Abstract
Ames dwarf mice are exceptionally long-lived due to a Prop1 loss of function mutation resulting in deficiency of growth hormone, thyroid-stimulating hormone and prolactin. Deficiency in thyroid-stimulating hormone and growth hormone leads to greatly reduced levels of circulating thyroid hormones and insulin-like growth factor 1, as well as a reduction in insulin secretion. Early life growth hormone replacement therapy in Ames dwarf mice significantly shortens their longevity, while early life thyroxine (T4) replacement therapy does not. Possible mechanisms by which early life growth hormone replacement therapy shortens longevity include deleterious effects on glucose homeostasis and energy metabolism, which are long lasting. A mechanism explaining why early life T4 replacement therapy does not shorten longevity remains elusive. Here, we look for a possible explanation as to why early life T4 replacement therapy does not impact longevity of Ames dwarf mice. We found that early life T4 replacement therapy increased body weight and advanced the age of sexual maturation. We also find that early life T4 replacement therapy does not impact glucose tolerance or insulin sensitivity, and any deleterious effects on oxygen consumption, respiratory quotient and heat production are transient. Lastly, we find that early life T4 replacement therapy has long-lasting effects on bone mineral density and bone mineral content. We suggest that the transient effects on energy metabolism and lack of effects on glucose homeostasis are the reasons why there is no shortening of longevity after early life T4 replacement therapy in Ames dwarf mice.
Keywords: Ames dwarf, thyroid hormone, thyroxine, T4, longevity, aging
Introduction
Thyroid hormones (THs) have been implicated in alterations in longevity.1–7 The THs, triiodothyronine (T3) and thyroxine (T4) are tyrosine-based hormones that are produced by the thyroid after stimulation by thyroid-stimulating hormone (TSH).8 T4 is the most prominent TH in circulation where it is typically bound to thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA) or albumin.8 T4 is converted to T3 at target tissue by type II iodothyronine deiodinase (D2).9 T3 mediates TH action by binding to thyroid-hormone receptors (TRs). T3 binding to TRs causes nuclear translocation of TRs, and subsequent binding to thyroid hormone response elements (TREs) that induce transcription of TH-specific genes.10 The metabolic effects of TH include regulation of body weight, temperature and metabolic rate, as well as potentiating the effects of catecholamines.11
Since its inception in the 1990s, Barker’s hypothesis of developmental origins of adult disease12 has gained considerable interest and amassed support from multiple independent studies. For instance, birth weight has been linked to adult onset of several diseases such as cardiovascular disease and diabetes.13,14 Further, maternal diabetes,15 or cholestasis,16 during pregnancy increases the offspring’s risk of developing metabolic disorders. These examples of early life programming can only indirectly be utilized as a predictive marker of aging (i.e. higher incidence of metabolic disease may decrease longevity). However, early life programming has been directly linked to extension of longevity. For example, early life calorie restriction by crowded litter increases both median and maximal lifespan relative to mice from normal sized litters.17
Ames dwarf mice have a Prop1 homozygous loss of function mutation, resulting in lack of differentiation of somatotrophs, lactotrophs and thyrotrophs in the anterior pituitary.18 Lack of differentiation in these endocrine cell lineages leads to deficiency of growth hormone (GH), prolactin (PRL) and TSH, with secondary effects including reduced circulating levels of THs, insulin-like growth factor 1 (IGF-1) and insulin.7,18–20 Further, Ames dwarf mice have a 40%–60% extension of longevity in males and females, respectively.7 Panici et al.21 demonstrated that early life GH replacement therapy in Ames dwarf mice significantly shortened their longevity. The decrease in longevity was accompanied by impaired glucose homeostasis and energy metabolism that persisted for at least one year after GH replacement therapy stopped, and possibly throughout the animal’s life (Sun et al., unpublished observations). In contrast, early life T4 replacement therapy had no impact on longevity.21 Interestingly, lifelong T4 replacement therapy in Snell dwarf mice, which are long-lived due to a mutation with effects essentially identical to those of the Ames dwarf mouse, shortens their longevity.5 Here, our objective was to elucidate a possible mechanism for why short-term TH replacement therapy early in life does not impact longevity of hypothyroid Ames dwarf mice. We find that short-term T4 replacement therapy in Ames dwarf mice produces no alterations in glucose homeostasis, and only transiently impairs energy metabolism.
