Abstract
Type 1 diabetes (T1D) is a CD4+ T cell-driven autoimmune disease resulting from the destruction of insulin-producing pancreatic beta cells. Clinical evidence and studies in non-obese diabetic (NOD) mice suggest that insulin is a major autoantigen. With this in mind, we developed insulin B10-23:IAg7 tetramer reagents to track insulin-specific CD4+ T cells in mice and interrogated the role of Programmed death-1 (PD-1) for peripheral tolerance. PD-1 is a T cell inhibitory receptor necessary to maintain tolerance and prevent T1D in NOD mice. PD-1 pathway inhibitors are increasingly used in the clinic for treating malignancies, and while many patients benefit, some develop adverse autoimmune events, including T1D. We therefore sought to understand the role of PD-1 in maintaining islet-specific tolerance in diabetes-resistant strains. B6.g7 mice express the same MHC Class II allele as NOD mice, have predominantly naïve insulin-specific CD4+ T cells in the periphery, and remain diabetes-free even after PD-1 pathway blockade. Here, we examined the trafficking potential of insulin-specific CD4+ T cells in NOD and B6.g7 mice with or without anti-PD-L1 treatment, and found that PD-L1 blockade preferentially increased the number of CD44highCXCR3+ insulin-specific cells in NOD but not B6.g7 mice. Additionally, we investigated whether pancreatic islets in NOD and B6.g7 mice expressed CXCL10, a lymphocyte homing chemokine and ligand for CXCR3. Anti-PD-L1 treated and control NOD mice had detectable CXCL10 expression in the islets, while B6.g7 islets did not. These data suggest that islet tolerance may be in part attributed to the pancreatic environment and in the absence of pancreas inflammation, chemotactic cytokines may be missing. This, together with our previous data showing that PD-1 pathway blockade preferentially affects effector but not anergic self-specific T cells has implications for the use of checkpoint blockade in treating tumor patients. Our work suggests that determining tumor- and self-specific CD4+ T cell activation status (naïve, effector or anergic) prior to initiation of immunotherapy would likely help to stratify individuals who would benefit from this therapy versus those who might have adverse effects or incomplete tumor control.
Keywords: type 1 diabetes, CD4+ T cells, insulin, anergy, PD-1, checkpoint blockade
Introduction
Type 1 diabetes (T1D) is caused by the immune-mediated destruction of insulin-producing pancreatic beta cells in the islets of Langerhans [1]. An estimated 3 million people currently suffer from T1D in the United States alone. In the last decade, incidence has risen by 23% among individuals younger than 20 years of age, and this alarming trend is expected to continue [2]. Daily insulin injections are the standard of care, but they are not a cure. Due to artificial blood glucose regulation, T1D patients remain at an increased risk of heart and kidney disease, blindness and peripheral neuropathy [3–5]. These complications have a significant impact on the quality of life and longevity [6]. Islet transplantation is an attractive therapeutic approach, but requires immunosuppression. Understanding how islet-reactive lymphocytes are activated, escape peripheral tolerance, and cause disease is necessary to design antigen-specific therapies to cure T1D.
Clinical evidence as well as studies using the non-obese diabetic (NOD) mouse model of spontaneous T1D demonstrate that CD4+ and CD8+ T cells are critical for beta cell destruction [7–13]. While a case study described T1D onset in a patient with X-linked agammaglobulinemia [14], new-onset T1D patients benefited from B cell depletion therapy, suggesting that B cells are also required for disease [15]. In fact, B cell-mediated autoantibody production against islet antigens precedes T1D onset and is currently the only immunological biomarker of disease progression [3]. Specifically, insulin autoantibody onset can predict time to overt T1D in mice [16] and to a lesser degree in humans. All patients who develop T1D before age 5 have insulin autoantibodies [17], suggesting that insulin is a critical autoantigen. In NOD mice, as many as 90% of insulin-specific CD4+ T cells target insulin B10-23 residue [18]. This peptide is required for T1D, as a single mutation in a T cell receptor contact site abrogates disease [19]. With that in mind, we and others developed insulin B10-23:MHC Class II tetramer reagents [20–23] to track insulin-specific CD4+ T cells during disease development and at onset in NOD mice, as well as interrogate the fate of this population in diabetes resistant B6.I-Ag7 (B6.g7) mice to understand tolerance mechanisms in play [24, 25].
