Abstract
Sphingolipidoses are a class of inherited diseases that result from the toxic accumulation of undigested sphingolipids in lysosomes and other cellular membranes. Sphingolipids are particularly enriched in cells of the nervous system, and their excessive accumulation during disease has a significant impact on the nervous system. Neuronal dysfunction followed by neurological compromise is a common feature in many of these diseases, however the underlying mechanisms that cause vulnerability of neurons are not fully understood. The plasma membrane plays a critical role in regulating cellular survival pathways, and its dysfunction has been implicated in neuronal failure in various adult onset neuropathies. In the context of sphingolipidoses, we hypothesized that the gradual accumulation of undigested lipids in plasma membranes would cause local disruptions in lipid raft domains, leading to deregulation of multiple signaling pathways important for neuronal survival and function. We propose that defects in downstream signaling as a result of membrane dysfunction is a common mechanism underlying neuronal vulnerability in sphingolipid storage disorders with neurological compromise.
Keywords: Krabbe's disease, sphingolipids, lysosomal storage disorder, neuronal vulnerability, lipid rafts, neurotrophin signaling, insulin-like growth factor signaling, synaptic dysfunction
INTRODUCTION AND HYPOTHESIS
The cell membrane acts primarily as a barrier to protect the cell's interior from the outside environment. In addition to this fundamental role, the membrane also participates in critical cellular functions such as signaling and cell-to-cell communication to promote survival. In the context of the neuron, the highly polarized plasma membrane plays a variety of important roles in signaling. The cell body and associated dendrites have cell-surface receptors that receive signals from the environment; axonal membranes mediate nerve impulse conduction and also regulate myelination via glial signaling, and synaptic membranes participate in the release and uptake of signaling molecules important for cellular communication and survival. Therefore, membrane integrity and function are critical for neuronal survival and growth, and their disruption could lead to neurodegeneration. Since a membrane's function is strongly influenced by its architecture, it is not difficult to imagine that in cases where membrane structure and composition is acutely altered, such as in sphingolipid storage disorders characterized by excessive accumulation of undigested lipids, there will be adverse consequences for neuronal signaling. In this context, we hypothesized that the gradual accumulation of undigested sphingolipids in plasma membranes would cause local disruptions in lipid raft domains resulting in deregulation of associated signaling pathways important for neuronal survival and function. We propose that defects in downstream signaling resulting from membrane dysfunction is a common mechanism underlying neuronal vulnerability in sphingolipid storage disorders with neurological compromise.
SPHINGOLIPIDS AND THEIR EFFECT ON NEURONAL SURVIVAL
Sphingolipids are a class of structural membrane lipids enriched in cells of the nervous system. In addition to influencing membrane structure and fluidity, certain sphingolipids also function as bioactive molecules impacting cellular signaling and proliferation (e.g. ceramide and sphingosine) (Zhou and Blom 2015). Given these critical roles, physiological levels of sphingolipids are highly regulated within a cell by the activities of metabolic enzymes through a series of synthesis and degradation steps. Failure to breakdown by-products generated in this metabolic pathway due to genetic deficiencies in various catabolic enzymes results in a class of inherited lipid storage diseases called sphingolipidoses, characterized by the toxic accumulation of undigested sphingolipids in lysosomes and other cellular membranes. Notable members of this class include Krabbe disease (primarily galactosylsphingosine accumulation), Niemann-Pick disease (sphingomyelin and cholesterol accumulation), Tay-Sachs disease (GM2 gangliosides accumulation), Gaucher disease (glucocerebrosides accumulation), Metachromatic Leukodystrophy (sulfatide accumulation) and Fabry disease (glycolipid accumulation).