Materials and methods
Animals
Male Ames dwarf (Prop1df/df) homozygous mice (df/df), and their normal littermates, were produced by mating heterozygous females and homozygous mutant males in our breeding colony at Southern Illinois University School of Medicine (SIUSOM). The Prop1df/df mutation is maintained on a heterogeneous genetic background. Animals were maintained under temperature- and light-controlled conditions (20℃–23℃, 12-h light–dark cycle (lights on at 7 a.m. and off at 7 p.m.)), and allowed ad libitum access to water and standard chow (LabDiet 5001, with 29% calories from protein, 13% calories from fat and 56% calories from carbohydrates). All animal protocols for this study were approved by the SIUSOM Laboratory Animal Care and Use Committee.
Thyroxine treatment
Male Ames dwarf mice, and their normal littermates, were treated with T4 (L-thyroxine; Sigma, St. Louis, MO, USA) as previously described.21 In short, T4 in 0.9% saline solution at pH 7.8 was administered by subcutaneous (s.c.) injection (0.1 µg/g body weight; 0.7 µg/50 µL dose) 3×/week (Monday, Wednesday, Friday) at 10 a.m. Control Ames dwarf mice were injected with 0.9% saline following the same schedule. All treatments were started one week after birth and continued for six weeks. All experiments conducted after the conclusion of treatment began at 10 a.m.
Body weight measurements and determination of sexual maturation
Body weight measurements were taken weekly, starting at two weeks of age. Sexual maturation was determined by balanopreputial separation.
Body composition measurements
Body composition was measured by dual-energy X-ray absorptiometry scanning using the PIXI-mus small animal densitometer (Lunar, Madison, WI, USA). This system generates low energy X-rays which are directed through the mouse to a radiation detector. The radiation is digitally processed and analyzed by the PIXI-mus software. Output parameters include percent body fat (% fat), bone mineral density (BMD—g/cm2) and bone mineral content (BMC—g).
Glucose tolerance testing
Mice were fasted for 16 h. Blood glucose was measured (time 0) by tail bleed. Glucose (Sigma, St. Louis, MO, USA) was injected by intraperitoneal (i.p.) injection at a dose of 2 g/kg body weight. Sequential glucose measurements were taken by glucometer (AgaMatrix, Salem, NH, USA) at 15, 30, 45, 60 and 120 min.
Insulin sensitivity testing
Blood glucose was measured (time 0) by tail bleed in non-fasted mice. Insulin (Sigma, St. Louis, MO, USA) was injected i.p. at a dose of 1 international unit (IU) per kg body weight. Sequential glucose measurements were taken by glucometer at 15, 30, 45, 60 and 120 min.
Indirect calorimetry
Indirect calorimetry was performed using the PhysioScan Metabolic System (AccuScan Instruments, Inc., Columbus, OH, USA). This system utilizes zirconia and infrared sensors to monitor oxygen (O2) and carbon dioxide (CO2), respectively, inside respiratory chambers where mice are housed individually. All comparisons are based on animals studied simultaneously in an effort to minimize the effect of environmental variation and calibration on data. After a 24-h acclimation period, mice were monitored in the metabolic chambers for 24 h, with ad libitum access to food and water. Gas samples were collected and analyzed every 10 min per animal. Output parameters include spontaneous locomotor activity (cm), oxygen consumption (VO2—mL/kg/min), respiratory quotient (RQ—VCO2/VO2) and heat production (cal/h/g body weight).
Body temperature measurements
Isoflurane-anesthetized mice were subcutaneously implanted with a transmitter that allowed remote measurement of body temperature by using a wand-type reader (model IPTT300, BioMedic Data Systems, Seaford, DE, USA). The microchip was implanted by using a 12-gauge needle delivery device, without a surgical incision. The wound was closed using surgical glue. Transmitters are 22 mm × 2 mm, and weigh approximately 0.125 g. The transmitters demonstrated accuracy to within 0.3℃ at an ambient temperature of 22℃ and, therefore, were not calibrated prior to use.
Statistical analysis
Analyses comparing more than two groups were performed by a two-way analysis of variance test. A Student’s t test was used when comparing two groups. Significance of the differences in body weight, insulin tolerance test (ITT) and glucose tolerance test (GTT) was calculated by comparing area under the curve. Values marked with a different superscript (a, b, c and d) are significantly different (P < 0.05). Values are reported as mean ± standard error of the mean (SEM) throughout the figures. All statistics and graphs were done using Prism 6 (GraphPad Software, San Diego, CA, USA).
