Abstract
Orexin A (OXA) and B (OXB) are neuropeptides which regulate appetite, energy expenditure, and arousal via G-protein coupled receptors termed as OXR1 and OXR2. The aim of this study was to characterize the effects of OXA and OXB on proliferation and differentiation of porcine preadipocytes. Porcine preadipocytes express both OXRs. OXA and OXB enhance porcine preadipocyte proliferation by 54.8% or 63.2 %, respectively. OXA and OXB potentiate differentiation of porcine preadipocytes, as judged by the increased lipid accumulation and expression of proadipogenic genes. Cellular lipid content after exposure of preadipocytes for six days to 100 nM OXA or OXB increased by 82.2% or 59.2%, respectively. OXA and OXB suppressed glycerol release by 23.9% or 24.9% in preadipocytes differentiated for six days. OXA (100 nM) increased peroxisome proliferator-activated receptor gamma (PPARγ) expression in cells differentiated for 24 h by 100.5%. PPARγ expression was also stimulated in preadipocytes differentiated in the presence of 10 nM (58.3%) or 100 nM OXA (50.6%) for three days. OXB potentiated PPARγ mRNA expression at 1 nM (59%), 10 nM (53.2%), and 100 nM (73.9%) in cells differentiated for three days. OXA increased CCAAT/enhancer binding protein alpha expression in preadipocytes differentiated for six days by 65%. OXB stimulated CCAAT/enhancer binding protein beta expression in preadipocytes differentiated for three days at 10 nM (149.5%) as well as 100 nM (207.2%). Lipoprotein lipase mRNA expression increased in cells treated with 10 nM OXA by 152.6% and 100 nM OXA by 162%. Lipoprotein lipase expression increased by 134% at 100 nM OXB. Furthermore, OXA (100 nM) and OXB (100 nM) increased leptin mRNA expression in preadipocytes differentiated for three days by 49.9% or 71.3%, respectively. These data indicate that orexin receptors may be relevant in the context of white adipose tissue formation.
Keywords: Differentiation, leptin, lipolysis, orexin, preadipocytes, proliferation
Introduction
Orexins A (OXA) and B (OXB) are 33 and 28 amino acid peptide hormones derived from the common precursor preproorexin.1,2 In blood, OXA peptide is more stable than OXB and in contrast to OXB, OXA can cross brain–blood barrier.3 Both peptides interact with two G-protein coupled receptors (OXR1, OXR2). OXA is bound by OXR1 and OXR2, whereas OXB is predominantly bound by OXR2.2 Brain orexin system regulates energy homeostasis by increasing food intake, arousal, as well as by promoting energy expenditure.2,4,5 There is convincing evidence that, in addition to brain actions, OXRs and their ligands are able to influence energy balance by modulating a variety of endocrine and metabolic functions of peripheral tissues. Orexins regulate glucose levels by controlling the secretion of glucocorticoids from adrenal glands,6 as well as glucagon7 and insulin8 from pancreatic alpha and beta cells, respectively. Recently, we and others showed that orexins regulate glucose and lipid metabolism by interacting with white adipose tissue.9–11 In human adipocytes, OXA stimulates peroxisome proliferator-activated receptor gamma (PPARγ) expression and suppresses lipolysis.9 Studies on rodent adipocytes confirmed these observations and revealed the ability of OXA to enhance glucose uptake, triacylglycerol accumulation, as well as adiponectin expression and secretion.10,11 In contrast to mature adipocytes, knowledge is scarce regarding the role of orexins in preadipocytes. It was shown that OXA stimulates proliferation and inhibits apoptosis in mouse 3T3-L1 and rat primary preadipocytes, whereas it fails to modulate adipogenesis.12,13 Despite data derived from murine and rat fat precursor cells, the effects of OXA and OXB have not been studied in other species.
While enhanced preadipocyte proliferation and differentiation may promote obesity, it was suggested that this can lead to improved insulin sensitivity and lipid profile in obese individuals.14,15
Currently, there is a great effort to identify novel approaches for the therapy of obesity and obesity-associated metabolic disorders. This strategy encompasses development and characterization of new animal and cellular models to study adipose tissue physiology and pathophysiology of obesity.16 Although rodent models are well characterized and widely utilized,16 the relevance of porcine model is growing. The rationale behind using porcine animal and cellular models is reflected by striking similarities between humans and pigs, including both being omnivores, fat deposition, as well as similar adipocytes size.17–20 Therefore, identification of novel factors able to influence preadipocytes proliferation and differentiation may significantly improve our understanding of porcine adipogenesis and understanding of obesity in general. Furthermore, characterization of novel targets with the capability to modulate fat tissue formation may contribute to the development of porcine breeding programs. Taking this into account, we therefore characterize here the role of OXA and OXB at modulating proliferation and differentiation of primary porcine adipocytes.
Materials and methods
Reagents
OXA and OXB were obtained from Sigma Aldrich (Deisenhofen, Germany). Media and supplements for cells culture were from Gibco-Life Technologies (Carlsbad, CA, USA). Anti-OXR1 and anti-OXR2 antibodies were from Alpha Diagnostics International (San Antonio, TX, USA). Anti-β-actin antibody was from Sigma Aldrich. Secondary antibodies were from Cell Signaling Technology (Danvers, MA, USA). Unless otherwise stated, all other reagents were purchased from Sigma Aldrich.
