Abstract
The presence of life-threatening neurological symptoms in more than two thirds of lysosomal storage disease (LSDs) underlines how vulnerable the nervous system is to lysosomal failure. Neurological dysfunction in LSDs has historically been attributed to the disruption of neuronal and glial homeostasis due to the progressive jamming of the endosomal/lysosomal pathway. In neurons, a dysfunctional endosomal-lysosomal system can elicit dire consequences. Considering that neurons are largely postmitotic after birth, one can clearly understand that the inability of these cells to proliferate obliterates any possibility of diluting stored lysosomal material by means of cellular division. At its most advanced stage, this situation constitutes a terminal factor in neuronal life, resulting in cell death. However, synaptic deficits in the absence of classical neuronal cell death appear to be a common feature during the early stages in many LSDs, particularly sphingolipidoses. In essence, failure of synapses to convey their messages, even without major structural damage of the neuronal bodies, is a form of physiological death. This concept of dying back neuropathology is not only highly relevant for understanding the dynamics of the neurological decline in these diseases, but more importantly, it may constitute an important target for molecular therapies to protect perhaps the “Achilles” point in the entire physiological architecture of brain and avoid an irreversible journey to neuronal demise.
Keywords: synapse, psychosine, sphingolipids, Krabbe’s disease, dying-back pathology, neurodegeneration
LOSS OF NEURONAL CONNECTIVITY IN SPHINGOLIPIDOSES: A HYPOTHESIS
A major understanding of the neurological defects in many sphingolipidoses has relied on the histopathological examination of post-mortem material collected from patients at terminal stages of the disease. In general, these studies provided a complex picture characterized by a combination of axonal, neuronal, and myelin defects, in addition to astrogliosis, and neuroinflammation. However, much less was known about the alterations occurring during initial stages of disease. In addition, defects are often manifested in multiple cell types, tissues, and organs due to the ubiquitous expression of lysosomal enzymes. The development of animal models for sphingolipidoses has been crucial for the characterization of early neuropathological changes. Animal studies have enabled researchers to identify changes in neuronal connectivity in many of these diseases, often long before the onset of neurological signs. For example, a marked functional and structural degeneration of neuromuscular junctions was observed in the murine models of Krabbe’s disease (Castelvetri et al. 2011; Falk et al. 2015; Schmitt 1981) and in the CNS synapses of the mouse models of Niemann Pick’s disease type A and C (Arroyo et al. 2014; Pressey et al. 2012). In many cases, defects included reduced radial axonal growth, decreased conduction velocity, axonal swelling, enlargement of axon hillocks with storage bodies, and decreased synaptic activity (Arroyo et al. 2014; Castelvetri et al. 2011; Falk et al. 2015; March et al. 1997; Matthes et al. 2012; Smith et al. 2011; Toscano et al. 1998; Walkley et al. 1991). At the later stages of disease, fragmentation of axons and loss of synaptic connections were also evident (Castelvetri et al. 2011; Falk et al. 2015; Ohara et al. 2004). Therefore, we speculate that distal alterations of the synaptic apparatus initiate a dying-back pathology, which in the absence of neuronal death, establishes the basis for early physiological damage to relevant neural circuitry, contributing to the generalized neurological decline observed in many sphingolipidoses.