Results
T4 replacement therapy accelerates growth and sexual maturation
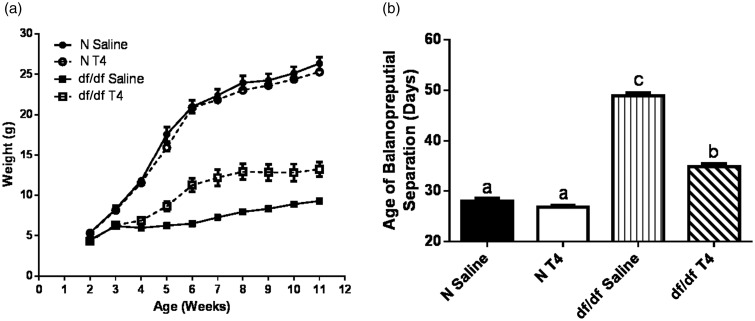
Ames dwarf mice have greatly reduced T3 and T4 levels as a result of their TSH deficiency.18,19 Decreased TH action, along with decreased GH action, causes these mutants to be ∼33% the size of their control littermates.19 Decreased TH action also causes a significant lag in the timing of sexual maturation in Ames dwarf mice.22 Therefore, we assessed the effects of early life T4 replacement therapy on Ames dwarf growth and sexual maturation. Since our study aims to elucidate why short-term T4 replacement therapy does not affect longevity, we utilized the same T4 dose used in a previously reported longevity study following short-term T4 treatment in juvenile Ames dwarf mice.21 As expected, early life T4 replacement therapy increased the body weight of dwarf mice (P < 0.0001); however, they did not reach the same body weight as their normal littermates (Figure 1(a)). Further, treatment with T4 significantly advanced the age dwarf mice underwent sexual maturation (P < 0.0001); however, they still matured later than their normal littermates (Figure 1(b)).
Figure 1.
T4 replacement therapy accelerates growth and sexual maturation. (a) Male Ames dwarf mice (df/df) body weight was monitored after treatment with T4 (n = 7) or saline (n = 10). Their normal littermates (N) were also monitored after treatment with T4 (n = 10) or saline (n = 9). (b) Sexual maturation in df/df and N mice was determined by balanopreputial separation (n = 9 for all groups)
T4 replacement therapy has lasting effects on bone but not body composition
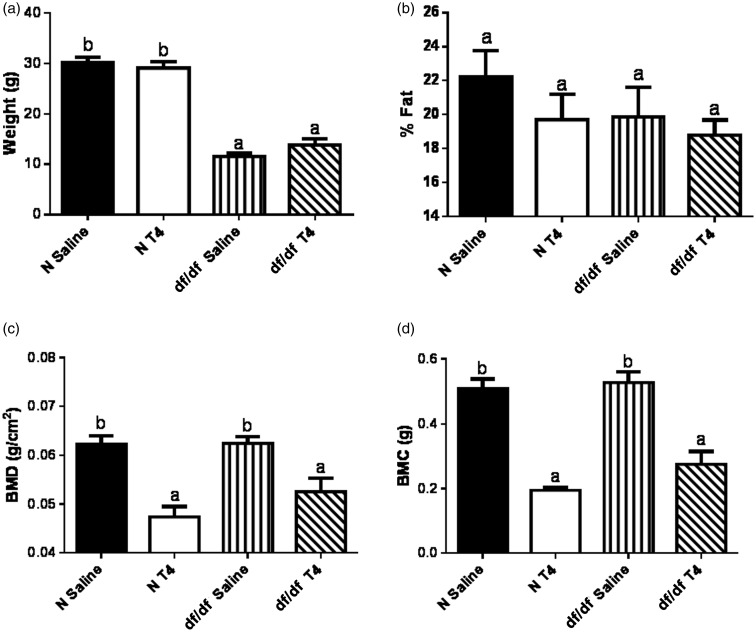
TH plays a major role in regulating body composition including body fat and bone composition.23–25 Therefore, we assessed the effects of early life T4 replacement therapy on Ames dwarf bone and body composition. Eight months following treatment with T4, body weight did not differ between the saline and T4-treated mice (Figure 2(a)), and there was no difference in percent body fat between these groups (Figure 2(b)). We did, however, observe a decrease in BMD in both dwarf mice (P = 0.0076) and their normal littermates that had been treated with T4 (P = 0.0002; Figure 2(c)). Further, we observed a decrease in BMC in T4-treated dwarf mice (P = 0.0004) as well as their normal littermates (P < 0.0001; Figure 2(d)).
Figure 2.
