Abstract
Intracellular metabolism is central to cell activity and function. CD4+CD25+ regulatory T (Treg) cells that express the transcription factor forkhead box P3 (FOXP3) play a pivotal role in the maintenance of immune tolerance to self. Recent studies have shown that metabolism and function of Treg cells are influenced significantly by local environmental conditions and the availability of certain metabolites. It has also been reported that defined metabolic programs associate with Treg cell differentiation, expression of FOXP3, and phenotype stabilization. This article reviews how metabolism modulates FOXP3 expression and Treg cell function, what environmental factors are involved, and how metabolic manipulation could alter Treg cell frequency and function in physiopathologic conditions.
1. Intracellular metabolism of Treg cells
CD4+CD25+FOXP3+ regulatory T (Treg) cells are critically involved in the maintenance of immune tolerance to self and in the control of immune and autoimmune responses (1). Similarly to conventional CD4+ T (Tconv) cells, Treg cells have a high degree of plasticity that associates with different transcriptional programs, which are in turn impacted by cellular metabolism.
During the past decade, significant advances have been made in furthering the understanding of the molecular regulation of gene expression in Treg cells (1-3). The integration of multiple cell signals can directly affect transcriptional programs and signalling pathways involved in cell proliferation, production of cytokines, and energy metabolism. In this context, it has been reported that glycolysis and fatty acid oxidation (FAO) may be used differently by Treg cells and Tconv cells (4). In vitro, differentiated mouse Treg cells display low glycolytic flux and oxidize lipids at higher rateas compared to other T cell subsets (via AMP-activated protein kinase [AMPK]), (5). For human Treg cells, hyporesponsive to T cell receptor (TCR) stimulation, the high glycolytic rate is supported by high mammalian target of rapamycin (mTOR) activity and does not associate with FAO (4). However, in vitro proliferating human Treg cells engage both glycolysis and FAO, whereas Tconv cell increase their metabolic activity by switching oxidative phosphorylation (OXPHOS) of the resting condition toward aerobic glycolysis to generate ATP (4). Aerobic glycolysis is far less efficient than OXPHOS and represents an unusual metabolic feature of proliferating T cells and cancer cells, a phenomenon known as “Warburg effect”. Despite its low efficiency in energy production, aerobic glycolysis provides essential materials to the synthesis of nucleic acids and phospholipids (4, 6).
In vivo, human and mouse Treg cells display high glycolytic rate associated with hyperactivation of the “environmental” sensor mTOR (7-9). mTOR comprises two multiprotein complexes: mTOR complex 1 (mTORC1), which contains the regulatory-associated protein of mTOR (RAPTOR), and mTOR complex 2 (mTORC2), which contains the rapamycin-insensitive companion of mTOR (RICTOR). mTORC1 is sensitive to the immunosuppressant drug rapamycin and represents an important regulator of cell growth and differentiation (10-11). Published evidence suggests that mTORC1 can act as a negative regulator of de novo differentiation of Treg cells, and as a positive determinant for their function (7, 8, 12). Mouse T cells in which mTORC1 has been ablated do not differentiate into Treg cells, requiring concomitant inhibition of mTORC2 signalling to generate Treg cells (13).
It must be noted that the metabolic differences between Treg and Tconv cells are significant. While Treg cells are highly dependent on mitochondrial metabolism with the flexibility to also oxidize lipid or glucose, Tconv cells mainly convert glucose to lactate (4, 5, 14). Treg cells appear to have a stronger respiratory capacity and preferentially oxidize glucose-derived pyruvate as compared to Tconv (15). The high expression of carnitine palmitoyltransferase 1a (CPT1a) - the rate-limiting enzyme of FAO that allows the entry of acyl groups into the mithocondria - supports the possibility that Treg cells can use multiple fuel sources (4, 5). Interestingly, mTOR controls several metabolic processes, including glucose metabolism but also fatty acid synthesis, which is important for Treg cells to acquire a full regulatory function. mTORC1 increases the expression of glucose transporters, including Glut1, on activated T cells, augments the intracellular concentration of glucose supporting glycolysis (16). TCR and CD28-induced Akt signaling playan important role for Glut1-mediated glucose transport (5). mTOR signaling also induces glycolysis via the oncogene c-MYC, a crucial regulator of metabolic reprogramming in T cells (14). Specific deletion of RAPTOR, an obligatory component of mTORC1, leads to alteration in cholesterol- and lipid-synthesis in Treg cells (8). The role of mTORC1 in lipogenesis is also supported by the findings that rapamycin blocks the expression of genes involved in lipid synthesis and alters nuclear localization of the master regulators of lipid homeostasis, sterol regulatory element-binding proteins (SREBPs) (17).
2. Metabolic status of Treg cells in relation to function
Cell metabolism is central for Treg cell differentiation and is tightly linked to their function, in addition to supporting responsiveness to cell stimulation. Depending on nutrient availability and microenvironmental cues, Treg cells can use alternate substrates and metabolic pathways for energy (Fig. 1). In the last decade, emphasis has been placed on the relationship between immune signaling and metabolic pathways that affect Treg cell function, particularly the role of mTOR complex that senses environmental nutrients and growth factors for the modulation of Treg cell function and differentiation (7, 8, 13, 18). mTORC1 couples TCR and IL-2 signaling to Treg cell suppressive activity (8) and, metabolically, drives cholesterol and lipid biosynthesis through the induction of genes including 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), squalene epoxidase (SQLE) and isopentyl-diphosphate δ isomerase 1 (IDI1), that are required for the expression of Treg cell markers such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and inducible costimulator (ICOS) (8, 19).
