Abstract
Until recently, lipids were considered inert building blocks of the cellular membranes. This changed three decades ago when lipids were found to regulate cell polarity and vesicle transport, and the “lipid raft” concept took shape. The lipid-driven membrane anisotropy in form of “rafts” that associate with proteins led to the view that organized complexes of lipids and proteins regulate various cell functions. Disturbance of this organization can lead to cellular, tissue, and organ malfunction. Sphingolipidoses, lysosomal storage diseases that are caused by enzyme deficiencies in the sphingolipid degradation pathway, were found to be particularly detrimental to the brain. These enzyme deficiencies result in accumulation of sphingolipid metabolites in lysosomes, although it is not yet clear how this accumulation affects the organization of lipids in cellular membranes. Krabbe disease or globoid cell leukodystrophy was one of the first sphingolipidosis for which the raft concept offered a potential mechanism. Krabbe disease is caused by mutations in the enzyme β-galactocerebrosidase, however, elevation of its substrate, galactosylceramide, is not observed or considered detrimental. Instead, it was found that a byproduct of galactosylceramide metabolism, the lysosphingolipid psychosine, is accumulated. The “psychosine hypothesis” has been refined by showing that psychosine disrupts “lipid rafts” and vesicular transport critical for the function of glia and neurons. The role of psychosine in Krabbe disease is an example of how the disruption of the sphingolipid metabolism can lead to elevation of a toxic lysosphingolipids resulting in disruption of cellular membrane organization and neurotoxicity.
From Thudichum to lipid rafts: biochemistry meets cell biology
The first comprehensive lipid analysis of the brain was published more than a century ago in the book entitled “A Treatise on the Chemical Constitution of the Brain” by the German chemist Johann Ludwig Wilhelm Thudichum (1829–1901)1–3. After he became a British citizen, most of Thudichum’s studies were achieved in England when he received a grant from the Privy Council of London. Thudichum’s work was amazing considering that he separated and characterized sphingomyelin, sphingosine, and other brain lipid fractions solely based on their solubility, hydrolysis with acids and bases, and precipitation of the hydrolyzed products as adducts with different salts. He also described for the first time a compound he termed “psychosin”, which he obtained as a basic hydrolysis product from the “phrenosin” (galactosylceramide) fraction. Remarkably, Thudichum’s elemental analysis and description of “psychosin” being composed of galactose and sphingosine was very similar to the compound now known as galactosylsphingosine or psychosine (Fig. 1). The correct structure of psychosine, with the only error in the positions of the hydroxyl- and amino groups, was resolved three decades after Thudichum by another biochemist, Ernst Klenk4–5. With the introduction of thin layer chromatography (TLC) in the 1950s, an analytical and preparative method to separate lipids due to their partitioning between a running solvent (a mobile phase) and a sorbent coated on a glass plate (a stationary phase), the complete structural characterization of sphingosine, psychosine, and many other sphingolipids was achieved by Kokichi Ohno and Herbert Carter. Carter also coined the term “sphingolipids” for lipids containing the long chain bases sphingosine and dihydrosphingosine (Fig. 1)1,4,6. The development of radioactive enzyme assays by Kunihiko Suzuki and Tadashi Miyatake led to the discovery that in Krabbe disease (globoid cell leukodystrophy), deficiency of the same lysosomal enzyme that hydrolyzes galactosylceramide, β-galactocerebrosidase or galactosylceramidase, results in accumulation of psychosine, which is also a substrate for this enzyme (Fig. 1)7. In 1972, Suzuki and Miyatake formulated the “psychosine hypothesis” that Krabbe disease is caused by accumulation of the neurotoxic lysosphingolipid psychosine, several years before Lars Svennerholm discovered elevation of psychosine in Krabbe brain tissue using mass spectrometry, currently the prevalent method for sphingolipid analysis8–10. However, despite these major advances in lipid analysis, it remained elusive why psychosine or other lysosphingolipids, like glucosylsphingosine and lysosphingomyelin are neurotoxic (Fig. 1).
Figure 1.