T4 replacement therapy has lasting effects on bone, but not body composition. Eight months after the last injection with T4, (a) body weight, (b) percent body fat, (c) bone mineral density (BMD) and (d) bone mineral content (BMC) of male Ames dwarf mice (df/df) treated with T4 or saline, along with their normal littermates (N) treated with T4 or saline, was measured (n = 7 for all groups)
T4 replacement therapy does not impact glucose homeostasis
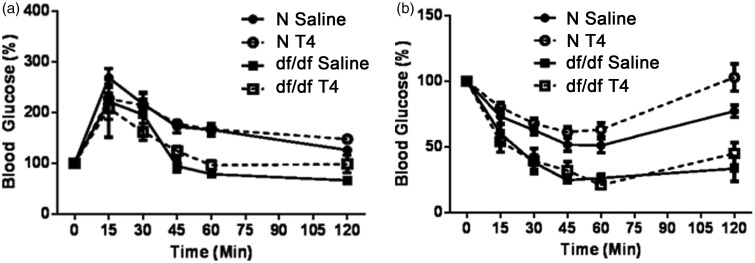
It has been suggested that a major factor in Ames dwarf longevity is improved glucose homeostasis.19,26,27 To test the effects of early life T4 replacement therapy on glucose homeostasis, a GTT was performed one week after the last T4 injection, and an ITT was performed one week later. As previously reported,19,26,27 male Ames dwarf mice were more glucose tolerant (P = 0.0026; Figure 3(a)), and insulin sensitive (P = 0.0004; Figure 3(b)) compared to their normal littermates. Early life T4 replacement therapy had no impact on glucose tolerance or insulin sensitivity in dwarf mice or their normal littermates. Ames dwarf mice treated with T4 remained glucose tolerant (P = 0.0253), and insulin sensitive (P = 0.0025) compared to their normal littermates treated with saline.
Figure 3.
T4 replacement therapy does not impact glucose homeostasis. (a) Male Ames dwarf mice (df/df) treated with T4 (n = 4) or saline (n = 6), along with their normal littermates (N) treated with T4 (n = 10) or saline (n = 9), underwent a glucose tolerance test (GTT) one week following treatment. (b) df/df mice treated with T4 (n = 5) or saline (n = 6), along with N mice treated with T4 (n = 10) or saline (n = 9), underwent an insulin tolerance test (ITT) two weeks following treatment
T4 replacement therapy transiently impairs energy metabolism
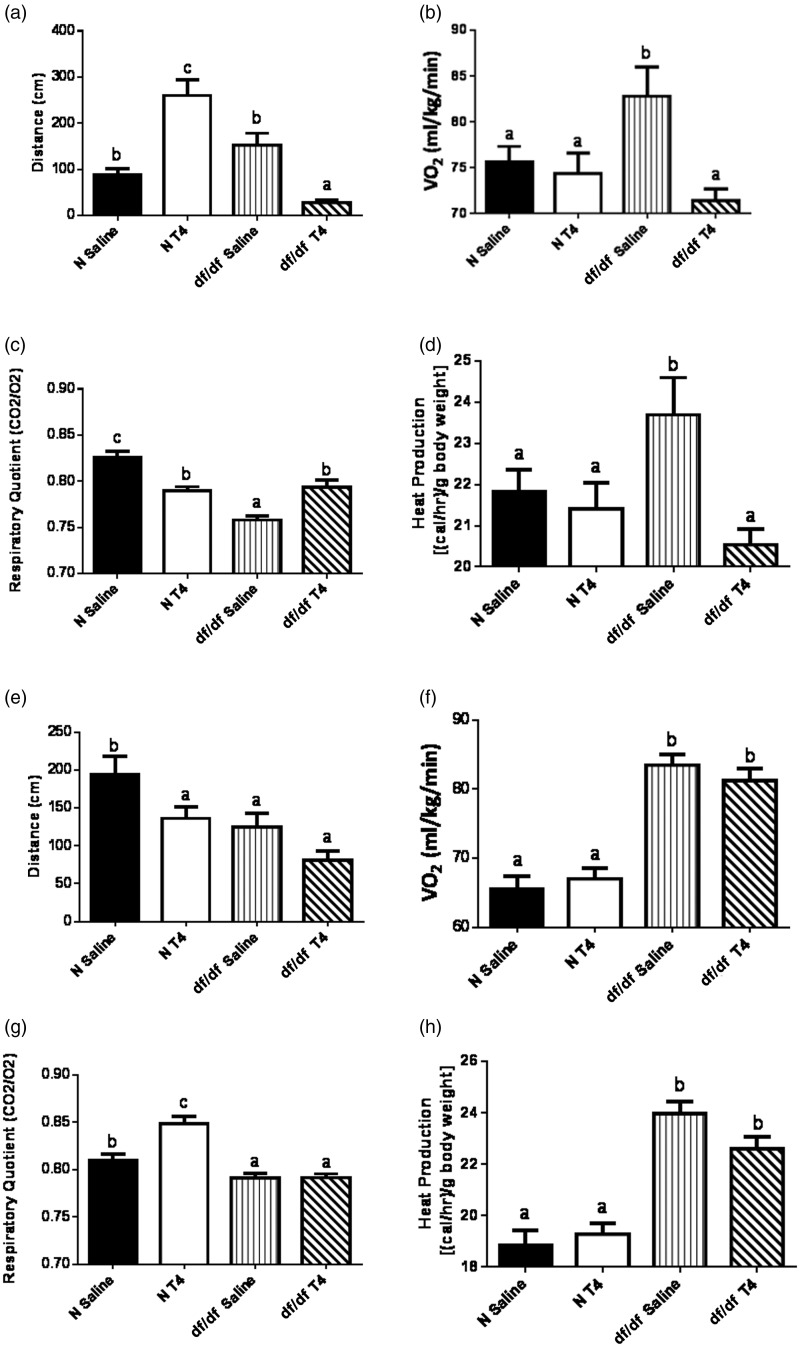
Another major factor believed to be involved in Ames dwarf longevity is their improved energy metabolism [increased VO2 per gram body weight, reduced RQ and increased heat production per gram body weight].28 To test the effects of early life T4 replacement therapy on Ames dwarf energy metabolism, we utilized indirect calorimetry to monitor spontaneous locomotor activity, VO2, RQ and heat production. Initial measurements were taken the day after the last T4 injection and were repeated four weeks later.