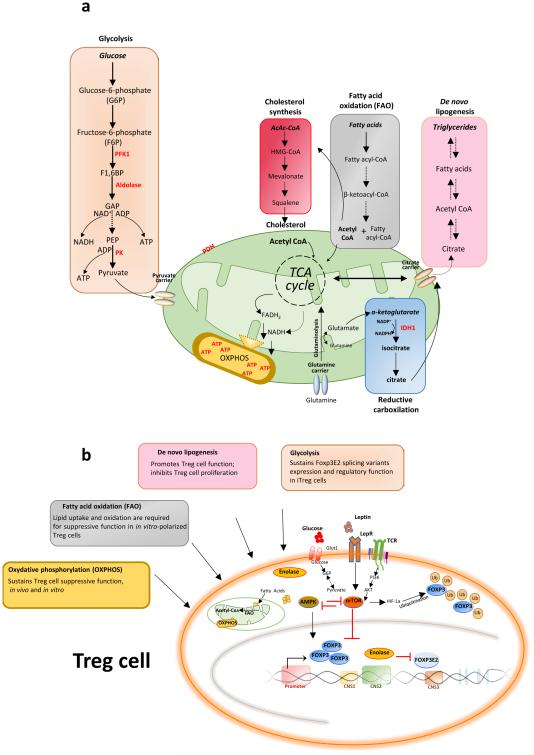
Figure 1. Schematic representation of the metabolic pathways in Treg cells and their effects on FOXP3 expression and cell function.
a, Main metabolic pathways in T cells. b, Cell-intrinsic metabolic programs and environmental factors that can modulate FOXP3 expression and Treg cell suppressive activity, in addition to differentiation, depending on nutrient availability and external or intracellular signals. The metabolic programs and their products can ultimately affect the Treg cell fate.
Abbreviations: ATP, adenosine triphosphate; CNS, conserved non-coding sequence; FAO, fatty acid oxidation; FOXP3, forkhead box P3; F6P, fructose 6-phosphate; G6P, glucose 6-phosphate; mTOR, mammalian target of rapamycin; OXPHOS, oxydative phosphorylation; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; TCA, tricarboxylic acid cycle; AMPK, AMP-activated protein kinase; LepR, leptin receptor; TCR, T cell receptor; HIF-1, Hypoxia-inducible factor 1; Ub, ubiquitination; iTreg, inducible Treg cells.
Freshly isolated Treg cells express high levels of mTOR and ATP because they actively proliferate in vivo (9). Their highly proliferative state reflects higher mTOR activity and ATP levels as compared with Tconv cells that do not proliferate in vivo. The high proliferative state of Treg cells makes them refractory to TCR stimulation in vitro and therefore anergic. mTOR overactivation in Treg cells can also depends on the capacity of Treg cells to secrete leptin, an adipocyte-derived cytokine that activates mTOR via its class I cytokine receptor that is expressed on Treg cells (Fig. 1b) (20). Inhibition of mTOR activity via leptin neutralization or transient rapamycin treatment, reverses Treg cell hyporesponsiveness inducing proliferation after TCR stimulation (20, 7). A possible explanation could be that Treg cells need to reduce transiently their metabolic rate to enter the cell cycle and proliferate, as suggested by a recent report that showed that transient reduction of glycolysis and mTOR activity (via leptin neutralization) in freshly–isolated human Treg cells before TCR stimulation reversed their anergic state in vitro (20, 7). Together, the data suggest that although high mTOR activity renders Treg cells refractory to TCR stimulation, it is also necessary for Treg cells to proliferate over time once the cell cycle is engaged (20, 7, 4). Another key issue for Treg cells that proliferate in vivo is that active glycolytic-lipogenic pathways (18) would allow rapid generation of ATP and the transfer of glucose-derived carbons into metabolic intermediates for synthesis of proteins, nucleic acids, and lipids. Modulation of phosphatidylinositol 3-kinase (PI3K) signaling can also alter cellular metabolism and FOXP3 expression. Genetic ablation of phosphatase and tensin homolog on chromosome 10 (PTEN), the primary negative regulator of PI3K, results in an increase in glycolysis, loss of FOXP3 expression, and the induction of effector T cells (21, 22). Recently Wei et al. showed that deletion of Atg7 or Atg5, two essential genes involved in autophagy, led to Treg cells loss (autophagy deficiency upregulated the metabolic regulators mTORC1 and glycolysis contributing to a defective Treg function) (23).
Glycolytic enzymes are also regulated by hypoxia-inducible factor-1α (HIF-1α) induced by TCR engagement (24, 25). HIF-1α is a transcription factor that, during hypoxia, binds to hypoxia-response elements and determines the transcription of genes that important for cell survival under low oxygen conditions (26), including those encoding enzymes required for glycolysis (27). HIF-1α also promotes the expression of Glut1 and enforces ATP synthesis by glycolysis, rather than OXPHOS by upregulating pyruvate dehydrogenase kinase 1 (PDK1), an enzyme that inhibits the entry of pyruvate into the TCA cycle (28-30). HIF-1α expression is also dependent on external cues that are integrated by mTOR signaling (31), and HIF-1α is required for optimal Treg function since HIF-1α -deficient Treg cells fail to control autoimmune colitis (32). However,the mTOR-HIF-1α axis also promotes Th17 differentiation and lack of HIF-1α can results in diminished Th17 development and enhanced Treg cell differentiation that can protect mice from autoimmune neuroinflammation (25). Finally, the key role of OXPHOS in the energy production of Treg cells derives from the observation that deletion of regulators of mitochondrial activity such as peroxisome proliferator-activated receptor γ coactivator 1a (Pgc1a) or sirtuin (Sirt) 3 inhibit Treg cell suppressive function in vitro and in vivo (33).
