Abstract
Background
Phthalates are widely used chemicals with ubiquitous exposure. Dibutyl-phthalate (DBP), a male reproductive toxicant in animals, is understudied in humans. Some mesalamine medications used to treat inflammatory bowel disease (IBD) have DBP in their coating, whereas other mesalamine formulations do not.
Objectives
Taking advantage of differences in mesalamine formulations, we investigated whether high-DBP exposure from mesalamine medications was associated with decreased semen parameters.
Methods
73 men with IBD taking mesalamine participated in a crossover-crossback prospective study. Men taking non-DBP containing mesalamine at baseline i.e., background exposure, crossed-over for four months to high-DBP mesalamine and then crossed-back for four months to their non-DBP mesalamine (B1HB2-arm;Background1-High-Background2) and vice versa for men taking high-DBP mesalamine at baseline (H1BH2-arm;High1-Background-High2). Men provided up to six semen samples (2: baseline, 2: crossover and 2: crossback).
Results
We estimated crossover, crossback and carryover effects using linear mixed models adjusted for abstinence time, age, season and duration on high-DBP mesalamine at baseline. Semen parameters in B1HB2-arm (26 men, 133 samples) decreased after high-DBP mesalamine exposure (crossover versus baseline), especially motility parameters, and continued to decrease further even after crossback to non-DBP mesalamine (crossback versus crossover).The cumulative carryover effect of high-DBP (crossback versus baseline) was a decrease of % total sperm motility by 7.61(CI:-13.1, -2.15), % progressive sperm motility by 4.23(CI:-8.05, -0.4) and motile sperm count by 26.0% (CI:-46.2%, 1.7%). However, H1BH2-arm (47 men, 199 samples) had no significant change during crossover or crossback.
Conclusions
Men newly exposed to high-DBP mesalamine for four months had a cumulative reduction in several semen parameters, primarily sperm motility, that was more pronounced and statistically significant even after exposure ended for four months.
Keywords: Phthalates, Mesalamine, Inflammatory Bowel Disease (IBD), Semen Quality
Graphical abstract
1. Introduction
Over the last several decades, accumulating evidence suggests a downward trend1-3 and geographic variability in semen quality4, a surrogate for male fertility. These trends raise concern that lifestyle or environmental exposures may affect semen quality and male fertility5. One class of environmental chemicals for which there is concern about potential adverse male reproductive health effects are phthalates6. In experimental animal studies, several phthalates including dibutyl-phthalate (DBP) were anti-androgens and male reproductive toxicants, adversely affecting testicular function7-11. The most studied window of exposure is in-utero exposure which led to male reproductive tract malformations in rats12-14. Less well-studied are puberty and adulthood exposure. Studies in rats have shown effects of postnatal exposure to DBP on the male reproductive tract15-18 and to butyl benzyl phthalate (BBzP)19. There are several epidemiologic studies in adult men that explored cross-sectional associations between background low-DBP environmental exposure, and other phthalates, with semen quality. Most of these studies were conducted in men recruited from infertility clinics20-27, and although some studies found associations of DBP22,24 and other phthalates20,21,24,25,28 with lower semen quality, others did not23,29,30.
In addition to widespread general population DBP exposure from personal care and consumer products31,32, some medications such as specific mesalamine formulations have enteric coatings that contain DBP31,33-36 despite the recent US Food and Drug Administration (FDA) recommendation against the use of phthalates in drug delivery vehicles37. Mesalamine or 5-aminosalicylic acid (5-ASA) is a commonly prescribed maintenance therapy for inflammatory bowel diseases (IBD), specifically ulcerative colitis (UC) and Crohn's disease (CD)38. Our research and others have shown that mesalamine medications with coatings that contain DBP contribute to high-DBP exposure as measured by concentrations of urinary monobutyl phthalate (MBP), the primary DBP metabolite39,40. Specifically, in individuals taking mesalamine medications that contain DBP, their urinary levels of MBP were approximately 1,000 times higher than the median levels reported for men in the US general population (National Health and Nutrition Examination Survey (NHANES))41. Therefore, patients with IBD taking DBP-containing mesalamine will have chronic high exposure to DBP because the medication is taken daily to treat IBD.