Since sphingolipids have important structural and functional roles in neuronal tissue, their excessive accumulation in sphingolipidoses have a significant impact on the nervous system function. A common complication in a majority of sphingolipidoses is defects in neuronal activity followed by neurologic decline (Cox and Cachon-Gonzalez 2012), however the underlying mechanism for neuronal vulnerability is not fully understood. One attractive explanation is that excessive sphingolipid accumulation on the plasma membrane, especially in lipid raft domains, leads to its structural disruption and interferes with cellular signaling important for neuronal survival and function. Hence, neuronal vulnerability would be a consequence of changes in signaling pathways that critically depend on membrane integrity. Such an example is found in a mouse model of Krabbe disease, twitcher mouse, where toxic accumulation of galactosylsphingosine (psychosine) disrupts lipid rafts resulting in the inhibition of survival signals such as PKC, Akt and ERK, defects in axonal transport and synaptic maintenance, and neuronal degeneration through a dying-back mechanism (White et al. 2009; Cantuti Castelvetri et al. 2013; Cantuti-Castelvetri et al. 2015; Castelvetri et al. 2011; Hawkins-Salsbury et al. 2013; Teixeira et al. 2014). Glucosylceramide accumulation in Gaucher disease (Hattersley et al. 2013), sulfatide accumulation in Metachromatic leukodystrophy (Moyano et al. 2014), globotriaosylceramide accumulation in Fabry disease (Labilloy et al. 2013), and cholesterol accumulation in Niemann-Pick Type C (Vainio et al. 2005) also preferentially take place in lipid rafts and cause altered lipid raft dynamics. Therefore, it is conceivable that they involve neuronal vulnerability through a similar mechanism that includes membrane disruption as a root cause for downregulation of survival signals leading to neurodegeneration.
THE INFLUENCE OF MEMBRANE ARCHITECTURE ON NEURONAL SIGNALING
The architectural make-up of a cell's membrane is determined by the types and the relative amounts of each of its building blocks, namely the different kinds of lipids (phospholipids, glycolipids and cholesterol) and their associated proteins (e.g. glycoproteins, membrane receptors). The membrane, however, is not uniform in its composition and shows regional diversity in its form and function, which is influenced by its local biophysical/biochemical properties. These include regional curvature of the membrane, local concentrations of membrane-associated proteins, and membrane fluidity that determines the degree of lateral movement and protein interactions in the lipid bilayer (for an extended discussion on the influence of sphingolipids on membrane dynamics, see D'auria and Bongarzone in this issue).
The cell membrane is a fluidic bilayer with local areas of increased rigidity resulting from enrichment of saturated lipids such as cholesterol, glycolipids and sphingolipids. These rigid domains are termed lipid rafts, nanometer scale highly dynamic structures that function as platforms for regulating cellular signaling (Simons and Ikonen 1997). Many receptors belonging to the tyrosine-kinase family (RTK) and their downstream effectors are known to be enriched in neuronal lipid rafts, such as the Insulin-like Growth Factor and insulin (IGF-R/IR) receptors, Nerve Growth Factor (NGF) receptor TrkA, brain-derived neurotrophic factor (BDNF) receptor TrkB, and glial-derived neurotrophic factor (GDNF) receptor c-Ret, and signaling intermediates such as PI3K, MAPK, and PKC (Pike 2003; Smart et al. 1999). In many cases, recruitment of the receptor into lipid rafts following activation, or receptor activation within the raft itself, is critical for the type and duration of downstream signaling and can lead to dramatically different cellular outcomes if the receptor was to be activated elsewhere. Additionally, disruption of lipid rafts through cholesterol depletion or other destabilizing agents has inhibitory effects especially on RTK-mediated signals, most notably the PI3K/Akt and ERK pathways in neurons (Fukui et al. 2015). These observations highlight the importance of plasma membrane compartmentalization and lipid raft integrity in maintaining signaling pathways important for cellular growth and survival.