In sum, in vivo Treg cell metabolism is dynamic and finely regulated to ensure function, being intimately connected to oscillatory cues such as the strength of TCR signal, cytokine milieu, and nutrient availability.
3. Effects of metabolism on FOXP3 expression
Earlier studies showed that the expression of genes involved in cell metabolism influence FOXP3 induction and IL-2 signaling (34-36). Using multiple pharmacological inhibitors and activators, it was shown that differential metabolic programs could regulate Treg cell lineage differentiation both in vivo and in vitro (25, 37, 38). Specifically, inhibition of glycolysis with the glucose analogue 2-deoxyglucose (2-DG) - a prototypical inhibitor of the glycolytic pathway - promoted induction of mouse Treg cells in vitro, in the presence of polarizing cytokines such as transforming growth factor (TGF)-β and IL-2 (5, 25). During glycolysis inhibition, the reduction of mTOR-dependent HIF-1α transcriptional program resulted in FOXP3 induction (Fig. 1b). Absence of HIF-1α led to increased Treg cell differentiation and protected mice from autoimmune diseases, being this factor capable to promote FOXP3 ubiquitination and subsequent proteasome degradation (Fig. 1b) (25, 39). Chronic treatment with rapamycin (which hampers glycolysis by inhibiting mTOR) induced de novo expression of FOXP3 and Treg cells expansion from naïve CD4+ T cells in the presence of high concentration of IL-2 (12, 40, 41). This strategy to expand tTreg cells could be seen as an “apparent paradox” because rapamycin inhibits mTOR but IL-2 can activate it. It is known that chronic rapamycin treatment alone inhibits Treg cell proliferation (7, 42, 43) yet rapamycin in the presence of high doses of IL-2 allowsTreg cells to expand more robustly than with IL-2 alone (40, 41). We reported the possibility that this phenomenon could be due to the fact that to enter the cell cycle, Treg cells need low mTOR phosphorylation (achieved with rapamycin treatment) and after entering the cell cycle, IL-2 can help to reactivate mTOR which is necessary for Treg cells to proliferate over time. This “oscillatory” phenomenon for Treg cell expansion would occur both in vitro and in vivo (20, 7, 4, 44).
Inhibition of glucose uptake and glucose oxidation by dichloroacetate (DCA) promoted Treg cells differentiation (37), and glycolysis appeared as necessary for the generation of human inducible Treg cells (iTregs) from Tconv in vitro in the absence of either exogenous polarizing cytokines (i.e. TGF-β), drugs (i.e. rapamycin), or strong TCR stimulation (38). We recently reported that the inhibition of glycolysis with 2-DG led to a differential expression of human FOXP3 splicing variants, including those required for the suppressive function of Treg cells, whereas the inhibition of lipid oxidation supported iTregs differentiation by increasing the expression of the FOXP3 splicing forms that support regulatory functions (38) (Fig. 1b). Other groups confirmed the notion that different human FOXP3 splicing variants have differential capacities to control the generation and function of Treg cells (45-48). In any case, FOXP3 expression is also impacted by pyruvate metabolism, a checkpoint in glucose metabolism (15). Pyruvate dehydrogenase (PDH) contributes to the transformation of pyruvate into mitochondrial acetyl-CoA for oxidative metabolism. Acetyl-CoA levels can also control FOXP3 stability. The acetylated state of FOXP3 is reciprocally regulated by the histone acetyltransferase p300 and the histone deacetylase Sirt1. Acetylation of FOXP3 increases stable protein levels by preventing polyubiquitination and proteasomal degradation (49).
On the other hand, a requirement of lipid uptake and oxidation for the expression of FOXP3 is testified by the use of etomoxir, a selective CPT1a inhibitor that significantly affects FOXP3 expression in mouse T cells (5). Lipid metabolism in Treg cells commitment was also apparent when using pharmacological inhibition of estrogen-related receptor-α (ERRα), which impairs Th1, Th2, Th17 responses and also Treg cell differentiation in vitro. The addition of fatty acids to in vitro cultures rescued differentiation of Treg cells but not Th cells because ERRα upregulated Glut1 protein, glucose uptake and mitochondrial processes (hampering FAO through CPT1a inhibition), thus favoring Treg cells and not Th cells (50). In conclusion, cell metabolism is highly dynamic in vivo and strongly related to in vitro experimental conditions that include TCR signal strength (dose and duration) and the presence of cytokines or drugs (51-53). Also, while the metabolic requirements of in vitro differentiating Treg cells under polarizing conditions have been actively analysed, less has been done to identify the metabolic determinants of Treg cells induction in vivo.
4. Environment and Treg cells
Treg cels metabolism requires cues that include TCR signal strength, cytokine milieu, and nutrients availability. An emerging concept is that Treg cells are functionally specialized and influenced in their development, maintenance, and function by the local environment represented by the local milieu of metabolites, adipocytokines, and gut microbiota (54).