Mesalamine is the active ingredient in Asacol® and Asacol®HD and DBP is an excipient in their enteric coating42. Other mesalamine formulations such as Pentasa®, Lialda®, Apriso®, and Delzicol® do not contain DBP36,43. Asacol®, widely used to treat IBD in adults and children, was a first line of therapy for patients with UC and often used in pregnant women with IBD43,44. The aim of the study was to investigate the effect of high-DBP exposure on semen quality, taking advantage of the difference in mesalamine formulations to conduct a crossover-crossback prospective study in adult men with IBD.
2. Materials and Methods
2.1. Study population
We conducted a crossover-crossback prospective study in adult men with IBD (Mesalamine And Reproductive health Study (MARS)). Eligibility for participants in the MARS was 18 to 55 years of age and taking oral mesalamine for at least the past three months. All men must have had a mild IBD score on the simple clinical colitis activity index45 (five or less for UC) or Harvey-Bradshaw index46 (four or less for CD). Men were recruited from gastroenterology clinics at three Boston hospitals; Beth Israel Deaconess Medical Center (BIMC), Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH) from October 2010 through October 2015. MARS was approved by the institutional review boards (IRBs) of Harvard T.H. Chan School of Public Health, BIMC, BWH and MGH. All men signed informed consents.
2.2. Study design
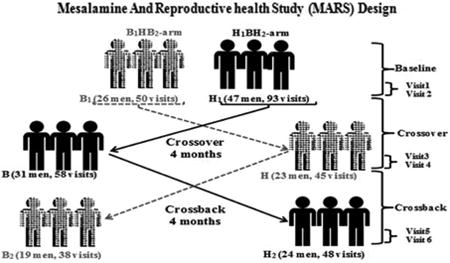
Eligible men were invited to participate in up to six visits; to account for within-person variability in semen parameters. Each man was asked to participate in two visits at baseline, after crossover and after crossback. At each of the two baseline visits, participants provided semen, urine and blood samples collected two weeks apart (visits 1 and 2). Men were then asked to crossover to another formulation of mesalamine; men who were taking non-DBP mesalamine at baseline crossed-over to DBP-containing mesalamine medication (i.e., Asacol®) and vice versa for men prescribed DBP-containing mesalamine at baseline crossed-over to non-DBP mesalamine. After crossover for four months, two sets of semen, urine and blood samples were collected two weeks apart (visits 3 and 4). Participants were then asked to crossback for four months to their original mesalamine medications after which two sets of semen, urine and blood samples were collected two weeks apart (visits 5 and 6) (Figure-1).
Figure 1. Mesalamine And Reproductive health Study (MARS) Design.
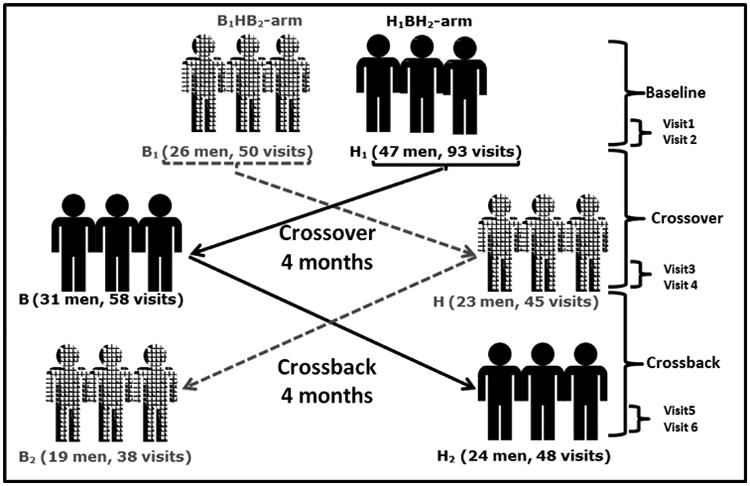
B1HB2-arm: B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline. H1BH2-arm: H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine-containing DBP at baseline. Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate.