Programmed death-1 (PD-1) is a T cell inhibitory receptor, and it is highly expressed on recently activated effector T cells as well as chronically stimulated (anergic) CD4+ and (exhausted) CD8+ T cells, thus limiting their antiviral and antitumor activity [26–28]. Blocking PD-1 signaling has the potential to reinvigorate anergic or exhausted cells. This spurred the development of PD-1 pathway inhibitors (checkpoint blockade) for the treatment of advanced malignancies [29, 30]. While some patients benefit from this treatment, it is unclear why others do not, or why some patients develop adverse events and proceed to develop autoimmune-like symptoms or overt autoimmunity, including T1D [31]. PD-1 SNPs have been shown to increase the risk of T1D development in several populations [32–35], suggesting that at least in a subset of patients, PD-1 plays a critical role in maintaining islet tolerance. Deficiency in, or blocking PD-1 from interacting with its ligand programmed death ligand-1 (PD-L1), accelerates T1D onset in NOD mice (Figure 1); [36–40]). However, the role PD-1/PD-L1 pathway plays in islet tolerance maintenance in non-autoimmune prone mouse strains is unclear. To address this gap in knowledge, we sought to determine whether the PD-1 signaling pathway regulated islet-specific CD4+ T cells in mice of varying autoimmune susceptibilities, as well as whether anergy maintenance required continuous PD-1 signaling [25].
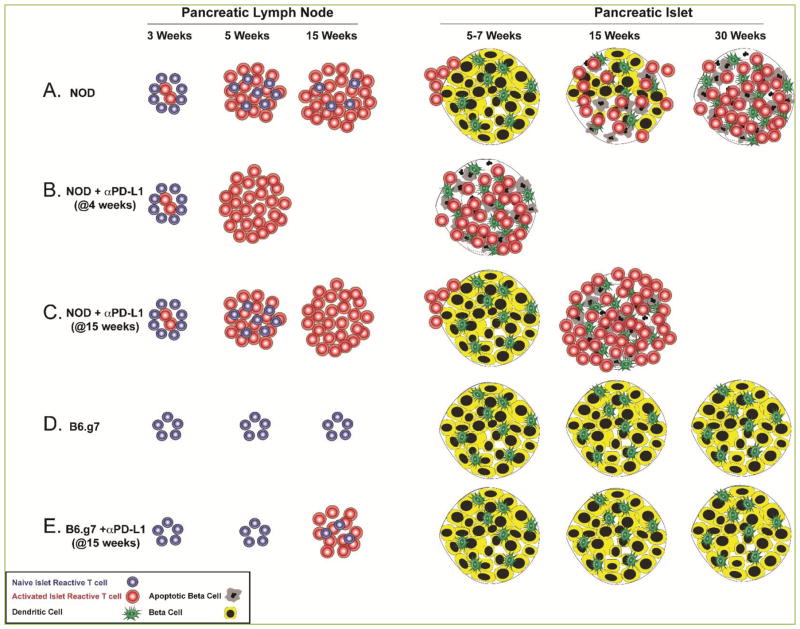
Figure 1. Model of islet antigen-specific CD4 T cell activation and islet inflammation timeline in NOD and B6.g7 mice.
