Abstract
Purpose
We performed a phase I study to determine the safety, maximum tolerated dose (MTD), and efficacy of weekly bolus recombinant human interleukin-21 (rIL-21) plus rituximab in patients with indolent B-cell malignancies.
Experimental Design
One week after a lead-in rituximab dose, cohorts of 3 patients were treated with 30, 100, or 150 μg/kg rIL-21 weekly for 4 weeks, concurrent with 4 weekly doses of rituximab. Patients with stable disease or better were eligible for a second course of therapy.
Results
Twenty-one patients with relapsed small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL, n=11), follicular lymphoma (n=9), or marginal zone lymphoma (n=1) were enrolled, with 19 completing at least 1 course of therapy. The MTD for rIL-21 was 100 μg/kg, based on observed toxicities including nausea, vomiting, diarrhea, hypotension, edema, and hypophosphatemia. Clinical responses were seen in 8 of 19 evaluable patients (42%; 3 CR/CRu, 5 PR), with 4 of longer duration than the patient’s previous response to rituximab-based treatment (median 9 versus 3 months).
Conclusions
Outpatient therapy of indolent B-cell malignancies with rituximab and weekly rIL-21 was well-tolerated and clinically-active, with durable complete remissions in a small subset of patients. Additional studies of rIL-21 and anti-CD20 antibodies in lymphoma and SLL/CLL are warranted.
Introduction
The advent of the anti-CD20 monoclonal antibody rituximab has contributed significantly to improving outcome in virtually all low-grade B-cell malignancies including follicular lymphoma (FL) and small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL)(1–4). The mechanism of rituximab in B-cell malignancies appears to include antibody dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells and monocytes, complement dependent cytotoxicity (CDC), and direct killing (5–8). While rituximab is an effective therapy in low-grade lymphoproliferative disorders, the most durable remissions have resulted from combination with cytotoxic chemotherapy. However, this approach has long-term consequences including immune suppression, infections, and secondary cancers. Identifying alternative immune-based combination therapies to enhance the durability of rituximab remissions among low-grade lymphoproliferative disorders therefore represents a major therapeutic goal. Recombinant interleukin-21 (rIL-21) represents one such potential therapeutic.
IL-21 is the most recently discovered member of the common γ-chain family of cytokines, which also includes IL-2, IL-4, IL-7, IL-9, and IL-15 (9). IL-21 is produced by activated CD4+ T-cells and possesses a variety of properties that make it an attractive candidate for the immunotherapy of lymphomas and other cancers (10–12). IL-21 stimulates the proliferation and cytotoxicity of CD8+ T-cells(13–19), promotes the activation of NK and NKT cells (13, 14, 20–22), and inhibits regulatory T-cell functions (17, 23, 24). IL-21 can also induce the proliferation, differentiation, or apoptosis of B-cells, depending on their co-stimulatory environment and developmental stage (25). The direct effect of IL-21 on B-cell lymphoproliferative disorders is also varied based upon their stage of differentiation. IL-21 antagonizes apoptosis in mature B-cell malignancies including multiple myeloma (26, 27) and Hodgkin lymphoma (28) whereas it directly promotes apoptosis in FL (29, 30), CLL (31–33), and diffuse large B-cell lymphoma (34). IL-21 thus represents the only γ-chain family cytokine possessing this favorable pro-apoptotic capacity against select B-cell lymphoproliferative disorders.
Given the direct apoptotic signaling properties of IL-21 and its ability to enhance ADCC, pre-clinical studies in both CLL and non-Hodgkin lymphoma (NHL) have been performed to justify its combination with rituximab. In CLL, rIL-21 was demonstrated to both enhance rituximab-mediated direct killing and autologous NK cell-based ADCC against primary CLL cells (32). Indeed, rIL-21 increased the lytic activity of NK cells against human B-cell lymphoma targets in the presence of rituximab, and prolonged the survival of mice bearing human lymphoma xenografts treated with rituximab(35). In primates, rIL-21 also enhanced depletion of normal B-cells by rituximab, while increasing circulating Fc receptor-bearing NK cells (36). We thus hypothesized that rIL-21 might improve the efficacy of rituximab in both CLL and low-grade lymphoma by enhancing both direct killing and ADCC.
Herein, we describe a phase I study of rIL-21 in combination with rituximab in select low-grade lymphoproliferative disorders including FL and SLL/CLL where we demonstrate the feasibility of outpatient administration and durable remissions in a subset of treated patients.
Materials and Methods
Patients
Eligible patients had indolent CD20+ B-cell lymphomas, either SLL/CLL, FL, or marginal zone lymphoma (MZL), measurable by computed tomography (CT) scans, relapsed after previous therapy (including rituximab for patients with FL); age ≥18 years; ECOG performance status 0 or 1; life expectancy ≥6 months; hemoglobin >10 g/dL; neutrophil count >1,500 cells/mm3; platelet count >75,000/mm3; and adequate hepatic and renal function. Patients with a history of central nervous system involvement, peripheral white blood cell count >50,000/mm3, systemic corticosteroids within one month of enrollment, or previous autologous or allogeneic hematopoietic stem cell transplant were excluded. The institutional review boards of each participating medical center approved the protocol, and patients gave written informed consent.