In brief, men who were prescribed non-DBP mesalamine with background exposure from other sources crossed-over to high-DBP mesalamine then crossed-back to non-DBP mesalamine (B1HB2-arm: Background1-High-Background2). Men who were prescribed high-DBP mesalamine crossed-over to non-DBP mesalamine then crossed-back to high-DBP mesalamine (H1BH2-arm: High1-Background-High2). The ‘wash-in’ and ‘wash-out’ periods between crossover and crossback were four months to extend beyond the 70 days average period of spermatogenesis47. Questionnaires about lifestyle factors, medical history and ejaculation abstinence time were administered at every visit.
Among the 47 men in the H1BH2-arm, 13 men participated only in a short protocol defined as up to four visits. These 13 men did not want to change medication but because the manufacturer was reformulating Asacol® to remove DBP, we anticipated that they would be ‘switched’ to a non-DBP mesalamine when this came to market. Warner Chilcott discontinued Asacol® in 2013 and introduced Delzicol® (non-DBP mesalamine) to the market. However, Asacol®HD (containing DBP) remained on the market. For 10 of the 13 men, their physician changed their medications to Asacol®HD, thus they never crossed-over to non-DBP mesalamine and only contributed to the baseline visits while three men changed medication to Delzicol® i.e. crossed-over. However, by design none of the men in the short protocol crossed-back.
Men were asked to abstain from ejaculation for 2-5 days before providing semen samples, collected by masturbation at the MGH andrology laboratory into a sterile container and analyzed using standardized clinical protocols and quality control (QC) as described previously22,48. Briefly, semen was allowed to liquefy at 37° C for 20 minutes. The physical properties of the semen were reported, including the sample volume, pH, color and viscosity. Ejaculate volume was measured using a graduated serological pipet. Sperm concentration and motility were assessed with a computer-aided semen analysis (CASA; 10HTM-IVOS, Hamilton-Thorne Research, Beverly, MA) which is used for routine diagnostic applications49. For semen concentration and motility assessment, 5 μl of semen from each sample was placed into a pre-warmed (37°C) Makler counting chamber (Sefi-Medical Instruments, Haifa, Israel). Minimum of 200 sperm cells from at least four different fields were analyzed from each specimen. Sperm motility was expressed as the percentage of total motile (progressive + non-progressive) and percentage of progressive motile spermatozoa and defined as World Health Organization (WHO-2010) grade “a” sperm (rapidly progressive with a velocity ≥25 μm/sec) plus “b” grade sperm (slow/sluggish progressive with a velocity of ≥5 μm/sec but < 25 μm/sec)49. Sperm morphology was measured on two slides for each specimen (with at least 200 cells assessed per slide) with a microscope using an oil-immersion 100× objective (Nikon, Tokyo, Japan). Sperm morphology was assessed using Kruger's strict criteria 50. To minimize variability, the laboratory followed a constant analysis set-up including play-back and QC plots if sperm counts < 20 or > 50 million/mL. Unusual values were re-evaluated. In addition to a quarterly competency technicians' evaluation, an outside evaluator performed proficiency testing, biannually. The technicians performing the semen assays were blinded to the study group.
2.3. Statistical Analysis
We created a six-level indicator variable cross-classifying each observation according to medication type (high-DBP or non-DBP mesalamine) and period (baseline, crossover and crossback) for the two study arms (H1BH2 and B1HB2). We considered for analysis, ejaculate volume, sperm concentration, motility (% motility and % progressive motility) and morphology. We also calculated total sperm count, motile sperm count, and morphologically normal sperm count. We used natural log-transformed sperm concentration, total sperm count, motile sperm count and morphologically normal count to satisfy model's normality assumption.
We performed descriptive statistics for participants' baseline and time-varying characteristics in both study arms. We also tested for any differences between the two arms using Kruskal-Wallis test for continuous variables and Fisher's exact test for categorical variables.