THE MOLECULAR ARCHITECTURE OF DYING-BACK PATHOLOGIES
Although many of the mutations causing LSDs have been identified, the mechanisms underpinning cellular defects are not fully understood. A ‘cytotoxicity hypothesis” for sphingolipidoses and other LSDs considers that the accumulation of undigested substrates interferes with lysosomal function, eventually impairing cellular homeostasis (Desnick et al. 1976). The resulting collapse of the cellular functions triggers cell death, largely via apoptosis. The extensive cell death characterizing the terminal stages in many of these diseases supports this catastrophic model. For example, accumulation of galactosylsphingosine or psychosine in Krabbe’s disease is thought to mediate the death of oligodendroglia and Schwann cells and the subsequent demyelination. However, if this model is correct, the prediction will be that neuronal death precedes the degeneration and loss of axons and dendrites in Krabbe's disease. However, studies in the twitcher mouse, the authentic mouse model for Krabbe’s disease, revealed axonal and synaptic changes in the absence of any significant neuronal death. For example, axonal spheroids, which positively stain for abnormally dephosphorylated neurofilaments, and decreased neurotransmitter release are observed during the first two weeks of life in fibers and neuromuscular junctions of the twitcher mouse(Cantuti-Castelvetri et al. 2015; Cantuti-Castelvetri et al. 2012). Twitcher neuromuscular junctions are smaller and stain positively for the apoptotic effector caspase 3. However, no changes in the total number of spinal cord motoneurons are observed until the late stages of the disease, when an increase in TUNEL+ (apoptotic) neurons is observed(Castelvetri et al. 2011). Similarly, axonal spheroids were observed in fibers of the central nervous system of the npc1−/− mouse, the mouse model of Niemann-Pick type C disease(Pressey et al. 2012) (Table I). This observation suggests that distal neurodegeneration is unlikely to be the consequence of neuronal death but rather caused by local insults, reflecting initial stages in the process of neurodegeneration (Castelvetri et al. 2011; Pressey et al. 2012).
Table I.
Summary of LSDs with signs of dying-back pathology
| Disease | Substrate | Gene | Neuronal defects | Model | Reference |
|---|---|---|---|---|---|
| Krabbe’s disease | galactosylsphingosine | galactosylceramidase | Axonal spheroids, loss of axonal calibre, structural and molecular defects in neuromuscular junctions, defects in membrane dynamics and accumulation of α-synuclein |
Twitcher mouse | (Cantuti-Castelvetri et al. 2015; Cantuti-Castelvetri et al. 2012; Cantuti Castelvetri et al. 2013; Castelvetri et al. 2011; Smith et al. 2011 Teixeira et al. 2014 White et al. 2009) |
| Pompe’s disease | glycogen | acid-alpha glucosidase |
Enlargement and fragmentation of the endplate. Aberrant levels of neurofilament and change in axonal diameter |
acid-alpha glucosidase−/− mouse |
(Falk et al. 2015) |
|
Niemann-Pick type C disease |
cholesterol | NPC1 | Degeneration of neuronal terminals, axonal spheroids filled with synaptic markers, defects in recycling of synaptic vesicles |
