; 212: 263–9. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem 2004; 279: 35914–22. [DOI] [PubMed] [Google Scholar]
- 28.Peng XD, Xu PZ, Chen ML, Hahn-Windqassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 2003; 17: 1352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overman A, Bumrungpert A, Kennedy A, Martinez K, Chuang C, West T, Dawson B, Jia W, McIntosh M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Internat J Obes (Lond) 2010; 34: 800–8. [DOI] [PubMed] [Google Scholar]
- 30.Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992; 97: 493–7. [DOI] [PubMed] [Google Scholar]
- 31.Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 2003; 51: 6509–15. [DOI] [PubMed] [Google Scholar]
- 32.Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985; 100: 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elfakhani M, Torabi S, Hussein D, Mills N, Verbeck G, Mo H. Mevalonate deprivation mediates the impact of lovastatin on the differentiation of murine 3T3-F442A preadipocytes. Exp Biol Med (Maywood) 2014; 239: 293–301. [DOI] [PubMed] [Google Scholar]
- 34.Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond) 2011; 35: 1165–72. [DOI] [PubMed] [Google Scholar]
- 35.Chuang CC, Martinez K, Xie G, Kennedy A, Bumrungpert A, Overman A, Jia W, McIntosh MK. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-{alpha}-mediated inflammation and insulin resistance in primary human adipocytes. Am J Clin Nutr 2010; 92: 1511–21. [DOI] [PubMed] [Google Scholar]
- 36.Chuang CC, Shen W, Chen H, Xie G, Jia W, Chung S, McIntosh MK. Differential effects of grape powder and its extract on glucose tolerance and chronic inflammation in high-fat-fed obese mice. J Agric Food Chem 2012; 60: 12458–68. [DOI] [PubMed] [Google Scholar]
- 37.He A, Liu X, Liu L, Chang Y, Fang F. How many signals impinge on GLUT4 activation by insulin? Cell Signal 2007; 19: 1–7. [DOI] [PubMed] [Google Scholar]
- 38.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 1994; 8: 1224–34. [DOI] [PubMed] [Google Scholar]
- 39.Liao Z, Wu Z, Wu M. Cirsium japonicum flavones enhance adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biol Pharm Bull 2012; 35: 855–60. [DOI] [PubMed] [Google Scholar]
- 40.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol 2010; 176: 1364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995; 270: 12953–6. [DOI] [PubMed] [Google Scholar]
- 42.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999; 402: 880–3. [DOI] [PubMed] [Google Scholar]
- 43.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab 2006; 4: 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A 2003; 100: 15712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouskila M, Pajvani UB, Scherer PE. Adiponectin: a relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity. Int J Obes (Lond) 2005; 29(Suppl 1): S17–23. [DOI] [PubMed] [Google Scholar]
- 46.Hu E, Liang P, Spiegelman B. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271: 10697–703. [DOI] [PubMed] [Google Scholar]
- 47.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–70. [DOI] [PubMed] [Google Scholar]
- 48.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1998; 1: 445–49. [DOI] [PubMed] [Google Scholar]
- 49.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early onset obesity in humans. Nature 1997; 387: 903–8. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Takaishi K, Cook W, McCorkle SK, Unger RH. “Insig-1 “brakes” lipogenesis in adipocytes and inhibits differentiation of preadipocytes. Proc Natl Acad Sci U S A 2003; 100: 9476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 2002; 110: 489–500. [DOI] [PubMed] [Google Scholar]
- 52.Tontonoz P, Kim JB, Graves RA, Spigelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol 1993; 13: 4753–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8: 774–85. [DOI] [PubMed] [Google Scholar]
- 54.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating Protein abundant in skeletal muscle, is partially relieved by amp-activated protein kinase activation. J Biol Chem 2008; 283: 9187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007; 5: 237–52. [DOI] [PubMed] [Google Scholar]
- 56.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 2007; 5: 293–303. [DOI] [PubMed] [Google Scholar]
- 57.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2005; 2: 263–72. [DOI] [PubMed] [Google Scholar]
- 58.Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 2005; 280: 37803–13. [DOI] [PubMed] [Google Scholar]
- 59.Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, Choi SY, Kwon HY. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J PhysiolPharmacol 2014; 18: 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kristensen JM, Treebak JT, Schjerling P, Goodyear L, Wojtaszewski JF. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin stimulated glucose uptake in mouse soleus muscle. Am J Physiol Endocrinol Metab 2014; 306: 1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji L, Zhang X, Liu W, Huang Q, Yang W, Fu F, Ma H, Su H, Wang H, Wang J, Zhang H, Gao F. AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS One 2013; 8: e69910–e69910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueda M, Hayashibara K, Ashida H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscle. Biofactors 2013; 39: 457–466. [DOI] [PubMed] [Google Scholar]
- 63.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010; 235: 1185–93. [DOI] [PubMed] [Google Scholar]