We used linear mixed effects models (LMEM) with a random intercept to account for within-person correlation among longitudinal measures of a given outcome arising from person-to-person heterogeneity across the study population. We estimated the DBP mesalamine crossover, crossback and carryover effects on semen parameters as absolute mean differences for the non-transformed semen parameters and as percent change for the log-transformed parameters.
Selection of covariates was based on directed acyclic graphs (Supplementary Figure-2) and statistical considerations (>10 % change in the effect estimate). The final model included abstinence time (< 2 days, 2≤ days <4 and ≥ 4 days), age at baseline (continuous), the season of the sample collection (warm and cold) and duration on high-DBP containing mesalamine at baseline (continuous in years). There were two samples missing abstinence times and we imputed the category of abstinence time based on the other semen samples provided by the same man (both men had the same abstinence time category for all their other semen samples).
In our analysis, we also considered that the duration of IBD as a chronic inflammatory condition may affect semen quality. In preliminary models, we considered adjustment for duration of IBD, severity score of the disease, IBD condition (UC/CD), race, history of reproductive diseases or surgeries, BMI and smoking but they were not confounders and thus not retained in the final models.
We assessed model sensitivity to the covariance structure implied by the random intercept model, using empirical standard errors that are robust to misspecification of the covariance structure51. As a sensitivity analysis, we further adjusted for body mass index (BMI <18.5, 18.5 ≤BMI<25, 25≤BMI<30 or ≥30)52 and smoking status (never, former and current) in addition to the above covariates. As a secondary analysis, we applied fixed effect models (FEM) that, rather than assume a random distribution for the person-specific intercepts, estimate these terms as ordinary fixed regression coefficients in the model. These models isolate the longitudinal within-person effect of exposure, adjusted for the same covariates as above51.
Although not a primary aim of our study, we explored the cross-sectional differences between the two arms at baseline by restricting the data to the average of the first two visits, applying linear regression adjusted for the covariates above. We further explored the effect of the duration under high-DBP mesalamine at enrollment on baseline semen parameters in the H1BH2-arm.
We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC) and considered two-sided alpha <0.05 as statistically significant.
3. Results
Out of 215 confirmed eligible men, 73 agreed to participate (34%) (Supplementary Figure-1) and provided 332 semen samples with an average of 4.6 semen samples per man [range: 1 to 6]. The B1HB2-arm included 26 men (133 semen samples) with an average 5.1 samples per man. The H1BH2-arm included 47 men (199 samples) with an average of 4.9 samples per man. The two study arms had comparable baseline fixed and time-varying characteristics (Table-1). The semen parameter distributions are presented (Table-2). Men enrolled in the short protocol provided 31 samples (26 baseline and 5 after crossover samples).
Table 1. Demographics of 73 men contributing 332 visits in the MARS Study by arm.
| B1HB2-arm (26 men, 133 visits) | H1BH2-arm (47 men, 199 visits) | Total (73 men, 332 visits) | ||
|---|---|---|---|---|
|
|
||||
| N(%)/Mean (SD),[Range] | N(%)/Mean (SD),[Range] | N(%)/Mean (SD),[Range] | P-valueb | |
| Baseline characteristics (at Visit 1), # men (N)a | ||||
|
| ||||
| Age (Years) | 33.9 (8.97), [20.2, 54.6] | 34.9 (9.66), [19.6, 55.7] | 34.6 (9.37), [19.6, 55.7] | 0.82 |
| Race | 0.27 | |||
| Caucasian N (%) | 25 (96%) | 38 (81%) | 63 (86%) | - |
| Black/African American | 1 (4%) | 2 (4%) | 3 (4%) | - |
| Asian | 0 | 4 (9%) | 4 (6%) | - |