Since TH increases energy expenditure,29 we assessed how early life T4 replacement therapy impacted Ames dwarf locomotor activity. T4 treatment in normal mice increased locomotor activity (P < 0.0001), while it decreased locomotor activity in dwarf mice (P < 0.0001; Figure 4(a)). Dwarf mice treated with T4 did travel less than control mice on saline (P = 0.0002). Subsequent locomotor activity measurements four weeks later showed the dwarf locomotor activity reverted to baseline (Figure 4(e)). Normal mice treated with T4 did show a decrease in their locomotor activity (P = 0.047) four weeks following initial testing, despite showing an increase immediately following T4 treatment. While we reported locomotor activity as a 24-h average, the diurnal patterns of activity did not differ between T4- and saline-treated groups. This leads us to believe the sleeping habits of the mice were unaffected by T4 treatment.
Figure 4.
T4 replacement therapy transiently impairs energy metabolism. The day after the last T4 injection, (a) spontaneous locomotor activity, (b) oxygen consumption, (c) respiratory quotient and (d) heat production of male Ames dwarf mice (df/df) treated with T4 or saline (n = 4), along with their normal littermates (N) treated with T4 or saline (n = 5), was measured. Four weeks later, (e) spontaneous locomotor activity, (f) oxygen consumption, (g) respiratory quotient and (h) heat production was measured in the same mice
VO2 is the volume of oxygen consumed by an animal over a predetermined period of time.28 Since TH increases VO2,30 we assessed how early life T4 replacement therapy impacted Ames dwarf VO2. Immediately following the period of T4 replacement therapy, dwarf mice had decreased VO2 (P = 0.002), while their normal littermates showed no alterations in VO2 (Figure 4(b)). Subsequent VO2 measurements four weeks later showed the dwarf VO2 reverted to baseline (Figure 4(f)).
RQ is a dimensionless ratio of carbon dioxide eliminated (VCO2) to oxygen consumed (VO2).28,31 RQ is indicative of the macromolecule energy source an animal utilizes. In a healthy animal, RQ values typically range from 1.0 (representative of pure carbohydrate oxidation) to 0.7 (representative of pure lipid oxidation).28,31 Since TH is lipolytic,32 we assessed how early life T4 replacement therapy impacted Ames dwarf macromolecule metabolism. Following T4 replacement therapy, dwarf RQ increased (P = 0.0004), while their normal littermates’ RQ decreased (P < 0.0001; Figure 4(c)). Subsequent RQ measurements four weeks later showed that dwarf RQ reverted to baseline, while their normal littermates showed an increase in their RQ (P = 0.005; Figure 4(g)).
Heat production is directly proportional to basal metabolic rate (BMR).28 Since TH increases BMR,33 we assessed how early life T4 replacement therapy impacted Ames dwarf BMR by measuring heat production. Following T4 replacement therapy, Ames dwarf mice heat production decreased (P = 0.0025), while their normal littermates’ heat production was unaffected by T4 treatment (Figure 4(d)). Subsequent heat production measurements four weeks later showed the dwarf heat production reverted to baseline (Figure 4(h)).