4.1 Effects of metabolites on Treg cell stability
Purine catabolism is an important metabolic process that regulates the balance of proinflammatory adenosine 5’-triphosphate (ATP) and immunosuppressive adenosine. Extracellular nucleotides such as the ATP released by T cells during TCR stimulation can contribute to autocrine modulation through the activation of purinergic P2X receptor (P2X7) that inhibits the Treg cell suppressive activity through FOXP3 inhibition (55). Stimulation of P2X7 inhibits tissue-specific immunosuppressive potential of Treg cells and facilitates conversion into Th17 cells during chronic inflammation. Pharmacological antagonism of P2X receptors or loss of P2X7 in Treg cells ameliorates tissue inflammation by preserving Treg cell function (55). Also, the CD39 ectoenzyme on human Treg cells produces adenosine monophosphate (AMP) from ATP or adenosine diphosphate (ADP), which is subsequently converted to extracellular adenosine by the CD73 ectoenzyme expressed on Tconv. Proper Treg cell function requires a coordinated expression of the adenosine 2A receptor (A2A) on activated T cells to enable adenosine-mediated immune suppression (56-58). Adenosine can bind A2A receptor and facilitate Treg cells generation and suppressive function (59).
Other metabolites that can affect Treg cell function are vitamins A, D, tryptophan and arginine. Retinoic acid (RA), the bioactive metabolite of vitamin A, promotes with TGF-β the conversion of naïve T cells into FOXP3+ Treg cells (60-62). RA stabilizes FOXP3 expression and prevents IL-1β/IL-6-driven conversion of Treg cells into Th1/Th17 cells (63). The active metabolite of vitamin D, calcitriol, also promotes growth of FOXP3+ and IL-10-producing Treg cells, increasing the frequency of FOXP3+ cells when combined with TGF-β (64, 65). Another amino acid that influences FOXP3 expression is glutamine. Glutaminolysis provides, in active T cells, carbon and nitrogen for other proliferation-associated biosynthetic pathways such as the hexosamine and nucleotide biosynthetic pathways involved in several T cell functions. Limited availability of extracellular glutamine shifted the balance from Th1 to Treg cells (66). TCR stimulation of naïve CD4+ T cells in the presence of low glutamine levels resulted in the conversion into FOXP3+ Treg cells under Th1 polarizing conditions. Furthermore, TGF-β-induced Treg cells exhibited Treg cell–specific demethylated region (TSDR) with a methylation status similar to that of Treg cells generated in the absence of glutamine (66). The explanation could be that glutamine is catabolized to generate α-ketoglutarate (α-KG), which in T cells decreases generation of FOXP3+ cells and supports energy production through the tricarboxylic acid (TCA) cycle - critical for Th1 cells commitment. Conversely, Song et al. reported that glutamine administration in a mouse aGVHD model significantly increased the fraction of Treg cells and inhibited GVHD-induced inflammation and tissue injury in the intestine, liver, skin and spleen (67).
Other metabolites stimulate the aryl hydrocarbon receptor (AHR), which controls the balance between Treg cells and Tconv. For example, kynurenine, a product of tryptophan catabolism generated by indoleamine 2,3-dioxygenase (IDO), is an AHR agonist important for the generation, expansion and suppressive function of stable Treg cells (68-70). Dietary metabolites that can bind AHR such as indole-3-carbanole (I3C) and 3, 3'-diindolylmethane (DIM), can increase Treg cells infiltration into the central nervous system and ameliorate experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (71). Moreover, in skin grafts, dendritic cells (DCs) that upregulated enzymes that consume different essential amino acids (EAAs) determine reduction of mTOR activity and the induction of FOXP3 in T cells as naïve T cells fail to proliferate in response to antigen and can be converted into Treg cells (72).
Finally, Morse et al. showed that an arginine-rich cell-penetrating phoshorodiamitade morpholino oligomer (PPMO), by targeting FOXP3, inhibited Treg cell function and induced effector T cell response (73), suggesting that PPMO antisense-based strategy might be used as a potential tool to improve immunotherapy.
4.2 Effects of adipokines on Treg cells
Molecules involved in the regulation of food intake and metabolism at the hypothalamic level, such as leptin and adiponectin, can also affect generation and proliferation of Treg cells. The leptin/leptin receptor (LepR) axis controls metabolic state and functional activity of Treg cells Treg cells express LepR and are present as a specific fat-resident population producing leptin (20, 74, 75). Leptin deficiency in mice associates with an increased frequency of Treg cells and the protection from multiple autoimmune diseases (20, 76-77). Also reduction of leptin levels induced by starvation or leptin neutralization, increased Treg cell frequency and reduced inflammation and autoimmune progression (7, 78). The mechanism of action of leptin on Treg and Tconv cells are interesting. LepR engagement induced mTOR activity in both cell compartments. However, in Tconv cells, thanks to their glucose metabolism, it supported proliferation and differentiation towards Th1/Th17 inflammatory phenotypes. On the contrary, leptin produced by Treg cells, in an autocrine loop, activated glycolysis and mTOR making these cells hyporesponsive to in vitro TCR activation.
Another adipocyte-derived hormone is adiponectin, that also has effects on immune cells. For example, adiponectin treatment of DCs resulted in decreased expression of CD80, CD86, MHC class II and IL-12p40; these effects favored an increase in the frequency of FOXP3+ Treg cells and reduced Tconv proliferation and IL-2 production (79). Adiponectin-deficient mice developed worse EAE and greater central nervous system (CNS) inflammation, demyelination and axon injury together with a defect in Treg cell number and function (80). In this context, Ramos-Ramírez et al. showed that adipose tissue resident Treg cells expressed higher levels of adiponectin receptor 1 (AdipoR1) than Treg cells in the spleen, and adipoR1 expression on adipose tissue Helios+ Treg cells was negatively correlated with epididymal fat (81). In conclusion, these data suggest that mediators involved in the regulation of food intake have affect Treg cell metabolism.