| Other | 0 | 3 (6%) | 3 (4%) | - |
| BMI (Kg/m2) | 25.3 (3.56), [19.4, 32.7] | 26.4 (6.39), [19.5, 54.0] | 26.0 (5.54), [19.4, 54.0] | 0.88 |
| BMI-categories N (%) | 0.99 | |||
| Normal weight (18.5 ≤BMI<25) | 14 (54%) | 24 (51%) | 38 (52%) | - |
| Overweight (25≤BMI<30) | 9 (35%) | 16 (34%) | 25 (34%) | - |
| Obese (BMI≥30) | 3 (11%) | 7 (15%) | 10 (14%) | - |
| Education N (%) | 0.82 | |||
| Below college | 3 (14%) | 6 (14%) | 9 (14%) | - |
| College graduate | 10 (48%) | 16 (38%) | 26 (41%) | - |
| Graduate degree | 8 (38%) | 20 (48%) | 28 (45%) | - |
| Smoking status N (%) | 0.009 | |||
| Never smoker | 22 (85%) | 36 (77%) | 58 (80%) | - |
| Former smoker | 1 (4%) | 11 (23%) | 12 (16%) | - |
| Current smoker | 3 (11%) | 0 (0%) | 3 (4%) | - |
| Warm season at baselinec | 5 (19%) | 20 (43%) | 25 (34%) | 0.07 |
| IBD diagnosis N (%) | 0.61 | |||
| Ulcerative colitis | 16 (62%) | 32 (68%) | 48 (66%) | - |
| Crohn's disease | 10 (38%) | 15 (32%) | 25 (34%) | - |
| IBD scored | 1.65 (1.38), [0, 4] | 1.19 (1.35), [0, 5] | 1.36 (1.37), [0, 5] | 0.13 |
|
| ||||
| Time-varying characteristics, #visitsa | ||||
|
| ||||
| Warm season c | 61 (46%) | 92 (46%) | 153 (46%) | 0.99 |
| Ejaculation abstinence time | 0.84 | |||
| < 2 Days | 10 (8%) | 19 (10%) | 29 (9%) | - |
| 2≤Days<4 | 115 (86%) | 169 (85%) | 284 (85%) | - |
| ≥4 Days | 8 (6%) | 11 (5%) | 19 (6%) | - |
B1HB2-arm, B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline; 16 men (62%) on Lialda®, 8 men (31%) on Pentasa®, one man (4%) on Apriso® and one man (4%) was not on mesalamine medication at the time of recruitment.
H1BH2-arm, H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine-containing DBP at baseline; 24 men (51%) on Asacol® and 23 men (49%) on Asacol®HD.
:N (%) for categorical/binary variables and mean (SD), [Range] for continuous variables
:P-values are based on Fisher exact test for categorical variables and Kruskal Wallis test for continuous variables.
:Season of sample collection, Warm: April Through September
10 men missing information on education; for 2 semen samples missing abstinence times, we imputed values for their categories based on the other samples given by the same man (both men had the same abstinence time category for all their other samples).
:BD score: simple clinical colitis activity index, includes bowel frequency and urgency, presence of blood in the stool and general wellbeing. Mild IBD score: 5 or less for UC and 4 or less for CD.
Abbreviations: MARS, Mesalamine And Reproductive health Study; BMI, body mass index; Kg, Kilogram; m, meter; SD, standard deviation; IBD, Inflammatory Bowel Disease; UC, ulcerative colitis; CD, Crohn's disease; N, number of men; DBP, dibutyl phthalate; B1HB2, Background1-High-Background2 DBP exposure; H1BH2, High1-Background-High2 DBP exposure.
Table 2. Semen quality parameters for 73 men (332 semen samples) in the MARS study.
| Semen quality parameters | Mean (SD) | Median [Range] | n (%) < WHO lower referencelimitsa |
|---|---|---|---|
| Ejaculate volume (mL) | 2.71 (1.21) | 2.5 [0.23, 7.10] | 45 (14%) |
| Sperm concentration (million/mL) | 74.4 (68.2) | 59.8 [2.7, 670] | 22 (7%) |
| Total sperm count (million) | 192 (151) | 168 [0.62, 828] | 35 (11%) |
| %Total sperm motility | 49.0 (20.6) | 51.0 [0, 88] | 111 (33%) |
| %Progressive sperm motility | 29.4 (14.2) | 30.0 [0, 77] | 177 (53%) |
| Motile sperm count (million)b | 111 (115) | 74.3 [0, 621] | - |
| % Normal sperm morphology | 7.01 (3.61) | 7 [0, 23] | 93 (28%) |
| Morphologically normal sperm count (million)c | 15.6 (17.7) | 9.63 [0, 131] | - |
Binary semen quality parameters less than WHO lower reference limits (2010)49: ejaculate volume<15 ml; sperm concentration <15 million/ml; total sperm count< 39 million; total motile sperm<40%; progressive motile sperm <32% and normal sperm morphology< 4% using “strict” Tygerberg method; n: # semen samples. There were 2 samples missing morphology (n=330).
