Abstract
Purpose
Ipilimumab is a first-in-class immune checkpoint inhibitor approved for treatment of metastatic melanoma but not studied in children until this phase 1 protocol.
Experimental Design
This study examined safety, pharmacokinetics, and immunogenicity, and immune correlates of ipilimumab administered to subjects ≤21 years old with recurrent or progressive solid tumors. Dose escalation cohorts received 1, 3, 5, or 10mg/m2 intravenously every 3 weeks in a 3 + 3 design. Response was assessed after 6 weeks and 12 weeks, and then every 3 months. Treatment was continued until disease progression or unacceptable toxicity.
Results
Thirty-three patients received 72 doses of ipilimumab. Patients enrolled had melanoma (n=12), sarcoma (n=17), or other refractory solid tumors (n= 4). Immune-related adverse events included pancreatitis, pneumonitis, colitis, endocrinopathies, and transaminitis with dose-limiting toxicities observed at 5mg/kg and 10mg/kg dose levels. Pharmacokinetics revealed a half–life of 8-15 days. At day 21, subjects had increased levels of cycling T cells, but no change in regulatory T cell populations. Six subjects had confirmed stable disease for 4-10 cycles (melanoma, osteosarcoma, clear cell sarcoma, and synovial sarcoma).
Conclusions
Ipilimumab was safely administered to pediatric patients using management algorithms for immune-related toxicities. The spectrum of immune-related adverse events is similar to those described in adults; however, many of the pediatric toxicities were evident after a single dose. Although no objective tumor regressions were observed with ipilimumab as a single agent, subjects with immune related toxicities had an increased overall survival compared to those who showed no evidence of breaking tolerance.
Keywords: Immunotherapy, Pediatric, melanoma, sarcoma, Checkpoint inhibitor
Introduction
During the course of tumor growth, T cells can recognize tumor-associated antigens and mediate anti-cancer immune responses. However, inhibitory signals delivered via immune checkpoints such Cytotoxic T lymphocyte Antigen 4 (CTLA-4) or programmed death receptor 1 (PD1) can dampen naturally acquired anti-tumor immune responses (reviewed in (1)). Ipilimumab is a fully human monoclonal antibody that binds CTLA-4 and blocks its interaction with B7-1 and B7-2 (CD80 and CD86), thus inhibiting this immune checkpoint.(2-4) In the presence of ipilimumab, B7-1 preferentially binds CD28, which provides costimulation that activates T cells.(3) The overall response rates reported for ipilimumab monotherapy in metastatic melanoma range from 4-15%(5-9). Based on pivotal phase 3 studies showing increased survival, ipilimumab was approved for the treatment of metastatic melanoma at 3mg/kg.(10) Several trials in adult patients with melanoma showed safety and potential increased efficacy with higher doses up to 10mg/kg.(5, 11, 12) Clinical responses following ipilimumab are often durable as evidenced by long-term follow-up of patients from single institution trials(13) and review of phase 2 clinical trials of ipilimumab revealed 4-year survival rates of 37.7-49.5% in treatment-naïve patients who received ipilimumab at 10mg/kg. (14)
Ipilimumab-mediated inhibition of CTLA4 signaling can also result in tissue-specific inflammation, as a result of expansion or activation of autoreactive cell populations, which leads to immune-mediated side effects.(15-17) The most commonly reported immune-related adverse events in patients treated with ipilimumab are rash, colitis, transaminitis, and endocrinopathies.(18) Prior to this study, it was unknown if CTLA-4 blockade would have similar immune-mediated effects in children.
Pediatric melanoma is a rare and aggressive malignancy.(19) Early stage disease can be surgically resected with long-term remissions; however, unresectable stage III or IV metastatic disease in the pediatric patient has been difficult to treat and lacks curative options.(20) Like malignant melanoma occurring in adults, T cell infiltrates are often found within the tumor, thus inhibition of the immune checkpoint is of interest as a potential treatment modality. Many other pediatric solid tumors including neuroblastoma, osteosarcoma, rhabdomyosarcoma, and synovial sarcoma have preclinical or clinical evidence of an immune mediated anti-tumor response.(21-25) This phase 1 study of ipilimumab was open to patients aged 1-21 with solid tumors, excluding primary brain tumors. Primary objectives were to determine safety and pharmacokinetics of the drug as well as to define the toxicity profile of this first immune checkpoint inhibitor to be given to children.