NPC1 −/− mouse | (Ong et al. 2001; Pressey et al. 2012; Xu et al. 2010) |
|
Niemann-Pick type A disease |
sphingomyelin | Acid sphingomyelinase |
Axonal spheroids, loss of dendrites and defects in vesicular traffic |
ASM −/− mouse and ASM −/− neurons |
(Galvan et al. 2008; Kuemmel et al. 1997; Sarna et al. 2001) |
|
Mucopolysaccharidosis III type B |
heparan sulfate | α-N-acetylglucosa minidase |
Accumulation of α-synuclein, ubiquitin and phosphorylated tau in the cell body, axons and dendrites. Axonal dystrophy |
Human post-mortem tissue, NAGLU−/− mouse |
(Hamano et al. 2008; Ohmi et al. 2009; Ohmi et al. 2011; Wilkinson et al. 2012) |
|
Neuronal ceroid lipofuscinoses |
autofluorescent pigments |
palmitoyl protein thioesterase (PPT1), CLN6, or cathepsin D (CLN10) |
Axonal spheroids and decrease in the pool of synaptic vesicles. Decrease in the levels of synaptic proteins |
PPT1−/−, CLN6−/− or CLN10−/− mice |
(Kielar et al. 2009; Partanen et al. 2008; Virmani et al. 2005) |
|
Metachromatic leukodystrophy |
sulfatides | arylsulfatase A | Axonal degeneration and accumulation of axoplasmic densities |
ARSA−/− mice overexpressor of either UDP-galactoseceramide galactosyltransferase (CGT) or cerebroside sulfotransferase (CST) |
(Eckhardt et al. 2007) |
| Gaucher’s disease | glucocerebroside and glucosylsphingosine |
β-d-glucosyl-N- acylsphingosine glucohydrolase |
Accumulation of α-synuclein. Decrease in striatal post-synaptic density size. Decrease in action potential amplitudes. Neurotoxic effect of glucosylsphingosine |
Inhibition of GBA with conduritol-β-epoxide (CBE). Induced pluripotent stem cells derived neurons from patients |
(Ginns et al. 2014; Schueler et al. 2003; Sun et al. 2015) |
Once “dying-back” neurodegeneration is initiated at the distal end of the axon, it has to slowly progress towards the neuronal soma (Figure 1). Various factors, not mutually exclusive, may contribute to this mechanism, including deficiencies in axonal transport, neurofilaments, microtubules, and deregulated signalling (For an extended discussion of signalling deficits, see Sural & Bongarzone, this issue). Axons are the largest compartment in most neurons, and hence, highly dependent on regulatory mechanisms to avoid axonal death (Coleman 2005). As most of the synthesis of proteins and lipids occurs in the cell body, neurons rely on a tightly regulated system of molecular motors that travel on microtubules to translocate membrane cargoes. Delivery of cargoes is regulated by specific kinases, such as GSK3β and casein kinase 2, which phosphorylate molecular motors and therefore, trigger the release of the motor and/or the cargo where they are most needed (Morfini et al. 2002; Pigino et al. 2009). Alterations in the activity of these and other kinases are considered critical factors for limiting the timely delivery of cargoes to/from distal axonal domains in many adult onset neurological conditions, and it could also bear relevance in LSDs (Morfini et al. 2009). Recently, we have addressed the contributing role of axonal transport to the pathogenesis of Krabbe disease, a LSD caused by the deficiency of galactosylceramidase and the