4.3 Effects of microbiota on Treg cells
Microbial-derived molecules such as short-chain fatty acids (SCFAs) and polysaccharide A (PSA) from Bacteroides fragilis seem to promote expansion and function of intestinal Treg cells (82-86). Among all SCFAs, butyrate is the strongest Treg cell inducer in vitro through G-protein coupled receptors (GPR). It inhibits histone deacetylase (HDAC) determining increased FOXP3 expression (82, 85). Indeed, when Tconv cell are cultured in Treg cell differentiation conditions, butyrate treatment enhances acetylation at histone H3 lysine 27 (H3K27) at the level of the FOXP3 promoter, CNS1 and CNS3 regions, thus leading to an increased FOXP3 expression (82). Moreover, purified PSA increases Treg cell frequency and the expression of regulatory molecules such as IL-10 and TGF-β (84), also via a direct interaction with the toll-like receptor (TLR)2 (86). In this context, Atarashi et al. showed that colonization of mice with murine fecal-derived Clostridia clusters IV and XIVa, or clusters IV, XIVa and XVIII isolated from human feces, expanded Treg cells and enhanced their activity in the colon. Mechanistically, those bacterial strains produce, in the intestine, high amount of TGF-β, a major cytokine that promotes Treg differentiation (87, 88).
5. Metabolism of Treg cells in autoimmunity
As discussed above, mTOR critically integrates multiple environmental stimuli to regulate T cell activation, differentiation and homeostasis. However, the same upstream stimuli that can activate mTOR in Tconv can have different effects on the function and differentiation of Treg cells (89, 90). In this context, mTOR controls differentiation and function, suggesting that its targeting could modulate Treg cell responses. Specifically, mTOR inhibition with rapamycin promoted Treg proliferation following acute treatment, while chronic treatment with rapamycin required IL-2 for Treg proliferation (7, 40, 41).
This aspect could relate to an increased activation of Akt by chronic rapamycin (91, 92) or to the fact that rapamycin suppressed mTORC2 and partly inhibited mTORC1 (93). Therefore, the positive effects of rapamycin and mTOR deletion on iTreg differentiation could be attributed to concomitant reductions in mTORC1 and mTORC2 activity (13), considering that RICTOR deletion delays lethality in mice with RAPTOR deficiency in Treg cells. It is interestingly to note that genetic Treg-specific deletion of RAPTOR determined a reduced proliferation of Treg cells (8), further confirming that in the absence of IL-2, chronic (pharmacological or genetic) mTOR inhibition negatively regulated Treg cells proliferation. Moreover, deletion of the gene encoding tuberous sclerosis 1 (TSC1), a negative regulator of mTOR, impaired Treg cell suppressive function and FOXP3expression (94).
Another consideration is that mTORC1 is a critical positive regulator of metabolic programs for Treg cells in vivo, and genetic deficiency in mice of RAPTOR in Treg cells leads to lymphadenopathy and multi-organ autoimmunity associated with T cell hyperactivity (8). This can be explained by the finding that RAPTOR regulates the expression of CTLA-4 and, partly, ICOS, and links the biogenesis of cholesterol with metabolic pathways that regulate proliferation of Treg cells (8). However, since those data derive from genetic knockout models, it cannot be excluded that those findings could be ascribed to activation of alternative, compensatory pathways.
However, excessive mTOR signaling can dampen Treg cell responses. Transient TCR stimulation induces phosphoinositide 3-kinase (PI3K)-Akt-mTOR signaling that antagonizes FOXP3 expression (35). Freshly-isolated Treg cells from relapsing-remitting multiple sclerosis (RRMS) patients show an mTOR overactivation that correlates with reduced IL-2 signaling, Treg cell proliferation, and FOXP3 expression (89), suggesting that the proposed mTOR “oscillatory” activity can be lost in autoimmune conditions and lead to altered Treg cell homeostasis/proliferation (89).
Finally, in EAE, the inhibition of acetyl-CoA carboxylase 1 (ACC1) promoted development of Treg cells while restraining Th17 cells (18) because Th17 cells, but not Treg cells, depend on ACC1-mediated de novo fatty acid synthesis and the glycolytic-lipogenic metabolic pathway to produce phospholipids for cellular membranes, whereas Treg cells readily take up exogenous fatty acids (18). These results indicate fundamental differences between Th17 cells and Treg cells regarding their dependency on ACC1-mediated de novo fatty acid synthesis in those autoimmune models (18).