Isolation of porcine preadipocytes
Preadipocytes were isolated from 5 to 7 days old male Zlotnicka piglets from an Experimental Station of the Poznan University of Life Sciences in Zlotniki, Poland. The Local Ethical Committee for Experiments on Animals approved all procedures (approval number 11/2010). For independent isolations of preadipocytes, three piglets were subjected to sedation with Stresnil (40 mg azaperon/mL) at a dose of 0.4 mg/kg body weight, before transport. After arrival at the experimental facilities, piglets were anesthetized by intramuscular injection of ketamine/xylazine mixture, followed by exsanguination. The method of tissue samples collection and stromal-vascular cells isolation was performed, as previously described,21 with several modifications. Dorsal subcutaneous adipose tissue samples obtained from three piglets were cut, mixed, and placed in a warm sterile Krebs-Ringer buffer (118 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 24.8 mM NaHCO3, and 10 mM Hepes) supplemented with 3% BSA, 5 mM glucose, and antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin). After purification and cutting with scissors, the fat pads were placed in collagenase type II (3 mg/mL) digestion medium and incubated at 37℃ for 45 min in shaking water bath. Cell suspension was then filtrated through sterile 250 µm nylon mesh to separate undigested tissue and centrifuged at 450 × g for 10 min at room temperature. Mature adipocytes enriched in the upper fraction of the tube were discarded. Red Blood Cell Lysing Buffer (Sigma Aldrich) was added to the pellet to remove erythrocytes, according to producer’s protocol. Final cell suspension was filtrated through a 20 µm nylon mesh and centrifuged at 450 × g for 10 min at RT. The pellet containing stromal-vascular cells was resuspended in DMEM/F12 medium and counted using Fuchs Rosenthal chamber. Prior to the experiments, cells were plated in multi-well plates for one day to pre-culture in DMEM/F12 medium, supplemented with 10% fetal bovine serum and antibiotics. Cell viability (limit of >95% viability) was assessed after preadipocytes isolation as well as in cell differentiated for one, three, or six days (we detected 2%–3% of death cell) using trypan blue staining.
Proliferation assay
Preadipocytes were cultured in 96-well plates (2 × 103 cells/well) in serum-free DMEM/F12 medium with or without OXA or OXB (both peptides at the final concentrations of 1 nM, 10 nM, and 100 nM) for 24 h. Thereafter, cell proliferation was assessed using a Cell Proliferation ELISA (BrdU) Assay (Roche Diagnostic, Penzberg, Germany), according to manufacturer’s protocol.
In brief, after incubation with OXA or OXB, BrdU solution was added (final concentration of 10 µM) and cells were cultured for 2 h. Thereafter, incubation medium was removed and cells were fixed with Fix Denat solution for 25 min, following by incubation with Anti-BrdU-POD conjugate for 70 min. Afterwards, cells were washed three times with PBS and substrate solution was added. After 20 min, reaction was stopped by addition of 25 µl of 1 M H2SO4. The absorbance of the samples was read using Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA) at 450 nm wave length.
Differentiation of preadipocytes
Subconfluent preadipocytes underwent differentiation, as previously described22,23 with several modifications. Preadipocytes were incubated in a serum-free DMEM/F12 medium containing differentiation agents (850 nM insulin, 10 nM dexamethasone and 2 nM triiodothyronine) and with or without OXA or OXB (1-100 nM). Incubation was carried out for one, three, or six days.
Western blot
Proteins were isolated from preadipocytes differentiated in six-well plates for six days using RIPA buffer, as described.24 Protein concentration was measured using BCA Protein Assay Kit (ThermoScientific, Rockford, IL, USA); 30 µg of protein was resolved on 5–10% Tris-HCl SDS-PAGE gel and blotted onto a nitrocellulose membrane (Amersham Biosciences, Freiburg, Germany). Thereafter, membrane was blocked by 5% BSA in tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature. Next, membrane was incubated with primary antibody (1:1000 dilution) for 18 h at 4℃. After incubation with primary antibody, membranes were washed with TBST (three times, 5 min each) and incubated with the secondary anti-rabbit IgG HRP-linked goat antibody (1:5000 dilution) in TBST for 1 h at room temperature. Next, membranes were washed three times (5 min each). Chemiluminescence reaction was performed using ECL Kit (GE Healthcare Europe, Freiburg, Germany). Signals were visualized using VersaDoc Imaging System (Bio-Rad Laboratories, Munich, Germany). Afterwards membrane was stripped and incubated with antibodies against β-actin (1:10,000 dilution). The signal intensity was quantified using Quantity One 1-D Analysis Software (Bio-Rad Laboratories).
Oil red O staining
Lipid accumulation was assessed as we previously described.25 In brief, preadipocytes were washed with PBS and fixed in 10% formaldehyde in PBS for 1 h. Fixed preadipocytes were washed with 60% isopropanol, completely dried and stained with Oil red O (ORO) working solution for 10 min at RT. ORO solution was prepared by mixing six parts of ORO stock solution (0.7 g/200 mL isopropanol) with four parts of distilled water. Thereafter, stained cells were washed four times with distilled water and photographed using LSM 510 inverted microscopy (Carl Zeiss, Germany) using Axio vision v. 4.6 software. For quantitative assays, cells were dried and ORO was eluted using 100% isopropanol. Absorbances of eluates were read using Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA) at 520 nm wave length and the results were normalized against pure isopropanol as a blank.
Determination of glycerol release
Lipolysis was analyzed by measurement of glycerol release to the medium using Free Glycerol Determination Kit (Sigma Aldrich) according to manufacturer’s procedure.
Real time PCR
Total RNA was isolated using Tripure Isolation Reagent (Roche Diagnostics). Purity and quantity of RNA were determined using NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Total RNA (0.5 µg) was transcribed into cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostic). cDNA was used for multiplex quantitative RT-PCR (qPCR) using gene-specific primers and TaqMan probes (Thermo Fisher Scientific, Waltham, MA, USA). List of assay identification numbers for primers/probes are given in Table 1. Fluorescence was measured using QuantStudio 12 K Flex (Life Technologies). The relative expression was analyzed using the ΔΔCt method. Expression of the target genes was normalized to the expression of the housekeeping gene TATA binding protein.
Table 1.