Motile count= Total Sperm Count × % Motile
Morphologically normal count =Total Sperm Count × % normal morphology
Abbreviations: MARS, Mesalamine And Reproductive health Study; WHO: World health organization.
Among the 60 full-protocol enrolled men, 51 (85%) men crossed-over medications, 23 (89%) in B1HB2-arm and 28 (82%) in H1BH2-arm; 43 (72%) men crossed-back medications, 19 (73%) in B1HB2-arm and 24 (71%) in H1BH2-arm. During follow-up, 9 (15%) men dropped out, 3 (12%) in B1HB2-arm and 6 (18%) in H1BH2-arm (p-value= 0.64). The median period between the second baseline visit (visit-2) and collection of crossover semen samples at visit-3 and similarly between visit-4 and visit-5 for crossback was 119 days. The median time period between visits on the same medication (visits-1&2), (visits-3&4) and (visits-5&6) was 16 days. The H1BH2-arm men were on their high-DBP mesalamine medication at entry for median 2.9 years and up to 24.1 years. The B1HB2-arm men were on their non-DBP mesalamine medication at baseline for median 1.0 year and up to 10.3 years.
None of the semen samples were azoospermic (Table-2); 188 (55%) samples were above the reference limits of the World Health Organization (WHO-2010)49 with regard to sperm concentration, total count, percent motile and percent normal morphology.
