Abstract
Urinary nephrin is a potential non-invasive biomarker of disease. To date, however, most studies of urinary nephrin have been conducted in animal models of diabetic nephropathy, and correlations between urinary nephrin-to-creatinine ratio and other parameters have yet to be evaluated in animal models or patients of kidney disease with podocyte dysfunction. We hypothesized that urinary nephrin-to-creatinine ratio can be up-regulated and is negatively correlated with renal nephrin mRNA levels in animal models of kidney disease, and that increased urinary nephrin-to-creatinine ratio levels are attenuated following administration of glucocorticoids. In the present study, renal nephrin mRNA, urinary nephrin-to-creatinine ratio, urinary protein-to-creatinine ratio, and creatinine clearance ratio were measured in animal models of adriamycin nephropathy, puromycin aminonucleoside nephropathy, anti-glomerular basement membrane glomerulonephritis, and 5/6 nephrectomy. The effects of prednisolone on urinary nephrin-to-creatinine ratio and other parameters in puromycin aminonucleoside (single injection) nephropathy rats were also investigated. In all models tested, urinary nephrin-to-creatinine ratio and urinary protein-to-creatinine ratio increased, while renal nephrin mRNA and creatinine clearance ratio decreased. Urinary nephrin-to-creatinine ratio exhibited a significant negative correlation with renal nephrin mRNA in almost all models, as well as a significant positive correlation with urinary protein-to-creatinine ratio and a significant negative correlation with creatinine clearance ratio. Urinary protein-to-creatinine ratio exhibited a significant negative correlation with renal nephrin mRNA. Following the administration of prednisolone to puromycin aminonucleoside (single injection) nephropathy rats, urinary nephrin-to-creatinine ratio was significantly suppressed and exhibited a significant positive correlation with urinary protein-to-creatinine ratio. In addition, the decrease in number of glomerular Wilms tumor antigen-1-positive cells was attenuated, and urinary nephrin-to-creatinine ratio exhibited a significant negative correlation in these cells. In conclusion, these results suggest that urinary nephrin-to-creatinine ratio level is a useful and reliable biomarker for predicting the amelioration of podocyte dysfunction by candidate drugs in various kidney disease models with podocyte dysfunction. This suggestion will also be validated in a clinical setting in future studies.
Keywords: Podocyte, renal, biomarkers, animal, models, disease
Introduction
Podocytes comprise a triple-layer structure with vascular endothelial cell and the glomerular basement membrane in renal glomerulus, forming a critical barrier for the filtration of large molecules such as proteins from blood into urine. Dysfunction of podocyte can, therefore, lead to proteinuria.1 Nephrin is a key structural molecule of the podocyte slit diaphragm that maintains podocyte function via cross talk with the actin cytoskeleton.2–5 In addition, nephrin is also a hub molecule that binds several important molecules in podocytes, such as podocin, CD2AP, β-arrestin2, and Fyn, and transmits variable signals to podocytes via mechanisms such as phosphorylation.6–10 Further, podocyte dysfunction might be correlated with the number of urinary podocytes, and the alteration of nephrin expression in renal tissue might be related to renal dysfunction, particularly decreases in estimated glomerular filtration ratio (eGFR).11 For example, as with podocyte presence, the presence of nephrin in urine may indicate damaged podocytes, as nephrin is intimately involved in podocyte dysfunction and is expressed on podocytes.12 Given the above, developing drugs capable of ameliorating or preventing podocyte dysfunction is extremely important. However, before administering such drugs to human patients, their efficacy must be tested in vivo in various animal models of kidney disease. These tests evaluate not only general parameters such as proteinuria and renal function but also non-invasive biomarkers that reflect the degree of podocyte dysfunction. Therefore, urinary nephrin may be suitable as a biomarker candidate not only in animal models but in human patients as well.
A decrease in renal nephrin levels and a subsequent increase in urinary levels have been hypothesized to occur when damaged renal podocytes leak into urine.13 As such, demonstrating a negative correlation between urinary nephrin-to-creatinine ratio (uNCR) and renal nephrin mRNA (rNRNA) and analyzing correlations with urinary protein-to-creatinine ratio (uPCR) or creatinine clearance ratio (Ccr) may be useful in clarifying the importance of uNCR as a biomarker. To our knowledge, however, animal studies of uNCR have mainly focused on diabetic nephropathy, while those in humans have focused on various kidney diseases,10,14–19 with no reports published regarding correlations between uNCR and rNRNA. Comprehensively testing correlations between parameters using various kidney disease models with podocyte dysfunction may, therefore, be useful as biomarkers for human patients in addition to animal models. In the present study, to demonstrate the importance of uNCR as a biomarker, we investigated the following animal models with podocyte dysfunction: adriamycin (ADR) nephropathy, puromycin aminonucleoside (PAN) nephropathy, anti-glomerular basement membrane glomerulonephritis (anti-GBM GN), and 5/6 nephrectomized (5/6 Nx) animals.20–29
Glucocorticoids are widely used to treat patients with glomerulonephritis or nephrotic syndrome. In pre-clinical studies, a single dose of glucocorticoids was reported to attenuate proteinuria in PAN nephropathy rats,30,31 but whether or not they suppressed uNCR in both humans and animals, including the PAN nephropathy rat model, has not been examined. However, the protections of podocytes by glucocorticoids in vitro and in vivo were recently reported.32,33
We demonstrate here the application of a useful and reliable biomarker for predicting the amelioration of podocyte dysfunction by candidate drugs to test our hypothesis that uNCR can be up-regulated and exhibits a negative correlation with rNRNA in various animal models of kidney disease with podocyte dysfunction, and that increases in uNCR might be attenuated by glucocorticoids.
Materials and methods
Ethics statement
Animals were housed under controlled temperature, humidity, and light (12-h light-dark cycle) and provided a standard commercial diet and water ad libitum. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Animal Ethical Committee of Astellas Pharma Inc.