List of assay identification numbers for primers/probes
| Gene symbol | Reporter/quancher | Assay ID | Catalog number |
|---|---|---|---|
| OXR1 (HCRTR1) | FAM/MGB-NFQ | Ss02839035_m1 | 4448892 |
| OXR2 (HCRTR2) | FAM/MGB-NFQ | Ss03373226_m1 | 4448892 |
| C/EBPα | FAM/MGB-NFQ | Ss03373315_s1 | 4448892 |
| C/EBPβ | FAM/MGB-NFQ | Ss03375347_u1 | 4448892 |
| PPARγ | FAM/MGB-NFQ | Ss03394828_m1 | 4448892 |
| LPL | FAM/MGB-NFQ | Ss03394610_m1 | 4448892 |
| Leptin | FAM/MGB-NFQ | Ss03392404 | 4448892 |
| TBP (LOC100125545) | VIC/MGB-NFQ | Ss03377123_u1 | 4448489 |
C/EBPα: CCAAT/enhancer binding protein alpha; C/EBPβ: CCAAT/enhancer binding protein beta; LPL: lipoprotein lipase; TBP: TATA binding protein; PPARγ: peroxisome proliferator-activated receptor gamma; LPL: Lipoprotein lipase; TBP: TATA binding protein.
Determination of leptin secretion
Leptin concentration in culture medium was analyzed using Multispecies Leptin RIA Kit (Merck Millipore, USA). This test has a 67% specificity for porcine leptin comparing with 100% specificity of the same antibody to human leptin. Intra- and interassay CVs were <6% and <9%, respectively.
Statistical analysis
Statistical analysis was performed using ANOVA, followed by Bonferroni's test. If the data failed the normality test, Dunn’s multiple comparisons test was used; P-values <0.05 (*). The Student’s t-test (parametric two-tailed t-test) was used for statistical significance determination between two groups. Results are shown as means ± SEM and were derived in representative experiments, in which cells were obtained from three individuals, performed at least in four replicates.
Results
OXR1 and OXR2 are expressed in porcine preadipocytes
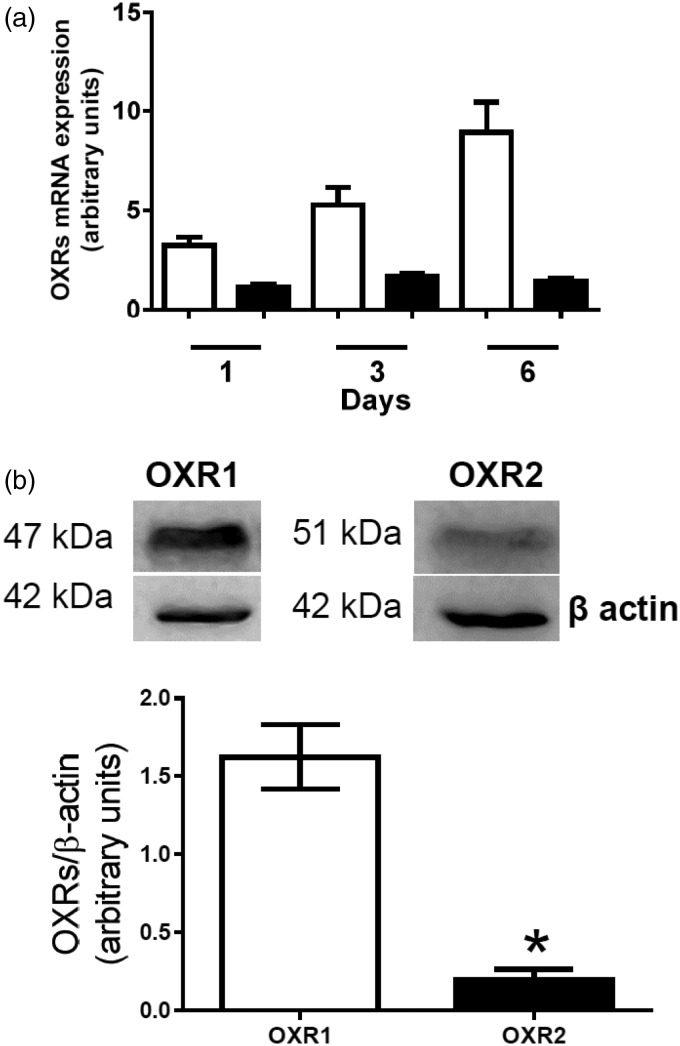
Both, OXR1 as well as OXR2 mRNA were detected in preadipocytes. OXR1 expression increased during differentiation reaching the maximal level on day 6 (Figure 1(a)). OXR2 mRNA expression was stable during the whole differentiation process. These results show that porcine preadipocytes express OXR1 and OXR2. The presence of OXR1 and OXR2 was confirmed on protein level in preadipocytes differentiated for six days (Figure 1(b)). The protein level of OXR1 was higher than OXR2 (P < 0.05).
Figure 1.