Study Design
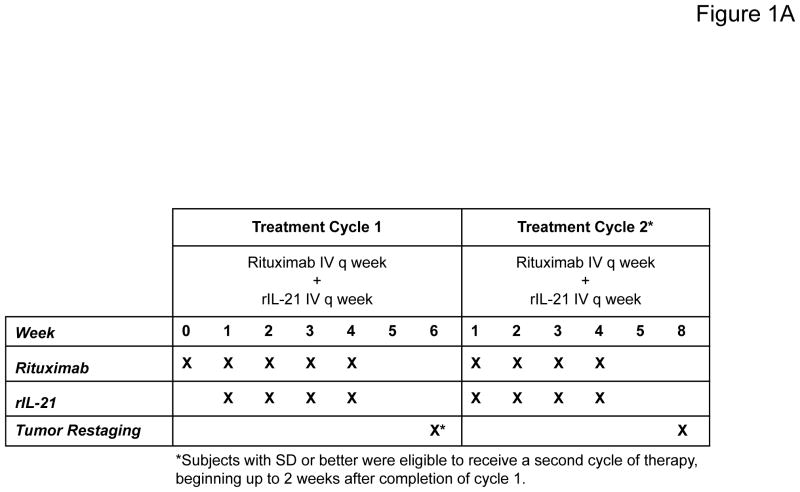
This open-label, dose-escalation study was conducted at 3 centers in the United States. The primary objective was to determine the MTD of rIL-21 administered once weekly for 4 weeks in combination with rituximab. The study design is shown in Figure 1A. Cohorts of 3 patients were sequentially enrolled in each dose level, with dose-escalation proceeding according to dose-limiting toxicity (DLT) during the first treatment cycle. DLTs were defined using National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0), and consisted of ≥grade 3 rIL-21-related toxicity except for transient (≤3 days) fatigue, fever, or rigor of grade 3; non-hematological lab abnormalities of grade 4 for any duration or of grade 3 (except hypophosphatemia) for ≥3 days; anemia, neutropenia, or thrombocytopenia of grade 4 for any duration or of grade 3 for ≥7 days; or any grade 3 laboratory abnormality resulting in withholding 2 doses. Lymphopenia was an anticipated effect of rIL-21 based on prior studies(37–39), and not considered dose-limiting unless associated with grade ≥3 clinical adverse events. Cohort expansion at the MTD was performed to characterize the safety profile and preliminary anti-tumor activity of rIL-21 plus rituximab.
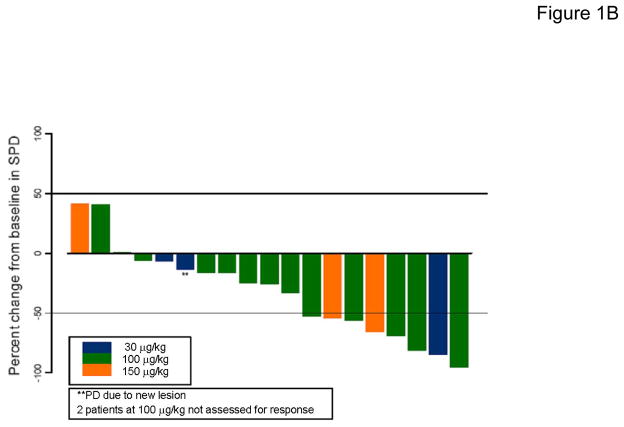
Figure 1. Trial design and clinical response results for rIL-21 plus rituximab.
(A) Schema for combination immunotherapy with rIL-21 plus rituximab. The week 0 dose of rituximab alone was given to separate any first-dose rituximab infusion reactions from those associated with IL-21 administration. (B) Maximum percent change in SPD for target lesions after treatment among patients evaluable for response (N=19). SPD = sum of the products of diameters for the 6 largest measurable index lesions.
Study Treatment
Each treatment cycle consisted of 4 weekly doses of rituximab plus rIL-21. Cycle 1 was preceded by one dose of single-agent rituximab (375 mg/m2) to distinguish first-dose rituximab infusion-related symptoms from rIL-21 infusion reactions and avoid potential compounding of these effects. Pre-medication with corticosteroids before rituximab was not permitted. Patients then received 4 weekly treatments with rituximab (375 mg/m2), followed at least 60 minutes later by rIL-21 (30, 100, or 150 μg/kg) via intravenous push. Patients with tumor response of stable disease (SD) or better as defined by standard criteria (40) 2 weeks after completion of Cycle 1 were eligible to begin a second 4-week treatment cycle (Cycle 2).