DISRUPTION OF LIPID RAFT FUNCTION AS A BASIS FOR NEURONAL VULNERABILITY IN SPHINGOLIPIDOSES
Survival of a neuron is largely determined by its synaptic activity, axonal transport dynamics, cellular energy metabolism and state of myelination. Dysregulation in any of these processes can leave neurons vulnerable and prone to degeneration. Many of these activities are regulated by signals received from the extracellular space via receptors enriched in neuronal lipid raft domains. Neurons have highly polarized membranes with specialized regions (the cell body/soma, the axon and the synaptic terminal) that perform distinct functions required for neuronal survival. Evidence suggest that each of these regions contains lipid raft domains (Faivre-Sarrailh and Rougon 1993; Hering et al. 2003; Zonta and Minichiello 2013), although there likely is inherent differences in the composition and function of these rafts that is determined by regional signaling needs (Fig. 1). A prediction from this assumption would be that activities performed by distinct regions of a neuron may have differential sensitivities to membrane disruption. This differential sensitivity would be influenced by the type of lipid rafts each region harbors, and how much their activity utilizes signaling pathways that depend on intact lipid raft function. For example, membrane disruption from accumulation of undigested lipids in sphingolipidoses may affect signaling pathways required for synaptic function more, or sooner, than signaling that takes place on the somatic side of the membrane.
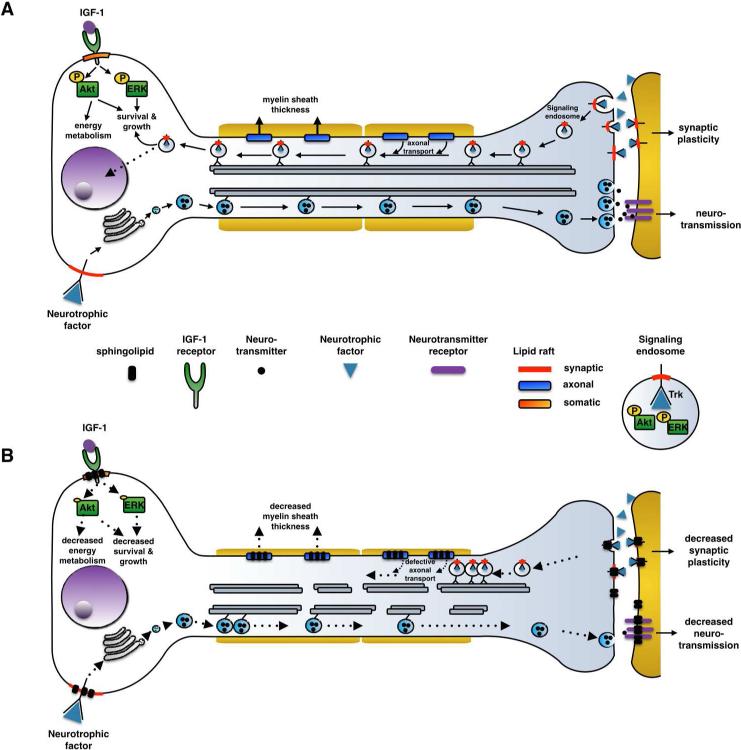
Figure 1.
Model of dysregulation of neuronal survival pathways in sphingolipidoses. A) In a healthy neuron somatic, axonal and synaptic membranes contain lipid rafts - possibly with differing compositions- that contribute to distinct survival functions based on regional signaling needs. In the synapse, target-derived neurotrophic factors activate raft associated Trk receptors on both the pre- and post-synaptic side. Ligand-bound Trk activates pathways important for synaptic plasticity in the post-synaptic neuron/dendrite. On the pre-synaptic side, Trk receptors are internalized by endocytosis and form ‘signaling endosomes’ complete with activated downstream effectors such as phospho-Akt and phospho-Erk. Signaling endosomes are then transferred to the cell body through retrograde axonal transport along microtubules, where they trigger a survival response. Axonal lipid rafts may contribute to this process by coordinating signaling molecules that regulate the functions of dynein and kinesin motors. In a separate role, axonal rafts could also mediate signals that regulate myelin sheath thickness around the axon. In contrast, somatic rafts mediate IGF-1 and neurotrophic receptor signaling that are important for cellular energy metabolism needed for growth and survival, and secretion of neurotransmitters respectively. B) In sphingolipidoses, the accumulation of undigested lipids in rafts disrupts signaling pathways important for the function of that particular membrane region. There could be regional differences in the extent of signaling activity that depends on intact lipid rafts, and could explain the differential sensitivity observed especially in the synapse, which is first to fail in function. Decreased receptor internalization at the synapse due to changes in biophysical characteristics of the membrane followed by microtubule destabilization and axonal transport defects lead to dying-back pathology, and eventually neuronal apoptosis.