ADR nephropathy models in mice and rats
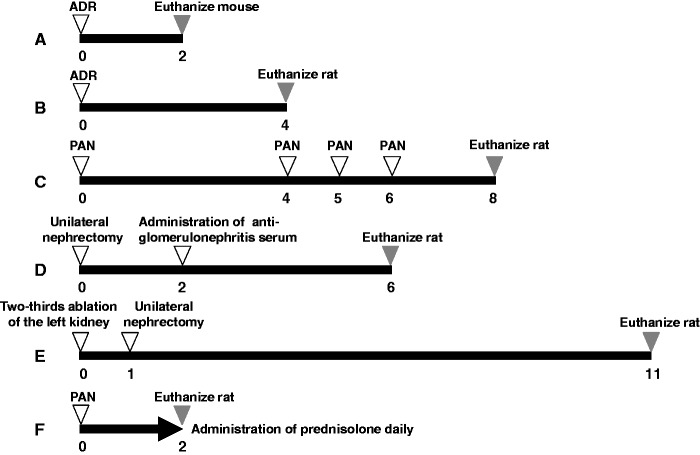
Male Balb/c mice were purchased from Charles River Japan (Yokohama, Japan). ADR nephropathy was induced at eight weeks of age (n = 13) by single injection of ADR (Sigma, St. Louis, MO, USA) at 11 to 12 mg/kg body weight in saline (Kyowa Hakko Kirin, Tokyo, Japan). Other mice (n = 4) received an identical volume of saline and were used as normal controls. Two weeks after ADR injection, mice were euthanized by isoflurane inhalation and dissected (Figure 1(a)).
Figure 1.
Protocol for animal models of renal injury. A: Adriamycin (ADR) nephropathy in mice, B: ADR nephropathy in rats, C: puromycin aminonucleoside (PAN) nephropathy in rats, D: Anti-glomerular basement membrane glomerulonephritis (anti-GBM GN) in rats, E: 5/6 nephrectomy (5/6 Nx) in rats, F: PAN (single injection) nephropathy rats administered prednisolone. Numbers indicate weeks
Male Wistar rats were purchased from Charles River Japan. ADR nephropathy was induced at eight weeks of age (n = 10) by single injection of ADR at 5 mg/kg body weight in saline. Control rats (n = 5) received an identical volume of saline. Four weeks after ADR injection, rats were euthanized by isoflurane inhalation and dissected (Figure 1(b)).
PAN nephropathy model in rats
Male five-week-old Sprague-Dawley rats were purchased from Japan SLC (Hamamatsu, Japan). PAN nephropathy (n = 6) was induced in rats by an intravenous injection of PAN (Sigma) at 90 mg/kg body weight in saline (Ohtsuka, Tokyo, Japan) and at 30 mg/kg at four, five, and six weeks later. The control group (n = 6) received an identical volume of saline. Eight weeks after the first injection of PAN, rats were euthanized by isoflurane inhalation and dissected (Figure 1(c)). For time course analysis, PAN nephropathy (n = 8) was induced in rats via intravenous injection of PAN (Sigma) at 100 mg/kg body weight in saline and at 33 mg/kg at four, five, and six weeks later. The control group (n = 8) received an identical volume of saline. uNCR and uPCR were measured every week after the first injection of PAN, for 10 weeks (Figure 4(a) and (b)).
Figure 4.
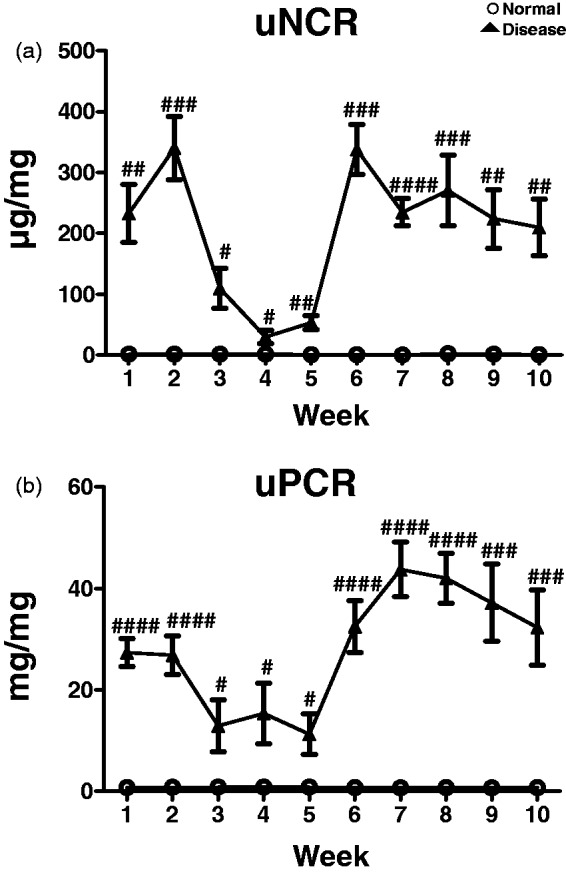
Time courses of uNCR (a) and uPCR (b) in PAN nephropathy rats. Values are mean ± S.E.M per group. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 (unpaired t test)
Anti-glomerular basement membrane glomerulonephritis model in rats
Male six-week-old WKY rats were purchased from Charles River Japan. Rats were prepared under isoflurane anesthesia by the ablation of half of the left kidney followed two weeks later by intravenous administration of rabbit anti-glomerular basement membrane serum (IBL, Fujioka, Japan) at a body weight of approximately 200 g (n = 8). Age-matched rats were used as a normal group (n = 4). Six weeks after unilateral semi-nephrectomy, rats were euthanized by isoflurane inhalation and dissected (Figure 1(d)).
Five-sixth nephrectomized model in rats
Experiments were performed as previously described.34 Briefly, male Wistar rats were 5/6 nephrectomized (n = 12). Age-matched rats were used as a normal group (n = 6). Under isoflurane anesthesia, 5/6 Nx rats were prepared via ablation of two-thirds of the left kidney, followed one week later by a right unilateral nephrectomy. Based on findings from previous investigations, we determined this surgical method to be superior to the traditional method (5/6 nephrectomy was completed in a single day) in improving individual variations in parameters, including urinary protein excretion and mortality rate, without inducing significant differences in disease state, such as urine volume or plasma creatinine level (Figure 1(e)).
Administration of prednisolone in PAN (single injection) nephropathy model in rats
Male five-week-old Sprague-Dawley rats were purchased from Japan SLC. Groups consisted of normal rats (n = 6) and PAN nephropathy rats administered vehicle (n = 6) or prednisolone (Nacalai Tesque, Kyoto, Japan) at 0.1 mg/kg (n = 6), 0.3 mg/kg (n = 6), or 1 mg/kg (n = 6). Prednisolone was administered once daily for two weeks immediately before PAN injection at 90 mg/kg body weight in saline. After the final drug administration at Week 2, rats were euthanized by isoflurane inhalation and dissected (Figure 1(f)).