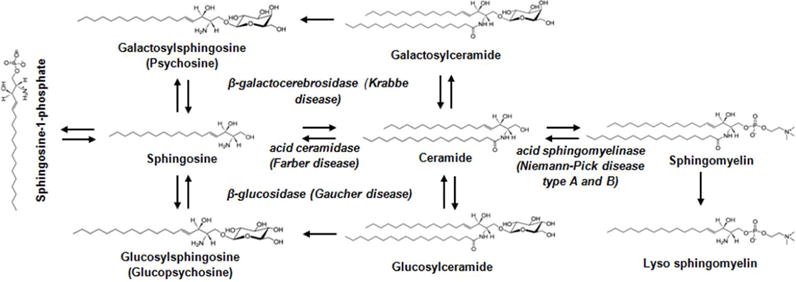
Sphingolipidoses affect enzymes from the sphingolipid degradation pathway. Metabolic steps in the sphingolipid degradation pathway affected by the mutations in the enzymes identified as the underlying cause of the respected lysosomal storage diseases.
In 1987, Yusuf Hannun and Robert Bell published a seminal paper showing evidence that the neurotoxicity of sphingosine and lysosphingolipids, including psychosine, is due to their inhibitory activity on protein kinases, particularly protein kinase C (PKC)11–12. This work initiated a plethora of studies defining protein targets for sphingolipids, including phosphatases (ceramide), kinases (ceramide, sphingosine, and psychosine), and receptors (sphingosine-1-phosphate)13–17. Despite solid evidence for sphingolipid binding to these proteins, it is still not fully understood which of these interactions is critical for cell biological processes and the pathophysiology of sphingolipidoses and other sphingolipid-related diseases. The major conceptual and methodological obstacle in defining the function of sphingolipid binding to proteins is to reconcile the embedding of a sphingolipid in cell membranes with its functional association with a cytosolic protein. While a lateral association of a lipid with a protein with a transmembrane domain is potentially easier to explain, it is enigmatic how cytosolic proteins specifically recognize and bind asphingolipid when the accessible portion is mainly the polar head group? In particular, cytosolic proteins would not be able to readily distinguish different hydrophobic tails of sphingolipids with the same head group. One plausible explanation how sphingolipids bind to proteins comes from the structural similarity of ceramide with diacylglycerol (DAG), for which the binding mechanism to PKC is well established. The structural similarity between DAG and ceramide suggests that ceramide may bind to the pertinent protein(s) by a similar mechanism18–19. This is achieved by a two-step binding process; firstly, PKC attaches to the polar surface of the membrane, which is then followed by probing the hydrophobic part with two prong-like protein domains digging deep into the inner leaflet20–21. One could imagine a similar “touch and dig” mechanism for a variety of proteins binding to sphingolipids; including the way sphingosine and lysosphingolipids bind PKC to inhibit it. This “touch and dig” binding mechanism implies that the respective target proteins are first attracted to membrane surfaces (“touch”) by a variety of lipid polar head groups (glycerol, serine, phosphate etc.) and then activated or inhibited by association with the hydrophobic portion of the lipid. The hydrophobic properties of the lipid are defined by the number, length, saturation, and hydroxylation of its hydrocarbon moieties. Alternatively, in a “touch and pull” mechanism as in sphingolipid hydrolyzing enzymes or the ceramide transport protein (CERT), the protein will lift the sphingolipid completely out of the membrane and embed it in its binding site15,22. Accordingly, these proteins do not have to stay associated with the membrane and can move the lipid within the cytosol or luminal space of membrane compartments.
The major enigma of both, the “touch and dig” and “touch and pull” mechanisms, is how the initial recognition of the membrane is achieved with some degree of specificity without forcing the protein to randomly scan membrane surfaces for binding to the appropriate lipid. Clustering of specific lipids in rafts or microdomains to facilitate scanning of proteins for selective ‘touch’ could be a plausible solution to this problem. The concept of lipid clustering and sorting was postulated about three decades ago by Gerrit van Meer and Kai Simons. They showed that fluorescently labeled glycosphingolipids are asymmetrically transported to the apical membrane of MDCK cells23–24. The “raft hypothesis” was formally introduced about a decade later by Simons and Ikonen in their seminal Nature publication25. Experimental evidence for “lipid microdomains”, however, can be traced to studies from the early 1970s, which showed that alkaline or cold Triton X-100-insoluble membrane fractions were enriched in cholesterol and particular sphingolipids26–27. These insoluble membrane fractions were later shown to be enriched with raft proteins, such as caveolins, and therefore, considered to be equivalent to the biological constitution of lipid rafts in living cells28–39. Recent evidence emerging from modern imaging methods such as imaging mass spectrometry or super resolution fluorescence microscopy shows that the lipid distribution in the cell membrane of fixed cells is functionally organized in microdomains or ‘rafts’40. However, proof for an equivalent functional organization in living cells is still elusive.