accumulation of large amounts of undegraded psychosine (Cantuti Castelvetri et al. 2013). Our group showed that psychosine promotes phosphorylation of kinesin light chains, slowing fast axonal transport in twitcher neurons by overactivation of GSK3β (Cantuti Castelvetri et al. 2013). Similar pathogenic defects may occur in other LSDs. For example, decreased neurotransmitter release and axonal accumulation of synaptic markers in Niemann-Pick’s disease type C neurons supports the idea that reduced transport of synaptic vesicles affects their delivery to the presynaptic terminal (Pressey et al. 2012).
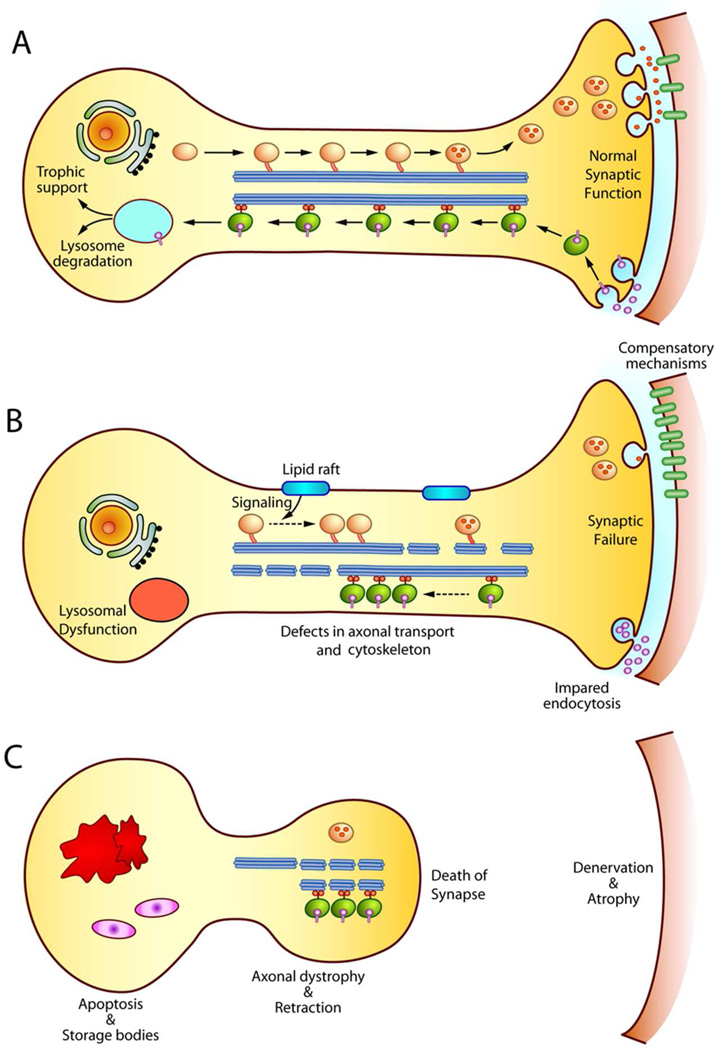
Figure 1. Model of axonal and synaptic damage in sphingolipidoses.
A) In a healthy neuron, axonal transport guarantees the delivery of synaptic vesicles to the synapse. Moreover, trophic signals are transported to the cell body to maintain the neuronal survival. B) In sphingolipidoses, lysosomal dysfunction leads to the storage of compounds that impair axonal transport and the axonal cytoskeleton, possibly via the deregulation of signalling cascades. Deregulation of membrane domains (i.e. lipid rafts) may be key in initiating abnormal signalling. Inefficient delivery of cargoes to distal domains and impaired retrograde transport of trophic signals weakens neuronal function in the absence of neuronal death. Compensatory mechanisms at the synapse (e.g. increase of post-synaptic neurotransmitter receptors) may be recruited to maintain synaptic connections. C) The progressive accumulation of membrane and axonal defects eventually leads to full synaptic failure, loss of neuronal connections, and the retraction of the processes. Apoptosis of the cell body occurs at later stages, once enough connections are lost. Denervated tissues undergo atrophy.
As axons extend for remarkably long distances, the axonal cytoskeleton plays a pivotal role in the maintenance of axonal stability. Cytoskeletal alterations have been observed in adult onset disorders of the nervous system (e.g. Charcot-Marie-Tooth, Alzheimer's disease, Parkinson's disease) and also in different LSDs (e.g. Krabbe's disease; Niemann-Pick's disease type C) (Bu et al. 2002; Cantuti-Castelvetri et al. 2012; Falk et al. 2015). The discovery of neurofilament abnormalities in LSDs argues for their involvement in axonopathy. For example, the expansion of the axonal calibre is highly influenced by the level and phosphorylation of neurofilaments (Elder et al. 1998; Perrot et al. 2008). Therefore, reductions in phosphorylated neurofilaments could contribute to the prevalence of small-diameter axons, a frequent ultrastructural observation in most neurological LSDs such as Krabbe’s disease (Bagel et al. 2013; Cantuti-Castelvetri et al. 2012; Falk et al. 2015). Axonal diameter is also a critical factor for regulating conduction velocities in nerves (Cole et al. 1994). Importantly, neurofilaments also provide mechanical resistance to axons. Thus, changes in neurofilament homeostasis may increase axonal vulnerability. Consistent with this idea, dephosphorylated neurofilaments tend to localize in axonal swellings and breaks (Cantuti-Castelvetri et al. 2012).