Sample processing
Urine samples collected over 24 h in metabolic cages were centrifuged at 3000–6000 × g, and the supernatant was used to measure nephrin, urinary protein, creatinine, and other parameters. Blood samples were collected from the abdominal vena cava under isoflurane anesthesia using a 19- to 23-gauge needle for rats or 23- to 25-gauge needle for mice and centrifuged at approximately 15,000 × g to measure levels of plasma creatinine and other compounds in the supernatant. Heparin was used as the anti-coagulation reagent. Kidneys were weighed after extraction from the body; the upper half of the renal tissue was immersed in 10% neutral-buffered formalin for histological evaluation, and the remaining tissue was frozen in liquid nitrogen and stored at −80℃ until processing for mRNA quantification.
Laboratory measurements
Nephrin levels in urine samples were measured using an ELISA kit from Exocell Inc. (Philadelphia, PA, USA) in accordance with the manufacturer’s instructions. The anti-nephrin antibody included with this kit cross-reacted with both mouse and rat nephrin. The quantity of urinary protein in each 24-h urine sample was measured using pyrogallol red (Wako, Osaka, Japan) and an Automatic Analyzer 7250 (Hitachi, Tokyo, Japan). Urinary or plasma creatinine level was measured using Detaminar L-CRE (Kyowa Medics, Tokyo, Japan) and an Automatic Analyzer 7250. Urinary nephrin and protein levels were normalized by correction for urinary creatinine level. Ccr (mL/min) was calculated as follows: ((urinary creatinine (mg/dL)/plasma creatinine (mg/dL)) × (urinary volume (mL)/24/60 (min)).
Quantification of rNRNA
Total renal RNA was extracted using an RNeasy Mini Kit (QIAGEN, Hilden, Germany) in accordance with the manufacturer’s instructions. Complementary DNA was synthesized using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA). Real-time PCR was used to quantify nephrin gene expression. The following primers were used: 5′-CCCAGGTACACAGAGCACAA-3′ and 5′-CTCACGCTCACAACCTTCAG-3′ for mouse nephrin, and 5′-CCGTTTTTGGTCCAAGTGAAG-3′ and 5′-CCGTTTTTGGTCCAAGTGAAG-3′ for rat nephrin. Reactions were performed using SYBR Green with an ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Data were normalized to endogenous hypoxanthine-guanine phosphoribosyltransferase (HPRT) or β-actin mRNA control.
Electron microscopy
Samples were cut into 1- to 2-mm blocks and fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) (4℃, pH 7.4, 0.1 M) for 24 h. Blocks were rinsed in the same PBS and then fixed in 2% osmium acid and embedded in Epon. Ultra-thin sections were cut and observed with a TEM (H-7600; Hitachi, Tokyo, Japan).
Histological study
Renal tissues fixed in 10% neutral-buffered formalin were embedded in paraffin, and 2 -µm-thick sections were cut for morphological study. These sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). All glomeruli and the entire microscopic area of each specimen were examined.
Wilms tumor antigen-1-positive cells in glomerulus
Mouse anti-Wilms tumor antigen-1 (WT1) antibody (Nichirei Biosciences, Tokyo, Japan) and Envision system-HRP labeled polymer (Dako Denmark, Glostrup, Denmark) were used for immunohistochemistry. The number of WT1-positive cells per glomerulus was counted for 50 glomeruli, and then the mean value was calculated.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Unpaired t test was used to analyze differences between two groups. Dunnett’s Multiple Comparison Test was used to compare multiple groups. All statistical and data analyses were conducted using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA), with p < 0.05 considered significant.
Results
ADR and PAN nephropathy models in mice and rats
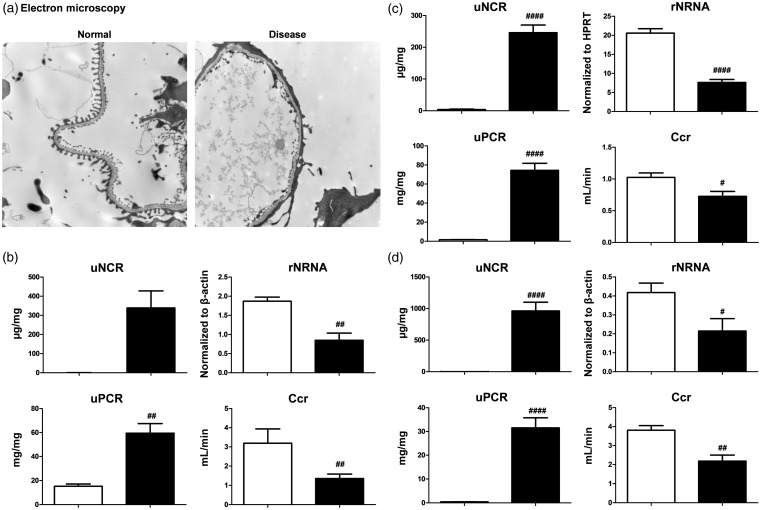
ADR and PAN nephropathy models exhibit histological features such as focal segmental glomerular sclerosis (FSGS) and podocyte dysfunction.20–24 uNCR might, therefore, reflect podocyte dysfunction. In fact, foot process effacement, which causes podocyte dysfunction, was confirmed in ADR nephropathy mice using electron microscopy (Figure 2(a)). In this model, uNCR tended to be higher and uPCR was significantly higher than in control mice, but rNRNA and Ccr were significantly lower than in control mice (Figure 2(b)). In ADR and PAN nephropathy rats, uNCR and uPCR were significantly higher than in control mice, but rNRNA and Ccr were significantly lower than in control rats (Figure 2(c) and (d)). Daily urinary nephrin excretion values showed similar trends to uNCR in all models (Supplementary Figures A to C).
Figure 2.