Apart from the proposed function of lipid rafts to assemble cell signaling platforms by gathering membrane-resident receptors, it is possible that they generate a particular polar surface potential of clustered lipid head groups that can be recognized by adequate lipid binding proteins prior to “digging” into the membrane or “pulling” out a lipid. Lysosphingolipids such as psychosine can disturb this microdomain organization or possibly aberrantly bind proteins that rely on the formation and function of normal lipid rafts and in that way unfold neurotoxicity. Indeed, several studies have provided evidence that psychosine disturbs the lipid raft organization of oligodendrocytes and neurons in Krabbe tissue41–42. It should be noted that the raft concept as discussed in this review involves both, the inner (cytoplasmic) and outer (exoplasmic) leaflet of the membrane, although most of the typical raft lipids such as sphingomyelin, cholesterol, and glycosphingolipids are predominantly distributed to the outer leaflet35. Lysosphingolipids such as psychosine are likely to be elevated in the luminal (equivalent to outer) leaflet of the lysosomal membrane. To affect cytosolic proteins lysosphingolipids will need to alter the raft organization of the inner membrane leaflet. To date, it is unclear how this is achieved. Simons suggests that sphingolipids in the outer leaflet are “coupled” to rafts in the inner leaflet via very long fatty acid chains that reach across the two membrane leaflets35. In the case of lysosphingolipids, however, this is not possible since they lack the fatty acid moiety. A potential solution is that accumulation of these lipids disturbs rafts in the outer leaflet, which then affects the inner leaflet through impaired coupling. Alternatively, lysosphingolipids “flip” across the membrane (transbilayer movement), which may require flippases that have been suggested for a variety of sphingolipids with polar head groups43–44. Consistent with the higher water solubility and rather low concentration of lysosphingolipids they may also act as “neurotoxic ligands” for specific receptor proteins, which has been suggested for psychosine and other lysosphingolipids45. On the other hand, a recent study showed that a stereo-insensitive and nonenantioselective enantiomer of psychosine has equal or even greater toxicity than the stereo-specific and enantioselective enantiomer that is suggested to specifically bind to a receptor protein46. Probably, the difference between effects resulting from receptor binding and a more generalized raft-perturbing or even membrane-lysing activity of psychosine depends on its concentration. In Krabbe brain white matter, the concentration of psychosine is up to 10 μM, while non-specific effects on the membrane were tested at 20 μM46–47. Currently, it is not clear at which threshold concentration of psychosine the raft-perturbing or even detergent-like effects are distinct from specific activities on potential protein binding.
Studies on Krabbe and other sphingolipidoses show that the current view on the neurotoxic mechanism has evolved from the original hypothesis that neurotoxicity emerges from lysosphingolipids inhibiting PKC to the more holistic idea that these lipids lead to disorganization of cellular membranes, particularly lipid rafts11,48. These views are not mutually exclusive and a closer look at different molecular and cellular levels of pathophysiology will quickly reveal a complex cascade of various insults including those on membranes, protein binding, and organellar function ultimately leading to neurotoxicity and disease pathology.
Lysosphingolipids and sphingolipidoses: mind your membranes
Krabbe disease or globoid cell leukodystrophy is one of six autosomal recessive sphingolipidoses with accumulation of a particular sphingolipid metabolite due to mutation or deficiency of the enzyme that degrades it. Similarly to psychosine in Krabbe disease, other lysosphingolipids were found 100 to1000-fold enriched in sphingolipidoses; e.g. Gaucher disease (glucosylsphingosine or glucopsychosine), Fabry disease (globotriaosylsphingosine), and Niemann Pick disease (lysosphingomyelin or sphingosylphosphocholin) (Fig. 1)48–52. As discussed above, there is solid evidence that the lysosphingolipid psychosine leads to membrane disorganization due to disruption of lipid rafts (Fig. 2)41,53. Twenty years ago, Suzuki proposed that the pathology of other sphingolipidoses may follow a similar molecular mechanism as Krabbe disease, although he did not discuss the effect of lysosphingolipids on membrane organization or rafts8. Whether disruption of membrane domains, as in Krabbe disease, plays a role in other sphingolipidoses, where lysosphingolipids accumulate, remains to be tested. Nevertheless, it is worth exploring if there is a more general model of a neurotoxic mechanism based on a multi-leveled cascade of molecular, organellar, and cellular pathologies that is initiated by membrane disorganization due to accumulation of a particular sphingolipid or its neurotoxic lysosphingolipid byproduct (Fig. 2). It is possible that lipid raft disruption due to accumulation of lysosphingolipids compromises the ability of sphingolipid binding proteins to scan for a cluster of polar head groups (see previous section), which leads to defunct binding or activation or inactivation of kinases and phosphatases or directly affecting receptors and channels.