Assessments
Toxicities were monitored throughout the study and up to 28 days after the last rIL-21 dose. Adverse events (AEs) and laboratory abnormalities were graded using CTCAE v3.0. Tumor restaging by CT imaging and bone marrow aspirate and biopsy were performed at screening, two weeks after completion of treatment cycle 1, and 4 weeks after completion of treatment cycle 2. Bone marrow examination was only required after treatment for patients with bone marrow involvement at screening and results consistent with complete response (CR) for all other evaluations. Serum was obtained at selected timepoints for rIL-21 pharmacokinetic evaluation and measurement of anti-rIL-21 antibodies by ELISA.
Correlative Cell Signaling and Immunologic Analyses
In vivo cell signaling analysis was performed in Patient 1007 with SLL/CLL and circulating tumor cells. Whole blood was collected prior to rIL-21 dosing on day 8 of therapy and also 2 hours post-treatment. CLL cells were purified and stained for IL-21 receptor, and lysates subjected to western blotting for STAT1, p-STAT1, STAT3, and p-STAT3 as previously described (32). Flow cytometric analysis of NK cells was performed on 4 patients (during 5 cycles of therapy), as follows. Heparinized blood was collected pre- and 3 days post-treatment, and peripheral blood mononuclear cells isolated by Ficoll-Hypaque sedimentation were stained using the following mAbs purchased from BD Biosciences (San Jose, CA): CD3-FITC (clone HIT3a), CD3-PE (clone UCHT1), CD16-FITC (clone 3G8), CD25-PE (clone M-A251), CD56-APC (clone B159), CD69-PE (clone FN50), and appropriate isotype controls. Analysis was performed using a BD FACScan flow cytometer with FCS Express software (De Novo Software, Los Angeles, CA). Mean fluorescence intensities (MFI) for each marker were compared among pre- and post-treatment samples using a paired 2-tailed t test.
Pharmacokinetics and Immunogenicity of rIL-21
Serum samples for evaluation of rIL-21 pharmacokinetics were obtained at selected timepoints and rIL-21 levels determined using a validated custom ELISA (lower limit of detection 0.28 ng/ml). A linear fixed effects model was used to formally test the influence of dose level and repeat dosing on exposure. Individual log10 AUC0-t estimates were fit to a model: log10 AUC0-t= Intercept + Dose + Visit.
Statistical Analysis
Data was summarized using descriptive statistics including the median and range for continuous measures and the number and percent for categorical measures. Best overall response was assessed by investigators per standard lymphoma response criteria(40). The overall response rate was defined as the proportion of eligible patients with a best response categorized as: complete response (CR), unconfirmed complete response (CRu), or partial response (PR). Based on the binomial distribution, it was determined that a sample size of 15 patients at the MTD would provide approximately 80% probability of observing a relevant safety event in one or more patients, assuming a true population incidence rate of 10% or greater. Given the early phase of this study, formal assessments of power for efficacy endpoints were not conducted.
Results
Patients
Twenty-one patients were enrolled and treated with rIL-21 plus rituximab, including 11 with SLL/CLL, 9 with FL, and 1 with extranodal MZL (Table 1). Eighteen patients (86%) had received ≥2 prior treatment regimens, and 8 (38%) had failed to respond to their last treatment. All but one patient had received prior rituximab, and over half (57%) had received 2 or more prior rituximab-containing regimens. Of the 20 patients who had received prior rituximab, 15 (75%) had either failed to respond to, or relapsed within 6 months of their last rituximab-based therapy (Table 2), meeting a common definition for rituximab-resistant disease (41).
Table 1.
Summary of Patient Characteristics
| Parameter | All Patients (N=21) |
|---|---|
| Age (years) | |
| Median | 63.0 |
| Range | 37–82 |
|
| |
| Gender, n (%) | |
| Female | 6 (29) |
| Male | 15 (71) |
|
| |
| Disease type, N (%) | |
| Small lymphocytic lymphoma / CLL | 11 (52) |
| Follicular lymphoma (grade 1 or 2) | 9 (43) |
| Extranodal marginal zone, MALT-type | 1 (5) |
|
| |
| Prior systemic therapeutic regimens | |
| 1 | 3 (14) |
| 2 | 6 (29) |
| 3 | 6 (29) |
| ≥ 4 | 6 (29) |
|
| |
| Prior rituximab-containing regimens | |
| 0 | 1 (5) |
| 1 | 8 (38) |
| 2 | 7 (33) |
| 3 | 3 (14) |
| 6 | 2 (10) |
|
| |
| Rituximab-resistant disease | 15 (75) |
|
| |
| Best response to last systemic therapy | |
| CR/CRu | 2 (10) |
| PR | 11 (52) |
| SD | 7 (33) |
| PD | 1 (5) |
Table 2.