Additionally, in the context of sphingolipidoses, one would expect the rate of inhibition in signaling to be influenced by the extent of membrane disruption, which in turn is affected by the rate of sphingolipid accumulation (endogenous synthesis and uptake from the extracellular space), and the capacity of the membrane to buffer the biophysical changes induced by this accumulation over time (i.e. dilution factor). Since sphingolipid accumulation preferentially disrupts lipid raft structure (White et al. 2009; Hawkins-Salsbury et al. 2013), neuronal signaling pathways that depend primarily on lipid raft integrity such as neurotrophic and IGF-receptor signaling will be expected to be affected the most.
Neurotrophic signaling
Neurotrophic factors (BDNF, NGF, NT-3/4 and GDNF) are essential for survival, growth and differentiation of neurons. They are secreted from the target tissue (e.g. post-synaptic neuron or muscle), and activate Trk family of tyrosine kinase receptors to initiate downstream signaling including the phosphatidyl inositol-3 (PI3)-kinase, Ras/MAPK, and phospholipase C-γ1 pathways (Reichardt 2006). Trk receptors specifically localize to synaptic membranes, and ligand binding triggers distinct local signaling events at pre- and post-synaptic terminals that are important for neuronal survival (Paratcha and Ibanez 2002). Post-synaptic Trk activation is important for dendritic spine growth, morphology, and synaptic plasticity (Yoshii and Constantine-Paton 2010). Pre-synaptic activation regulates neurotransmitter release, axonal growth and neuronal survival (Jovanovic et al. 2000). Once activated at the pre-synaptic terminal, ligand-bound Trk receptors along with associated downstream signaling molecules are internalized via endocytosis to form signaling endosomes. Retrograde transport of signaling endosomes from the synapse to the cell body is regulated by dynein motors and microtubules, and this method of long-range delivery of survival signals is crucial for synaptic maintenance and cellular connectivity mediated by neurotrophic action. Impaired trafficking of endosomal vesicles resulting from defects in axonal transport has been implicated in diverse neurodegenerative disorders that show dying-back pathology including Krabbe disease and Alzheimer's disease, emphasizing the importance of this process for neuronal survival (Cantuti Castelvetri et al. 2013; Castelvetri et al. 2011; Kanaan et al. 2013) (see Cantuti-Castelvetri and Bongarzone in this issue for an expanded discussion on axonal transport defects in dying-back pathologies).
IGF-1/insulin signaling
Insulin and Insulin-like growth factor (IGF-1) are important for neuronal growth and survival in vitro and in vivo, and their actions are mediated through the PI3K/Akt and MAPK/ERK pathways (Parrizas et al. 1997). Insulin and IGF-1 promote dendritic growth and formation of synapses as well as inhibit apoptosis, and IGF-1 has a specific role in axon guidance and synaptic plasticity (Cheng et al. 2003; Scolnick et al. 2008). Similar to insulin, IGF-1 also regulates brain glucose metabolism through IGF-1 receptor-mediated Akt phosphorylation and GLUT4 expression (Cheng et al. 2000), which is important for maintaining cellular energy levels to accommodate rapid neuronal growth especially in the developing brain. IGF-1 receptor localizes mainly to lipid rafts (Huo et al. 2003), and downstream pathway activation is acutely sensitive to lipid raft disruption through cholesterol depletion (Romanelli et al. 2009). Cholesterol depletion also causes loss of dendritic synapses and spines (Hering et al. 2003), highlighting the contribution of lipid rafts to dendritic and axonal growth through IGF-1 receptor activity. Therefore, it is not surprising that dysfunction in IGF/insulin signaling is implicated in neurodegenerative diseases such as Parkinson's and Alzheimer's diseases (Bassil et al. 2014).