(A) In NOD mice, insulin-specific CD4+ T cells encounter antigen in the pancreatic lymph node, expand and acquire the potential to traffic to the pancreas between 3 and 5 weeks of age. Pancreatic inflammation becomes more severe as mice age and peaks at disease onset, between 10 and 30 weeks (15 weeks as illustrated here). (B) Anti-PD-L1 treatment at 4 weeks of age significantly enhances T cell activation, thus leading to accelerated diabetes onset within 20 days in 90% of treated animals [38]. (C) PD-1 pathway blockade at a later time point when existing insulitis is extensive (at 15 weeks for example) [25], leads to a very rapid disease onset in NOD mice within 1–3 days [38]. (D) In B6.g7 mice, insulin-specific CD4+ T cells do not expand as mice age and ~80% of the islets remain uninfiltrated [25]. (E) PD-1 pathway blockade at 15 weeks of age leads to an increased number of activated insulin-specific CD4+ T cells in the pancreatic lymph node, but insulin reactive T cells do not traffic to the pancreas and the extent of islet inflammation does not change [25].
To illustrate previous work combined with results presented here, we have generated a model figure depicting the relative number of antigen specific cells, expansion, trafficking to the pancreas, and islet destruction (Figure 1). Compared to NOD mice (Figure 1A), B6.g7 mice had a detectable population of insulin-specific CD4+ T cells in the secondary lymphoid organs (SLO), but these cells remained predominantly naïve throughout the lifetime of the animal (Figure 1D) and expressed low levels of PD-1[25]. PD-1 pathway blockade led to an increased number of activated insulin-specific CD4+ T cells in the pancreatic lymph node of both NOD (Figure 1B, C) and B6.g7 mice (Figure 1E), but B6.g7 mice remained disease- and infiltrate-free [25] (Figure 1E). PD-1/PD-L1 deficiency on a diabetes-prone background (NOD) led to accelerated disease as previously reported, and increased numbers of insulin-specific but not foreign antigen-specific CD4+ T cells in the SLO and pancreas [25].
Since the majority of islet-specific CD4+ T cells in NOD mice are anergic [24], we also investigated whether PD-1 pathway blockade restores function to these cells. Anergic cells were defined as cells producing little to no IFNγ and expressing CD73 and folate receptor 4 (FR4) on their surface [24, 41]. Effector cells were defined as FR4−CD73− cells capable of producing IFNγ following stimulation [24, 41]. Following anti-PD-L1 treatment, effector cells produced significantly more IFNγ on a per cell basis. While PD-1 blockade led to a modest increase in the amount of IFNγ produced by anergic cells, IFNγ production was still blunted in comparison to effector cells [25]. This suggested that PD-1 pathway blockade preferentially impacted the effector cell subset, and did not uniformly break T cell anergy.
In the current study, we evaluated the insulin-specific CD4+ T cell trafficking potential from NOD and B6.g7 mice with and without PD-1 pathway blockade. Collectively, our findings suggest that PD-1 blockade was not sufficient to result in B6.g7 lymphocyte homing to the pancreas due to lower expression of CXCR3 on insulin-specific CD4+ T cells, and an absence of a CXCL10 chemotactic gradient.
Materials and Methods
Mice
NOD mice were purchased from Taconic (Germantown, NY). B6.g7, NOD.PD-1−/− (PD-1 KO), and NOD.PD-L1−/− (PD-L1 KO) mice were generated as described [25, 42] and housed in specific-pathogen free conditions at the University of Minnesota. All animal experiments were approved by the Institutional Animal Care and Use Committee.
PD-1 pathway blockade
9 or 15 week old NOD and B6.g7 mice were treated with 250μg anti-PD-L1 (clone MIH6; [42]) or isotype control (Rat IgG2a; BioXCell) at day −3 and −1 prior to harvest for CXCR3 and CXCL10 analysis.