Characteristics and Outcomes of Individual Patients
| Patient number | Diagnosis | Response History
|
Response on Study
|
|||
|---|---|---|---|---|---|---|
| Number of prior therapies | Number of prior rituximab therapies | Best response to last rituximab-based therapy/ duration (months) | Number of rIL-21 + rituximab cycles | Best response to rIL-21 + rituximab/ duration (months) | ||
| 30 μg/kg Dose Cohort | ||||||
|
| ||||||
| 1001 | FL | 2 | 1 | PR / 6 | 1 | PD / — |
| 1002 | FL | 3 | 3 | SD / 9 | 2 | SD / 10+ |
| 1004 | FL | 2 | 1 | CR / 5 | 2 | CR / 9 |
|
| ||||||
| 100 μg/kg Expansion Cohort | ||||||
|
| ||||||
| 1005 | FL | 5 | 1 | CR / 63 | 2 | CRu / 25+ |
| 1007 | SLL/CLL | 6 | 3 | SD / 2 | 2 | PR / <2 |
| 1008 | MZL | 3 | 1 | PR / 3 | 2 | PR / 10 |
| 1014 | SLL | 3 | 2 | PR / 3 | 2 | SD / 6 |
| 1019 | SLL | 3 | 2 | PR / 5 | 2 | SD / 12 |
| 1021 | FL | 2 | 2 | PR / 12 | 1 | —a |
| 1022 | SLL | 2 | 2 | SD / 2 | 2 | SD / Unknown |
| 1025 | SLL | 4 | 2 | PD / — | 1 | SD / 1 |
| 1026 | FL | 2 | 1 | PR / 3 | 2 | PR / 9 |
| 1031 | SLL | 8 | 6 | SD / 8 | 1 | SD / 2 |
| 1032 | SLL/CLL | 1 | 1 | CR / 40 | 2 | CR / 28+ |
| 1033 | FL | 10 | 6 | SD / 6 | 1 | SD / 4 |
| 1034 | SLL | 3 | 1 | PD / — | 0.5 | —b |
| 1035 | SLL | 1 | 1 | PR / 6 | 2 | SD / 5 |
| 1036 | FL | 2 | 2 | SD / 6 | 2 | SD / 3 |
|
| ||||||
| 150 μg/kg Dose Cohort | ||||||
|
| ||||||
| 1010 | SLL | 1 | 0 | — | 2 | PR / 28 |
| 1011 | FL | 3 | 3 | PR / 2 | 2 | PR / 10 |
| 1012 | SLL | 7 | 2 | PR / 2 | 1 | SD / 3 |
CR, complete response; CRu, complete response unconfirmed; FL, follicular lymphoma; MZL, extranodal marginal zone lymphoma; PD, progressive disease; PR, partial response; SD, stable disease; SLL, small lymphocytic lymphoma (without circulating tumor cells); SLL/CLL, small lymphocytic lymphoma with circulating tumor cells,
Patient received 4 doses of rIL-21 and died prior to restaging
Patient withdrew after 2 doses of rIL-21 and died 5 months later
Three patients were enrolled and treated in each dose escalation cohort, and 12 additional patients were enrolled and treated with 100 μg/kg rIL-21 during cohort expansion. Of the 21 patients enrolled, 19 completed the study protocol, receiving all 4 doses of rIL-21 in Cycle 1 and all assessments. Two patients in the expansion cohort did not complete the study; one patient withdrew prematurely for personal reasons, and one patient with pre-existing coronary artery disease died from cardiac complications after completing Cycle 1 but before restaging. Fourteen patients (67%) completed Cycle 2. Four patients had rIL-21 plus rituximab delayed by one week for an AE or laboratory abnormality.
Safety Experience: Dose Escalation
Three dose levels of weekly rIL-21 in combination with rituximab were evaluated; 30, 100, and 150 μg/kg. During Cycle 1 dose escalation, all AEs were grade 1 or 2 in severity and no DLTs were observed. Common AEs (observed in >3 patients, Cycles 1 and 2) included influenza-like symptoms, fatigue, headache, dizziness, pruritus, night sweats, nausea, and fever. Two patients in the 100 μg/kg cohort experienced transient grade 3 neutropenia not associated with fever or infection. Two patients experienced grade 3 lymphopenia, one of whom also had grade 3 leukopenia; these laboratory changes were expected effects of study treatment. During Cycle 2, one patient treated at 150 μg/kg rIL-21 experienced grade 3 nausea, vomiting, and diarrhea; and grade 2 hypotension, all considered possibly related to study drug. A second patient treated at 150 μg/kg rIL-21 experienced grade 2 lower extremity edema during Cycle 2, considered possibly related to study drug. The third patient in this cohort developed grade 3 fatigue and hypophosphatemia attributable to study drug. Although no patients treated at doses up to 150 μg/kg rIL-21 experienced any DLT during Cycle 1, based on the toxicities experienced by the patients treated at 150 μg/kg during Cycle 2, the Safety Monitoring Committee considered this dose to be above the MTD of a feasible outpatient therapy and recommended 100 μg/kg for evaluation during cohort expansion.
