Abstract
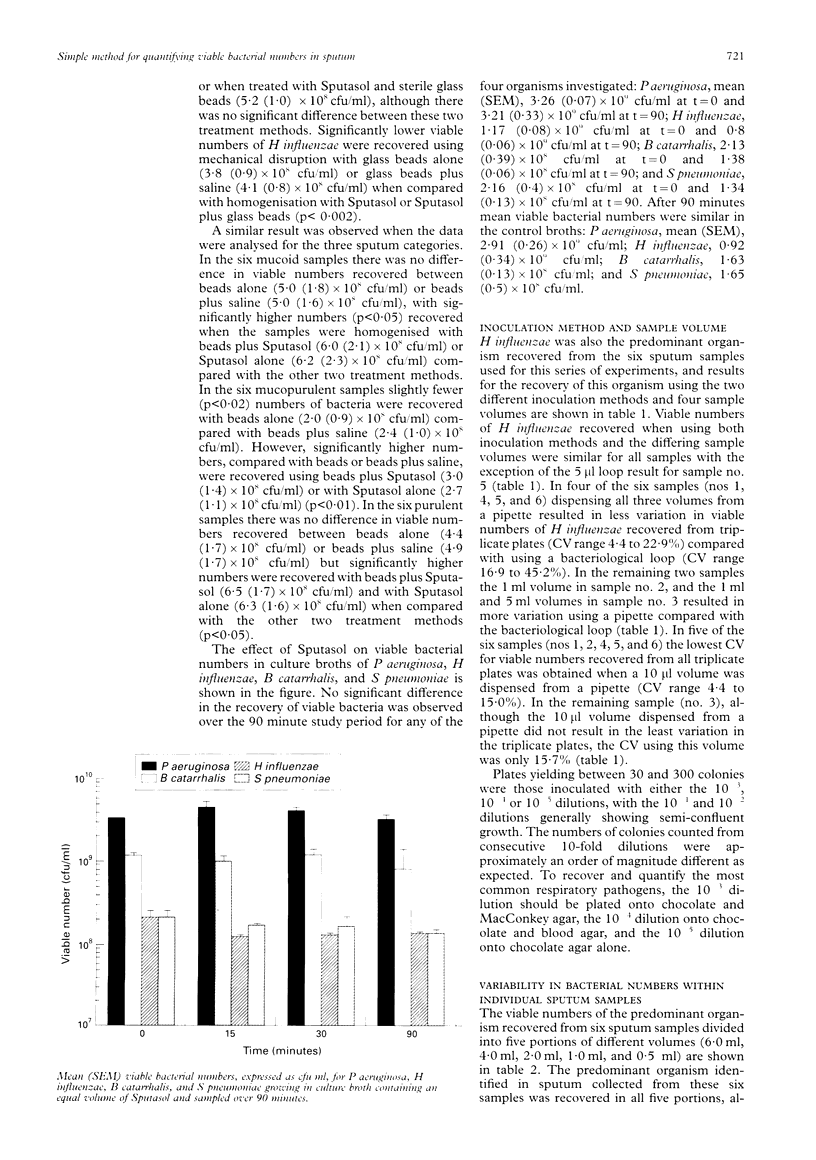
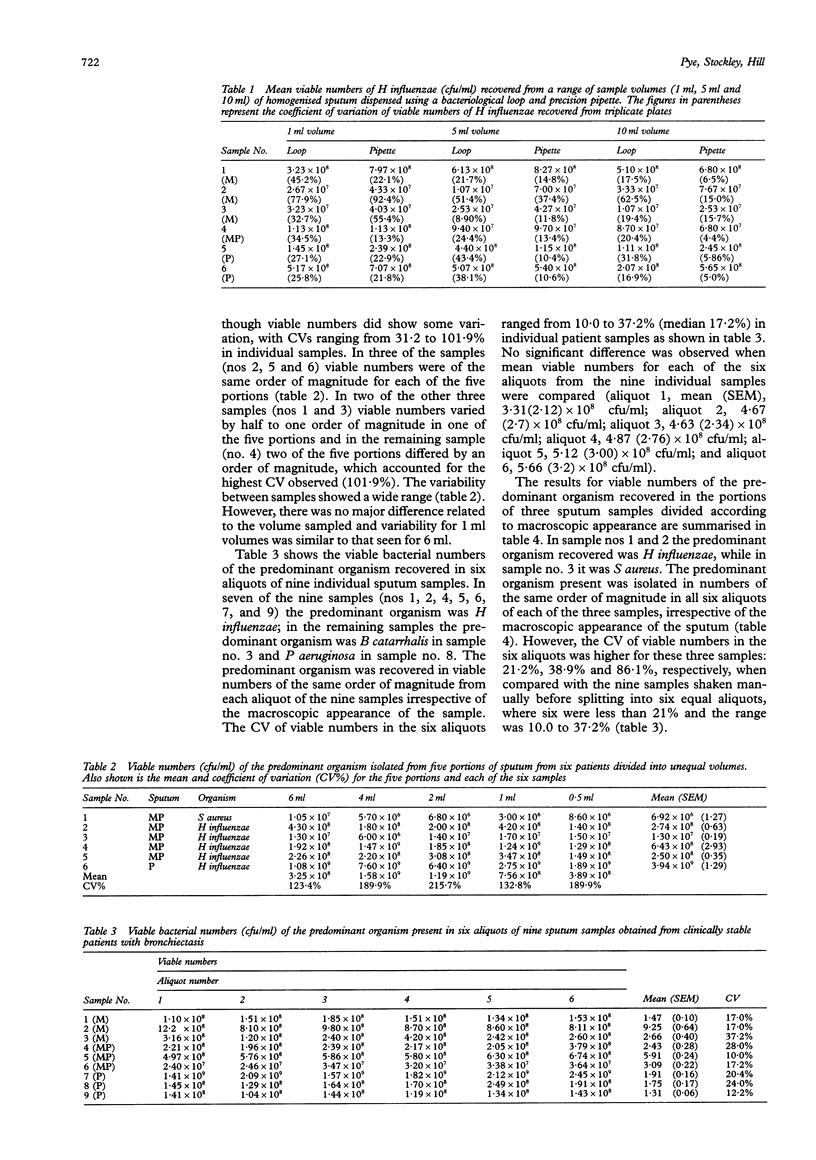
AIMS--To establish a simple method of quantitative culture for determining the viable bacterial numbers present in expectorated sputum samples. METHODS--Sputum samples were homogenised with dithiothreitol, sterile saline or glass beads to determine which method recovered the greatest number of viable bacteria. Culture broths were also incubated with dithiothreitol and sampled over time to determine its effect on bacterial viability. Sputum samples homogenised with dithiothreitol were diluted in sterile saline and sampled using either standard bacteriological loops or a precision pipette to determine which method resulted in the least variation. RESULTS--Homogenisation of sputum using dithiothreitol increased the recovery of viable bacteria compared with sterile glass beads and/or saline, with no apparent effect on bacterial viability when incubated with culture broths. By inoculating agar plates with 10(-3), 10(-4) and 10(-5) dilutions of the homogenised sputum sample, all potential pathogens could easily be identified. A 10 microliter sample volume dispensed by precision pipette and spread with a "hockey stick" resulted in the least variation between plates (less than 16%) and an even distribution of bacterial colonies. Numbers of viable bacteria recovered from different aliquots of individual sputum samples were generally of the same order of magnitude. CONCLUSIONS--This method represents a relatively quick and simple technique for accurately quantifying viable bacteria present in sputum samples. The use of a small portion appears to be representative of the sample as a whole.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers A. C., Fletcher R. D. Accuracy of calibrated-loop transfer. J Clin Microbiol. 1983 Jul;18(1):40–42. doi: 10.1128/jcm.18.1.40-42.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Finegold S. M. Bacteriology of expectorated sputum with quantitative culture and wash technique compared to transtracheal aspirates. Am Rev Respir Dis. 1978 Jun;117(6):1019–1027. doi: 10.1164/arrd.1978.117.6.1019. [DOI] [PubMed] [Google Scholar]