6. Metabolism and Treg cells in cancer
Treg cells represent a major obstacle to effective anti-tumor immunity and immunotherapy. Indeed, the presence of Treg cells correlates with poor prognosis in different tumor types (95, 96). The presence of specific metabolites in the microenvironment profoundly affects Treg cells suppressive function and lineage stability (97). For example, IDO metabolizes tryptophan to kynurenine, an endogenous ligand able to activate AHR which contributes to Treg cell induction (98, 99). Many types of cancers overexpress IDO, either in tumour cell or in cancer-associated cells, including macrophages, DCs and endothelial cells. In the tumour microenvironment, IDO activity reduces local tryptophan availability in the proximity of Treg cells. Low concentration of tryptophan activates a stress responses pathway in Treg cells through the protein kinase general control nonderepressing-2 (GCN2), which inhibits mTORC2 and prevents its ability to phosphorylate Akt. The inhibition of Akt contributes to maintain the Treg suppressive function (100, 101). Also, GCN2 activation in T cells may switch CD4+ T cell differentiation toward a regulatory-type phenotype (102, 103). Last, mouse tumor-draining lymph nodes contain IDO+ DCs that activate Treg cells which sustain the intra-tumoral suppressive microenvironment (104). In papillary thyroid carcinomas (PTCs), IDO expression correlates with Treg cell density in the tumor site (105). The anti-tumor activity of Treg cells is also linked to their expression of CD39 and CD73 that generates adenosine from extracellular nucleotides. Adenosine is a potent inhibitor of T cell responses and the A2A receptor is a major anti-inflammatory adenosine receptor involved in the protection from tissue damage (106, 107). CD39+CD73+ iTregcells hydrolyze ATP to 5'-AMP and adenosine and mediate suppression of immune cells that express adenosine receptors. These iTreg, expanding in response to tumor antigens and cytokines such as TGF-β or IL-10, are presumably responsible for the suppression of anti-tumor immune responses and successful tumor escape (108).
7. Metabolic targeting of Treg cell number and function in pathologic conditions
Metabolic intervention could have relevant implications on Treg cells in the development of strategies of intervention when immune tolerance is compromised. In this context, high fat diet (HFD)-fed mice treated with the mTOR inhibitor rapamycin displayed significant changes of inflammatory profiles in both adipose tissue and liver, together with increased Treg cell function (109). Rapamycin protected against insulin resistance, increased energy expenditure and reduced weight gain in diet-dependent obese mice (109). Moreover, in type 1 diabetes (T1D), rapamycin expanded Treg cells and increased their capability to suppress Tconv (110, 111). Combined treatment of rapamycin with IL-10 also inhibited T1D development and induced Treg cells and long-term immune tolerance in non-obese diabetic (NOD) mice (112). In EAE, mTOR inhibition, at peak of disease, ameliorated clinical course and reduced CNS demyelination and axonal loss associated with an expansion of Treg cells (113), and in vivo transient inhibition of mTOR enhanced Treg cell proliferation and ameliorated EAE (7). Recent studies in mice and humans reported a wide efficacy of metformin, an AMPK activator classically used for hyperglycaemia and T2D treatment, in the treatment of autoimmune disorders. Treatment with metformin inhibited mTOR phosphorylation and increased FOXP3 expression, positively affecting the Treg/Th17 cell balance in humans with multiple sclerosis and in mice with inflammatory bowel disease (114, 115). Pathways others than mTOR can be relevant as well as pioglitazone (PIO) - a drug used for type 2 diabetes that stimulates peroxisome proliferator-activated receptor (PPAR)-γ - also restored number and function of visceral adipose tissue (VAT) Treg cells (74). Also, the inhibition of acetyl-CoA carboxylase-1 (ACC1) - a key enzyme for the regulation of fatty acid metabolism - promoted Treg cell development and impaired Th17 cell formation in EAE (18).
Lastly, the possibility to modulate immune response through manipulation of gut microbiota has been considered, particularly targeting the Bacteroidetes and Firmicudes phyla that appear to stimulate Treg cells and restrain Th17 cells in autoimmunity. As anticipated before, there are reports that suggested that colonization of mice with the human commensal B. fragilis induced IL-10 production and FOXP3 expression, preventing colitis (84, 116). Oral administration of polysaccharide A (PSA) from B. fragilis prevented EAE by stimulating dendritic cells to convert naïve T cells into Treg cells (117, 118). Several Lactobacillus strains also ameliorated experimental colitis in mice via Treg cell induction (119, 120).
7. Conclusions
The field that links immunity and metabolism is rapidly expanding. Interestingly, non-immunological disorders with a strong metabolic component such as obesity and type 2 diabetes have been linked to immune dysregulation, suggesting that metabolic alterations can be induced by or be consequence of an altered state of immune tolerance. In addition, immune-mediated disorders such as multiple sclerosis display conspicuous metabolic alterations.
Treg cells can represent a bridge linking metabolism and immunity, due to their unique sensitivity to changes in the intracellular and extracellular milieu that reflects in metabolic cell changes. Experimental evidence (121, 122) shows that metabolic imbalance (i.e., overweight and obesity) can increase the risk of development of immune-mediated diseases such T1D and as multiple sclerosis. Unfortunately, a limitation in studying Treg cell metabolism is represented by their plasticity and differences according to source (human vs mouse), in vitro culture conditions (i.e., exogenous cytokines such as IL-2 and TGF-β), and TCR engagement.
Pharmacological approaches that can target Treg cell metabolism have recently been considered, with the hope to possible be used as means of restoration of impaired function (115). However, the field is still preliminary, particularly because those pharmacological agents might have effects not only on Treg cells but also on all other immune cell subsets.
Acknowledgements
The authors would like to thank Drs. Marianna Santopaolo and Alessandra Colamatteo for reviewing the manuscript.
G.M. is supported by grants from the European Union IDEAS Programme European Research Council Starting Grant “menTORingTregs” n. 310496, the Fondazione Italiana Sclerosi Multipla (FISM) n. 2012/R/11 and the European Foundation for the Study of Diabetes (EFSD)/JDRF/Lilly Programme 2015. V.D.R. is supported by the Ministero della Salute grant n. GR-2010-2315414, the Fondazione Italiana Sclerosi Multipla (FISM) n. 2014/R/21 and AIRC-Cariplo TRIDEO Grant n. 17447. M.G. is supported by grant from Juvenile Diabetes Research Foundation (JDRF) n. 1-PNF-2015-115-5-B. A.L.C. is partly supported by the National Institutes of Health Grant AI109677.