Detection of orexin receptors expression in porcine preadipocytes. (a) OXR1 (white bars) and OXR2 (black bars) mRNA expression was analyzed one, three, or six days of differentiation. Results are shown as mean ± SEM, derived from n = 5 replicates. The experiment was repeated three times independently. (b) Western blot detection of OXR1 and OXR2 in preadipocytes differentiated for six days. Results are shown as mean ± SEM, derived from n = 3 replicates obtained from three independent preadipocytes isolations
OXA and OXB stimulate porcine preadipocyte proliferation
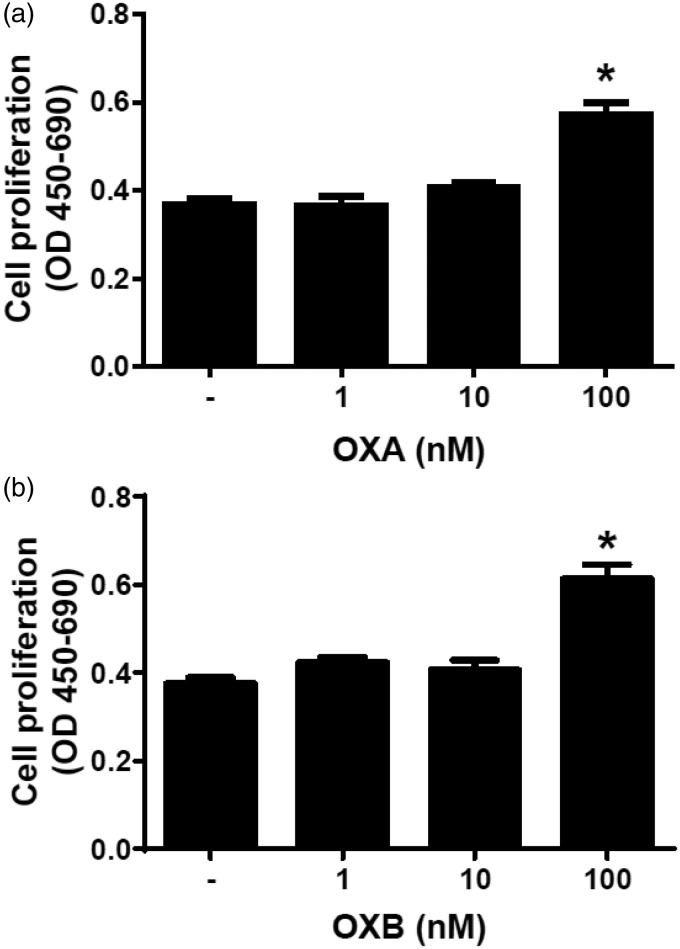
Next, we examined the effects of 24 h exposure of OXA and OXB on preadipocyte proliferation. Both, OXA (100 nM) (Figure 2(a)) as well as OXB (100 nM) (Figure 2(b)) stimulated preadipocyte proliferation (P < 0.05). Both peptides at 1 nM and 10 nM failed to affect cell proliferation, however. These data indicate that highest doses of OXA and OXB enhance proliferation of porcine preadipocytes.
Figure 2.
Effects of OXA and OXB on porcine preadipocytes cell proliferation. Cell proliferation was assessed in preadipocytes treated either with 1–100 nM OXA (a) or 1-100 nM OXB (b), for 24 h. Results are shown as mean ± SEM, derived from n = 8 replicates. The experiment was repeated two times, independently
OXA and OXB enhance differentiation of porcine preadipocytes into mature adipocytes
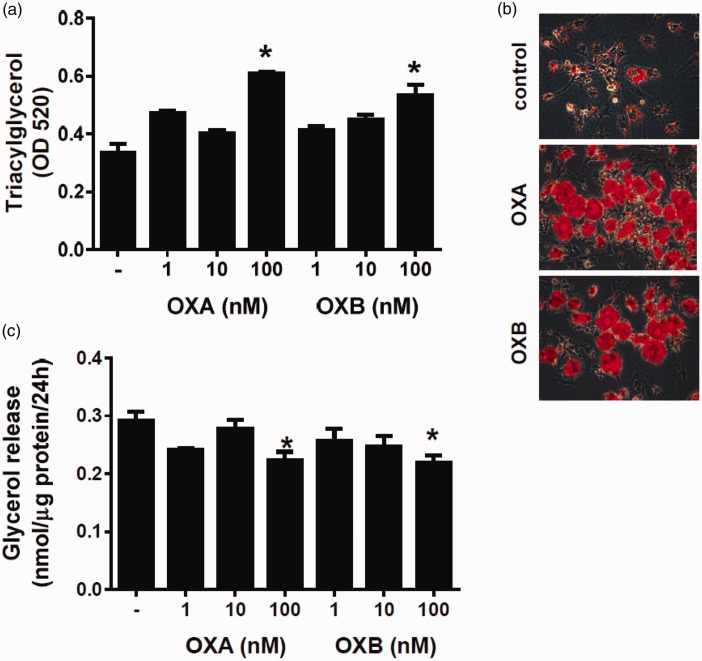
We then studied the influence of OXA and OXB on differentiation of porcine preadipocytes into mature adipocytes. To address this question, we assessed triacylglycerol accumulation, glycerol secretion, and morphological changes in preadipocytes, continuously exposed to OXA or OXB for six days. As showed in Figure 3(a), OXA (P < 0.05) and OXB (P < 0.05) at 100 nM increased intracellular triacylglycerol content in preadipocytes. Adipogenic properties of OXA and OXB were confirmed by studying the morphology of preadipocytes. As shown in Figure 3(b), preadipocytes exposed either to 100 nM OXA or 100 nM OXB for six days displayed larger lipid droplets as compared to vehicle-treated preadipocytes. An increased lipid accumulation in response to 100 nM OXA and 100 nM OXB was associated by a suppressed glycerol release (P < 0.05) from preadipocytes (Figure 3(c)).
Figure 3.