Figure 2.
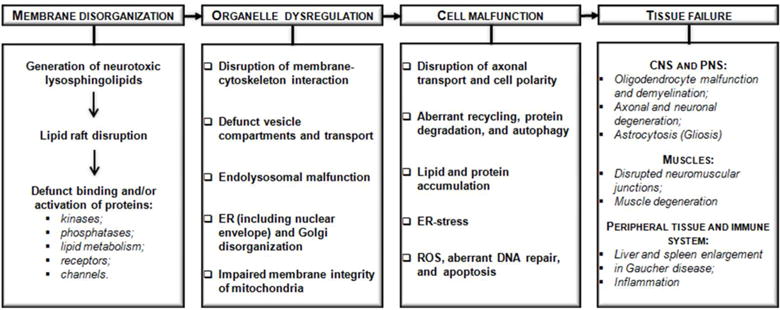
Lysosphingolipid neurotoxicity on different levels of cellular organization.
The second level of molecular dysfunction in sphingolipidoses involves a variety of organelles (ER, including nuclear envelope, Golgi, endolysosomal compartment, and mitochondria), the function of which critically relies on the organization of their membranes, membrane-cytoskeleton interaction, and vesicle transport. For example, psychosine has been shown to induce dispersion of Golgi and endosomal vesicles, as well as to affect mitochondria54–55. Some aspects of psychosine toxicity resemble that of ceramide accumulation based on the observation that psychosine and ceramide induce similar malfunctions: disruption of axonal transport and growth53,56, elevated proteasomal protein degradation56–58, and increased autophagy59–60. Importantly, ceramide has been reported to regulate lipid rafts, which may explain many of its biological effects at the organellar and cellular levels30,61. It is possible that the effects of psychosine on the organelle level are also due to its disruption of membrane rafts as discussed above.
Psychosine has been shown to affect oligodendrocytes, but remarkably also to impair the fast axonal transport in neurons53. Since neurons are not known to synthesize significant quantities of galactosylceramide62, psychosine is likely to be transported from glial cells to neurons. Most recently, work from one of our laboratories has discovered the transfer of ceramide between astrocytes using extracellular vesicles as carriers63. It is plausible, that exosomes or other extracellular vesicles are able to carry a variety of neurotoxic lipids, such as psychosine and other lysosphingolipids, probably as a cleansing process of the vesicle donor cells, which may cause collateral damage to the neighboring cells due to spreading of a toxic lipid. Another set of similarities between psychosine and ceramide is their activity on PKCs and glycogen synthase kinase 3 (GSK3), as well as their effect on calcium release and generation of reactive oxygen species in mitochondria18,53,64–73. Even though there is no striking structural similarity between psychosine and ceramide (Fig. 1), some of the pathological effects on the cellular level suggest that the mechanism for neurotoxicity is related, possibly through dysregulation of lipid rafts.
Despite plausible similarities in the mechanisms potentially underlying neurotoxicity in sphingolipidoses, different sphingolipidoses affect different cell types and tissues (Fig. 2). Macrophages in the brain turning into large multinucleated cells, the so-called globoid cells, are one of the first visible signs of Krabbe pathology, therefore the disease is also known as globoid cell leukodystrophy. Uniquely among the sphingolipidoses affecting the nervous system, Krabbe disease is shown to damage oligodendrocytes and Schwann cells, and to involve neuronal degeneration mostly after demyelination. Impairment of neuromuscular junctions and muscle degeneration, probably in the wake of failed innervation, has also been described in Krabbe disease56,59. On the other hand, Gaucher disease, a deficiency of glucosylceramidase, and Farber disease, a deficiency of ceramidase, mainly affect peripheral organs such as spleen and liver (Figs. 1 and 2). This specificity is enigmatic if one tries to understand tissue failure on the basis of membrane disorganization as a common mechanism of neurotoxic lysosphingolipids. There are clear limitations in a purely lipid raft point of view and additional modifiers determining cell- or tissue specific neurotoxicity need to be considered. These modifiers are probably proteins interacting with lysosphingolipids the expression of which is regulated in a cell- or tissue specific manner or different lipid makeup of the membranes of different cell types in a specific tissue. Understanding these interactions and differences will require further development of sophisticated techniques for lipid analysis and visualization that will elevate lipid biochemistry to the level of cell biology.