Although not statistically significant, in a cross-sectional analysis of the baseline visits, sperm motility and morphology parameters for men in H1BH2-arm were higher than among men in B1HB2-arm (Figure-2). However, among men in the H1BH2-arm at the baseline, there was a negative association between the number of years on high-DBP containing mesalamine and semen parameters, steeper in the early years as compared to the later years (data not shown).
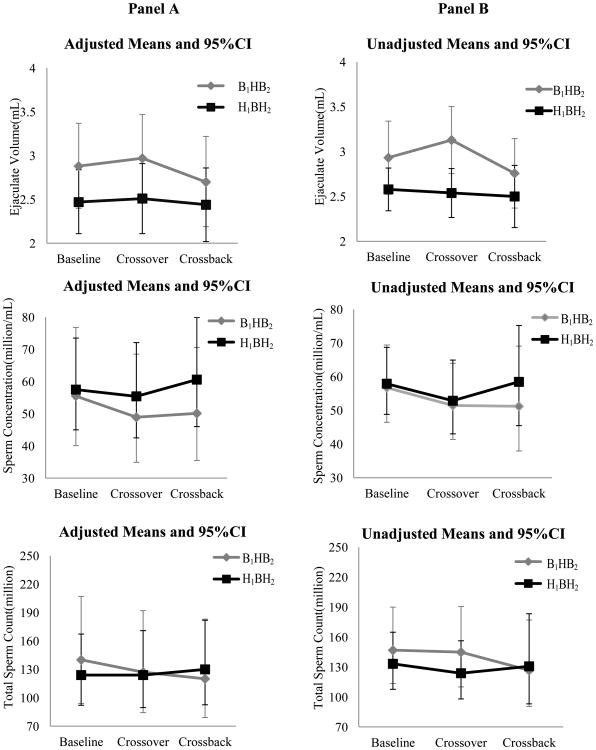
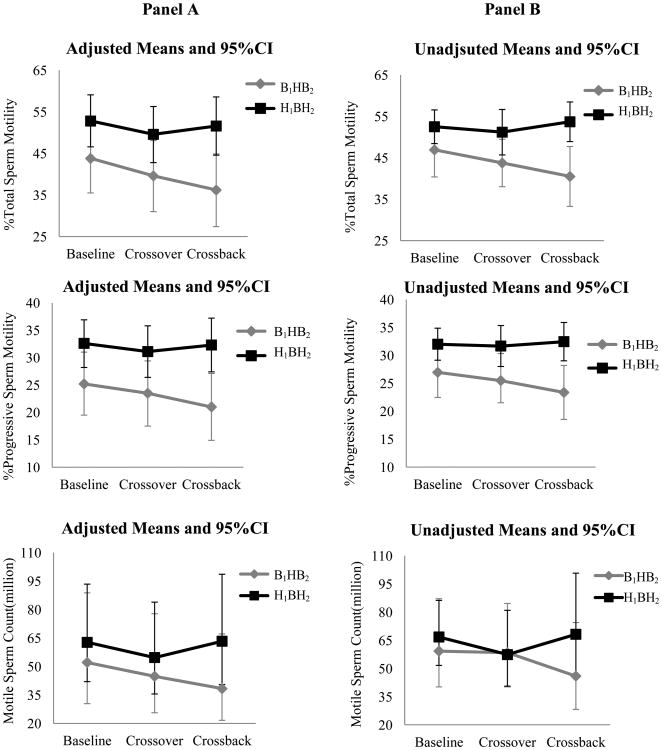
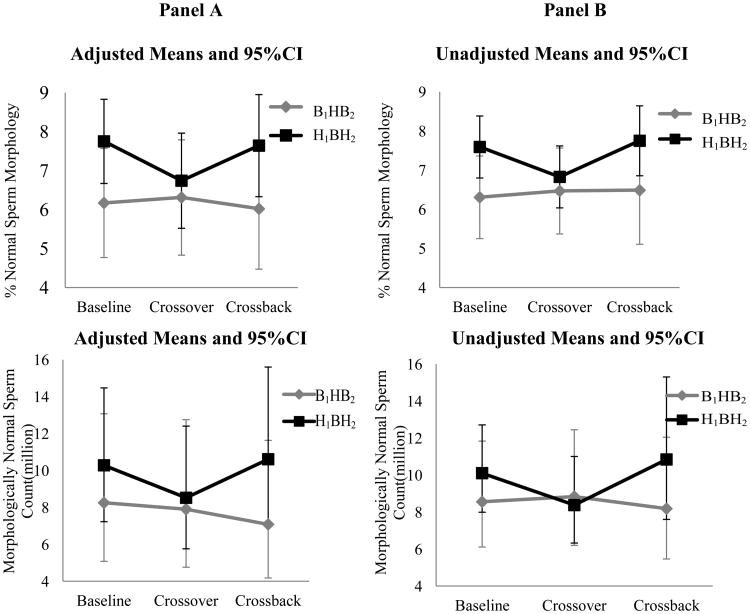
Figures 2. Adjusted and unadjusted means (95%CI) of semen parameters at baseline, crossover and crossback for the 2 arms-MARS.
Panel A shows the adjusted means and 95% CI and Panel B shows the unadjusted means and 95% CI [adjusted for abstinence time (categorical), age (continuous), season (warm versus cold) and duration on DBP-containing mesalamine medication at baseline (in years)]. For the log-transformed outcomes (sperm concentration, total sperm count, motile sperm count and morphologically normal sperm count), the geometric means and 95% CI are presented. B1HB2-arm, B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline. H1BH2-arm, H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine-containing DBP at baseline. Abbreviations: MARS, Mesalamine And Reproductive health Study; 95% CI, 95% Confidence Interval; DBP, dibutyl phthalate.