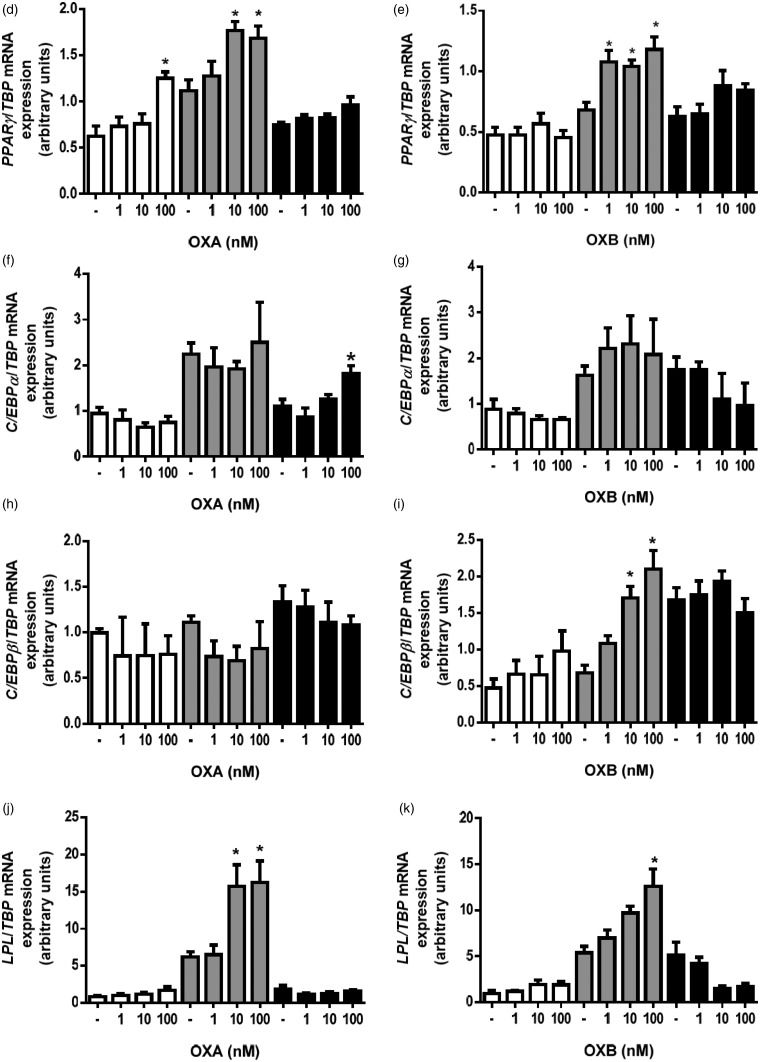
Effects of OXA and OXB (1–100 nM) on triacylglycerol accumulation, glycerol secretion and adipogenic genes expressions during differentiation of porcine preadipocytes. Quantification of intracellular triacylglycerol content in cells incubated with or without OXA or OXB (a) for six days. (b) Representative images (ORO staining) of porcine preadipocytes differentiated without (upper panel) or with 100 nM OXA (middle panel) or 100 nM OXB (lower panel) for six days. Images were taken at 400-fold magnification. (c) Changes in glycerol secretion levels from preadipocytes differentiated with or without OXA and OXB for six days. Effects of OXA and OXB on proadipogenic genes expression in porcine preadipocytes. PPARγ (d–e), C/EBPβ (f-g) and LPL (h-i) expressions were evaluated in cell exposed to 1–100 nM OXA and OXB for one (open bars), three (gray bars), and six days (black bars) after the onset of differentiation process. Results are shown as mean ± SEM, derived from at least n = 4 replicates. The experiment was repeated three times, independently. (A color version of this figure is available in the online journal.)
Next, we examined changes of adipogenic gene expression in preadipocytes incubated in the differentiation medium with or without OXA or OXB (1-100 nM) for one, three, or six days. As shown in Figure 3(d), OXA at 100 nM stimulated (P < 0.05) PPARγ expression 24 h after initiation of differentiation. PPARγ expression also increased (P < 0.05) in preadipocytes exposed to 10 or 100 nM OXA for three days. OXA, at all tested doses, did not influence PPARγ expression in preadipocytes differentiated for six days. OXB increased PPARγ mRNA expression at 1 nM and 10 nM (P < 0.05) as well as at 100 nM (P < 0.05), assessed three days after the initiation of the differentiation process (Figure 3(e)). OXB failed to modulate PPARγ expression in preadipocytes differentiated for 24 h or six days. CCAAT/enhancer binding protein alpha (C/EBPα) expression increased in preadipocytes treated with 100 nM OXA for six days (P < 0.05) was and remained unchanged in other experimental groups (Figure 3(f) and (g)). CCAAT/enhancer binding protein beta (C/EBPβ) expression was not affected by OXA as detected one, three, or six days after the onset of the differentiation (Figure 3(h)). OXB at 10 as well as 100 nM stimulated (P < 0.05) C/EBPβ expression in preadipocytes differentiated for three days, however, no changes were observed after one and six days after initiation of adipogenesis at all tested doses (Figure 3(i)).
Furthermore, both peptides enhanced lipoprotein lipase (LPL) expression in cells, differentiated for three days (Figure 3(j) to (k)). LPL mRNA expression increased in cells were exposed to 10 nM OXA (P < 0.05) or 100 nM OXA (P < 0.05). OXB (100 nM) increased LPL expression (P < 0.05). There were no changes in LPL mRNA expression in preadipocytes exposed either to OXA or OXB for one or six days.
Overall, these data collectively show that both OXA as well as OXB can enhance lipid accumulation and the expression of proadipogenic genes in time- and dose-dependent fashion.
OXA and OXB stimulate leptin mRNA expression but fail to modulate leptin secretion
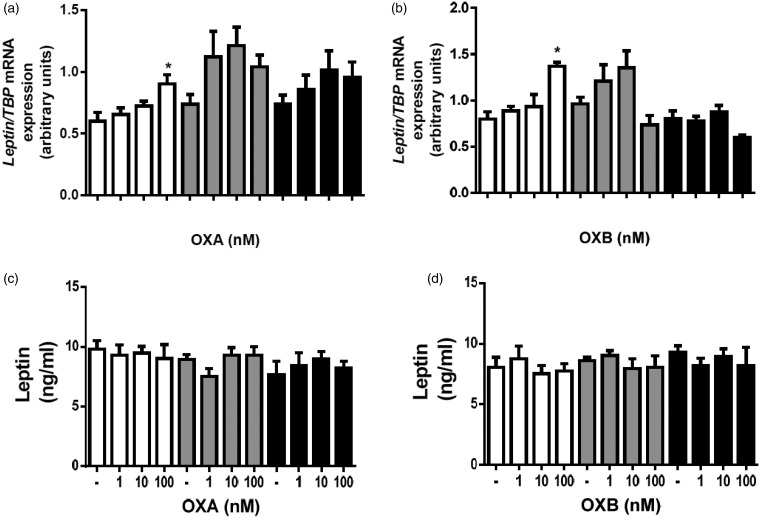
Next, we examined the effects of OXA and OXB on leptin mRNA expression and secretion. OXA (100 nM) and OXB (100 nM) increased leptin mRNA expression (P < 0.05) in preadipocytes differentiated for three days (Figure 4(a) and (b)). In case of both peptides, at all tested doses, there appears to be no influence on leptin secretion from preadipocytes (Figure 4(c) and (d)).