Safety Experience: Cohort Expansion
Fifteen patients were treated with rIL-21 at the MTD of 100 μg/kg plus rituximab including 3 patients in dose escalation and 12 patients in the expansion cohort; 10 of these patients received 2 cycles of therapy. All patients were included in the safety analysis. Table 3 displays all adverse events occurring in at least 10% of patients treated with 100 μg/kg. Most adverse events were mild or moderate. The frequency of adverse events increased with increasing dose of rIL-21, as displayed in Supplemental Table 1. Grade ≥3 events considered at least possibly related to rIL-21 occurred in a total of 4 patients and included grade 3 influenza-like illness, fatigue, rash, arthralgia, nocturnal pain of the fingers, and grade 4 thrombocytopenia. One patient developed a grade 4 non-ST elevation myocardial infarction treated with percutaneous stent placement complicated by asystole, respiratory distress, subsequent stent thombosis and fatal bradycardic arrest. These events were considered possibly related to rIL-21 as they occurred after the fourth dose of rIL-21. However, the relationship is confounded by the patient’s prior history of hypertension, hypercholesterolemia, coronary artery disease, myocardial infarction, and supraventricular tachycardia.
Table 3. Common Non-Hematologic Adverse Events.
* by Severity (Patients Treated with 100 μg/kg rIL-21; N = 15)
| Preferred Term | Any Grade n (%) |
Grade 1 n (%) |
Grade 2 n (%) |
Grade 3 n (%) |
|---|---|---|---|---|
| Influenza-like illness | 11 (73) | 8 (53) | 2 (13) | 1 (7) |
| Fatigue | 9 (60) | 4 (27) | 4 (27) | 1 (7) |
| Nausea | 6 (40) | 5 (33) | 1 (7) | — |
| Pyrexia | 6 (40) | 4 (27) | 2 (13) | — |
| Headache | 5 (33) | 4 (27) | 1 (7) | — |
| Pruritus | 4 (27) | 3 (20) | 1 (7) | — |
| Diarrhea | 4 (27) | 4 (27) | — | — |
| Dizziness | 3 (20) | 3 (20) | — | — |
| Insomnia | 3 (20) | 2 (13) | 1 (7) | — |
| Night sweats | 3 (20) | 3 (20) | — | — |
| Pain in extremity | 3 (20) | 1 (7) | 1 (7) | 1 (7) |
| Constipation | 3 (20) | 3 (20) | — | — |
| Muscle spasms | 2 (13) | 1 (7) | 1 (7) | — |
| Chills | 2 (13) | 1 (7) | 1 (7) | — |
| Decreased appetite | 2 (13) | 2 (13) | — | — |
| Edema, peripheral | 2 (13) | 1 (7) | 1 (7) | — |
| Rash, pruritic | 2 (13) | — | 2 (13) | — |
| Cough | 2 (13) | 1 (7) | 1 (7) | — |
| Neuropathy | 2 (13) | 2 (13) | — | — |
| Palpitations | 2 (13) | 1 (7) | 1 (7) | — |
| Rash, erythematous | 2 (13) | 1 (7) | — | 1 (7) |
Adverse events occurring in at least 10% of patients treated at 100 μg/kg rIL-21.
Grade 3/4 treatment-emergent laboratory abnormalities are presented in Table 4. Transient cytopenias were the most common laboratory abnormalities, as seen in prior rIL-21 trials(37–39). Four of 15 patients treated at 100 μg/kg experienced grade ≥3 lymphopenia, and 6 had grade ≥3 neutropenia. In most cases, neutropenia resolved to grade 2 or better by the next study visit; there were no associated fevers or infections and only one patient had a dose delay of one week due to neutropenia. One patient had grade 4 thrombocytopenia, yet after a single platelet transfusion and one-week delay of his third dose of rIL-21 plus rituximab, he successfully completed Cycle 1 and also completed Cycle 2.
Table 4.
Grade 3/4 Treatment-Emergent Laboratory Abnormalities
| * Lab Abnormality | 30 μg/kg N=3 |
100 μg/kg N=15 |
150 μg/kg N=3 |
Total N=21 n (%) |
|---|---|---|---|---|
| Lymphopenia | 1 | 4 | — | 5 (24) |
| Neutropenia | — | 6 | — | 6 (29) |
| Leukopenia | — | 2 | — | 3 (14) |
| Thrombocytopenia | — | 1 | — | 1 (5) |
| Anemia | — | 1 | — | 1 (5) |
| Increased AST | — | 2 | 0 | 2 (10) |
| Increased ALT | — | 1 | 0 | 1 (5) |
| Hypophosphatemia | — | 1 | 1 | 2 (10) |
| Hypoalbuminemia | 1 | — | — | 1 (5) |
Worst case per patient as graded by CTCAE v3.0 criteria
Hypoalbuminemia was the most common change in serum chemistry with 12 and 2 patients overall having a 1-grade and 2-grade worsening from baseline, respectively. Two patients with normal phosphorous at baseline developed grade 3 hypophosphatemia. While most patients had no elevations in liver transaminases, two developed grade 3 AST elevation, with one accompanied by grade 3 ALT elevation. In both cases, these occurred after the fourth dose of rIL-21 plus rituximab during the treatment cycle, and resolved without specific intervention. One of these patients subsequently received Cycle 2 without recurrence of transaminase elevations. Over a three-year period during and after the study’s completion, no long-term unexpected adverse effects have been attributed to treatment with rIL-21.