THE EFFECT OF SPHINGOLIPID ACCUMULATION ON SYNAPTIC MEMBRANE FUNCTION
Synaptic activity plays an important role in neuronal survival by regulating the release of neurotransmitters into the extracellular space, and receiving survival signals from surrounding tissues. Lipid rafts are enriched in synaptic membranes, and their integrity is essential for neurotrophic signaling in maintaining synaptic activity (Nagappan and Lu 2005).
Neurotrophic receptors (TrkA, TrkB, TrkC, c-Ret) are enriched in synaptic lipid rafts, and raft disruption by cholesterol depletion causes a decrease in neurotrophic signaling leading to synaptic dysfunction (Hering et al. 2003). In primary cerebellar granule neurons, BDNF binding to TrkB receptor triggers its movement into lipid rafts and activates ERK signaling on the pre-synaptic side, which is required for neurotransmitter release and synaptic plasticity (Suzuki et al. 2004). BDNF also activates the PLCγ pathway and PKC to promote survival in primary neurons (Zirrgiebel et al. 1995). Similarly, in sensory and sympathetic neurons, NGF-induced TrkA activation in lipid rafts is required for efficient stimulation of ERK1/2, Akt and PLCγ for survival (Huang and Reichardt 2001). In peripheral motor neurons, c-Ret activation by GDNF leads to different cellular responses depending on whether the receptor is activated within or outside of lipid rafts, emphasizing the importance of signaling pathway compartmentalization for neuronal function (Paratcha et al. 2001).
There is evidence of dysregulation of these pathways by sphingolipids, presumably as a result of lipid raft disruption. Our lab has shown that in the twitcher mouse model for Krabbe disease, psychosine accumulation in lipid rafts inhibits PKC (White et al. 2009) an important factor for neuronal survival. Additionally, lipid raft recruitment of the NGF receptor TrkA is impaired in twitcher mutant dorsal root ganglia neurons resulting in defective activation of ERK1/2 and Akt pathways (Teixeira et al. 2014). A decrease in dynein levels and microtubule stabilization seen in these mutant neurons also point to defects in retrograde transport, which would affect signaling endosomes. In another example, in vitro overexpression of ganglioside GM1 causes decreased membrane fluidity, and triggers TrkA movement out of lipid rafts resulting in the inhibition of autophosphorylation and downstream ERK1/2 signaling (Nishio et al. 2004). Sulfatide accumulation in an in vitro model for Metachromatic Leukodystrophy causes PDGF receptor to move out of lipid rafts and a significant decrease in Akt phosphorylation (Pituch et al. 2015).
IGF-1 receptor activity, which also depends on intact lipid rafts, may be similarly affected at synaptic rafts. Decreased receptor activation at synapses, along with defective transport of signaling endosomes, could explain the synaptic failure seen in twitcher neurons and may be a shared mechanism of neurodegeneration in multiple sphingolipidoses.
THE EFFECT OF SPHINGOLIPID ACCUMULATION ON AXONAL MEMBRANE FUNCTION
The main function of an axon is to maintain active communication between the cell body and the synaptic terminal, therefore axonal integrity is essential for neuronal survival. Axonal microtubules transport cargo vesicles containing signaling molecules that promote survival, such as retrograde signaling endosomes generated by neurotrophic signaling at the synapse and anterograde vesicles containing neurotransmitters generated at the cell body. Defects in axonal transport cause synaptic dysfunction and lead to dying-back pathology in many neurodegenerative diseases (Roy et al. 2005).