Figure 4.
Effects of OXA and OXB on leptin mRNA expression and leptin secretion in preadipocytes. Changes in leptin mRNA expression in preadipocytes differentiated with or without OXA (a) and OXB (b) for differentiated for one (open bars), three (gray bars) or six days (black bars). Effects of OXA (c) and OXB (d) on leptin secretion in preadipocytes differentiated with or without OXA (a) and OXB (b) for differentiated for one (open bars), three (gray bars) or six days (black bars). Results are shown as mean ± SEM, derived from at least n = 4 replicates. The experiment was repeated three times, independently.
Discussion
In the present study, we identify OXA and OXB as novel stimuli of porcine white preadipocyte proliferation as well as their differentiation into mature adipocytes.
We found that porcine preadipocytes express both OXR1 and OXR2. The presence of both isoforms of OXRs in white fat precursor cells is in line with the results of our former study, demonstrating expression of OXR1 and OXR2 in murine 3T3-L1 preadipocytes.10 Furthermore, likewise in 3T3-L1 preadipocytes,10 expression of OXR1 mRNA increased during the differentiation of porcine preadipocytes into mature adipocytes. Since we additionally confirmed the presence of OXR1 and OXR2 on protein level, these data collectively suggest that both OXRs may be involved in predipocyte growth and differentiation. Nevertheless, the levels of OXR1 mRNA as well as protein were higher compared to OXR2, which is consistent with higher expression of OXR1 in human white adipocytes.9 Importantly, OXR1 predominantly binds OXA, therefore the higher levels of this type of orexin receptor may suggest that OXA is more relevant in controlling preadipocyte functions. This notion is supported by our current data. As discussed below, the effects of OXA were more effective on preadipocytes differentiation as compared to OXB.
Several studies demonstrated that orexin receptors modulate cell growth, as well as apoptotic cell death. OXR1 and OXR2 activation was associated with a suppression of cell proliferation; however, a stimulation of cell growth was reported as well.12,26,27
The vast amount of data indicated that OXR1 or OXR2 signaling suppresses cell growth and induces apoptotic cell death, which is based upon experiments using tumor cell lines, e.g., colon cancer, neuroblastoma cells, or pancreatic AR42J cells.26–28 These data indicate that orexins may trigger cell death selectively in tumor cells, while stimulating proliferation of healthy cells. However, recent results showed that OXA can enhance proliferation of rat insulinoma or SGC-7901 human cancer gastric cells.27,29,30 Noteworthy, in all studies, orexin concentrations as well as times of incubations were very similar. Overall, stimulation of cell death in cancer cells is not general, and cell specific effects of OXA may be possible.
Since the number of mature adipocytes depends on preadipocytes pool,14 we studied the effects of orexins on proliferation of porcine preadipocytes. In the present study, we found that both OXA and OXB augmented porcine preadipocyte proliferation. Stimulation of white preadipocyte growth by OXA is in line with previous observations indicating that OXA increases 3T3-L1 as well as primary rodent preadipocytes growth via ERK1/2-dependent mechanism.12,13 In contrast, OXB was reported to suppress proliferation of 3T3-L1 preadipocytes.12 Since OXB binds mainly to OXR2, it is likely that activation of this receptor isoform in murine and porcine preadipocytes may lead to opposite effects on cell growth. Studies on OXR2 cascade signal transduction in murine and porcine preadipocytes are required to answer this question.
Apart from preadipocyte proliferation and dietary energy supply, fat tissue mass precisely modulated the ability of preadipocytes to differentiate into mature fat cells.31 In the present study, we assessed the effects of OXA on porcine preadipocyte differentiation. Initially, we found that both OXA as well as OXB enhanced triacylglycerol accumulation in porcine preadipocytes. Notably, stimulation of lipid accumulation by OXA or OXB was accompanied by reduced glycerol secretion, confirming antilipolytic activates of OXA previously reported in human and murine adipocytes.9,10 Since accumulation of triacylglycerol is a hallmark of adipogenesis,32 these findings suggest that both peptides can stimulate differentiation of preadipocytes into mature adipocytes. To further confirm this hypothesis, we assessed expression of differentiation markers (PPARγ, C/EBPα, C/EBPβ, and LPL32) in preadipocytes exposed to OXA or OXB. Moreover, we evaluated the expression and secretion of leptin as a major satiety signal produced by adipocytes.33 Our study shows that predominantly OXA as well as OXB (to a lesser degree) increased expressions of PPARγ, C/EBPα, C/EBPβ, and LPL mRNAs in a dose- and time-dependent manner. Interestingly, in our experiments, the highest levels of PPARγ and other genes were detected three days after initiation of differentiation process. These findings are inconsistent with previous data showing that PPARγ and LPL are continuously increasing during differentiation.34 However, there are also studies available showing that PPARγ expression increases at an early stage of differentiation only. For instance, Wang et al.35 found the maximal levels of PPARγ and C/EBPα expression were detected three days after initiation of differentiation process. This is consistent with our current observation that PPARγ expression decreases from day 3 to day 6. Furthermore, others reported that PPARγ2 expression reached the maximal level four days after initiation of adipogenesis and was lower in preadipocytes differentiated for eight days.36 Contradictory results describing expression pattern of main transcription factors controlling preadipocytes differentiation could result from several experimental factors. It cannot be excluded that composition of differentiation medium and/or animal breed may cause different pattern of adipogenic genes expression.
Furthermore, we show that OXA and OXB stimulate leptin mRNA expression. Notably, both peptides stimulated leptin mRNA expression only one day after onset of differentiation process.
Stimulation of leptin mRNA expression by orexins in preadipocytes suggests that elevation of serum leptin levels in rodents in response to OXA37,38 may result from the ability of orexin to stimulate leptin mRNA expression in adipocytes. However, since the increased leptin mRNA expression was not accompanied by enhanced leptin secretion in our study, this can be considered as a hypothesis only. Evaluation of adipose tissue leptin mRNA expression in OXA-treated animals would be appropriate to verify this hypothesis.