References
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Luo CT, Li MO. Transcriptional control of regulatory T cell development and function. Trends Immunol. 2013;34:531–539. doi: 10.1016/j.it.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painter MW, Davis S, Hardy RR, Mathis D, Benoist C. Transcriptomes of the B and T lineages compared by multiplatform microarray profiling. J. Immunol. 2011;186:3047–3057. doi: 10.4049/jimmunol.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procaccini C, Carbone F, Di Silvestre D, Brambilla F, De Rosa V, Galgani M, Faicchia D, Marone G, Tramontano D, Corona M, Alviggi C, Porcellini A, La Cava A, Mauri P, Matarese G. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity. 2016;44:406–421. doi: 10.1016/j.immuni.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, Rathmell JC. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+FOXP3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J. Clin. Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 12.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+FOXP3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J. Leukoc. Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 13.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorens B, Mueckler M. Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, Tschirner SK, Gorinski N, Gohmert M, Mayer CT, Huehn J, Ponimaskin E, Abraham WR, Müller R, Lochner M, Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 19.Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl. Acad. Sci. USA. 2011;108:15201–15206. doi: 10.1073/pnas.1103746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. CM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, Wu C, Vogel P, Neale G, Green DR, Chi H. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 2016;17:277–285. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaelin WG., Jr. The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem. Biophys Res. Commun. 2005;338:627–638. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 25.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1α dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 28.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, Jing Y, Yang H, Chen R, Chang L, Zhang Y, Goto J, Onda H, Chen T, Wang MR, Lu Y, You H, Kwiatkowski D, Zhang H. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, De Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FOXP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, Jiao J, Veasey SC, Sims CA, Baur JA, Wallace DC, Hancock WW. Essential role of mitochondrial energy metabolism in FOXP3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in FOXP3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 35.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Antoniadis N, Liakopoulos V, Stefanidis I. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. J. Basic Clin. Physiol. Pharmacol. 2013;24:271–276. doi: 10.1515/jbcpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- 38.De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, De Simone S, Procaccini C, La Rocca C, Carrieri PB, Maniscalco GT, Salvetti M, Buscarinu MC, Franzese A, Mozzillo E, La Cava A, Matarese G. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FOXP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 41.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 42.Oh B, Yoon J, Farris A, Kirk A, Knechtle S, Kwun J. Rapamycin interferes post-depletion regulatory T cell homeostasis and enhances DSA formation corrected by CTLA4-Ig. Am. J. Transplant. 2016 doi: 10.1111/ajt.13789. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Camirand G, Lin Y, Froicu M, Deng S, Shlomchik WD, Lakkis FG, Rothstein DM. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J. Immunol. 2011;186:2809–2818. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbatini M, Ruggiero G, Palatucci AT, Rubino V, Federico S, Giovazzino A, Apicella L, Santopaolo M, Matarese G, Galgani M, Terrazzano G. Oscillatory mTOR inhibition and Treg increase in kidney transplantation. Clin. Exp. Immunol. 2015;182:230–240. doi: 10.1111/cei.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaur G, Goodall JC, Jarvis LB, Hill Gaston JS. Characterisation of Foxp3 splice variants in human CD4+ and CD8+ T cells--identification of Foxp3Δ7 in human regulatory T cells. Mol. Immunol. 2010;48:321–332. doi: 10.1016/j.molimm.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Joly AL, Liu S, Dahlberg CI, Mailer RK, Westerberg LS, Andersson J. Foxp3 lacking exons 2 and 7 is unable to confer suppressive ability to regulatory T cells in vivo. J. Autoimmun. 2015;63:23–30. doi: 10.1016/j.jaut.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Mailer RK, Joly AL, Liu S, Elias S, Tegner J, Andersson J. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015;5:14674. doi: 10.1038/srep14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 50.Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, Dwyer MA, Nelson ER, Pollizzi KN, Ilkayeva O, Giguere V, Zuercher WJ, Powell JD, Shinohara ML, McDonnell DP, Rathmell JC. Estrogen-related receptor-α is a metabolic regulator of effector T cell activation and differentiation. Proc. Natl. Acad. Sci. USA. 2011;108:18348–1853. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miskov-Zivanov N, Turner MS, Kane LP, Morel PA, Faeder JR. The duration of T cell stimulation is a critical determinant of cell fate and plasticity. Sci. Signal. 2013;6:ra97. doi: 10.1126/scisignal.2004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front. Immunol. 2015;6:61. doi: 10.3389/fimmu.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 56.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 2008;1:S457–464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by FOXP3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 58.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+CD25+FOXP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J. Immunol. 2014;192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 61.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces FOXP3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. USA. 2014;111:E3432–3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabryšová L, Christensen J, Gupta A, Saglani S, Bush A, O'Garra A, Brown Z, Hawrylowicz CM. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct FOXP3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012;42:2697–2708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chambers ES, Suwannasaen D, Mann EH, Urry Z, Richards DF, Lertmemongkolchai G, Hawrylowicz CM. 1α,25-dihydroxyvitamin D3 in combination with transforming growth factor-β increases the frequency of FOXP3+ regulatory T cells through preferential expansion and usage of interleukin-2. Immunology. 2014;143:52–60. doi: 10.1111/imm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, Mongellaz C, Floess S, Fritz V, Matias MI, Yong C, Surh N, Marie JC, Huehn J, Zimmermann V, Kinet S, Dardalhon V, Taylor N. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 2015;8:ra97. doi: 10.1126/scisignal.aab2610. [DOI] [PubMed] [Google Scholar]