Detection of antigen-specific T cells
Insulin-specific CD4+ T cells were detected using insB10-23r3:I-Ag7 tetramer reagent and dual color staining with magnetic enrichment as described [24, 25]. Briefly, single cell suspensions were incubated for one hour at room temperature with 10 nM of tetramers conjugated to phycoerythrin (PE) and allophycocyanin (APC) in medium containing Fc receptor block (2.4G2) and 0.05% sodium azide. Spleen and non-draining lymph node samples were subjected to magnetic enrichment following a 30 minute incubation with both anti-PE and anti-APC microbeads at 4°C (Miltenyi Biotec). Single cell suspensions from the pancreas were isolated using collagenase P digestion (Roche) and discontinuous Percoll gradients (44%/67%) [24, 25]. Following tetramer staining, single cell suspensions from pancreas and pancreatic lymph node were subjected to surface staining for flow cytometric analysis.
Flow cytometry
Staining was performed for 30 minutes at 4°C using anti-PD-1 (J43), CD4 (GK1.5), CD3 (145-2C11), B220 (RA3-6B2), CD11b (M1/70), CD11c (N418), CD44 (IM7), CXCR3 (CXCR3-173) (eBioscience) and anti-CD8α (53-6.7) antibodies (Biolegend). Samples were acquired using BD LSRII or Fortessa instruments (BD) and analyzed with Flow Jo software (Treestar). Insulin-specific CD4+ T cells were identified as singlet+, CD3+ lineage (B220, CD11b, CD11c)− CD4+ CD8α−, insB10-23r3: I-Ag7-PE and -APC tetramer double positive [24, 25].
Immunofluorescence Microscopy
Pancreata were harvested for immunofluorescence and prepared as described [42]. Antibodies included guinea pig anti-swine insulin (Dako, Denmark), goat anti-mouse CXCL10 (R&D systems), donkey anti-guinea pig AF488 and bovine anti-goat AF647 (Jackson Immunoresearch), and hamster anti-mouse CD3-PE (eBioscience). Slides were mounted using Prolong Gold with DAPI (Life Technologies) and imaged on a Leica epifluorescent DM5500 microscope (Germany).
Results and Discussion
Emerging results from genome wide association studies point to PD-1 as a critical risk factor in T1D pathogenesis [32–35]. Additionally, PD-1 pathway inhibitors used to treat advanced malignancies have led to T1D development in several patients, indicating that at least in a subset of individuals, PD-1 is required for restraining islet-reactive T cells [31]. Increasing use of checkpoint blockade therapies warrants a better understanding of how PD-1 regulates islet-reactive CD4+ T cells in contexts of varying autoimmune susceptibilities. To this end, we examined insulin-specific CD4+ T cells following checkpoint blockade in diabetes-prone and diabetes-resistant mice expressing the same MHC class II molecule.
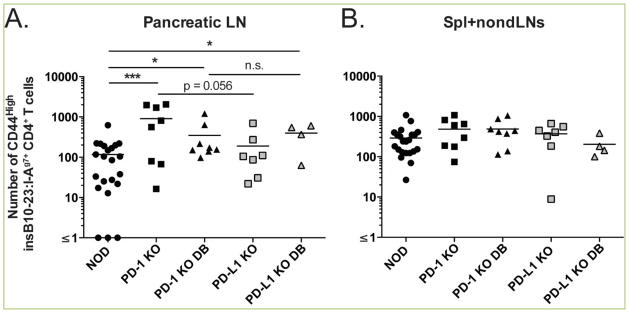
Genetic loss of PD-1 or PD-L1 in NOD mice led to an increased number of insulin-specific but not foreign antigen-specific CD4+ T cells [25]. Not only was there an increase in the abundance of insulin-specific cells in both PD-1 KO and PD-L1 KO mice, these cells exhibited increased expression of the activation marker CD44 compared to age-matched NOD mice (Figure 2). The number of CD44high insulin-specific CD4+ T cells was significantly higher in the pancreatic lymph nodes of prediabetic (907.9±310.4 cells) and diabetic PD-1 KO mice (347.2±137.0 cells) (p<0.001 and p<0.01, respectively) compared to prediabetic NOD mice (117.0±29.5 cells) (Figure 2A). Similarly, diabetic PD-L1 KO mice had a significantly greater number of CD44high insulin-specific CD4+ T cells (395.9±122.2 cells) compared to age-matched NOD mice (p <0.01) (Figure 2A). The number of activated insulin-specific CD4+ T cells in the spleen and non-draining lymph nodes (spl+non-dLNs) was not different across these strains (Figure 2B). Additionally, CD44 expression on foreign antigen-specific CD4+ T cells was comparable (data not shown).