Taken together, our results indicate that OXA and OXB are capable to stimulate porcine preadipocyte proliferation and differentiation, thereby potentially contributing to changes of fat tissue composition and energy homeostasis. Stimulation of preadipocyte proliferation and differentiation suggests that orexin receptors signaling in adipocytes may trigger enhanced fat accumulation, leading to obesity. Indeed, there are studies available, indicating that blockade of OXR1 results in alleviation of obesity in rodents.39,40 However, a vast majority of studies showed that orexin receptor signaling protects against obesity and improves insulin sensitivity in rodents.41–43 It was postulated that contradictory data describing the effects of orexin system on energy homeostasis may result from site-specific application of orexin as well as different pattern of orexin receptors expression.43
Interestingly, it was shown that stimuli of white adipogenesis can improve lipid homeostasis and insulin sensitivity in rodents.15 Thus, it cannot be excluded that improved insulin sensitivity in response to orexin treatment reported in rodents may result from augmented preadipocyte proliferation and differentiation. However, it must to be mentioned that in our recent study we found that OXA fails to affect murine as well as rat preadipocytes differentiation, as judged by changes in PPARγ expression and lipid accumulation.13 These observations suggest that OXRs are not relevant in regulating adipogenesis in rodents. Nevertheless, contrary to our data, a recently published work reported that OXA stimulates Pparγ mRNA expression in 3T3-L1 preadipocytes.11 Shen et al. studied the effects of OXA on Pparγ expression alone. In our study, however, the effects of OXA on Pparγ expression were assessed in the presence of 3-isobutyl-1-methylxanthin, which is a potent stimulus of PPARγ expression in preadipocytes.44 Therefore, it cannot be excluded that OXA failed to further potentiate Pparγ expression in 3T3-L1 adipocytes when combined with 3-isobutyl-1-methylxanthin. Nevertheless, since stimuli of PPARγ expression are able to enhance differentiation of white preadipocytes,45 the potential role of OXA in rodent preadipocytes cannot be completely ruled out and requires to be verified by independent research groups.
One of the limitations of our study is that the effects of OXA and OXB on expression of adipogenic markers were studied on mRNA level, only. Thus, further studies are required to investigate whether changes in mRNA levels are accompanied by changes in protein levels. Nevertheless, it is worth to note that both and OXB affected cell morphology and lipid accumulation. This allows us to claim that both peptides promote adipogenic processes in porcine preadipocytes.
Furthermore, the detailed evaluation of underlying mechanisms explaining the effects of orexins on porcine preadipocyte growth and differentiation is missing. However, it is known that OXA can activate PKB as well as ERK1/2 kinases.7,10,13 Since stimuli of PKB and ERK1/2 can induce preadipocyte proliferation and affect their differentiation,13,46,47 it is likely that the ability of orexins to stimulate adipogenesis of preadipocytes depends on these protein kinases.
In summary, we identified that OXA and OXB are able to stimulate proliferation of porcine primary preadipocytes. Furthermore, using porcine preadipocytes, we showed for the first time that orexins stimulate white preadipocyte differentiation. Our data appear that OXA and OXB may modulate energy balance by promoting adipogenesis.
Acknowledgements
This study was supported by the Ministry of Sciences and Higher Education/National Science Centre grant NN311508339.
Author contributions
TW, MS, DS, IH, PAK, and MB conducted the experiments and analyzed the data. TW and MS wrote the manuscript. KWN designed the study. MZS and KWN edited and revised the manuscript. All authors approved the final version of the manuscript for publication. TW and MS contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998; 95: 322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92: 573–85. [DOI] [PubMed] [Google Scholar]
- 3.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 1999; 289: 219–23. [PubMed] [Google Scholar]
- 4.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 1999; 96: 10911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Osaka T, Inoue S. Energy expenditure by intracerebroventricular administration of orexin to anesthetized rats. Neurosci Lett 2001; 315: 49–52. [DOI] [PubMed] [Google Scholar]
- 6.Malendowicz LK, Jedrzejczak N, Belloni AS, Trejter M, Hochol A, Nussdorfer GG. Effects of orexins A and B on the secretory and proliferative activity of immature and regenerating rat adrenal glands. Histol Histopathol 2001; 16: 713–17. [DOI] [PubMed] [Google Scholar]
- 7.Goncz E, Strowski MZ, Grotzinger C, Nowak KW, Kaczmarek P, Sassek M, Mergler S, El-Zayat BF, Theodoropoulou M, Stalla GK, Wiedenmann B, Plockinger U. Orexin-A inhibits glucagon secretion and gene expression through a Foxo1-dependent pathway. Endocrinology 2008; 149: 1618–26. [DOI] [PubMed] [Google Scholar]
- 8.Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK. Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci 2000; 66: 449–54. [DOI] [PubMed] [Google Scholar]
- 9.Digby JE, Chen J, Tang JY, Lehnert H, Matthews RN, Randeva HS. Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J Endocrinol 2006; 191: 129–36. [DOI] [PubMed] [Google Scholar]
- 10.Skrzypski M, T Le T, Kaczmarek P, Pruszynska-Oszmalek E, Pietrzak P, Szczepankiewicz D, Kolodziejski PA, Sassek M, Arafat A, Wiedenmann B, Nowak KW, Strowski MZ. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia 2011; 54: 1841–52. [DOI] [PubMed] [Google Scholar]
- 11.Shen Y, Zhao Y, Zheng D, Chang X, Ju S, Guo L. Effects of orexin A on GLUT4 expression and lipid content via MAPK signaling in 3T3-L1 adipocytes. J Steroid Biochem Mol Biol 2013; 138: 376–83. [DOI] [PubMed] [Google Scholar]
- 12.Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K, Namyslowski G, Misiolek M, Sodowski K, Kato I, Kuwahara A, Zabielski R. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 2007; 58: 53–64. [PubMed] [Google Scholar]