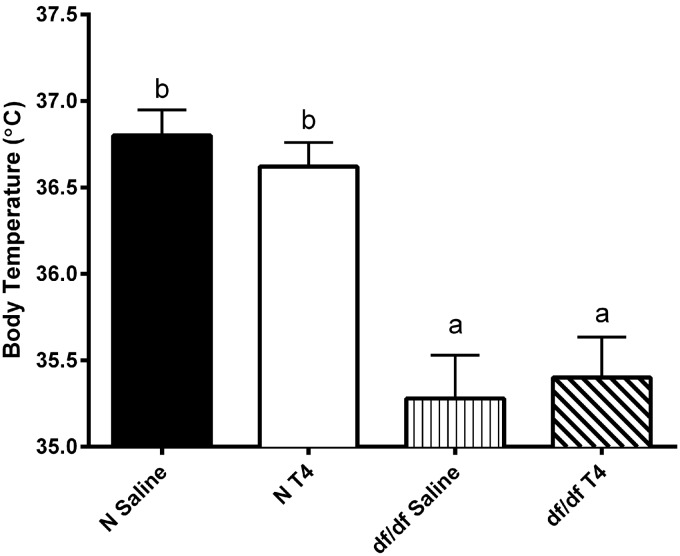
T4 replacement therapy does not alter body temperature
It has been previously reported that Ames dwarf mice have a lower body temperature than their normal littermates.34 It is believed that the lower body temperature is beneficial to the dwarf mice and may aid in their extended longevity. Therefore, we determined whether early life T4 replacement therapy affected body temperature one week following injections. We found that, as previously reported, Ames dwarf mice have a lower body temperature than their normal littermates (P = 0.0008; Figure 5). Interestingly, normal and dwarf mice treated with T4 did not have a difference in body temperature as compared to their saline-injected counterparts.
Figure 5.
T4 replacement therapy does not impact body temperature. Body temperature of male Ames dwarf mice (df/df) treated with T4 or saline (n = 5), along with their normal littermates (N) treated with T4 or saline (n = 5), was measured one week following treatment
Discussion
Ames dwarf mice are long-lived due to a Prop1 loss of function mutation, rendering them unable to produce GH, PRL and TSH.18 The deficiency of GH results in suppression of circulating IGF-1 levels, while deficiency of TSH results in greatly reduced levels of T3 and T4.18,19 While there are many instances of early life programming as a predictive marker of future diseases,14–16 there are fewer instances of early life programming as a predictive marker of aging.17 Early life GH replacement therapy in Ames dwarf mice significantly shortens their longevity, while early life T4 replacement therapy has little to no effect on their longevity.21 Our lab recently demonstrated that the effects of early life GH replacement therapy on longevity of Ames dwarf mice might be caused by at least two mechanisms: impairment of glucose homeostasis and a negative impact on energy metabolism (Sun et al., unpublished observations). Both of these impairments last for over a year after GH treatment is stopped, and may possibly last throughout the mouse’s life. Since lifelong T4 replacement therapy in Snell dwarf mice shortens longevity,5 but short-term replacement therapy in Ames dwarf mice does not,21 we hypothesized that any deleterious effects of early life T4 replacement therapy may be transient.
It is well documented that TH plays a role in development and body composition.23–25 As expected, TH administration to severely hypothyroid Ames dwarf mice not only increased body weight, but also accelerated the rate with which they underwent sexual maturation. The change in body size as a result of T4 replacement therapy was transient and had disappeared after eight months. We speculate that this is may be due to TH simply shifting the timeframe of the peripubertal growth spurt. Interestingly, T4 replacement therapy did not alter body composition in dwarf or normal mice; however, alterations in bone composition were long-lasting. Even after eight months had passed since the mice were exposed to exogenous T4, both dwarfs and their normal littermates had decreased BMD and BMC. Elevated TH levels increase bone remodeling by activating osteoclast and osteoblast activity, which favors high bone turnover and bone loss.24,25 The increased bone loss and turnover results in decreased BMD and BMC.24,25 This may explain the decrease in BMD and BMC seen in the normal mice treated with T4. Interestingly, decreased BMD has also been documented in hypothyroid human patients undergoing TH replacement therapy.35
Improved insulin sensitivity and glucose tolerance are believed to be critical to the extended lifespan of Ames dwarf mice.19,26,36 To test how TH impacts glucose homeostasis, we performed a GTT and ITT. Early life T4 replacement therapy had minimal effects on both glucose tolerance and insulin sensitivity. Moreover, dwarf mice treated with T4 remained glucose tolerant and insulin sensitive compared to their normal saline-treated counterparts. This is a major difference from the effects of early life GH replacement therapy, which impairs both glucose tolerance and insulin sensitivity for at least one year after GH treatment had stopped, and perhaps the duration of the mouse’s life (Sun et al., unpublished observations).