Although the contribution of axonal membranes to signaling pathways that regulate vesicular transport is currently understudied, one can speculate that axonal lipid rafts could mediate such signaling events. Lipid rafts exist on axonal membranes and they are enriched for GPI anchored proteins such as Thy-1 (Faivre Sarrailh and Rougon 1993). It remains to be explored whether these rafts also contain receptors/signaling intermediates that regulate the activity of molecules (e.g. GSK3β, p38, JNK) required for the transport of vesicles by molecular motors dynein and kinesin (Horiuchi et al. 2007; Morfini et al. 2002; Morfini et al. 2013). Our lab has shown that in twitcher mice psychosine leads to abnormal activation of PP1 protein phosphatase and GSK3β de-phosphorylation in axons, causing transport defects resulting from phosphorylation of the kinesin light chain (Cantuti Castelvetri et al. 2013). Although the mechanisms underlying abnormal PP1/GSK3β activation is currently unknown, disruption of axonal lipid rafts by psychosine may contribute to the dysregulation of signaling pathways that converge on PP1/GSK3β inhibition, such as MAPK and PI3K pathways, both of which can be activated in lipid rafts and inhibit GSK3β activity.
Axonal lipid rafts may also play a role in the myelination process. Axonal neuregulin proteins are essential for the survival and differentiation of Schwann cells, and promote axo-glial interaction and myelination (Esper and Loeb 2004). Neuregulin-1 proteins are localized to lipid rafts in synaptosomes (Cabedo et al. 2002; Frenzel and Falls 2001), and neuregulin binding to ErbB4 type receptor at postsynaptic densities trigger receptor localization into lipid rafts (Ma et al. 2003). Although there is currently not much evidence that neuregulins localize to and signal from axonal rafts, it is certainly possible this is the case. Hence, raft disruption in sphingolipidoses could interfere with axonal stimulation of neuregulin-mediated myelination, and contribute to abnormal myelination and weakened re-myelination.
THE EFFECTS OF SPHINGOLIPID ACCUMULATION ON CELLULAR ENERGY UTILIZATION
Cells need energy to function and neurons are no exception. Cellular energy homeostasis is regulated by peripheral insulin action, and also through insulin-like actions of IGF-1 in the central nervous system. IGF-1/InR receptor activity critically depends on lipid raft integrity (Romanelli et al. 2009; Taghibiglou et al. 2009), therefore it is not surprising that GM3 accumulation in Type I Gaucher patients is associated with peripheral insulin resistance (Langeveld et al. 2008), and cholesterol accumulation in Niemann-Pick Type C causes impaired insulin signaling (Vainio et al. 2005). Psychosine accumulation interferes with IGF-1 signaling in twitcher oligodendrocytes, and causes a dose dependent decrease in both Akt and ERK1/2 phosphorylation in vitro (Zaka et al. 2005), although a role in neurons is yet to be elucidated.
Peripheral neurons in particular have a high demand on cellular energy for axonal transport since they need to move vesicles over long distances to and from the cell body. Therefore, one can appreciate the importance of maintaining cellular energy homeostasis especially for peripheral neurons with long axons, as well as other types of neurons in the central nervous system. In the case of sphingolipid storage diseases, a defective energy metabolism in central and peripheral neurons due to a decrease in lipid raft-mediated insulin/IGF-1 pathways may be a contributing factor to neuronal vulnerability when combined with other regional insults such as decreased synaptic activity and insufficient vesicular transport.
SIGNIFICANCE.
Many sphingolipidoses have a neurologic component, yet neuronal dysfunction and selective vulnerability in these diseases are poorly understood. In this essay, we discuss the idea that excessive sphingolipid accumulation causes plasma membrane dysfunction resulting in deregulation of multiple downstream signaling pathways important for neuronal survival. Membrane dysfunction could represent a unified mechanism underlying neurologic compromise, therefore an important consideration for therapy would be to identify novel upstream targets to obtain the most neuroprotection. Additionally, studying membrane dysfunction in genetic sphingolipidoses could provide a unique opportunity to find a common mechanism for neuronal impairment in a range of unrelated neurodegenerative diseases.