Pharmacokinetics and Immunogenicity of rIL-21
Supplemental Figure 1 shows the mean rIL-21 serum concentrations after the first dose for the 30, 100, and 150 μg/kg dose levels. Mean AUC0-t after the first dose were 86.9, 258, and 310 h·ng/mL for the 30, 100, and 150 μg/kg dose groups during dose escalation, respectively, suggesting a dose-dependence for this parameter. Estimates from the 100 μg/kg expansion cohort had a CV% of 131%, and this intersubject variability prevented easy interpretation. Mean serum concentrations of rIL-21 after cycle 1 dose1, cycle 1 dose 4, and cycle 2 dose 4 were superimposable, suggesting the PK of rIL-21 does not change with repeated dosing. A linear fixed effects model was used to formally test the influence of dose level and repeat dosing on exposure. Parial tests with the inclusion of Visit and Dose in the model were found to have p-values of 0.7150 and <0.05 respectively, indicating a clear effect of dose level but no effect of repeat dosing on exposure. No specific antibodies to rIL-21 were identified in any subject.
Clinical Responses
Two patients, one who died prior to tumor evaluation and one who withdrew prior to completing Cycle 1 (both treated at 100 μg/kg), were excluded from efficacy analyses. Following treatment, a decrease in the SPD of target lesions was seen in 16 (84%) evaluable patients (Figure 1B), and objective clinical responses were seen in 8 (42%) evaluable patients, including 3 CR/CRu and 5 PR (Table 2). Of note, in 4 of these 8 responders, remissions were of longer duration than the patient’s previous response to rituximab-based treatment (Table 2, Patients 1004, 1008, 1026, and 1011, median 9 months versus 3 months). Among the 15 patients with rituximab-resistant disease, the objective response rate was 33%. Two patients who attained CR/CRu (Patients 1005 and 1032) have remained without disease progression for 25+ and 28+ months, respectively. The response of Patient 1032 is particularly noteworthy. This patient had presented with normal karyotype SLL/CLL and symptomatic, bulky adenopathy in 2003 and was treated with pentostatin, cyclophosphamide, and rituximab, attaining CR in May 2004. In November 2007 he presented with recurrent fatigue, enlarged abdominal nodes (largest 5 × 2 cm) and 40% bone marrow involvement with CLL. After one cycle of rIL-21 plus rituximab, CR was achieved by CT and bone marrow criteria, and Cycle 2 administered. High sensitivity 4-color flow cytometry confirmed absence of clonal CLL cells in the bone marrow, and he remains asymptomatic with ongoing CR at 28 months. Another noteworthy response in SLL/CLL was Patient 1010, who achieved a PR and remained without disease progression for 28 months, despite previously failing experimental therapy with an oral vitamin A derivative.
Correlative Cell Signaling and Immunologic Analyses
In vitro exposure of primary CLL cells to rIL-21 can result in pro-apoptotic signaling characterized by increased phosphorylation of STAT3 and Bim (32). Patient 1007 with SLL/CLL had tumor cells circulating in the peripheral blood, offering us the opportunity to explore such signaling in vivo during therapy with rIL-21. Blood was collected immediately before and 2 hours after initiation of rIL-21 therapy, and purified tumor cells analyzed for phosphoprotein expression. As shown in Supplemental Figure 2, tumor cells expressed IL-21Rα, and following in vivo exposure to IL-21, demonstrated increased phosphorylation of STAT3 but not STAT1. However, this patient’s tumor cells lacked ex vivo apoptosis induction in response to IL-21 treatment (data not shown). Analysis of peripheral blood NK cells obtained pre- and 3 days post-rIL-21 (during 5 treatment cycles in 4 patients) demonstrated significantly increased expression of the CD69 activation marker during therapy (MFI 8.0±2.4 versus 17.0±4.7, p=0.0045), while no consistent changes were noted in expression of CD16/FcγRIIIa (data not shown).
Discussion
Based on the capacity of rIL-21 to both inhibit the viability of malignant B-cells and activate NK cell ADCC effectors, we sought to evaluate the feasibility and safety of combining rIL-21 with rituximab anti-CD20 antibody therapy in patients with indolent lymphoproliferative disorders. In this phase I, dose-finding trial, we found that outpatient therapy with weekly rIL-21 at 100 μg/kg or lower was well-tolerated in combination with standard rituximab therapy. The most common AEs, including flu-like symptoms, fatigue, and headache, were mostly mild to moderate in severity. Transient laboratory abnormalities including lymphopenia, leukopenia, neutropenia, elevated hepatic transaminases, and hypophosphatemia were observed in a minority of patients, consistent with previous trials of rIL-21 in melanoma and renal cell carcinoma (37–39).