Next generation lipid analysis: understanding lipid-protein interaction
The major advantage of protein over lipid analyses at the level of cell biology is the wealth of techniques available to visualize the distribution of proteins in living cells. In addition, visualization of the lipid rafts in living cells is hampered by their small size and transient dynamic nature at physiological temperature74. This is not just a technical problem, but a serious conceptual obstacle for lipid rafts when it comes to explaining their biological function. If lipid rafts decay faster than raft proteins respond then the biological effect of rafts on these proteins is likely to be negligible. The elevation of lysosphingolipids may do just that: making lipid rafts short-lived or change their composition thereby rendering them dysfunctional. However, currently there are only very limited techniques available to test this. So far, only alkaline- or detergent-insoluble membranes, isolated by density gradient ultracentrifugation and tested for the distribution of raft-associated proteins, are taken as evidence for lysosphingolipid-induced disruption of lipid rafts in living cells41,53,75. Prerequisite is that insoluble membrane fractions are truly representative of lipid rafts and not altered by the methods used for their isolation. Further, if distinct lipid rafts or domains are formed in different cellular compartments any isolation technique based on cell lysis will obliterate this distinction. Most recently, a novel imaging technique using high resolution imaging mass spectrometry of metabolically labeled (15N) sphingolipids in combination with total internal reflection fluorescence microscopy (TIRFM) of Bodipy-conjugated sphingolipids has identified microdomains that are enriched with sphingolipids40. Intriguingly, these microdomains are not altered by glutaraldehyde fixation and they are regulated by the actin cytoskeleton. The interaction of sphingolipids with the cytoskeleton and its effect on cell polarity has been the research interest of one of our laboratories for more than ten years72,76–82. This group developed an anti-ceramide antibody that was used to visualize the distribution of ceramide in the ER, Golgi, the apical and leading cell membrane of polarized and migrating cells, and for the first time, an apical ceramide-enriched compartment (ACEC) at the base of primary and motile cilia83. Intriguingly, ceramide was not homogeneously distributed in these membranes, but it was enriched in contact sites that attached to the cytoskeleton, particularly microtubules with a high degree of tubulin acetylation77,82. Applying another technique originally developed in their laboratory, lipid-mediated magnetic activated cell sorting (LIMACS), the Bieberich group used the anti-ceramide antibody and magnetic beads to isolate ceramide-enriched vesicles that we found to be associated with acetylated tubulin, and the small Rho GTPases cdc42 and Rab1182. These results suggested that microdomains or rafts, particularly when enriched with ceramide, are involved in the regulation of the cytoskeleton and that disruption of their function will have impact on cell polarity and vesicle transport.
Any disorganization of raft lipids will affect their interaction with the cytoskeleton, regardless of whether rafts control the dynamics of the cytoskeleton or vice versa. In principle, sphingolipid regulation of the cytoskeleton and dysregulation by lysosphingolipids can proceed via direct binding to cytokeletal proteins or regulatory proteins such as microtubule-associated proteins or kinases and phosphatases affecting cytoskeletal dynamics. Recently, it was shown that ceramide binds and activates GSK3, a protein kinase that is also activated by psychosine53,72. Using a bifunctional ceramide analog, the ceramide-GSK3 interaction was visualized in glial cells72. Bifunctional or biorthogonal lipid analogs contain a diazirine group for UV crosslinking of the analog to a binding protein and an alkyne group for derivatization with fluorophores or biotin using click chemistry84–86. In another approach, azido-derivatized ceramide and ceramide analogs (S18) have been used with click chemistry to label ceramide-binding proteins (e.g., azido-S18 interaction with aPKC) in living cells87. In addition, bifunctional lipid analogs have been used to identify or visualize fatty acid, cholesterol, sphingosine, and ceramide binding proteins, and will certainly allow for a similar task with lysosphingolipids such as psychosine72,85,88–89.