ADR and PAN nephropathy models. (a) Foot process effacement, which causes podocyte injury, in ADR nephropathy mice was confirmed using electron microscopy. Magnification × 5000. (b–d) Urinary nephrin-to-creatinine ratio (uNCR), renal nephrin mRNA (rNRNA), urinary protein (uPCR), and creatinine clearance ratio (Ccr) were compared between normal (empty bar) and disease (black bar) groups of ADR nephropathy mice (b), ADR nephropathy rats (c), and PAN nephropathy rats (d). Values are mean ± S.E.M per group. #p < 0.05, ##p < 0.01, ####p < 0.0001 (unpaired t test)
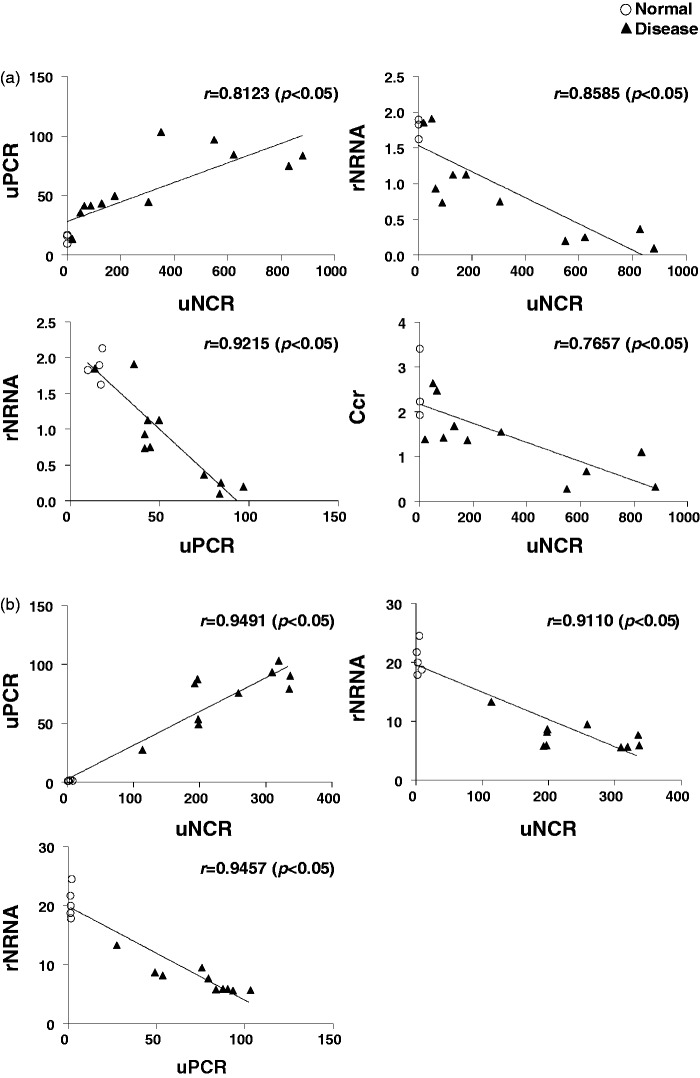
In ADR nephropathy mice and PAN nephropathy rats, uNCR exhibited a significant positive correlation with uPCR, and rNRNA exhibited a significant negative correlation with uNCR and uPCR. Further, uNCR exhibited a significant negative correlation with Ccr (Figure 3(a) and (c)). In ADR nephropathy rats, uNCR exhibited a significant positive correlation with uPCR, and rNRNA exhibited a significant negative correlation with uNCR and uPCR, which was similar to findings in ADR nephropathy mice and PAN nephropathy rats (Figure 3(b)). In contrast, the correlation between uNCR and Ccr was not analyzed, as the value of plasma creatinine barely increased (data not shown).
Figure 3.
Correlations between uNCR and uPCR, or uNCR and rNRNA, or uPCR and rNRNA, or uNCR and Ccr in ADR nephropathy mice (a), ADR nephropathy rats (b), and PAN nephropathy rats (c)
Figure 4(a) and (b) shows the time course for uNCR and uPCR in PAN nephropathy rats. Notable, the time course of uNCR was almost identical to that of uPCR. We hope to validate the correlation of the time course between these two parameters in a population of kidney disease patients in the future.
Anti-GBM GN model and 5/6 Nx model in rats
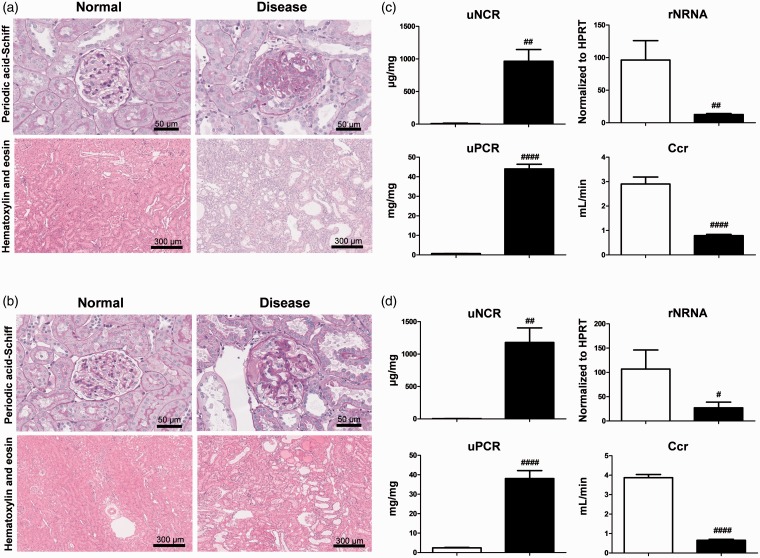
Anti-GBM GN is a glomerulonephritis model with podocyte dysfunction.25–27 The model used in the present study, however, was not only administered rabbit anti-glomerulonephritis serum, but the left kidney was also ablated. In this novel model of glomerulonephritis, the rest of the kidney rapidly develops irreversible glomerulosclerosis and dysfunction. The 5/6 Nx model is a CKD model that exhibits podocyte dysfunction.28,29 Both of these models exhibit glomerulosclerosis, tubular injuries, and interstitial fibrosis (Figure 5(a) and (b)). In both models, uNCR and uPCR levels were significantly higher than in the control group. In contrast, rNRNA and Ccr levels in these models were significantly lower than in the control group (Figure 5(c) and (d)). Daily urinary nephrin excretion values showed similar trends to uNCR in all models (Supplementary Figures D and E).
Figure 5.
Anti-GBM GN and 5/6 Nx models. (a, b) Representative histology for hematoxylin and eosin-stained, or periodic acid-Schiff-stained sections exhibiting glomerulosclerosis and tubular interstitial injuries in anti-GBM GN (a) and 5/6Nx (b) rats. Scale bar is shown in each photo. (c, d) uNCR, rNRNA, uPCR, and Ccr were compared between normal (empty bar) and disease (black bar) groups for anti-GBM GN rat (c) and 5/6 Nx rat (d). Values are mean ± S.E.M per group. #p < 0.05, ##p < 0.01, ####p < 0.0001 (unpaired t test). (A color version of this figure is available in the online journal.)