- 13.Skrzypski M, Kaczmarek P, Le TT, Wojciechowicz T, Pruszynska-Oszmalek E, Szczepankiewicz D, Sassek M, Arafat A, Wiedenmann B, Nowak KW, Strowski MZ. Effects of orexin A on proliferation, survival, apoptosis and differentiation of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett 2012;586:4157–64. [DOI] [PubMed]
- 14.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev 2001; 2: 239–54. [DOI] [PubMed] [Google Scholar]
- 15.Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem 2012; 287: 6421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin 2012; 33: 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houpt KA, Houpt TR, Pond WG. The pig as a model for the study of obesity and of control of food intake: a review. Yale J Biol Med 1979; 52: 307–29. [PMC free article] [PubMed] [Google Scholar]
- 18.Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring) 2015; 23: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J 2006; 47: 243–58. [DOI] [PubMed] [Google Scholar]
- 20.Bai L, Pang WJ, Yang YJ, Yang GS. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol Cell Biochem 2008; 307: 129–40. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay TG, White ME, Wolverton CK. Glucocorticoids and the differentiation of porcine preadipocytes. J Anim Sci 1989; 67: 2222–9. [DOI] [PubMed] [Google Scholar]
- 22.Hausman GJ. The influence of dexamethasone and insulin on expression of CCAAT/enhancer binding protein isoforms during preadipocyte differentiation in porcine stromal-vascular cell cultures: evidence for very early expression of C/EBPalpha. J Anim Sci 2000; 78: 1227–35. [DOI] [PubMed] [Google Scholar]
- 23.Boone C, Gregoire F, Remacle C. Culture of porcine stromal-vascular cells in serum-free medium: differential action of various hormonal agents on adipose conversion. J Anim Sci 2000; 78: 885–95. [DOI] [PubMed] [Google Scholar]
- 24.Skrzypski M, Sassek M, Abdelmessih S, Mergler S, Grotzinger C, Metzke D, Wojciechowicz T, Nowak KW, Strowski MZ. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell Signal 2014; 26: 41–8. [DOI] [PubMed] [Google Scholar]
- 25.Wojciechowicz T, Skrzypski M, Kolodziejski PA, Szczepankiewicz D, Pruszynska-Oszmalek E, Kaczmarek P, Strowski MZ, Nowak KW. Obestatin stimulates differentiation and regulates lipolysis and leptin secretion in rat preadipocytes. Mol Med Rep 2015; 12: 8169–75. [DOI] [PubMed] [Google Scholar]
- 26.Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, Avondo V, Pouzet C, Yanagisawa M, Laboisse C, Laburthe M, Voisin T. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem 2004; 279: 45875–86. [DOI] [PubMed] [Google Scholar]
- 27.Voisin T, Firar AE, Avondo V, Laburthe M. Orexin-induced apoptosis: the key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology 2006; 147: 4977–84. [DOI] [PubMed] [Google Scholar]
- 28.Laburthe M, Voisin T. The orexin receptor OX(1)R in colon cancer: a promising therapeutic target and a new paradigm in G protein-coupled receptor signalling through ITIMs. Br J Pharmacol 2012; 165: 1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Zhao Y, Zheng D, Ju S, Shen Y, Guo L. Orexin A Affects INS-1 Rat Insulinoma Cell Proliferation via Orexin Receptor 1 and the AKT Signaling Pathway. Int J Endocrinol 2013; 2013: 854623–854623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhao Y, Ju S, Guo L. Orexin A upregulates the protein expression of OX1R and enhances the proliferation of SGC-7901 gastric cancer cells through the ERK signaling pathway. Int J Mol Med 2015; 35: 539–45. [DOI] [PubMed] [Google Scholar]
- 31.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell 2010; 9: 667–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78: 783–809. [DOI] [PubMed] [Google Scholar]
- 33.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002; 967: 379–88. [DOI] [PubMed] [Google Scholar]
- 34.Ding ST, McNeel RL, Mersmann HJ. Expression of porcine adipocyte transcripts: tissue distribution and differentiation in vitro and in vivo. Comp Biochem Physiol B Biochem Mol Biol 1999; 123: 307–18. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Zhou G, Shu G, Wang L, Zhu X, Gao P, Xi Q, Zhang Y, Yuan L, Jiang Q. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cell Physiol Biochem 2013; 31: 981–96. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Chen Y, Zhang Y, Zhang Y, Chen L, Mo D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int J Biol Sci 2012; 8: 1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Switonska MM, Kaczmarek P, Malendowicz LK, Nowak KW. Orexins and adipoinsular axis function in the rat. Regul Pept 2002; 104: 69–73. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Shim HM, Na AY, Bae JH, Im SS, Song DK. Orexin A regulates plasma insulin and leptin levels in a time-dependent manner following a glucose load in mice. Diabetologia 2015; 58: 1542–50. [DOI] [PubMed] [Google Scholar]
- 39.Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept 2002; 104: 153–9. [DOI] [PubMed] [Google Scholar]
- 40.White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides 2005; 26: 2331–8. [DOI] [PubMed] [Google Scholar]
- 41.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab 2009; 9: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann N Y Acad Sci 2012; 1264: 72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab 2011; 14: 478–90. [DOI] [PubMed] [Google Scholar]
- 44.Kim SP, Ha JM, Yun SJ, Kim EK, Chung SW, Hong KW, Kim CD, Bae SS. Transcriptional activation of peroxisome proliferator-activated receptor-gamma requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem Biophys Res Commun 2010; 399: 55–9. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 1996; 16: 4128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagnon A, Chen CS, Sorisky A. Activation of protein kinase B and induction of adipogenesis by insulin in 3T3-L1 preadipocytes: contribution of phosphoinositide-3,4,5-trisphosphate versus phosphoinositide-3,4-bisphosphate. Diabetes 1999; 48: 691–8. [DOI] [PubMed] [Google Scholar]
- 47.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 2002; 277: 46226–32. [DOI] [PubMed] [Google Scholar]