Conclusions
Lipid analysis has come a long way from the first biochemical characterization of psychosine more than 100 years ago to the psychosine and lysophingolipid hypothesis as causative principle in Krabbe disease and other sphingolipidoses, respectively. And yet, novel concepts and technology are needed to advance our knowledge from the idea of lipid microdomains or rafts to an understanding of lipid-protein interaction within them and the effects on the cellular function and disease. Neurotoxicity caused by lysosphingolipids dysregulating the interaction of raft lipids with cytoskeletal proteins can certainly be a major focus of the research effort. Ultimately, a deeper understanding of lipids as diagnostic markers and therapeutic targets will demonstrate that lipids are more than just being fats or inert structural ‘bricks’ in the membranes, but a bioactive players on their own right and further fuel our effort to take sphingolipid biochemistry to the level of cell biology.
Significance statement.
Krabbe disease or globoid cell leukodystrophy is a lysosomal sphingolipidosis caused by deficiency of the enzyme β-galactocerebrosidase leading to accumulation of the substrate galactosylceramide and its lysosphingolipid derivative galactosylsphingosine or psychosine. Currently, lysosomal accumulation of psychosine is viewed as the cause of neurotoxicity of Krabbe disease (psychosine hypothesis). This review discusses a potential mechanism by which psychosine and other lysosphingolipids may disrupt the function of lipid microdomains or rafts, leading to neurotoxicity in Krabee disease and other sphingolipidoses.
Acknowledgments
This work was funded by NIH grant R01AG034389 and R56NS095215. Support by the Department of Neuroscience and Regenerative Medicine, Medical College of Georgia, Augusta University (Dr. Lin Mei, Chair) is acknowledged.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
The two authors, S.D. and E.B. wrote the original review and edited the revisions of the manuscript.
References
- 1.Pruett ST, et al. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J Lipid Res. 2008;49:1621–1639. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thudichum JWL. A Treatise of the Chemical Constitution of the Brain. Bailliere, Tindall, and Cox; London: 1884. [Google Scholar]
- 3.Thudichum JWL. Die chemische Konstitution des Gehirns des Menschen und der Tiere. Verlag von Franz Pietzcker; Tuebingen: 1901. [Google Scholar]
- 4.Sakagami T. Studies on Psychosine. J Biochem. 1958;45:281–283. [Google Scholar]
- 5.Klenk ED, W Uber Sphingosin. Z physiol Chem. 1931;198:25–32. [Google Scholar]
- 6.Carter HE, Glick FJ, Norris WP, Phillips GE. Biochemistry of the Sphingolipides. J Biol Chem. 1947;170:285–294. [Google Scholar]
- 7.Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun. 1972;48:539–543. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- 9.Vanier M, Svennerholm L. Chemical pathology of Krabbe disease: the occurrence of psychosine and other neutral sphingoglycolipids. Adv Exp Med Biol. 1976;68:115–126. doi: 10.1007/978-1-4684-7735-1_8. [DOI] [PubMed] [Google Scholar]
- 10.Dawson G. Measuring brain lipids. Biochim Biophys Acta. 2015;1851:1026–1039. doi: 10.1016/j.bbalip.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- 12.Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 13.Snook CF, Jones JA, Hannun YA. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Yamaji T, Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic. 2015;16:101–122. doi: 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- 15.Sandhoff K. My journey into the world of sphingolipids and sphingolipidoses. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:554–582. doi: 10.2183/pjab.88.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzmann G, Arenz C, Sandhoff K. Labeled chemical biology tools for investigating sphingolipid metabolism, trafficking and interaction with lipids and proteins. Biochim Biophys Acta. 2014;1841:1161–1173. doi: 10.1016/j.bbalip.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Ernst AM, Brugger B. Sphingolipids as modulators of membrane proteins. Biochim Biophys Acta. 2014;1841:665–670. doi: 10.1016/j.bbalip.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Hannun YA, Bell RM. Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin Chim Acta. 1989;185:333–345. doi: 10.1016/0009-8981(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 19.Gallegos LL, Newton AC. Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm. IUBMB Life. 2008;60:782–789. doi: 10.1002/iub.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 21.Stahelin RV, et al. The origin of C1A-C2 interdomain interactions in protein kinase Calpha. J Biol Chem. 2005;280:36452–36463. doi: 10.1074/jbc.M506224200. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai K, et al. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 23.van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987;105:1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 25.