Methods
The study population included patients aged 2-21 years old with recurrent or refractory solid tumors. Patients must have completed irradiation or chemotherapy at least 3 weeks prior to enrollment, and biological therapy at least 1 week prior to treatment. Other inclusion criteria included adequate performance and end organ function, specifically Karnofsky or Lansky score >50%, hemoglobin concentration >8g/dL, absolute granulocyte count >1000/mm3, platelet count >75,000/mm3, aspartate transaminase (AST) and alanine transaminase (ALT) ≤2.5-fold the upper limit of normal (ULN), as well as bilirubin and serum creatinine within normal limits. Patients with primary brain malignancies were excluded from the trial but asymptomatic patients with subcentimeric or treated brain metastases were eligible for enrollment.
Study Design and Treatment
NCI 08-C-0007 (NCT01445379) opened as a single center phase 1 study at the National Institutes of Health Clinical Center and was subsequently expanded to include enrollment at Memorial Sloan-Kettering Cancer Center and Dana-Farber Cancer Institute. Designed to test the safety and pharmacokinetics up to 10mg/kg, the dose escalation portion of the trial enrolled patients in a 3+3 fashion followed by an expansion cohort in two age groups (1-11yo and 12-21yo) for further safety and pharmacokinetic evaluation. Ipilimumab was supplied as a sterile, single use solution in phosphate-buffered saline and infused IV over 90 minutes using a DEHP and latex-free IV administration set with a Braun 1.2 micron in-line filter. Induction therapy comprised 4 cycles of ipilimumab IV every 3 weeks. If there was no evidence of progressive disease or dose limiting toxicity (DLT), maintenance therapy was initiated 3 weeks following induction with infusions of the same dose every 12 weeks.
Dose-limiting toxicities
Toxicity was graded according to the NCI Common Toxicity Criteria (CTCAE v3.1). The highest tolerated dose was defined as the maximum dose of ipilimumab administered at which no more than 1 of 6 patients experienced a DLT.
Pharmacokinetics
This trial used the same pharmacokinetic assay method as adult trials.(26) Blood samples (5 mL) were collected from a site other than the infusion line for determination of serum ipilimumab concentrations at completion of infusion (day 1) and also on days 2, 4, 8, 15, and 21. Pre-dose samples were obtained prior to cycle 2, cycle 4 and prior to each cycle of maintenance. Blood was processed to obtain serum and aliquots were frozen at -80C until assayed. Samples were analyzed for ipilimumab concentration and immunogenicity by qualified enzyme-linked immunosorbent assays (ELISAs) as previously described.(26) Individual pharmacokinetic (Cmin, Cmax, CL, AUC (0–T), AUC (0–21), Thalf, Tmax, Vss) and immunogenicity assessments were analyzed.
Evaluation of clinical activity
Baseline imaging was obtained within 14 days prior to the first ipilimumab dose, at 6 weeks and 12 weeks during induction and then every 12 weeks until withdrawal from the study. Imaging for response was appropriate for disease and response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines with modification to allow continued dosing of ipilimumab for up to 50% increase in size of target lesions.
Flow Cytometry
Whole blood was obtained to assess Immune cell subsets at baseline, pre-cycle 2 and pre-cycle 3. Peripheral blood mononuclear cells were isolated and stained for CD3, CD4, CD8, HLA-DR, foxP3, CD19, CD20 and ki67 in a CLIA certified clinical laboratory.