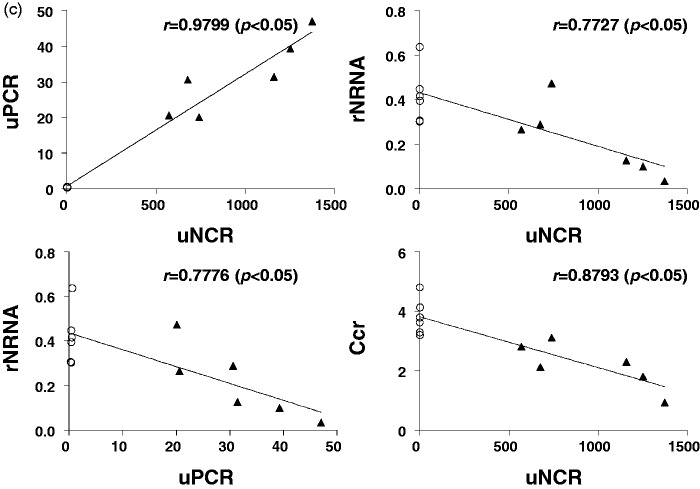
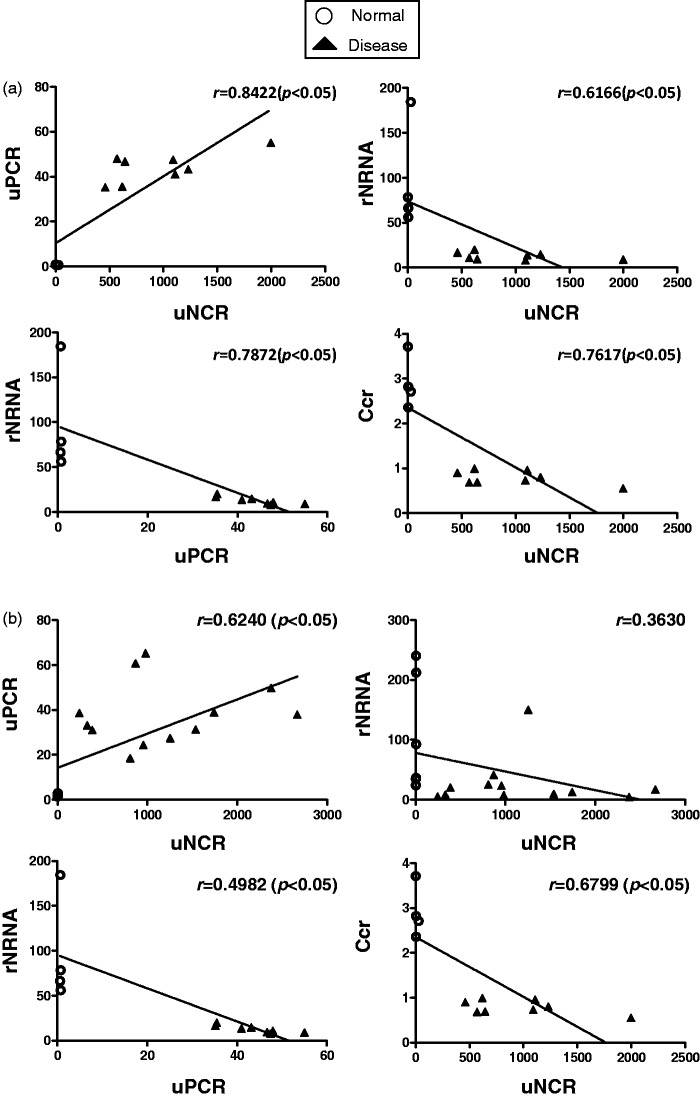
In anti-GBM GN rats, uNCR exhibited a significant positive correlation with uPCR, and rNRNA exhibited a significant negative correlation with both uNCR and uPCR. Further, uNCR exhibited a significant negative correlation with Ccr (Figure 6(a)). In 5/6 Nx rats, uNCR exhibited a significant positive correlation with uPCR. rNRNA tended to exhibit a non-significant negative correlation with uNCR, which might be due to cells of renin lineage supplying podocytes to the glomeruli in this model.35 rNRNA exhibited a significant negative correlation with uPCR. Further, uNCR exhibited a significant negative correlation with Ccr (Figure 6(b)).
Figure 6.
Correlations between uNCR and uPCR, or uNCR and rNRNA, or uPCR and rNRNA, or uNCR and Ccr for anti-GBM GN (a) and 5/6 Nx (b) rats
Administration of prednisolone to PAN nephropathy rats
PAN nephropathy rats receiving a single dose of PAN is a model of minimal change disease (MCD).23,36,37 Although glucocorticoids attenuate disorders such as proteinuria in this rat model,30,31 whether or not these compounds improve uNCR is unknown. However, the protection of podocytes by glucocorticoids has been recently reported in vitro and in vivo.32,33 The effect of glucocorticoids on parameters such as uNCR following prednisolone administration was, therefore, tested in this rat model. Following prednisolone administration, uNCR and uPCR both exhibited significant improvement (Figure 7(a) and (b)). uNCR, therefore, exhibited a significant positive correlation with uPCR (Figure 7(c)).
Figure 7.
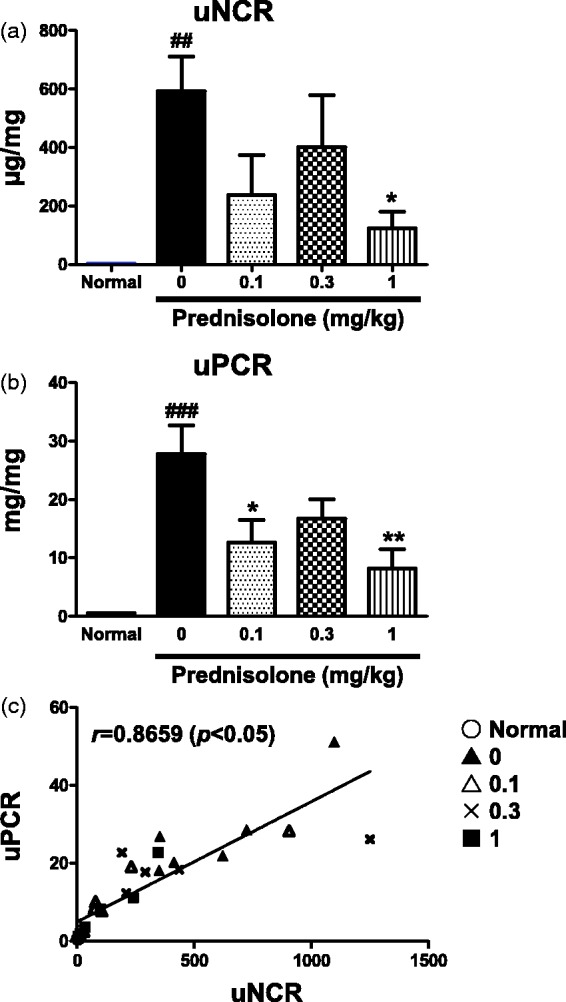
Administration of prednisolone to PAN (single injection) nephropathy rats. uNCRs (a) and uPCR (b) are shown per group. Correlation between uNCR and uPCR (c). Values are mean ± S.E.M. per group. ###p < 0.001, ####p < 0.0001 versus normal group (Unpaired t test), *p < 0.05, **p < 0.01 versus vehicle group shown with 0 mg/kg prednisolone (Dunnett’s Multiple Comparison Test)
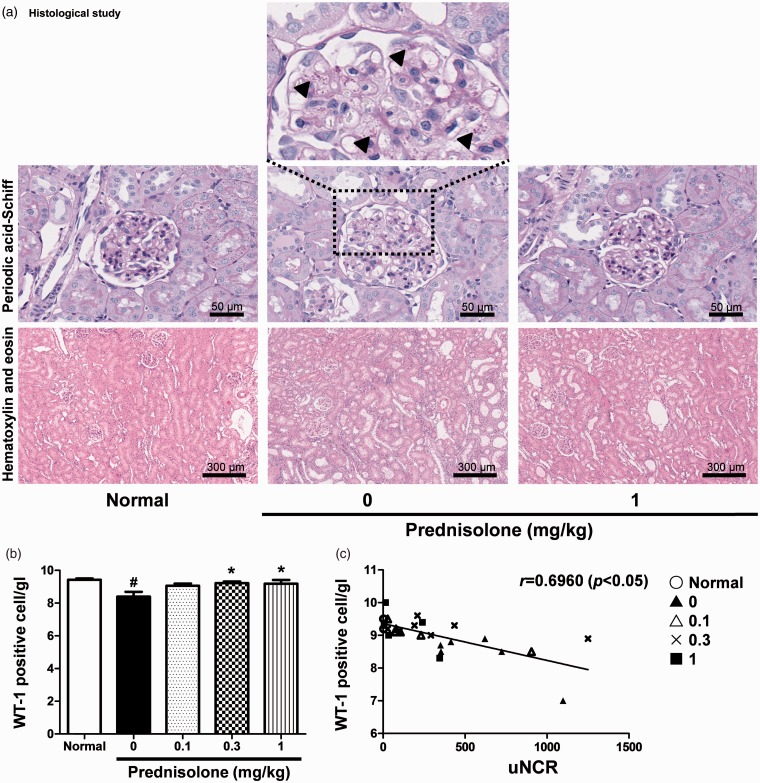
Although glomerulosclerosis was not observed in this model, a proportion of rats exhibited hyaline droplet deposition in podocytes or proliferation of parietal epithelial cells (Figure 8(a)). Tubular interstitial and these podocyte injuries were improved following prednisolone administration (Figure 8(a)). The WT1 molecule in the glomerulus is generally considered a useful marker for podocytes due to its dominant expression in podocytes.38,39 We, therefore, hypothesized that the number of WT1-positive cells per glomerulus (WT1[+] cells/gl) was negatively correlated with uNCR. WT1(+) cells/gl were improved following prednisolone administration (Figure 8(b)). uNCR exhibited a significant negative correlation with WT1(+) cells/gl (Figure 8(c)).
Figure 8.
Representative images of glomeruli and tubular interstitial region showing the effect by administration of prednisolone. (arrowheads show the depositions of hyaline droplet in podocyte) (a). The number of WT1-positive cells per glomerulus (b). Correlation between uNCR and the number of WT1-positive cell per glomerulus (c). Values are mean ± S.E.M. per group. #p < 0.05 versus normal group (unpaired t test), *p < 0.05 versus vehicle group (Dunnett’s Multiple Comparison Test). (A color version of this figure is available in the online journal.)