Figure 2. NOD PD-1/PD-L1 deficiency leads to increased activation of insulin-specific CD4+ T cells.
The frequency of activated (CD44high) insulin specific T cells was determined from (A) pancreatic lymph nodes and (B) Spleen and non-draining lymph nodes of 5–6 week old PD-1 KO (n=8), PD-L1 KO (n=4–7), and age-matched littermate NOD controls (n=22) by flow cytometry prior to or at disease onset (“DB”=diabetes) using insB10-23r3:I-Ag7 tetramer reagents. Shown are compiled data from four independent experiments.
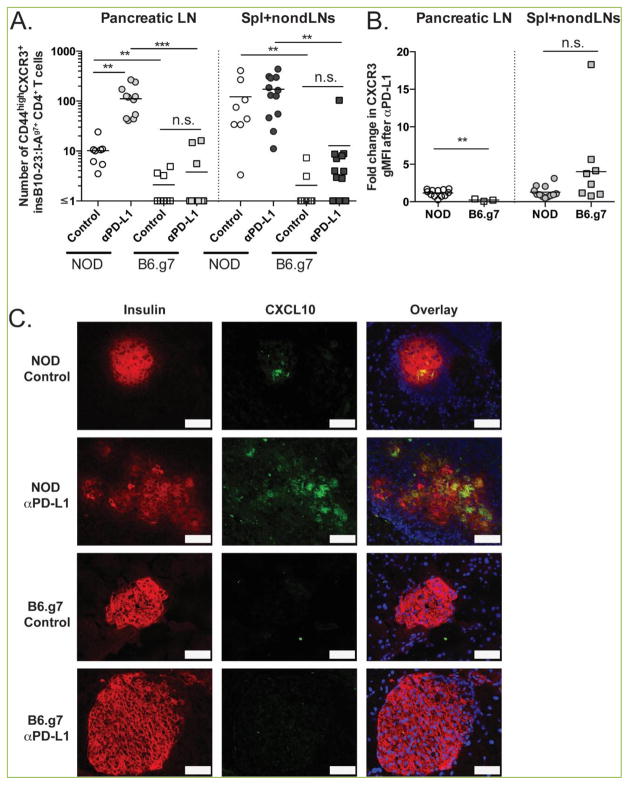
PD-1 pathway blockade led to an increase in the number of activated insulin-specific CD4+ T cells in the pancreatic lymph nodes of NOD and B6.g7 mice [25] (Figure 1B, E), but B6.g7 mice remained insulitis- and diabetes-free [25] (Figure 1E). We sought to determine whether anti-PD-L1 treatment altered the trafficking potential of insulin-specific cells in NOD or B6.g7 mice. Pancreas homing is regulated by the CXCR3-CXCL10 axis [43, 44]. Therefore, we assessed the expression levels of CXCR3 on insulin-specific CD4+ T cells in NOD and B6.g7 mice that were treated with anti-PD-L1 or control at days -3 and -1 prior to harvest. Anti-PD-L1 led to a significant increase in the number of CD44highCXCR3+ cells in the pancreatic lymph nodes of NOD (10.16±2.25 vs. 111.5±23.7, p<0.01), but not B6.g7 mice (2.09±0.55 vs. 3.78±1.62) (Figure 3A). Additionally, the number of insulin-specific CD4+ T cells with the potential to traffic was higher in NOD pancreatic lymph nodes compared to B6.g7 in control (p <0.01) and anti-PD-L1 treated mice (p<0.001). While anti-PD-L1 treatment did not lead to a significant increase in CD44highCXCR3+ cells in NOD spleen and nondraining lymph nodes, the number of cells with trafficking potential was larger than in B6.g7 mice (p<0.01 between control and anti-PD-L1 treated mice). The fold increase in the geometric mean florescence intensity (gMFI) of CXCR3 was higher on CD44high insulin-specific cells after anti-PD-L1 treatment in the pancreatic lymph node of NOD mice (p<0.01) (Figure 3B). These results suggest that NOD islet-reactive cells were more susceptible to PD-1 pathway blockade allowing enhanced activation (CD44) and greater ability to traffic to tissues with inflammation including the pancreas (CXCR3).