Along with improved insulin sensitivity and glucose tolerance, Ames dwarf mice have improved energy metabolism as indicated by increased VO2 and reduced RQ.28 Early life T4 replacement therapy did impair energy metabolism in Ames dwarf mice. This impairment, however, was only transient as compared to the long-lasting impairment from GH replacement therapy (Sun et al., unpublished observations). As expected, normal mice treated with T4 increased their locomotor activity. It was surprising that the dwarf mice decreased their locomotor activity. Further, early life T4 replacement therapy in Ames dwarf mice increased their RQ, while decreasing their VO2 and heat production. At first, these changes may seem counterintuitive since TH typically has the opposite effects on metabolism. However, it is important to remember that Ames dwarf mice are deficient in several hormones, two of which (GH and TH) play a major role in energy metabolism. Despite lacking these hormones, Ames dwarf mice have increased VO2 and heat production, along with decreased RQ. T4 replacement therapy normalized their metabolic phenotype to that of their normal littermates.
It is believed that the lower body temperature in Ames dwarf mice may be a possible mechanism for extension of longevity. As previously reported,34 Ames dwarf mice showed a decrease in their body temperature compared to their normal littermates. Following treatment with T4, there was no difference in body temperature compared to saline controls in both normal and dwarf mice. The lack of alteration in body temperature is consistent with the observed lack of alteration in longevity following short-term treatment with T4.21
Ames dwarf mice are long-lived due to depletion of several key hormones including GH and TH. It has been demonstrated that early life GH and TH replacement therapy in these long-lived mice elicit different responses; GH shortens longevity, while TH has no impact on longevity. Our lab recently identified possible mechanisms by which early life GH replacement therapy shortens the longevity of Ames dwarf mice (Sun et al., unpublished observations). Here, we elucidate possible reasons why early life TH does not shorten longevity in Ames dwarf mice. First, it does not impact glucose tolerance or insulin sensitivity. Second, any impairment in energy metabolism is only transient. These findings are consistent with the observation that short-term (early life) TH replacement therapy does not shorten longevity, although lifelong TH replacement therapy does shorten longevity in Snell dwarf mice, which share the endocrine phenotype with Ames dwarf mice.5,7,18,19 We recognize that utilization of only male mice is a limit to our study; however, male and female Ames dwarf mice have similar responses to GH and T4 treatment21 and similar extension longevity.7 Therefore, we believe we would observe the same results in female mice as described in this manuscript. Further testing would need to be done to test this hypothesis.
Acknowledgements
The National Institute of Aging grant (AG0119899) supported this work. We would like to thank Dr. Rita Trammell for generously providing transmitters to measure body temperature, and for graciously aiding in the placement of the transmitters. We would also like to thank Dr. Carmel Fratianni for her insights into our work.