Results
Patient Characteristics
A total of 33 patients aged 28 months to 21 years were enrolled in this multicenter trial. Thirty-one patients were fully evaluable for toxicity during the first two cycles of induction therapy. Two patients expired from rapidly progressive disease within 6 weeks of the initial dose of ipilimumab and were replaced for dose escalation, leaving 31 patients that were fully evaluable for toxicity. Twelve patients had unresectable stage IIIc or stage IV melanoma (Table 1). A heterogeneous group of patients with progressive or refractory sarcoma was also enrolled including osteosarcoma (n=8), synovial sarcoma (n=2), clear cell sarcoma (n=2; also referred to as melanoma of soft parts), rhabdomyosarcoma (n=2), pleomorphic sarcoma (n=1), clear cell sarcoma of the kidney (n=1), and undifferentiated sarcoma (n=1). Three patients with renal or bladder carcinomas and one patient with neuroblastoma were also treated. Patients without other curative standard therapies were eligible, and the majority had received at least two lines of therapy for relapsed or refractory disease. Of the patients with melanoma, six had previous biologic therapy with high dose IL2 and/or IFNα. Two patients with synovial sarcoma had previously received adoptive T cell therapy. Three patients with melanoma went on to receive adoptive therapy with tumor Infiltrating T cells (TIL) following ipilimumab, but none had received TIL therapy prior to enrolling on this study.
Table 1. Patient Characteristics.
| Total | 1mg/kg | 3mg/kg | 5mg/kg | 10mg/kg | ||
|---|---|---|---|---|---|---|
| Number treated | 33 | 3 | 3 | 14 | 13 | |
|
| ||||||
| Age | Range (years) | 2-21 | 2-21 | 4-21 | 3-20 | 7-19 |
| Mean (years) | 13.4 | 14.5 | 12.6 | 12.7 | 14.2 | |
|
| ||||||
| Sex | Male | 14 (42%) | 1 | 2 | 3 | 8 |
| Female | 19 (58%) | 2 | 1 | 11 | 5 | |
|
| ||||||
| Disease | Melanoma | 12 (36%) | 1 | 1 | 7 | 3 |
| Sarcoma | 17 (52%) | 2 | 2 | 6 | 7 | |
| Renal/Bladder Carcinoma | 3 (9%) | - | - | 1 | 2 | |
| Neuroblastoma | 1 (3%) | - | - | - | 1 | |
|
| ||||||
| Prior Therapy | Chemotherapy: 1-2 regimens | 9 (27%) | - | - | 5 | 4 |
| Chemotherapy: 3-4 regimens | 14 (42%) | 2 | 2 | 4 | 6 | |
| Radiation | 17 (52%) | 2 | 2 | 6 | 7 | |
| Biologic (IL2, IFNα or adoptive T cell therapy) | 11 (33%) | 1 | 2 | 3 | 5 | |
Dose escalation
The first two dose levels (1mg/kg and 3 mg/kg) accrued 3 patients each without any DLT. One subject at 3 mg/kg dose level had a transient grade 2 transaminitis but no other subjects in these first two dose levels had an immune related adverse event (irAE) ≥grade 2. At 5mg/kg, one patient with melanoma developed pancreatitis requiring hospitalization and steroid therapy following a single dose of ipilimumab. Because of this DLT, the dose level was expanded to include 6 evaluable patients. No further DLTs occurred at the 5mg/kg dose level during the safety evaluation period; however, several irAE were observed but not considered DLTs due to their onset beyond the first two cycles (Table 2). The maximum dose tested was 10mg/kg, the highest dose being explored in adult studies. Two evaluable patients developed DLT at this dose level. A child with neuroblastoma developed grade 3 colitis following the first dose and a child with melanoma developed grade 3 transaminitis following the second dose of ipilimumab. Both DLTs were in children <12years old, while 3 adolescent patients in the cohort tolerated the 10 mg/kg without toxicity. Following these DLTs, the 10mg/kg dose level expansion was continued for adolescent patients aged 12-21yo while an expanded cohort of 6 children <12 years was studied at 5mg/kg.
Table 2. Treatment with ipilimumab by dose level and associated immune related toxicities.