Figure 3. PD-1 pathway blockade increases the number of CD44high CXCR3+ insulin-specific CD4+ T cells in NOD but not B6.g7 mice.
NOD and B6.g7 mice were treated at day −3 and −1 with anti-PD-L1 (MIH6 clone; 250 μg/mouse) or isotype control (Rat IgG2a; 250 μg/mouse). (A) The number of CD44highCXCR3+ insulin-specific CD4+ T cells (identified by double-staining with insB10-23r3: I-Ag7-tetramers as described in Methods) in the secondary lymphoid organs of control and anti-PD-L1-treated NOD and B6.g7 mice. (B) Fold increase in geometric mean fluorescent intensity (gMFI) of CXCR3 after PD-L1 blockade from insulin-specific CD4+ T cells. Shown are compiled data from four independent experiments with n=2–3 per treatment group. (C) Immunofluorescent staining of CXCL10 expression in pancreas tissue from NOD and B6.g7 mice from anti-PD-L1 or isotype treated animals. Scale bar (white) corresponds to 50 μm. Images are representative of three independent experiments with >100 islets/treatment group.
The inflammatory cytokine interferon gamma (IFNγ) is known to be important for disease onset [45, 46]. CXCL10, or interferon regulated protein of 10Kd, is a chemokine that is made in response to IFNγ and inflammation [43, 47]. We hypothesized that B6.g7 islets lacked chemotactic gradients necessary to recruit the autoreactive cells. To test this, we examined islet CXCL10 expression by histology to correlate the level of islet infiltration with chemokine production. CXCL10 was absent from B6.g7 islets even after PD-L1-blockade, while CXCL10 was readily detectible in the islets of age-matched treated and untreated NOD mice (Figure 3C). Collectively, these findings indicate that there are at least two levels of islet tolerance: T cell-intrinsic and pancreas-microenvironment specific. We hypothesize that the initial wave of islet inflammation depends on CCL2, CCL5, and CXCL9 secreted in response to inflammatory cytokines such as IL-1β and TNF-α [48], and that CCR2+ or CCR5+ T cell-derived IFNγ drives CXCL10 expression in pancreatic beta cells to recruit CXCR3+ insulin-specific T cells. Further studies are needed to determine whether altering the pancreatic environment in B6.g7 mice would be sufficient to allow for T cell infiltration, islet destruction and diabetes development.
Our previous work suggests that the susceptibility to PD-1 blockade-mediated functional restoration depends on the differentiation state of CD4+ and CD8+ T cells [25]. Effector, but not anergic autoreactive CD4+ and CD8+ T cells were reinvigorated after PD-1 blockade, suggesting that blocking PD-1 signaling alone is not sufficient to rescue effector function for all anergic cells [25]. Therefore, blocking the PD-1/PD-L1 pathway alone might not be sufficient to reinvigorate all anergic tumor-specific cells, but it also might not be sufficient to restore full effector cell function of previously anergic, autoreactive T cells and/or allow T cell homing to target organ(s). Determining tumor- and self-specific CD4+ T cell activation status prior to initiation of checkpoint blockade therapy would likely help to stratify individuals who would benefit from this therapy versus those who might have adverse effects or incomplete tumor control.