Discussion
In this study, we comprehensively analyzed the correlations between rNRNA, uNCR, uPCR, and Ccr using FSGS-like, glomerulonephritis, and CKD models with podocyte dysfunction. We successfully demonstrated the utility of uNCR as a biomarker in the examined animal models. Results validated our hypothesis that uNCR can be up-regulated and is negatively correlated with rNRNA in these kidney disease models and that increased uNCR are attenuated following prednisolone administration.
uNCR levels increased in all kidney disease models tested (Figures 2 and 5). Previous reports suggested that podocyte dysfunction occurred in these models.20–29 uNCR also increased in patients suffering from nephrotic syndrome, glomerulonephritis, diabetic nephropathy, or preeclampsia, which are human kidney disease equivalents to kidney disease in the animal models tested in this study.11,14–18,40 To date, nephrin mRNA in urine samples from animal models of kidney disease or patients has also been analyzed.41,42 As a general rule, urinary mRNA is easily degraded by ribonucleases in urine.43 The majority of urinary nephrin mRNA might, therefore, exist in podocytes that detach from glomeruli and enter the urine. In the present study, however, uNCR was observed in the supernatant of urine samples following separation out of degraded podocytes that had undergone detachment or apoptosis. Of note, we were able to detect increases in uNCR in various kidney disease models with podocyte dysfunction, in addition to a diabetic nephropathy model in which uNCR increases have been reported.40,44,45
In the present study, uNCR was negatively correlated with rNRNA (Figures 3 and 6). In addition to nephrin, podocin, podocalyxin, WT1, and synaptopodin are established podocyte-specific molecules in the glomerulus, and their presence has been reported in urine samples from kidney disease models, such as diabetic nephropathy, and from patients with various diseases in which podocyte dysfunction has been observed.46,47 We selected nephrin as a sensitive biomarker of podocyte injury due to its role as a main structural molecule of the podocyte slit diaphragm and close relation to podocyte function. In contrast, rNRNA was selected as an indicator of nephrin expression in glomerulus, as rNRNA expression accompanies earlier and more sensitive alterations than renal nephrin protein (rNP) expression.48 Regarding widely used techniques to detect rNP, immunofluorescence and Western blotting are laborious and time-consuming, and ELISA kits do not offer easy detection. The measurement of rNRNA via quantitative PCR is, therefore, also considered superior to that of rNP in terms of quantitation. uNCR might exhibit a significant negative correlation with rNRNA, as both parameters were selected from a number of potential candidates. The alteration of rNRNA expression has also been reportedly correlated with the degree of podocyte dysfunction.49 uNCR might, therefore, represent a non-invasive biomarker that reflects the extent of podocyte dysfunction. To our knowledge, however, this is the first comprehensive analysis of rNRNA, uNCR, uPCR, and Ccr using various kidney disease patients with podocyte dysfunction. We speculate that uNCR utility as a biomarker in human patients will be validated in future studies.