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 26.Gurd JW, Evans WH, Perkins HR. Chemical characterization of the proteins and glycoproteins of mouse liver plasma membranes solubilized by sequential extraction with aqueous and organic solvents. Biochem J. 1972;126:459–466. doi: 10.1042/bj1260459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butters TD, Hughes RC. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. Biochem J. 1974;140:469–478. doi: 10.1042/bj1400469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aureli M, Grassi S, Prioni S, Sonnino S, Prinetti A. Lipid membrane domains in the brain. Biochim Biophys Acta. 2015;1851:1006–1016. doi: 10.1016/j.bbalip.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 31.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 32.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 34.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 35.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnino S, Prinetti A. Membrane domains and the “lipid raft” concept. Curr Med Chem. 2013;20:4–21. [PubMed] [Google Scholar]
- 37.Aureli M, Grassi S, Sonnino S, Prinetti A. Isolation and Analysis of Detergent-Resistant Membrane Fractions. Methods Mol Biol. 2016;1376:107–131. doi: 10.1007/978-1-4939-3170-5_10. [DOI] [PubMed] [Google Scholar]
- 38.Dawson G. Glycosignaling: a general review. Adv Neurobiol. 2014;9:293–306. doi: 10.1007/978-1-4939-1154-7_13. [DOI] [PubMed] [Google Scholar]
- 39.Ledeen RW, Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem Sci. 2015;40:407–418. doi: 10.1016/j.tibs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Frisz JF, et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci U S A. 2013;110:E613–622. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White AB, et al. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White AB, et al. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease. J Neurosci Res. 2011;89:352–364. doi: 10.1002/jnr.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.van Meer G. Dynamic transbilayer lipid asymmetry. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins–Salsbury JA, et al. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J Lipid Res. 2013;54:3303–3311. doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 48.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 49.Ferraz MJ, et al. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim Biophys Acta. 2014;1841:811–825. doi: 10.1016/j.bbalip.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Svennerholm L, Erikson A, Groth CG, Ringden O, Mansson JE. Norrbottnian type of Gaucher disease–clinical, biochemical and molecular biology aspects: successful treatment with bone marrow transplantation. Dev Neurosci. 1991;13:345–351. doi: 10.1159/000112184. [DOI] [PubMed] [Google Scholar]
- 51.Berger A, Rosenthal D, Spiegel S. Sphingosylphosphocholine, a signaling molecule which accumulates in Niemann-Pick disease type A, stimulates DNA-binding activity of the transcription activator protein AP-1. Proc Natl Acad Sci U S A. 1995;92:5885–5889. doi: 10.1073/pnas.92.13.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferraz MJ, et al. Lyso-glycosphingolipid abnormalities in different murine models of lysosomal storage disorders. Mol Genet Metab. 2016;117:186–193. doi: 10.1016/j.ymgme.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Cantuti Castelvetri L, et al. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J Neurosci. 2013;33:10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem. 2003;86:1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]
- 55.Kanazawa T, Takematsu H, Yamamoto A, Yamamoto H, Kozutsumi Y. Wheat germ agglutinin stains dispersed post-golgi vesicles after treatment with the cytokinesis inhibitor psychosine. J Cell Physiol. 2008;215:517–525. doi: 10.1002/jcp.21328. [DOI] [PubMed] [Google Scholar]
- 56.Cantuti-Castelvetri L, et al. Mechanism of neuromuscular dysfunction in Krabbe disease. J Neurosci. 2015;35:1606–1616. doi: 10.1523/JNEUROSCI.2431-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friant S, Meier KD, Riezman H. Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. EMBO J. 2003;22:3783–3791. doi: 10.1093/emboj/cdg375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroesen BJ, et al. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J Biol Chem. 2003;278:14723–14731. doi: 10.1074/jbc.M210756200. [DOI] [PubMed] [Google Scholar]
- 59.Ribbens JJ, Moser AB, Hubbard WC, Bongarzone ER, Maegawa GH. Characterization and application of a disease-cell model for a neurodegenerative lysosomal disease. Mol Genet Metab. 2014;111:172–183. doi: 10.1016/j.ymgme.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang W, Ogretmen B. Autophagy paradox and ceramide. Biochim Biophys Acta. 2014;1841:783–792. doi: 10.1016/j.bbalip.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grassme H, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 62.Okahara K, et al. Ceramide galactosyltransferase expression is regulated positively by Nkx2.2 and negatively by OLIG2. Glycobiology. 2014;24:926–934. doi: 10.1093/glycob/cwu042. [DOI] [PubMed] [Google Scholar]