Microtubules are also key components of axonal physiology, contributing to mechanical resistance and acting as highway tracks for motor-assisted transport. Compelling evidence also implicates microtubule associated proteins (MAPs) in late onset neurological conditions, including Alzheimer's and Parkinson's diseases (Frasier et al. 2005; Goedert et al. 1992) as well as in LSDs including mucopolysaccharidosis III type C, and Krabbe's disease (Martins et al. 2015; Teixeira et al. 2014). Accumulation of hyperphosphorylated tau is a common finding in Niemann-Pick type C and other LSDs neurons (Bu et al. 2002; Martins et al. 2015).
A MODEL TO UNDERSTAND HOW SYNAPTIC FAILURE STARTS AND PROGRESSES IN SPHINGOLIPIDOSES
In addition to the previous contributing factors, dying back neurodegeneration requires local insults to the distal component of the neuronal processes (e.g. pre-, post-synaptic membranes or both). Axonal defects slowly progress backward, towards the neuronal soma, hence the name dying-back pathology (Figure 1). In sphingolipidoses, little is known about the initial insult(s) to the synaptic structure, especially considering that axons do not contain lysosomes. Thus, a key question is how substrates such as psychosine, normally non-toxic in healthy conditions, acquire toxic properties for axons in LSDs(Suzuki 1998). Part of the answer may rely on observations done in adult onset neurodegenerative diseases. For example, synapses are particularly vulnerable to soluble oligomeric forms of α-synuclein, huntingtin, or β-amyloid in patients with Parkinson’s, Huntington’s, or Alzheimer’s disease (Lasagna-Reeves et al. 2011; Morfini et al. 2009; Suopanki et al. 2006), suggesting that the presence of toxic compounds is sufficient to promote synaptic failure. In the case of sphingolipidoses, we hypothesize that some of the undigested lipids may reach the synaptic apparatus via vesicular transport. Once assembled in axonal and/or dendritic membranes, the local increase of these lipids affects the composition, fluidity, and curvature of the membranes. Cellular processes based on membrane dynamics, like endocytosis and synaptic vesicle fusion, may be significantly affected by these changes in membrane behaviour. This model is further complicated by the heterogeneity of the accumulated material (i.e. the type of lipid being accumulated), and the likelihood of eliciting distinct and lipid-specific effects on membrane behaviour (For an expanded discussion on membrane alterations, please read D'auria & Bongarzone, this issue). Such changes may impair the physiological architecture of the synapse, thereby reducing neurotransmitter receptor activities, docking and release of synaptic vesicles, and/or vesicle recycling (Allen et al. 2007; Rohrbough and Broadie 2005).
Kinetically, these early changes are slow, which could explain the paucity in detecting major neuronal changes during early disease stages in multiple sphingolipidoses. However, the conjunction of changes in membrane behaviour (e.g. fluidity, curvature) with structural and physiological defects (e.g. axonal cytoskeleton, vesicular transport) may inexorably lead to the progressive weakening of synaptic connections. Together with demyelination and blockage of nerve conduction, the progressive increase in synaptic failure and subsequent dying-back pathology, leads to significant impairment of neural connectivity in sphingolipidoses without overt neuronal loss.
CONCLUSIONS
The study of the pathogenic mechanisms in sphingolipidoses and other neurological LSDs is often complicated by the slow and insidious nature of the synaptic pathology, which can be masked by the more dramatic effects of demyelination or inflammation. We underline the relevance for reframing the current view of these diseases by including the physiological relevance of a progressive impairment of neuronal connections. Studying this aspect might prove helpful in identifying new molecular targets and the design of neuroprotective small molecules. Pharmacological preservation of neuronal networks is an appealing therapeutic option that combined with more traditional approaches such as gene therapy and cell therapy, may provide synergistic alleviation from neurological deficits in patients affected by sphingolipidoses and other neurological LSDs.
SIGNIFICANCE.