uNCR was significantly suppressed and exhibited a significant positive correlation with uPCR following prednisolone administration to PAN (single injection) nephropathy rats (Figure 7(a) and (c)). uNCR might, therefore, serve as a useful biomarker for the prediction of a good response to prednisolone. A single-injection PAN nephropathy model, which is used to test the effect of prednisolone, can reflect human MCD. However, histopathological change in the glomerulus and tubular interstitium was observed, and the number of WT1(+) cells/gl was considered to reflect the decreased number of podocytes in the glomerulus (Figure 8(a) and (b)). This model was, therefore, considered to be in a more severe state than classical human MCD. uNCR in this model was significantly suppressed following prednisolone administration, suggesting that this drug might be effective in patients with kidney disease of equivalent severity to this model. The mechanism by which prednisolone improves podocyte dysfunction might involve stabilization of the actin cytoskeleton and inhibition of apoptosis in podocytes; specifically, prednisolone inhibits expression of Notch1 and p53 proteins following attenuation of the decrease in miR-30 family proteins which occurs when podocytes are injured.32
WT1(+) cells/gl, consisting mainly of podocytes in glomerulus, is considered to negatively correlate with the number of urinary podocytes.13 Detection of urinary podocytes without contaminants is possible when rodent urine in the bladder is used.37 However, measurement of these cells is considered difficult due to the presence of contaminants such as excrement and food in the pooled urine of rodents. For these reasons, we investigated whether or not uNCR retained in the supernatant after removal of contaminants and cells by centrifugation, instead of the number of urinary podocytes, correlates to WT1(+) cells/gl. In the present study, uNCR exhibited a significant negative correlation with WT1(+) cells/gl (Figure 8(c)) and is, therefore, considered to reflect the quantity of nephrin protein that might be separated from degraded podocytes suffering from mainly detachment and apoptosis. The progression of kidney disease, including decreased eGFR, might be due to the decreased number of glomerular podocytes.50 In these experiments, uNCR exhibited a significant negative correlation with Ccr (Figures 3 and 6). Podocyte dysfunction might, therefore, contribute to reduced renal function.
In the present study, uNCR was up-regulated, and rNRNA, uNCR, uPCR, and Ccr exhibited a significant correlation with podocyte dysfunction in our comprehensive analyses using FSGS-like, glomerulonephritis, and CKD models. Of these correlations, uNCR mostly exhibited a significant negative correlation with rNRNA. uNCR was significantly attenuated following prednisolone administration. This strongly suggests that the alteration of uNCR in these kidney disease models might be a biomarker for the prediction of improved podocyte dysfunction following the administration of drug candidates. It is, however, a study limitation that we cannot exclude tubular reuptake or metabolism of nephrin. This needs to be addressed in future studies before using nephrin as a reliable biomarker of podocyte loss.
Supplementary Material
Acknowledgments
The authors thank Drs. Akihiko Iwai, Minori Saitoh, and Wataru Uchida (Astellas Pharma Inc.) for their valuable comments and continuing encouragement.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
YW and MA designed the project and edited the paper. MA, HM, HM, MK, KT, and ME designed the studies, performed the data collection, and carried out the initial analyses. HK, TM, YT, YI, and AI reviewed the whole manuscript. YW and MA contributed equally to this work.
References
- 1.Welsh GI, Saleem MA. The podocyte cytoskeleton – key to a functioning glomerulus in health and disease. Nat Rev Nephrol 2012; 8: 14–21. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Liang W, Yang Y, Pan Y, Yang Q, Chen X, Singhal PC, Ding G. IQGAP1 regulates actin cytoskeleton organization in podocytes through interaction with nephrin. Cell Signal 2015; 27: 867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A. Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem 2011; 286: 39933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon R, Singh A, Welsh GI, Coward RJ, Satchell S, Ni L, Mathieson PW, Bakker WW, Saleem MA. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol 2008; 19: 2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlondorff J. Nephrin AKTs on actin. The slit diaphragm-actin cytoskeleton signaling network expands. Kidney Int 2008; 73: 524–6. [DOI] [PubMed] [Google Scholar]
- 6.Denhez B, Lizotte F, Guimond MO, Jones N, Takano T, Geraldes P. Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. J Biol Chem 2015; 290: 350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zha D, Chen C, Liang W, Chen X, Ma T, Yang H, Goor H, Ding G. Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-alpha-parvin complex. BMB Rep 2013; 46: 230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azhibekov TA, Wu Z, Padiyar A, Bruggeman LA, Simske JS. TM4SF10 and ADAP interaction in podocytes: role in Fyn activity and nephrin phosphorylation. Am J Physiol Cell Physiol 2011; 301: C1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon J, Leibiger I, Moede T, Walter B, Faul C, Maiguel D, Villarreal R, Guzman J, Berggren PO, Mundel P, Ricordi C, Merscher-Gomez S, Fornoni A. Dynamin-mediated nephrin phosphorylation regulates glucose-stimulated insulin release in pancreatic beta cells. J Biol Chem 2012; 287: 28932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi T, Uchida K, Uchida S, Sasaki S, Nitta K. Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin Exp Nephrol 2011; 15: 688–93. [DOI] [PubMed] [Google Scholar]
- 11.Ng DP, Tai BC, Tan E, Leong H, Nurbaya S, Lim XL, Chia KS, Wong CS, Lim WY, Holthofer H. Nephrinuria associates with multiple renal traits in type 2 diabetes. Nephrol Dial Transplant 2011; 26: 2508–14. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int 2005; 67: 2239–53. [DOI] [PubMed] [Google Scholar]
- 13.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 2005; 16: 1733–41. [DOI] [PubMed] [Google Scholar]
- 14.Kandasamy Y, Smith R, Lumbers ER, Rudd D. Nephrin – a biomarker of early glomerular injury. Biomark Res 2014; 2: 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu B, Li XF, Zhu XL, Lin Y, Zhong S, Zhu CF, Tang XL, Hu YQ, Cheng XX, Wang YJ. ELISA analysis of urinary nephrin and podocalyxin standardized by aquaporin-2 in adult patients with nephrotic syndrome. J Nephrol 2014; 27: 411–7. [DOI] [PubMed] [Google Scholar]
- 16.Yang GY, Lee KA, Park MH, Park HS, Ha EH, Chun SH, Kim YJ. Urinary nephrin: a new predictive marker for pregnancies with preeclampsia and small-for-gestational age infants. Obstet Gynecol Sci 2013; 56: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son GH, Kim JH, Hwang JH, Kim YH, Park YW, Kwon JY. Urinary excretion of nephrin in patients with severe preeclampsia. Urinary nephrin in preeclampsia. Hypertens Pregnancy 2011; 30: 408–13. [DOI] [PubMed] [Google Scholar]