- Butt H. L., Clancy R. L., Cripps A. W., Murree-Allen K., Saunders N. A., Sutherland D. C., Hensley M. J. Bacterial colonisation of the respiratory tract in chronic bronchitis. Aust N Z J Med. 1990 Feb;20(1):35–38. doi: 10.1111/j.1445-5994.1990.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Currie D. C., Higgs E., Metcalfe S., Roberts D. E., Cole P. J. Simple method of monitoring colonising microbial load in chronic bronchial sepsis: pilot comparison of reduction in colonising microbial load with antibiotics given intermittently and continuously. J Clin Pathol. 1987 Aug;40(8):830–836. doi: 10.1136/jcp.40.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Doherty C., Glass S. Rational parameters for antibiotic therapy in patients with cystic fibrosis. Infection. 1987 Jul-Aug;15(4):300–307. doi: 10.1007/BF01644142. [DOI] [PubMed] [Google Scholar]
- Hammerschlag M. R., Harding L., Macone A., Smith A. L., Goldmann D. A. Bacteriology of sputum in cystic fibrosis: evaluation of dithiothreitol as a mucolytic agent. J Clin Microbiol. 1980 Jun;11(6):552–557. doi: 10.1128/jcm.11.6.552-557.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. L., Morrison H. M., Burnett D., Stockley R. A. Short term response of patients with bronchiectasis to treatment with amoxycillin given in standard or high doses orally or by inhalation. Thorax. 1986 Jul;41(7):559–565. doi: 10.1136/thx.41.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S. R., Zastrow J. E., Kory R. C. Sputum liquefying agents: a comparative in vitro evaluation. J Lab Clin Med. 1969 Aug;74(2):346–353. [PubMed] [Google Scholar]
- Kalin M., Lindberg A. A., Tunevall G. Etiological diagnosis of bacterial pneumonia by gram stain and quantitative culture of expectorates. Leukocytes or alveolar macrophages as indicators of sample representativity. Scand J Infect Dis. 1983;15(2):153–160. doi: 10.3109/inf.1983.15.issue-2.05. [DOI] [PubMed] [Google Scholar]
- Kilbourn J. P., Campbell R. A., Grach J. L., Willis M. D. Quantitative bacteriology of sputum. Am Rev Respir Dis. 1968 Nov;98(5):810–818. doi: 10.1164/arrd.1968.98.5.810. [DOI] [PubMed] [Google Scholar]