| 1mg/kg | 3mg/kg | 5mg/kg | 10mg/kg | |
|---|---|---|---|---|
| Immune related ≥Grade 2 Toxicities (Week of onset)* | 0 | 0 |
Gr 4 pancreatitis (Wk 2) Gr 2 transaminitis (Wk 3) Gr 2 rash (Wk 6) Gr 3 transaminitis (Wk 7) Gr 3 hypophysitis (Wk 12) Gr 2 thyroiditis (Wk 36) |
Gr 3 colitis (Wk 1)
Gr 3 pleural effusions +pneumonitis (Wk 2) Gr 3 transaminitis (Wk 4) Gr 2 myalgias (Wk 6) Grade 3 Colitis (Wk 7) Gr 2 thyroiditis (Wk 12) Gr 3 colitis (Wk 19) |
|
| ||||
| Patients with Confirmed Stable Disease > 6wks (# cycles received) | None | None | Melanoma (15) Renal Cell Carcinoma (4) Synovial Sarcoma (4) |
Clear Cell Sarcoma (6) Osteosarcoma (5) Osteosarcoma (4) Neuroblastoma (1) |
bolded toxicities represent events that qualified as Dose Limiting Toxicities
Overall, 18 (55%) of subjects developed any grade irAE and 9 (27%) developed grade 3 or 4 irAE with gastrointestinal and liver toxicities being most common. The incidence of irAEs is within the range observed on adult studies, except for skin manifestations, which occurred at a lower frequency in pediatric and adolescent patients (Table 3). Nine subjects developed irAE after just one dose of ipilimumab. Following management guidelines and early intervention recommendations, there were no fatal irAEs observed in the subjects on this trial. There were no ophthalmologic inflammatory changes identified on evaluation each cycle.
Table 3. Toxicities Attributable to Ipilimumab at all dose levels.
| All grades | Grades 3 / 4 | |||
|---|---|---|---|---|
| # | % | # | % | |
| All patients with irAE | 18 | 55% | 9 | 27% |
| Colitis/Diarrhea | 4 | 12% | 3 | 9% |
| Rash | 4 | 12% | 0 | 0% |
| Transaminitis | 3 | 9% | 2 | 6% |
| Endocrinopathies | 3 | 9% | 1 | 3% |
| Other irAE | 3 | 9% | 3 | 9% |
| >1 irAE | 2 | 6% | 2 | 6% |
Toxicity in adolescent patients aged 12-21
Nine adolescents were treated at the 10mg/kg dose level. Two of nine subjects had grade 3 irAE (colitis and vomiting in one patient, pleural effusion and pneumonitis in a second patient). The only grade 3/4 non-immune related adverse events occurred at 10mg/kg dose level with one subject developing grade 4 creatine kinase elevation, uric acidemia and grade 3 neutropenia that were possibly related to ipilimumab. Two other subjects exhibited grade 1 or 2 toxicities that were likely related to drug administration including myalgias and autoimmune thyroiditis.
Outside of the DLT evaluation period, three adolescent patients had clinically significant irAE. After 4 doses of ipilimumab at 5mg/kg, a subject with renal cell carcinoma developed hypophysitis, heralded by severe headache, vision changes, and diabetes insipidus without clinical adrenal crisis. Hypophysitis was confirmed by MRI and required steroids for resolution of symptoms. The patient was able to taper off steroids approximately one month later but remained with panhypopituitarism that required hormone replacement (Fig 1a). One subject with clear cell sarcoma received ipilimumab at 5mg/kg and developed colitis during week 12 that initially responded to steroids. Approximately 4 weeks into a steroid taper, the patient presented with a colon perforation requiring surgical intervention. An adolescent with osteosarcoma developed grade 3 colitis two weeks into maintenance therapy with 10mg/kg. The colitis was not sufficiently controlled with corticosteroids and required infliximab for resolution. In each of these cases, the malignancy had been stable prior to development of late DLT but no further ipilimumab was administered.
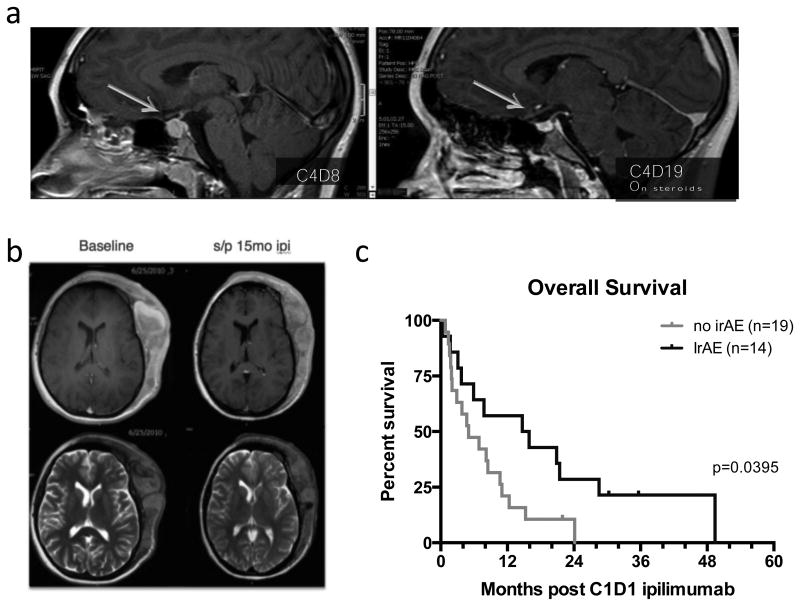
Figure 1. Clinical findings.