Author contributions
All authors participated in the experimental design, interpretation and analysis of the data, or writing and review of the manuscript; JD, YF, CH and SM conducted experiments; JD, YF, CH, LS and AB aided in experimental design; JD, YF, and AB aided in data interpretation; JD wrote the manuscript; AB and YF revised and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Buffenstein R, Woodley R, Thomadakis C, Daly TJ, Gray DA. Cold-induced changes in thyroid function in a poikilothermic mammal, the naked mole-rat. Am J Physiol Regul Integr Comp Physiol 2001; 280: R149–55. [DOI] [PubMed] [Google Scholar]
- 2.Hulbert AJ, Hinds DS, MacMillen RE. Minimal metabolism, summit metabolism and plasma thyroxine in rodents from different environments. Comp Biochem Physiol A Comp Physiol 1985; 81: 687–93. [DOI] [PubMed] [Google Scholar]
- 3.Kwiecinski GG, Damassa DA, Gustafson AW. Control of sex steroid-binding protein (SBP) in the male little brown bat: relationship of plasma thyroxine levels to the induction of plasma SBP in immature males. J Endocrinol 1986; 110: 271–8. [DOI] [PubMed] [Google Scholar]
- 4.Bozhkov AI, Nikitchenko YV. Thermogenesis and longevity in mammals. Thyroxine model of accelerated aging. Exp Gerontol 2014; 60: 173–82. [DOI] [PubMed] [Google Scholar]
- 5.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci 2004; 59: 1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 2001; 98: 6736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature 1996; 384: 33–33. [DOI] [PubMed] [Google Scholar]
- 8.Bowers J, Terrien J, Clerget-Froidevaux MS, Gothie JD, Rozing MP, Westendorp RG, van Heemst D, Demeneix BA. Thyroid hormone signaling and homeostasis during aging. Endocr Rev 2013; 34: 556–89. [DOI] [PubMed] [Google Scholar]
- 9.Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev 2001; 22: 451–76. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Koenig RJ. Gene regulation by thyroid hormone. Trends Endocrinol Metab 2000; 11: 207–11. [DOI] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359. [DOI] [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601. [DOI] [PubMed] [Google Scholar]
- 13.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol 2006; 46: 4–14. [DOI] [PubMed] [Google Scholar]
- 14.Dover GJ. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans Am Clin Climatol Assoc 2009; 120: 199–207. [PMC free article] [PubMed] [Google Scholar]
- 15.Plagemann A. Maternal diabetes and perinatal programming. Early Hum Dev 2011; 87: 743–7. [DOI] [PubMed] [Google Scholar]
- 16.Papacleovoulou G, Abu-Hayyeh S, Nikolopoulou E, Briz O, Owen BM, Nikolova V, Ovadia C, Huang X, Vaarasmaki M, Baumann M, Jansen E, Albrecht C, Jarvelin MR, Marin JJ, Knisely AS, Williamson C. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J Clin Invest 2013; 123: 3172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 2009; 64: 711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 1996; 384: 327–33. [DOI] [PubMed] [Google Scholar]
- 19.Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 2013; 93: 571–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, Bartke A. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci 2004; 59: 784–8. [DOI] [PubMed] [Google Scholar]
- 21.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 2010; 24: 5073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartke A. Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size. Results Probl Cell Differ 2000; 29: 181–202. [DOI] [PubMed] [Google Scholar]
- 23.Ruchala M, Stangierski A, Krauze T, Moczko J, Guzik P. Treatment of severe thyroid function disorders and changes in body composition. Endokrynol Pol Epub ahead of print 17 February 2016. doi: 10.5603/EP.a2016.0025. [DOI] [PubMed]
- 24.Tsourdi E, Rijntjes E, Kohrle J, Hofbauer LC, Rauner M. Hyperthyroidism and hypothyroidism in male mice and their effects on bone mass, bone turnover, and the Wnt inhibitors Sclerostin and Dickkopf-1. Endocrinology 2015; 156: 3517–2. [DOI] [PubMed] [Google Scholar]
- 25.Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk—a meta-analysis. Thyroid 2003; 13: 585–93. [DOI] [PubMed] [Google Scholar]
- 26.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 2005; 146: 3718–23. [DOI] [PubMed] [Google Scholar]
- 27.Bartke A, Coschigano K, Kopchick J, Chandrashekar V, Mattison J, Kinney B, Hauck S. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci 2001; 56: B340–9. [DOI] [PubMed] [Google Scholar]
- 28.Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci 2009; 64: 443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez M, Alvarez CV, Nogueiras R, Dieguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 2013; 19: 418–27. [DOI] [PubMed] [Google Scholar]
- 30.Kvetny J, Matzen LE. Thyroid hormone induced oxygen consumption and glucose-uptake in human mononuclear cells. Thyroidology 1989; 1: 5–9. [PubMed] [Google Scholar]
- 31.Hill CM, Fang Y, Miquet JG, Sun LY, Masternak MM, Bartke A. Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell. Epub ahead of print 17 March 2016. doi: 10.1111/acel.12467. [DOI] [PMC free article] [PubMed]
- 32.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 2000; 24(Suppl. 2): S109–12. [DOI] [PubMed] [Google Scholar]
- 33.Elliott KH, Welcker J, Gaston AJ, Hatch SA, Palace V, Hare JF, Speakman JR, Anderson WG. Thyroid hormones correlate with resting metabolic rate, not daily energy expenditure, in two charadriiform seabirds. Biology Open 2013; 2: 580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001; 226: 552–8. [DOI] [PubMed] [Google Scholar]
- 35.Karimifar M, Esmaili F, Salari A, Kachuei A, Faragzadegan Z, Karimifar M. Effects of Levothyroxine and thyroid stimulating hormone on bone loss in patients with primary hypothyroidism. J Res Pharm Pract 2014; 3: 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci 2011; 366: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]