- 63.Wang G, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller G, et al. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. Embo J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang G, et al. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 66.Lozano J, et al. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 67.Wang YM, Seibenhener ML, Vandenplas ML, Wooten MW. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. doi: 10.1002/(SICI)1097-4547(19990201)55:3<293::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 69.Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Calpha. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- 70.Kitatani K, Idkowiak-Baldys J, Hannun YA. Mechanism of inhibition of sequestration of protein kinase C alpha/betaII by ceramide. Roles of ceramide-activated protein phosphatases and phosphorylation/dephosphorylation of protein kinase C alpha/betaII on threonine 638/641. J Biol Chem. 2007;282:20647–20656. doi: 10.1074/jbc.M609162200. [DOI] [PubMed] [Google Scholar]
- 71.Vartanian T, Dawson G, Soliven B, Nelson DJ, Szuchet S. Phosphorylation of myelin basic protein in intact oligodendrocytes: inhibition by galactosylsphingosine and cyclic AMP. Glia. 1989;2:370–379. doi: 10.1002/glia.440020509. [DOI] [PubMed] [Google Scholar]
- 72.Kong JN, et al. Regulation of Chlamydomonas flagella and ependymal cell motile cilia by ceramide-mediated translocation of GSK3. Mol Biol Cell. 2015 doi: 10.1091/mbc.E15-06-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strasberg PM, Callahan JW. Lysosphingolipids and mitochondrial function. II. Deleterious effects of sphingosylphosphorylcholine. Biochem Cell Biol. 1988;66:1322–1332. doi: 10.1139/o88-153. [DOI] [PubMed] [Google Scholar]
- 74.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 75.Teixeira CA, et al. Early axonal loss accompanied by impaired endocytosis, abnormal axonal transport, and decreased microtubule stability occur in the model of Krabbe’s disease. Neurobiol Dis. 2014;66:92–103. doi: 10.1016/j.nbd.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnamurthy K, Wang G, Silva J, Condie BG, Bieberich E. Ceramide Regulates Atypical PKC{zeta}/{lambda}-mediated Cell Polarity in Primitive Ectoderm Cells: A NOVEL FUNCTION OF SPHINGOLIPIDS IN MORPHOGENESIS. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 77.Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E. Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. J Neurochem. 2008;106:718–733. doi: 10.1111/j.1471-4159.2008.05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang G, Krishnamurthy K, Bieberich E. Regulation of primary cilia formation by ceramide. J Lipid Res. 2009 doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bieberich E. Ceramide in stem cell differentiation and embryo development: novel functions of a topological cell-signaling lipid and the concept of ceramide compartments. J Lipids. 2011;2011:610306. doi: 10.1155/2011/610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He Q, et al. Characterization of an apical ceramide-enriched compartment regulating ciliogenesis. Mol Biol Cell. 2012;23:3156–3166. doi: 10.1091/mbc.E12-02-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He Q, et al. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol Biol Cell. 2014;25:1715–1729. doi: 10.1091/mbc.E13-12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J Lipid Res. 2007;48:968–975. doi: 10.1194/jlr.D600043-JLR200. [DOI] [PubMed] [Google Scholar]
- 84.Haberkant P, van Meer G. Protein-lipid interactions: paparazzi hunting for snapshots. Biol Chem. 2009;390:795–803. doi: 10.1515/BC.2009.074. [DOI] [PubMed] [Google Scholar]
- 85.Haberkant P, et al. In vivo profiling and visualization of cellular protein-lipid interactions using bifunctional fatty acids. Angew Chem Int Ed Engl. 2013;52:4033–4038. doi: 10.1002/anie.201210178. [DOI] [PubMed] [Google Scholar]
- 86.Haberkant P, Holthuis JC. Fat & fabulous: bifunctional lipids in the spotlight. Biochim Biophys Acta. 2014;1841:1022–1030. doi: 10.1016/j.bbalip.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Walter T, et al. Incorporation and visualization of azido-functionalized N-oleoyl serinol in Jurkat cells, mouse brain astrocytes, 3T3 fibroblasts and human brain microvascular endothelial cells. Chem Commun (Camb) 2016;52:8612–8614. doi: 10.1039/c6cc02879a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat Methods. 2013;10:259–264. doi: 10.1038/nmeth.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haberkant P, et al. Bifunctional Sphingosine for Cell-Based Analysis of Protein-Sphingolipid Interactions. ACS Chem Biol. 2016;11:222–230. doi: 10.1021/acschembio.5b00810. [DOI] [PubMed] [Google Scholar]