- 18.Jim B, Ghanta M, Qipo A, Fan Y, Chuang PY, Cohen HW, Abadi M, Thomas DB, He JC. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One 2012; 7: e36041–e36041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Li M, Liu Q, Chen B, Ji Z. Detection of urinary podocytes and nephrin as markers for children with glomerular diseases. Exp Biol Med (Maywood) 2015; 240: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulkko J, Patrakka J, Lal M, Tryggvason K, Hultenby K, Wernerson A. Neph1 is reduced in primary focal segmental glomerulosclerosis, minimal change nephrotic syndrome, and corresponding experimental animal models of adriamycin-induced nephropathy and puromycin aminonucleoside nephrosis. Nephron Extra 2014; 4: 146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan YG, Che XY, Sun W, Huang YR, Meng XJ, Chen HL, Shi XM, Tu Y, Wu W, Liu YL. Low-dose of multi-glycoside of Tripterygium wilfordii Hook. f., a natural regulator of TGF-beta1/Smad signaling activity improves adriamycin-induced glomerulosclerosis in vivo. J Ethnopharmacol 2014; 151: 1079–89. [DOI] [PubMed] [Google Scholar]
- 22.Zickri MB, Fattah MM, Metwally HG. Tissue regeneration and stem cell distribution in adriamycin induced glomerulopathy. Int J Stem Cells 2012; 5: 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara M, Yamagata K, Capaldi RA, Koyama A. Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney Int 2006; 69: 1146–52. [DOI] [PubMed] [Google Scholar]
- 24.Shiiki H, Sasaki Y, Nishino T, Kimura T, Kurioka H, Fujimoto S, Dohi K. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology 1998; 66: 221–9. [DOI] [PubMed] [Google Scholar]
- 25.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 2010; 298: F702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidaka T, Suzuki Y, Yamashita M, Shibata T, Tanaka Y, Horikoshi S, Tomino Y. Amelioration of crescentic glomerulonephritis by RhoA kinase inhibitor, Fasudil, through podocyte protection and prevention of leukocyte migration. Am J Pathol 2008; 172: 603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 2011; 17: 1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stitt-Cavanagh EM, Faour WH, Takami K, Carter A, Vanderhyden B, Guan Y, Schneider A, Breyer MD, Kennedy CR. A maladaptive role for EP4 receptors in podocytes. J Am Soc Nephrol 2010; 21: 1678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Shi W, Ma J, Sloan A, Faul C, Wei C, Reiser J, Yang Y, Liu S, Wang W. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J Mol Med (Berl) 2012; 90: 1407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura T, Ebihara I, Fukui M, Tomino Y, Koide H. Effects of methylprednisolone on glomerular and medullary mRNA levels for extracellular matrices in puromycin aminonucleoside nephrosis. Kidney Int 1991; 40: 874–81. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Ebihara I, Fukui M, Takahashi T, Tomino Y, Koide H. Altered glomerular steady-state levels of tumour necrosis factor-alpha mRNA during nephrotic and sclerotic phases of puromycin aminonucleoside nephrosis in rats. Clin Sci (Lond) 1993; 84: 349–56. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol 2014; 25: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 2013; 304: F1375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo M, Tahara A, Hayashi K, Abe M, Inami H, Ishikawa T, Ito H, Tomura Y. Renoprotective effects of novel interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 through anti-inflammatory action in 5/6 nephrectomized rats. Naunyn Schmiedebergs Arch Pharmacol 2014; 387: 909–19. [DOI] [PubMed] [Google Scholar]
- 35.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 2015; 309: F341–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda A, Fujimoto S, Iwatsubo S, Kawachi H, Kitamura K. Effects of mineralocorticoid and angiotensin II receptor blockers on proteinuria and glomerular podocyte protein expression in a model of minimal change nephrotic syndrome. Nephrology (Carlton) 2010; 15: 321–6. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Shah SV, Baliga R. Cytochrome P-450 as a source of catalytic iron in minimal change nephrotic syndrome in rats. Am J Physiol Renal Physiol 2001; 280: F88–94. [DOI] [PubMed] [Google Scholar]
- 38.Tao J, Polumbo C, Reidy K, Sweetwyne M, Susztak K. A multicolor podocyte reporter highlights heterogeneous podocyte changes in focal segmental glomerulosclerosis. Kidney Int 2014; 85: 972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, Tiwari S. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One 2013; 8: e60177–e60177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li LL, Chen ZQ, Wang YH, Zhang JH, Yin ZW, Li LL, Zhang XY, Wang FL. Relationship between urinary nephrin and urinary albumin changes in diabetic rats and effects of yiqiyangyinhuayutongluo recipe. J Tradit Chin Med 2012; 32: 278–82. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda A, Sato Y, Iwakiri T, Komatsu H, Kikuchi M, Kitamura K, Wiggins RC, Fujimoto S. Urine podocyte mRNAs mark disease activity in IgA nephropathy. Nephrol Dial Transplant 2015; 30: 1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant 2012; 27: 4079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naskalski JW, Kapusta M. Ribonuclease in human serum and urine expressed in arbitrary standard units of activity. Folia Med Cracov 1994; 35: 31–8. [PubMed] [Google Scholar]
- 44.Alter ML, Kretschmer A, Von Websky K, Tsuprykov O, Reichetzeder C, Simon A, Stasch JP, Hocher B. Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin Lab 2012; 58: 659–71. [PubMed] [Google Scholar]
- 45.Chang JH, Paik SY, Mao L, Eisner W, Flannery PJ, Wang L, Tang Y, Mattocks N, Hadjadj S, Goujon JM, Ruiz P, Gurley SB, Spurney RF. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS One 2012; 7: e33942–e33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Kihara I. Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol 2010; 41: 1265–75. [DOI] [PubMed] [Google Scholar]
- 47.Zheng M, Lv LL, Ni J, Ni HF, Li Q, Ma KL, Liu BC. Urinary podocyte-associated mRNA profile in various stages of diabetic nephropathy. PLoS One 2011; 6: e20431–e20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aaltonen P, Luimula P, Astrom E, Palmen T, Gronholm T, Palojoki E, Jaakkola I, Ahola H, Tikkanen I, Holthofer H. Changes in the expression of nephrin gene and protein in experimental diabetic nephropathy. Lab Invest 2001; 81: 1185–90. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X, Kennedy C, Breyer MD, Moeckel GW, Harris RC. Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 2007; 18: 551–9. [DOI] [PubMed] [Google Scholar]
- 50.Zhu L, Zhang Q, Shi S, Liu L, Lv J, Zhang H. Synergistic effect of mesangial cell-induced CXCL1 and TGF-beta1 in promoting podocyte loss in IgA nephropathy. PLoS One 2013; 8: e73425–e73425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.