Acknowledgments
This work was supported by National Institutes of Health Grants R01AI106791 and NIH P01 AI35296 (to B.T.F.) and by Juvenile Diabetes Research Foundation Grant 2-2011-662 (to B.T.F.) and 3-2014-215 (J.A.S.). The authors thank James Heffernan, Nathanael Sahli, Kevin Osum, Jason Mitchell, Adam Burrack, Jason Schenkel, Christine Nelson, Vaiva Vezys and Marc Jenkins for their assistance with this project.
Abbreviations
- T1D
type 1 diabetes
- NOD
non-obese diabetic
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- SLO
secondary lymphoid organs
- FR4
folate receptor 4.
Footnotes
Conflicting Interests
The authors have declared that no conflict of interests exist.
Author contributions
T.M., K.E.P. and B.T.F. designed, performed, and analyzed the data. J.A.S. designed and generated the IAg7 tetramers. B.T.F directed the studies. T.M. and B.T.F. prepared the figures. J.A.S. and B.T.F. prepared the model figure. T.M. and B.T.F wrote the manuscript. J.A.S. and K.E.P. edited the manuscript.
References
- 1.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37:402–408. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 4.James S, Gallagher R, Dunbabin R, Perry L. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: systematic literature review. BMC Res Notes. 2014;7:593–603. doi: 10.1186/1756-0500-7-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 8.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 9.Serreze DV, Leiter EH, Christianson GH, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 10.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 12.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–274. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- 13.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 14.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med. 2001;345:1036–1040. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 15.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abiru N, Yu L, Miao D, Maniatis AK, Liu E, Moriyama H, et al. Transient insulin autoantibody expression independent of development of diabetes: comparison of NOD and NOR strains. J Autoimmun. 2001;17:1–6. doi: 10.1006/jaut.2001.0530. [DOI] [PubMed] [Google Scholar]
- 17.Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: prediction, significance, and detection of distinct disease subtypes Cold Spring Harb. Perspect Med. 2012;2:85–103. doi: 10.1101/cshperspect.a012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 21.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JS. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208:2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauken KE, Linehan JL, Spanier JA, Sahli NL, Kalekar LA, Binstadt BA, et al. Cutting edge: type 1 diabetes occurs despite robust anergy among endogenous insulin-specific CD4 T cells in NOD mice. J Immunol. 2013;191:4913–4917. doi: 10.4049/jimmunol.1301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauken KE, Nelson CE, Martinov T, Spanier JA, Heffernan JR, Sahli NL, et al. Cutting Edge: Identification of Autoreactive CD4+ and CD8+ T Cell Subsets Resistant to PD-1 Pathway Blockade. J Immunol. 2015;194:3551–3555. doi: 10.4049/jimmunol.1402262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 27.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2010;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 29.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni R, Ihara K, Miyako K, Kuromaru R, Inuo M, Kohno H, et al. PD-1 gene haplotype is associated with the development of type 1 diabetes mellitus in Japanese children. Hum Genet. 2007;121:223–232. doi: 10.1007/s00439-006-0309-8. [DOI] [PubMed] [Google Scholar]
- 33.Flores S, Beems M, Oyarzun A, Carrasco E, Perez F. Programmed cell death 1 (PDCD1) gene polymorphisms and type 1 diabetes in Chilean children. Rev Med Chil. 2010;138:543–550. [PubMed] [Google Scholar]
- 34.Pizarro C, Garcia-Diaz DF, Codner E, Salas-Perez F, Carrasco E, Perez-Bravo F. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes Metab Res Rev. 2014;30:761–766. doi: 10.1002/dmrr.2552. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62:492–497. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188:170–81. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during Type 1 Diabetes. Diabetes. 2013;62:2859–2869. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 44.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171:6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 45.Yi Z, Li L, Garland A, He Q, Wang H, Katz JD, et al. IFN-gamma receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells. Eur J Immunol. 2012;42:2010–2018. doi: 10.1002/eji.201242374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, et al. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 48.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]