Failure of synapses to transmit messages between neurons is a major factor in establishing neurodegeneration, and dying back neuropathology in adult onset neurological conditions. The contribution of these mechanisms to pathogenesis in childhood sphingolipidoses remains under-estimated. However, current studies show the occurrence of similar structural and mechanistic changes in both groups of diseases. Understanding the dynamics and molecular mechanisms of synaptic failure may provide the opportunity to identify targets for molecular therapies that protect the physiological architecture of brain.
Acknowledgments
This work was partially funded by grants from NIH (R01NS065808 and R21NS087474) and the Legacy for Angels Foundation to ERB.
REFERENCES
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8(2):128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Arroyo AI, Camoletto PG, Morando L, Sassoe-Pognetto M, Giustetto M, Van Veldhoven PP, Schuchman EH, Ledesma MD. Pharmacological reversion of sphingomyelin-induced dendritic spine anomalies in a Niemann Pick disease type A mouse model. EMBO Mol Med. 2014;6(3):398–413. doi: 10.1002/emmm.201302649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagel JH, Sikora TU, Prociuk M, Pesayco JP, Mizisin AP, Shelton GD, Vite CH. Electrodiagnostic testing and histopathologic changes confirm peripheral nervous system myelin abnormalities in the feline model of niemann-pick disease type C. J Neuropathol Exp Neurol. 2013;72(3):256–262. doi: 10.1097/NEN.0b013e318286587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22(15):6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Maravilla E, Marshall M, Tamayo T, D'Auria L, Monge J, Jeffries J, Sural-Fehr T, Lopez-Rosas A, Li G, Garcia K, van Breemen R, Vite C, Garcia J, Bongarzone ER. Mechanism of neuromuscular dysfunction in Krabbe disease. J Neurosci. 2015;35(4):1606–1616. doi: 10.1523/JNEUROSCI.2431-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Zhu H, Givogri MI, Chidavaenzi RL, Lopez-Rosas A, Bongarzone ER. Psychosine induces the dephosphorylation of neurofilaments by deregulation of PP1 and PP2A phosphatases. Neurobiol Dis. 2012;46(2):325–335. doi: 10.1016/j.nbd.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti Castelvetri L, Givogri MI, Hebert A, Smith B, Song Y, Kaminska A, Lopez-Rosas A, Morfini G, Pigino G, Sands M, Brady ST, Bongarzone ER. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J Neurosci. 2013;33(24):10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelvetri LC, Givogri MI, Zhu H, Smith B, Lopez-Rosas A, Qiu X, van Breemen R, Bongarzone ER. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011;122(1):35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JS, Messing A, Trojanowski JQ, Lee VM. Modulation of axon diameter and neurofilaments by hypomyelinating Schwann cells in transgenic mice. J Neurosci. 1994;14(11 Pt 2):6956–6966. doi: 10.1523/JNEUROSCI.14-11-06956.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6(11):889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Thorpe SR, Fiddler MB. Toward Enzyme Therapy for Lysosomal Storage Diseases. Physiological Reviews. 1976;56(1):57–99. doi: 10.1152/physrev.1976.56.1.57. [DOI] [PubMed] [Google Scholar]
- Elder GA, Friedrich VL, Jr, Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA. Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol. 1998;143(1):195–205. doi: 10.1083/jcb.143.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DJ, Todd AG, Lee S, Soustek MS, ElMallah MK, Fuller DD, Notterpek L, Byrne BJ. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum Mol Genet. 2015;24(3):625–636. doi: 10.1093/hmg/ddu476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasier M, Walzer M, McCarthy L, Magnuson D, Lee JM, Haas C, Kahle P, Wolozin B. Tau phosphorylation increases in symptomatic mice overexpressing A30P alpha-synuclein. Exp Neurol. 2005;192(2):274–287. doi: 10.1016/j.expneurol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener. 2011;6:39. doi: 10.1186/1750-1326-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March PA, Thrall MA, Brown DE, Mitchell TW, Lowenthal AC, Walkley SU. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol. 1997;94(2):164–172. doi: 10.1007/s004010050689. [DOI] [PubMed] [Google Scholar]