a. Hypophysitis developed in one adolescent with renal cell carcinoma on trial, heralded by vision changes, headache, and polyuria on cycle 4 day 8. MRI revealed pituitary enlargement that resolved within 2 weeks of corticosteroid treatment (cycle 4 day 19).
b. Best overall response in an adolescent with unresectable scalp melanoma with visible improvement and 30% decrease in size of this target mass.
c. Despite lack of complete or partial responses, overall survival in patients with grade 2 or greater immune related adverse events (irAE) was increased compared to overall survival in patients who only had grade 1 irAE or did not have any irAE.
Toxicity in young child cohort (2-12yo)
A total of 11 subjects younger than 12 years old were treated on this phase I study. Six patients received drug at 5mg/kg, which was determined to be the MTD for children 2-12yo. One patient with a background of severe allergic reactions had an anaphylactic reaction after receiving approximately 4mL of the first infusion. Ipilimumab was stopped and steroids were given. The patient was removed from study, but tolerated subsequent doses of ipilimumab with steroid pretreatment off study. The remaining five children <12years of age tolerated ipilimumab 5mg/kg without any toxicities. During the dose escalation phase, two children <12 years developed immune related DLT toxicity at 10mg/kg. One patient with neuroblastoma developed grade 3 colitis on day 8 of treatment and was found to have concurrent norovirus. Of note, this patient had normalization of urine catecholamines following this single dose of ipilimumab and stable disease for 2 months until starting other neuroblastoma directed therapy. The second patient had a grade 3 transaminitis that resolved within 3 weeks without steroid treatment.
Pharmacokinetics and Immunogenicity
Pharmacokinetics are summarized in Table 4. The serum half-life of ipilimumab ranged from 8-15 days with a mean that is lower than the 15 day mean half-life identified in the phase 1/2 study of ipilimumab in adults with melanoma.(26) There were no significant differences found between PK values for young children and adolescents or young adults on this study. Toxicities did not appear to be related to changes in clearance. Mean Tmax occurred between 1.58-2.44 hours and 1.56-1.93 hours post dose for all dosages for <12yo and >12yo, respectively. The serum half-life increased slightly with increasing dose levels. No immunogenicity against ipilimumab developed on study.
Table 4. Summary of ipilimumab PK parameters by age during cycle 1.