Reductions in tumor burden were seen in the great majority of patients during treatment, with 84% of evaluable patients showing decreases in radiographically-measurable lesions (Figure 1B), and 42% meeting objective response criteria (3 CR/CRu, 5 PR). While rituximab alone was expected to have some activity in this study population, several lines of evidence suggest that the addition of rIL-21 may have contributed to these outcomes. First, 4 of the responses were seen in patients whose prior response to rituximab-based therapy lasted less than 6 months, meeting a common definition for rituximab-resistant disease (41). Moreover, these responses were typically of longer duration than the patient’s previous response to rituximab-based therapy. Second, the response rate among patients with rituximab-resistant disease was 33%. And third, the durable complete clinical response achieved in patient 1032 with bulky SLL/CLL would not be expected using single agent rituximab, as attainment of CR to rituximab in this setting is rare (42, 43). Thus, rIL-21 may have contributed to the favorable clinical outcomes of some patients on this trial.
The weekly rIL-21 dosing schedule used in this trial was chosen to coincide with standard rituximab dosing, and differed from the schedule established in earlier phase I/II trials of rIL-21 patients with metastatic melanoma or renal cell carcinoma (37–39). While these previous trials established an MTD of 30 μg/kg using daily dosing for 5 days followed by 9 days of rest (“5+9” schedule), we found the MTD to be 100 μg/kg when utilizing once weekly dosing. In accordance with data from a previous phase I study38, dosing with 100 or 150 μg/kg yielded peak rIL-21 levels of approximately 200–350 ng/ml and a half-life of several hours. Since both schedules are well-tolerated, both may be worth exploring in future trials employing anti-CD20 antibodies.
Accumulating data suggest that select B-cell malignancies may represent additional attractive targets for rIL-21 therapy. Unlike solid tumors, many B-cell neoplasms express IL-21 receptors, and are thus susceptible to IL-21’s potentially growth-inhibitory effects. Several groups have demonstrated that rIL-21 can promote apoptosis in freshly-isolated CLL cells in vitro (31–33), and that IL-21 receptor expression and tumor apoptosis can be enhanced by immune modulation with CD40 ligation or CpG treatment (31, 33). Gowda et al further demonstrated that rIL-21 sensitizes CLL cells to the cytotoxic effects of both fludarabine and rituximab, and increases NK cell ADCC against rituximab-coated CLL cells (32). Follicular and mantle cell lymphoma cells can also be susceptible to direct apoptosis induction by rIL-21 (30, 44). Sarosiek and colleagues (34) recently reported that rIL-21 efficiently induced caspase-dependent apoptosis of diffuse large B-cell lymphoma (DLBCL) cell lines and primary tumors without affecting the viability of normal human B cells. The degree of apoptosis induced in primary tumor samples was greater among DLBCL cases than FL specimens. In vivo, the growth of DLBCL xenograft tumors was also inhibited by rIL-21 treatment. Thus, B-cell malignancies, particularly SLL/CLL and DLBCL, should be considered high priorities in the continuing clinical development of rIL-21 therapy.
Supplementary Material
Translational Relevance.
IL-21 is a common γ-chain cytokine that can stimulate T cell and NK cell-mediated anti-tumor immunity, and also directly promote apoptosis in select B cell malignancies including follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, and diffuse large B-cell lymphoma. Given these properties, and its ability to enhance antibody-dependent cellular cytotoxicity against tumor cells, IL-21 might improve the efficacy of anti-CD20 monoclonal antibodies by enhancing both direct and antibody-mediated killing. This phase I dose-finding study of rituximab plus rIL-21 in indolent B cell malignancies establishes a well-tolerated weekly outpatient treatment schedule that may be useful in further studies of this cytokine in combination with antibody therapy. Our in vivo results, together with prior in vitro observations, suggest that B cell malignancies should be considered high priority targets in the development of IL-21-based cancer therapies.
Acknowledgments
Supported by ZymoGenetics, Inc. Seattle, WA, and NCI P01 CA95426 (J.C.B. and N.M.). The authors thank the patients who participated in this trial, research nurses Donna Fernando and Kathleen O’Neill, and Drs. Ajay Gopal and Andrei Shustov for patient referrals.