- Koch C., Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993 Apr 24;341(8852):1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- MAY J. R. The bacteriology of chronic bronchitis. Lancet. 1953 Sep 12;265(6785):534–537. doi: 10.1016/s0140-6736(53)90274-8. [DOI] [PubMed] [Google Scholar]
- Medici T. C., von Graevenitz A., Shang H., Böhni E., Wall M. Gram stain and culture of morning and 24 h sputum in the diagnosis of bacterial exacerbation of chronic bronchitis: a dogma disputed. Eur Respir J. 1988 Dec;1(10):923–928. [PubMed] [Google Scholar]
- Monroe P. W., Muchmore H. G., Felton F. G., Pirtle J. K. Quantitation of microorganisms in sputum. Appl Microbiol. 1969 Aug;18(2):214–220. doi: 10.1128/am.18.2.214-220.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J. A., Cheng A., Chan H. S., Poon D., French G. The bacteriology of bronchiectasis in Hong Kong investigated by protected catheter brush and bronchoalveolar lavage. Am Rev Respir Dis. 1989 Jan;139(1):14–17. doi: 10.1164/ajrccm/139.1.14. [DOI] [PubMed] [Google Scholar]
- Pirtle J. K., Monroe P. W., Smalley T. K., Mohr J. A., Rhoades E. R. Diagnostic and therapeutic advantages of serial quantitative cultures of fresh sputum in acute bacterial pneumonia. Am Rev Respir Dis. 1969 Dec;100(6):831–838. doi: 10.1164/arrd.1969.100.6.831. [DOI] [PubMed] [Google Scholar]
- Stockley R. A. Chronic bronchitis: the antiproteinase/proteinase balance and the effect of infection and corticosteroids. Clin Chest Med. 1988 Dec;9(4):643–656. [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M. Effect of antibiotic treatment on sputum elastase in bronchiectatic outpatients in a stable clinical state. Thorax. 1984 Jun;39(6):414–419. doi: 10.1136/thx.39.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. J., Martin D. E. Quantitative sputum culture as a means of excluding false positive reports in the routine microbiology laboratory. J Clin Pathol. 1972 Aug;25(8):697–700. doi: 10.1136/jcp.25.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Roberts M. C., Owens L., Fife M., Smith A. L. Selective media for the quantitation of bacteria in cystic fibrosis sputum. J Med Microbiol. 1984 Apr;17(2):113–119. doi: 10.1099/00222615-17-2-113. [DOI] [PubMed] [Google Scholar]