| Age < 12 years | Age 12-21 years | |||||||
|---|---|---|---|---|---|---|---|---|
| PK Parameters | 1mg/kg (n=1) |
3mg/kg (n=2) |
5mg/kg (n=5) |
10mg/kg (n=4) |
1mg/kg (n=2) |
3mg/kg (n=1) |
5mg/kg (n=8) |
10mg/kg (n=9) |
| Cmax (ug/mL) | 17.30 (N/A) |
51.86 (17.40) |
93.35 (13.43) |
193.4 (16.93) |
20.26 (12.38) |
81.50 N/A |
90.56 (25.15) |
203.3 (22.21) |
| AUC(0-21) (ug*hr/mL) | 2554.4 (N/A) |
5273.1 (16.41) |
16317.5 (19.84) |
37053.1 (17.54) |
1356.9 (347.60) |
16484.1 (N/A) |
12681.1 (72.51) |
36751.3 (13.59) |
| AUCinf (ug*hr/mL) | 3126.3 (N/A) |
5913.1 (7.79) |
24676.8 (38.72) |
56313.1 (27.35) |
6542.7 (N/A)* |
25105.7 (N/A) |
23170.9 (53.14) |
52666.3 (24.27) |
| CL (mL/hr/kg) | 0.3199 (N/A) |
0.5074 (7.79) |
0.2026 (38.72) |
0.1776 (27.35) |
0.1528 (N/A)* |
0.1195 (N/A) |
0.2158 (53.14) |
0.1899 (24.265) |
| T1/2 (day) | 8.865 (N/A) |
7.010 (3.07) |
14.33 (5.30) |
14.05 (3.44) |
14.72 (N/A)* |
13.79 (N/A) |
9.816 (5.09) |
12.74 (5.07) |
| Tmax (hours) | 1.580 (N/A) |
2.440 (0.98) |
1.808 (0.35) |
1.637 (0.14) |
1.685 (0.09) |
1.93 (N/A) |
1.562 (0.11) |
1.558 (0.06) |
All values are represented as geometric mean (%CV). Abbreviations: Cmax, maximum concentration; AUC(0-21), area under the concentration-time curve from 0 to 21 days; AUCinf, area under the concentration-time curve from 0 to infinity; CL, clearance; T1/2, plasma half-life; Tmax, time at which Cmax is observed
only one sample available for analysis
Evaluation of clinical activity
A total of four patients had confirmed stable disease and two patients had unconfirmed stable disease. There were no complete responses or partial responses by standard RECIST criteria. One patient with melanoma enrolled at the 5mg/kg dose level had prolonged stable disease and derived clinical benefit as evidenced by decreased size of a visible scalp tumor mass and normalization of proptosis (Figure 1b). This patient developed autoimmune thyroiditis at 3 months, but continued to receive ipilimumab for a total of 12 months prior to development of new bone and liver lesions. At that time point, the patient received re-induction therapy with another 4 doses of ipilimumab at 5mg/kg and showed stabilization of disease for 12 weeks before being removed from study for other cancer directed therapy. Of note, anti-thyroglobulin titers, which had been decreasing during maintenance dosing, increased during re-induction therapy. We observed an increased overall survival in patients who developed grade 2 or greater immune related adverse events compared to patients who did not have any immune toxicities (figure 1c).
Immunomodulatory Activity
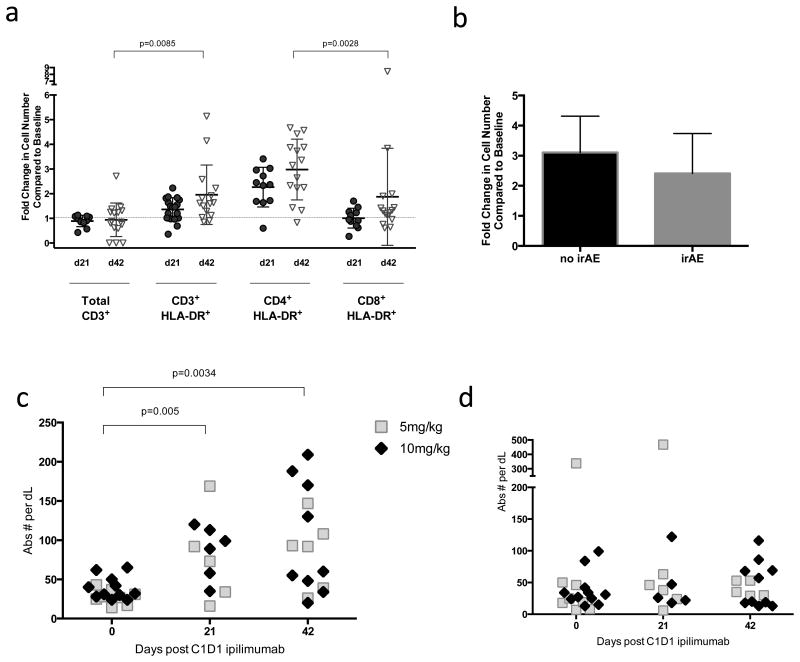
Significantly increased numbers of circulating cycling (ki67+) and activated (HLA-DR+) CD3 T cells were identified in patients after one or two cycles of ipilimumab (Figure 2). The fold increase in activated CD3+ cells was mainly due to activated CD4+ T cells as CD8+ T cells did not significantly increase in number following ipilimumab administration (Figure 2a and Supplemental Figure 1). The increase in activated CD4+HLA-DR+ T cells did not correlate with development of an irAE (figure 2b). While increases were observed in cycling T cells (Figure 2c) there was no increase in absolute numbers of FoxP3+ regulatory T cells was identified in any subject compared to baseline (Figure 2d). There was no difference in immune activation when comparing patients with melanoma to sarcoma or when comparing adolescents to younger age children.