Footnotes
ClinicalTrials.gov Identifier: NCT00347971
References
- 1.Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70:1445–76. doi: 10.2165/11201110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Jaglowski SM, Byrd JC. Rituximab in chronic lymphocytic leukemia. Seminars in hematology. 2010;47:156–69. doi: 10.1053/j.seminhematol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Sousou T, Friedberg J. Rituximab in indolent lymphomas. Seminars in hematology. 2010;47:133–42. doi: 10.1053/j.seminhematol.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal L, Gafter-Gvili A, Leibovici L, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: systematic review and meta-analysis of randomized trials. Journal of the National Cancer Institute. 2009;101:248–55. doi: 10.1093/jnci/djn478. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Seminars in hematology. 2010;47:124–32. doi: 10.1053/j.seminhematol.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner GJ. Rituximab: mechanism of action. Seminars in hematology. 2010;47:115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Kitada S, Flinn IW, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–43. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 8.Jaglowski SM, Alinari L, Lapalombella R, Muthusamy N, Byrd JC. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 2010 doi: 10.1182/blood-2010-04-001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 10.Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13:6926–32. doi: 10.1158/1078-0432.CCR-07-1238. [DOI] [PubMed] [Google Scholar]
- 11.Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–40. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- 12.Andorsky DJ, Timmerman JM. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther. 2008;8:1295–307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- 13.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 14.Strengell M, Matikainen S, Siren J, et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–9. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 15.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–9. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–9. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–35. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Lizee G, Lou Y, et al. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol. 2007;19:1213–21. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 19.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivori S, Cantoni C, Parolini S, et al. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol. 2003;33:3439–47. doi: 10.1002/eji.200324533. [DOI] [PubMed] [Google Scholar]
- 21.Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123:575–83. doi: 10.1111/j.1365-2567.2007.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coquet JM, Kyparissoudis K, Pellicci DG, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 23.Peluso I, Fantini MC, Fina D, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–9. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 24.Gowda A, Ramanunni A, Cheney C, et al. Differential effects of IL-2 and IL-21 on expansion of the CD4+ CD25+ Foxp3+ T regulatory cells with redundant roles in natural killer cell mediated antibody dependent cellular cytotoxicity in chronic lymphocytic leukemia. mAbs. 2010;2:35–41. doi: 10.4161/mabs.2.1.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–7. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- 26.Brenne AT, Ro TB, Waage A, Sundan A, Borset M, Hjorth-Hansen H. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood. 2002;99:3756–62. doi: 10.1182/blood.v99.10.3756. [DOI] [PubMed] [Google Scholar]
- 27.Menoret E, Maiga S, Descamps G, et al. IL-21 stimulates human myeloma cell growth through an autocrine IGF-1 loop. J Immunol. 2008;181:6837–42. doi: 10.4049/jimmunol.181.10.6837. [DOI] [PubMed] [Google Scholar]
- 28.Scheeren FA, Diehl SA, Smit LA, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood. 2008;111:4706–15. doi: 10.1182/blood-2007-08-105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akamatsu N, Yamada Y, Hasegawa H, et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Lett. 2007;256:196–206. doi: 10.1016/j.canlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.de Totero D, Capaia M, Fabbi M, et al. Heterogeneous expression and function of IL-21R and susceptibility to IL-21-mediated apoptosis in follicular lymphoma cells. Experimental hematology. 2010;38:373–83. doi: 10.1016/j.exphem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 31.de Totero D, Meazza R, Zupo S, et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood. 2006;107:3708–15. doi: 10.1182/blood-2005-09-3535. [DOI] [PubMed] [Google Scholar]
- 32.Gowda A, Roda J, Hussain SR, et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody dependent cellular cytotoxicity in primary chronic lymphocytic cells. Blood. 2008;111:4723–30. doi: 10.1182/blood-2007-07-099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahrsdorfer B, Blackwell SE, Wooldridge JE, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712–9. doi: 10.1182/blood-2006-03-014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarosiek KA, Malumbres R, Nechushtan H, Gentles AJ, Avisar E, Lossos IS. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood. 2010;115:570–80. doi: 10.1182/blood-2009-08-239996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kindsvogel W, Hughes SD, Bannink M, et al. IL-21 enhances rituximab-mediated killing of B-lymphoma cell lines in vitro and in vivo. Journal of Clinical Oncology. 2004;22:Abstract 2581. [Google Scholar]
- 36.Hughes SD, Ponce RA, Krejsa C, et al. IL-21 Improves Rituximab-Mediated B Cell Depletion. Blood. 2005;106:345. [Google Scholar]
- 37.Davis ID, Brady B, Kefford RF, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–9. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 38.Davis ID, Skrumsager BK, Cebon J, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–6. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 39.Thompson JA, Curti BD, Redman BG, et al. Phase I Study of Recombinant Interleukin-21 in Patients With Metastatic Melanoma and Renal Cell Carcinoma. J Clin Oncol. 2008;26:2034–9. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 40.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 41.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–14. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–24. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 44.Gelebart P, Zak Z, Anand M, Dien-Bard J, Amin HM, Lai R. Interleukin-21 effectively induces apoptosis in mantle cell lymphoma through a STAT1-dependent mechanism. Leukemia. 2009;23:1836–46. doi: 10.1038/leu.2009.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.