- Martins C, Hulkova H, Dridi L, Dormoy-Raclet V, Grigoryeva L, Choi Y, Langford-Smith A, Wilkinson FL, Ohmi K, DiCristo G, Hamel E, Ausseil J, Cheillan D, Moreau A, Svobodova E, Hajkova Z, Tesarova M, Hansikova H, Bigger BW, Hrebicek M, Pshezhetsky AV. Neuroinflammation, mitochondrial defects and neurodegeneration in mucopolysaccharidosis III type C mouse model. Brain. 2015;138(Pt 2):336–355. doi: 10.1093/brain/awu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes F, Stroobants S, Gerlach D, Wohlenberg C, Wessig C, Fogh J, Gieselmann V, Eckhardt M, D'Hooge R, Matzner U. Efficacy of enzyme replacement therapy in an aggravated mouse model of metachromatic leukodystrophy declines with age. Hum Mol Genet. 2012;21(11):2599–2609. doi: 10.1093/hmg/dds086. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21(3):281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29(41):12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Ukita Y, Ninomiya H, Ohno K. Axonal dystrophy of dorsal root ganglion sensory neurons in a mouse model of Niemann-Pick disease type C. Exp Neurol. 2004;187(2):289–298. doi: 10.1016/j.expneurol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38(1):27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci U S A. 2009;106(14):5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey SN, Smith DA, Wong AM, Platt FM, Cooper JD. Early glial activation, synaptic changes and axonal pathology in the thalamocortical system of Niemann-Pick type C1 mice. Neurobiol Dis. 2012;45(3):1086–1100. doi: 10.1016/j.nbd.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci. 2005;6(2):139–150. doi: 10.1038/nrn1608. [DOI] [PubMed] [Google Scholar]
- Schmitt HP. Changes in the voluntary muscles and the peripheral nerves in an autopsy case of MPS type II (Hunter) Neuropediatrics. 1981;12(1):83–91. doi: 10.1055/s-2008-1059642. [DOI] [PubMed] [Google Scholar]
- Smith B, Galbiati F, Castelvetri LC, Givogri MI, Lopez-Rosas A, Bongarzone ER. Peripheral neuropathy in the Twitcher mouse involves the activation of axonal caspase 3. ASN Neuro. 2011;3(4) doi: 10.1042/AN20110019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suopanki J, Gotz C, Lutsch G, Schiller J, Harjes P, Herrmann A, Wanker EE. Interaction of huntingtin fragments with brain membranes--clues to early dysfunction in Huntington's disease. J Neurochem. 2006;96(3):870–884. doi: 10.1111/j.1471-4159.2005.03620.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Twenty five years of the "psychosine hypothesis": a personal perspective of its history and present status. Neurochem Res. 1998;23(3):251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- Teixeira CA, Miranda CO, Sousa VF, Santos TE, Malheiro AR, Solomon M, Maegawa GH, Brites P, Sousa MM. Early axonal loss accompanied by impaired endocytosis, abnormal axonal transport, and decreased microtubule stability occur in the model of Krabbe's disease. Neurobiol Dis. 2014;66:92–103. doi: 10.1016/j.nbd.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano E, Perretti A, Balbi P, Silvestro E, Andria G, Parenti G. Detection of subclinical central nervous system abnormalities in two patients with mucolipidosis III by the use of motor and somatosensory evoked potentials. Neuropediatrics. 1998;29(1):40–42. doi: 10.1055/s-2007-973532. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Baker HJ, Rattazzi MC, Haskins ME, Wu JY. Neuroaxonal dystrophy in neuronal storage disorders: evidence for major GABAergic neuron involvement. J Neurol Sci. 1991;104(1):1–8. doi: 10.1016/0022-510x(91)90208-o. [DOI] [PubMed] [Google Scholar]