Figure 2. T cell correlative data.
a. Fold change on day 21 (circles) and day 42 (open triangles) in absolute number of T cells (CD3+) and activated T cell subsets expressing HLA-DR.
b. Fold change in CD4+HLADR+ cells does not differ between patients who had irAE vs those who did not have any irAE.
c. Absolute numbers of CD3+ki67+ cycling T cells are increased at day 21 or day 42 following first dose of ipilimumab compared to baseline in patients receiving 5mg/kg (open squares) and 10mg/kg (diamonds).
d. No change was observed in absolute numbers of CD4+CD25+FoxP3+ cells at day 21 or day 42 following first dose of ipilimumab in patients receiving 5mg/kg (open squares) and 10mg/kg (diamonds).
Study subjects underwent baseline analysis of endocrine function including TSH, T4, GH, and ACTH and followed longitudinally on study. Two patients developed increasing levels of anti-thyroglobulin antibodies following administration of ipilimumab. One patient had low-level anti-thyroglobulin antibodies at baseline without clinical or laboratory manifestations. In both cases, the patients had elevated TSH on study but normal thyroid hormone levels.
Discussion
In this pediatric phase I trial, ipilimumab was tolerated with similar toxicities and pharmacokinetics as reported in adult studies. No grade 2 or higher immune related toxicities were identified at doses of 3mg/kg or less, thus demonstrating that pediatric patients tolerate ipilimumab administration at the FDA approved dose of 3mg/kg. Because early studies suggested a dose related response in adult trials(5, 13, 14), dose escalation to 10mg/kg was undertaken in this pediatric study. We observed an increase in toxicities at 5mg/kg and 10mg/kg, with all grade 3 or 4 irAE occurring in these higher dose levels. Although an increased rate of irAE observed in children <12 years at the 10mg/kg dose level resulted in expansion of separate cohorts for children less than or greater than 12 years of age, we cannot conclude from this study that the toxicity of ipilimumab varies with age. Importantly however, at higher doses, ipilimumab clearly induced a break in immune tolerance, which was often seen early in induction, with many of immune related events occurring in the first 6 weeks. It is difficult to state an exact recommended phase 2 dose because the approved dose is lower than the maximum tolerated dose for the pediatric age groups in this trial.
The lack of objective responses in this trial could be due to the small sample size in this phase 1 trial. With an objective response rate of 13% across a large 855 patient expanded access study of adults with metastatic melanoma, we could statistically have missed any active signal in this relatively small phase I study with only 12 subjects with melanoma.(9) Overall survival was not a primary objective in this non-randomized trial and there are few reports of metastatic pediatric melanoma to provide a historical survival comparison, so we are unable to say whether ipilimumab contributed to any meaningful increase in overall survival in melanoma patients treated on this study. Stabilization of disease was observed in several sarcomas, including clear cell sarcoma and osteosarcoma consistent with preclinical studies suggesting a role for T cells in control of osteosarcoma. Interestingly, the overall survival appears to have been better in patients who developed irAE compared to those who did not have any irAE suggesting a possible common mechanism of activity in this heterogenous group of tumors (Figure 1).
Disease-related factors could contribute to the absence of clinical activity observed. The majority of patients on this trial had large tumor burden, which could have a negative impact on T cell-mediated cytotoxicity. Anti-tumor activity following checkpoint inhibition also relies on a recognizable antigen and the presence of already activated T cells that can recognize and kill the tumor. Melanomas in adults have multiple mutations that may be serve as neoantigens for T cell response(27), and recent data suggests that mutated proteins may be important for immune responses to non-melanoma tumors(28, 29). Pediatric tumors typically have low levels of tumor-associated mutations(30, 31), and could prove to be less immunogenic overall. Furthermore, the microenvironment of the sarcomas and other pediatric tumors enrolled on the trial may provide other obstacles to an effective T cell response (e.g. MDSC, Treg) so that effective immune checkpoint therapy may require combination therapies for maximal impact (25, 32).