ACKNOWLEDGMENTS
This work was partially funded by grants from the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health to TSF (F32NS082005), and to ERB (R01NS065808 and R21NS087474) and from the Legacy for Angels Foundation to ERB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
TSF devised and wrote the manuscript, ERB provided critical input.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1–18. doi: 10.1016/j.pneurobio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Cabedo H, Luna C, Fernandez AM, Gallar J, Ferrer-Montiel A. Molecular determinants of the sensory and motor neuron-derived factor insertion into plasma membrane. J Biol Chem. 2002;277(22):19905–19912. doi: 10.1074/jbc.M201587200. [DOI] [PubMed] [Google Scholar]
- Cantuti Castelvetri L, Givogri MI, Hebert A, Smith B, Song Y, Kaminska A, Lopez-Rosas A, Morfini G, Pigino G, Sands M, Brady ST, Bongarzone ER. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J Neurosci. 2013;33(24):10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Maravilla E, Marshall M, Tamayo T, D'Auria L, Monge J, Jeffries J, Sural-Fehr T, Lopez-Rosas A, Li G, Garcia K, van Breemen R, Vite C, Garcia J, Bongarzone ER. Mechanism of neuromuscular dysfunction in Krabbe disease. J Neurosci. 2015;35(4):1606–1616. doi: 10.1523/JNEUROSCI.2431-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelvetri LC, Givogri MI, Zhu H, Smith B, Lopez-Rosas A, Qiu X, van Breemen R, Bongarzone ER. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011;122(1):35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Mervis RF, Niu SL, Salem N, Jr., Witters LA, Tseng V, Reinhardt R, Bondy CA. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res. 2003;73(1):1–9. doi: 10.1002/jnr.10634. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci USA. 2000;97:10236–10241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM, Cachon-Gonzalez MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226(2):241–254. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- Esper RM, Loeb JA. Rapid axoglial signaling mediated by neuregulin and neurotrophic factors. J Neurosci. 2004;24(27):6218–6227. doi: 10.1523/JNEUROSCI.1692-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Rougon G. Are the glypiated adhesion molecules preferentially targeted to the axonal compartment? Mol Neurobiol. 1993;7(1):49–60. doi: 10.1007/BF02780608. [DOI] [PubMed] [Google Scholar]
- Frenzel KE, Falls DL. Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts. J Neurochem. 2001;77(1):1–12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. [DOI] [PubMed] [Google Scholar]
- Fukui K, Ferris HA, Kahn CR. Effect of cholesterol reduction on receptor signaling in neurons. J Biol Chem. 2015;290(44):26383–26392. doi: 10.1074/jbc.M115.664367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley KJ, Hein LK, Fuller M. Lipid composition of membrane rafts, isolated with and without detergent, from the spleen of a mouse model of Gaucher disease. Biochem Biophys Res Commun. 2013;442(1-2):62–67. doi: 10.1016/j.bbrc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Parameswar AR, Jiang X, Schlesinger PH, Bongarzone E, Ory DS, Demchenko AV, Sands MS. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J Lipid Res. 2013;54(12):3303–3311. doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23(8):3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17(15):1313–1317. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem. 2003;278(13):11561–11569. doi: 10.1074/jbc.M211785200. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3(4):323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA. Axonal degeneration in Alzheimer's disease: when signaling abnormalities meet the axonal transport system. Exp Neurol. 2013;246:44–53. doi: 10.1016/j.expneurol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labilloy A, Youker RT, Bruns JR, Kukic I, Kiselyov K, Halfter W, Finegold D, do Monte SJ, Weisz OA. Altered dynamics of a lipid raft associated protein in a kidney model of Fabry disease. Mol Genet Metab. 2013;111(2):184–192. doi: 10.1016/j.ymgme.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld M, Ghauharali KJ, Sauerwein HP, Ackermans MT, Groener JE, Hollak CE, Aerts JM, Serlie MJ. Type I Gaucher disease, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab. 2008;93(3):845–851. doi: 10.1210/jc.2007-1702. [DOI] [PubMed] [Google Scholar]
- Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Lu B, Xiong WC, Salter MW, Weinberg RJ, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23(8):3164, 3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. Embo J. 2002;21(3):281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, Tavassoli E, Bagnato C, Tiwari A, Hayward LJ, Pigino GF, Watterson DM, Huang CF, Banker G, Brown RH, Jr., Brady ST. Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS One. 2013;8(6):e65235. doi: 10.1371/journal.pone.0065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano AL, Li G, Lopez-Rosas A, Mansson JE, van Breemen RB, Givogri MI. Distribution of C16:0, C18:0, C24:1, and C24:0 sulfatides in central nervous system lipid rafts by quantitative ultra-high-pressure liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;467:31–39. doi: 10.1016/j.ab.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28(9):464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Nishio M, Fukumoto S, Furukawa K, Ichimura A, Miyazaki H, Kusunoki S, Urano T, Furukawa K. Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J Biol Chem. 2004;279(32):33368–33378. doi: 10.1074/jbc.M403816200. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF. Lipid rafts and the control of neurotrophic factor signaling in the nervous system: variations on a theme. Curr Opin Neurobiol. 2002;12(5):542–549. doi: 10.1016/s0959-4388(02)00363-x. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29(1):171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3'-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272(1):154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Pituch KC, Moyano AL, Lopez-Rosas A, Marottoli FM, Li G, Hu C, van Breemen R, Mansson JE, Givogri MI. Dysfunction of platelet-derived growth factor receptor alpha (PDGFRalpha) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. J Biol Chem. 2015;290(11):7040–7053. doi: 10.1074/jbc.M115.636498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli RJ, Mahajan KR, Fulmer CG, Wood TL. Insulin-like growth factor-I-stimulated Akt phosphorylation and oligodendrocyte progenitor cell survival require cholesterol-enriched membranes. J Neurosci Res. 2009;87(15):3369, 3377. doi: 10.1002/jnr.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109(1):5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Scolnick JA, Cui K, Duggan CD, Xuan S, Yuan XB, Efstratiadis A, Ngai J. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron. 2008;57(6):847–857. doi: 10.1016/j.neuron.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19(11):7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167:1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghibiglou C, Bradley CA, Gaertner T, Li Y, Wang Y, Wang YT. Mechanisms involved in cholesterol-induced neuronal insulin resistance. Neuropharmacology. 2009;57(3):268, 276. doi: 10.1016/j.neuropharm.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Teixeira CA, Miranda CO, Sousa VF, Santos TE, Malheiro AR, Solomon M, Maegawa GH, Brites P, Sousa MM. Early axonal loss accompanied by impaired endocytosis, abnormal axonal transport, and decreased microtubule stability occur in the model of Krabbe's disease. Neurobiol Dis. 2014;66:92–103. doi: 10.1016/j.nbd.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Bykov I, Hermansson M, Jokitalo E, Somerharju P, Ikonen E. Defective insulin receptor activation and altered lipid rafts in Niemann-Pick type C disease hepatocytes. Biochem J. 2005;391(Pt 3):465–472. doi: 10.1042/BJ20050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29(19):6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70(5):304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaka M, Rafi MA, Rao HZ, Luzi P, Wenger DA. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Mol Cell Neurosci. 2005;30(3):398–407. doi: 10.1016/j.mcn.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Zhou K, Blom T. Trafficking and Functions of Bioactive Sphingolipids: Lessons from Cells and Model Membranes. Lipid Insights. 2015;8(Suppl 1):11–20. doi: 10.4137/LPI.S31615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. Characterization of TrkB receptor mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65(5):2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]
- Zonta B, Minichiello L. Synaptic membrane rafts: traffic lights for local neurotrophin signaling? Front Synaptic Neurosci. 2013;5:9. doi: 10.3389/fnsyn.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]