- 67.Song EK, Yim JM, Yim JY, Song MY, Rho HW, Yim SK, Han YH, Jeon SY, Kim HS, Yhim HY, Lee NR, Kwak JY, Sohn MH, Park HS, Jang KY, Yim CY. Glutamine protects mice from acute graft-versus-host disease (aGVHD) Biochem. Biophys. Res. Commun. 2013;435:94–99. doi: 10.1016/j.bbrc.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 68.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curran TA, Jalili RB, Farrokhi A, Ghahary A. IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells. Immunobiology. 2014;219:17–24. doi: 10.1016/j.imbio.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br. J. Pharmacol. 2013;169:1305–1321. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.S.P. Cobbold, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morse MA, Hobeika A, Serra D, Aird K, McKinney M, Aldrich A, Clay T, Mourich D, Lyerly HK, Iversen PL, Devi GR. Depleting regulatory T cells with arginine-rich, cell-penetrating, peptide-conjugated morpholino oligomer targeting FOXP3 inhibits regulatory T-cell function. Cancer Gene Ther. 2012;19:30–37. doi: 10.1038/cgt.2011.63. [DOI] [PubMed] [Google Scholar]
- 74.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 77.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J. Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 2012;188:2070–2073. doi: 10.4049/jimmunol.1102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK. Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int. Immunopharmacol. 2011;11:604–609. doi: 10.1016/j.intimp.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Piccio L, Cantoni C, Henderson JG, Hawiger D, Ramsbottom M, Mikesell R, Ryu J, Hsieh CS, Cremasco V, Haynes W, Dong LQ, Chan L, Galimberti D, Cross AH. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur. J. Immunol. 2013;43:2089–2100. doi: 10.1002/eji.201242836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramos-Ramírez P, Malmhäll C, Johansson K, Lötvall J, Bossios A. Weight gain alters adiponectin receptor 1 expression on adipose tissue-resident Helios+ regulatory T cells. Scand. J. Immunol. 2016;83:244–254. doi: 10.1111/sji.12419. [DOI] [PubMed] [Google Scholar]
- 82.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky A. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Round JL, Mazmanian SK. Inducible FOXP3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2014;506:254. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 86.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 89.Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 90.Galgani M, Procaccini C, De Rosa V, Carbone F, Chieffi P, La Cava A, Matarese G. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J. Immunol. 2010;185:7474–7479. doi: 10.4049/jimmunol.1001674. [DOI] [PubMed] [Google Scholar]
- 91.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 92.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 93.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park Y, Jin HS, Lopez J, Elly C, Kim G, Murai M, Kronenberg M, Liu YC. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 96.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 97.Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 2014;259:115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 101.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM, Powell JD, Yagita H, Mellor AL, Blazar BR, Munn DH. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci. Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naïve T cells. J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 104.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moretti S, Menicali E, Voce P, Morelli S, Cantarelli S, Sponziello M, Colella R, Fallarino F, Orabona C, Alunno A, de Biase D, Bini V, Mameli MG, Filetti S, Gerli R, Macchiarulo A, Melillo RM, Tallini G, Santoro M, Puccetti P, Avenia N, Puxeddu E. Indoleamine 2,3-dioxygenase 1 (IDO1) is up-regulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J. Clin. Endocrinol. Metab. 2014;99:E832–840. doi: 10.1210/jc.2013-3351. [DOI] [PubMed] [Google Scholar]
- 106.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 107.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 108.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Makki K, Taront S, Molendi-Coste O, Bouchaert E, Neve B, Eury E, Lobbens S, Labalette M, Duez H, Staels B, Dombrowicz D, Froguel P, Wolowczuk I. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One. 2014;9:e92684. doi: 10.1371/journal.pone.0092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 111.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55:1571–1580. doi: 10.2337/db05-1576. [DOI] [PubMed] [Google Scholar]
- 113.Esposito M, Ruffini F, Bellone M, Gagliani N, Battaglia M, Martino G, Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J. Neuroimmunol. 2010;220:52–63. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the Th17/Treg balance. PLoS One. 2015;10:e0135858. doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 2016;73:520–528. doi: 10.1001/jamaneurol.2015.4807. [DOI] [PubMed] [Google Scholar]
- 116.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 117.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009;83:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 118.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 119.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β-bearing regulatory cells. J. Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 120.Livingston M, Loach D, Wilson M, Tannock GW, Baird M. Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response. Immunol. Cell Biol. 2010;88:99–102. doi: 10.1038/icb.2009.71. [DOI] [PubMed] [Google Scholar]
- 121.Galgani M, Nugnes R, Bruzzese D, Perna F, De Rosa V, Procaccini C, Mozzillo E, Cilio CM, Elding Larsson H, Lernmark A, La Cava A, Franzese A, Matarese G. Meta-immunological profiling of children with type 1 diabetes identifies new biomarkers to monitor disease progression. Diabetes. 2013;62:2481–2491. doi: 10.2337/db12-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Munger KL, Bentzen J, Laursen B, Stenager E, Koch-Henriksen N, Sørensen TI, Baker JL. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler. 2013;19:1323–1329. doi: 10.1177/1352458513483889. [DOI] [PMC free article] [PubMed] [Google Scholar]