Immune-related response criteria had not been developed at the start of trial so standard RECIST criteria were used to determine responses. Analysis revealed that no patients would have been able to remain on therapy after radiologic progression utilizing modified RECIST that takes into account the possibility of delayed responses. Due to concern that the pediatric tumor types on phase 1 trials tend to progress at first imaging, patients were imaged at 6 weeks rather than waiting to the end of induction at 12 weeks which had been standard on previous melanoma trials. Indeed the majority of patients were taken off treatment at 6 weeks for progressive disease.
This study provides a foundation for the use of future immune checkpoint inhibitors in children. Algorithms to deal with immune-related toxicities were applied safely to pediatric patients. As with all immunotherapy checkpoint inhibitor trials, management includes education of patient/family and medical care teams to intervene early and appropriately following the onset of immune related symptoms. Given the toxicities and inability to predict toxicity or response, we do not see a role for single agent ipilimumab in the pediatric tumors; however, the application of anti-CTLA-4 in combination with other checkpoint inhibitors or immune-modifying agents may hold promise and further pediatric studies are anticipated. Starting ipilimumab doses in combination trials using levels tolerated in adult trials would be supported by the findings of this study.
Supplementary Material
Acknowledgments
Funding: This research was supported in part by the Intramural Research Program of the NIH, NCI. There is no extramural grant number to include.
Footnotes
Note: no changes have been made to the authors since the original submission
Publisher's Disclaimer: Disclaimers: None
Contributor Information
Melinda S. Merchant, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD
Matthew Wright, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Kristin Baird, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Leonard H. Wexler, Departments of Pediatrics and Medicine, Memorial Sloan Kettering Cancer Center, New York, NY and Weill-Cornell Medical College, New York, NY
Carlos Rodriguez-Galindo, Pediatric Oncology, Dana Farber Cancer Institute, Boston, MA.
Donna Bernstein, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Cindy Delbrook, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Maya Lodish, National Institute of Child Health and Human Development, NIH, Bethesda, MD.
Rachel Bishop, National Eye Institute, NIH, Bethesda, MD.
Jedd D. Wolchok, Departments of Pediatrics and Medicine, Memorial Sloan Kettering Cancer Center, New York, NY and Weill-Cornell Medical College, New York, NY
Howard Streicher, Investigational Drug Branch, National Cancer Institute, NIH, Bethesda, MD.
Crystal L. Mackall, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Annals of surgical oncology. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The Lancet Oncology. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 6.O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21:1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 7.Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Investigational new drugs. 2011;29:489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 8.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 9.Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. Journal of translational medicine. 2014;12:116. doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber J, Hamid O, Amin A, O’Day S, Masson E, Goldberg SM, et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer immunity. 2013;13:7. [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 13.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:2174–80. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. The oncologist. 2007;12:864–72. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 17.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocrine-related cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. The oncologist. 2013;18:733–43. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin MT, Xing Y, Hayes-Jordan AA, Lally KP, Cormier JN. Melanoma incidence rises for children and adolescents: an epidemiologic review of pediatric melanoma in the United States. Journal of pediatric surgery. 2013;48:2207–13. doi: 10.1016/j.jpedsurg.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Paradela S, Fonseca E, Pita-Fernandez S, Kantrow SM, Diwan AH, Herzog C, et al. Prognostic factors for melanoma in children and adolescents: a clinicopathologic, single-center study of 137 Patients. Cancer. 2010;116:4334–44. doi: 10.1002/cncr.25222. [DOI] [PubMed] [Google Scholar]
- 21.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3264–70. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England journal of medicine. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant MS, Melchionda F, Sinha M, Khanna C, Helman L, Mackall CL. Immune reconstitution prevents metastatic recurrence of murine osteosarcoma. Cancer immunology, immunotherapy : CII. 2007;56:1037–46. doi: 10.1007/s00262-006-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science translational medicine. 2014;6:237r. doi: 10.1126/scitranslmed.3007974. a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 27.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013;122:1105–13. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.