In the adjusted analyses (Table-3 & Figure-2), on average among men in the B1HB2-arm who were newly-exposed to high-DBP mesalamine after crossover had decreased (though non-statistically significant) semen parameters, apart from ejaculate volume and % normal morphology. Their % total sperm motility decreased by 4.19 (95% confidence interval (CI): -9.25, 0.86), % progressive sperm motility by 1.76 (CI: -5.29, 1.78) and motile sperm count by 13.7% (CI: -35.7%, 15.8). After crossback to their non-DBP mesalamine, all semen parameters (except sperm concentration) continued to decrease further. The % total sperm motility further decreased by 3.41 (CI: -8.69, 1.86), % progressive sperm motility by 2.47 (CI: -6.16, 1.22) and motile sperm count by 14.3% (CI: -36.9%, 16.5%). The cumulative carryover effect of high-DBP exposure (i.e., crossback compared to baseline) was a decrease in all semen parameters; % total sperm motility decreased by 7.61 (CI: -13.1, -2.15), % progressive sperm motility by 4.23 (CI: -8.05, -0.4) and motile sperm count by 26.0% (CI: -46.2%, 1.7%). In contrast, in the H1BH2-arm, semen parameters did non-significantly change after crossover or crossback.
Table-3.A. Adjusted effect (95%CI) of crossover, crossback and carryover on semen parameters among men starting on mesalamine medication not containing dibutyl phthalate (B1HB2-arm)a.
| B1HB2-arm [Background (B1)- High (H)- Background (B2) dibutyl phthalate exposure]: 26 men, 133 semen samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fitted Means (95%CI) | Comparisons | ||||||||
| Semen quality parameter |
|
|
|||||||
| Baseline (B1) |
Crossover (H) |
Crossback (B2) |
Crossover effect (95%CI) H-B1 |
P value |
Crossback effect (95%CI) B2-H |
P value |
Carryover effect (95%CI) B2-B1 |
P value |
|
| Ejaculate volume (mL) | 2.88 (2.4, 3.37) | 2.97 (2.46, 3.47) | 2.70 (2.19, 3.22) | 0.09 (-0.22, 0.39) | 0.57 | -0.27 (-0.58, 0.05) | 0.10 | -0.18 (-0.51, 0.15) | 0.29 |
|
| |||||||||
| Sperm concentration (million/mL)b | 55.5 (40.1, 76.8) | 48.9 (34.9, 68.5) | 50.1 (35.5, 70.6) | -11.9% (-27.6%, 7.23%) | 0.21 | 2.36% (-16.6%, 25.6%) | 0.82 | -9.8% -27.0%, 11.5%) | 0.34 |
|
| |||||||||
| Total sperm count (million)b | 140 (94, 207) | 127 (84.3, 192) | 120 (79.1, 183) | -8.96% (-28.7%, 16.2%) | 0.45 | -5.45% (-26.7%, 22.0%) | 0.67 | -13.9% (-33.9%, 12.1%) | 0.26 |
|
| |||||||||
| %Total sperm motility | 43.8 (35.5, 52) | 39.6 (31, 48.1) | 36.2 (27.4, 44.9) | -4.19 (-9.25, 0.86) | 0.10 | -3.41 (-8.69, 1.86) | 0.20 | -7.61 (-13.1, -2.15) | 0.007 |
|
| |||||||||
| %progressive sperm motility | 25.2 (19.5, 31) | 23.5 (17.5, 29.4) | 21.0 (14.9, 27.1) | -1.76 (-5.29, 1.78) | 0.33 | -2.47 (-6.16, 1.22) | 0.19 | -4.23 (-8.05, -0.4) | 0.03 |
|
| |||||||||
| Motile sperm count (million)b | 52.1 (30.4, 88.8) | 44.8 (25.6, 77.8) | 38.3 (21.6, 67.1) | -13.7% (-35.7%, 15.8%) | 0.32 | -14.3% (-36.9%, 16.5%) | 0.32 | -26.0% (-46.2%, 1.7%) | 0.06 |
|
| |||||||||
| %Normal sperm morphology | 6.17 (4.77, 7.57) | 6.31 (4.83, 7.79) | 6.02 (4.47, 7.56) | 15.8%) 0.13 (-1.06, 1.33) | 0.82 | -0.29 (-1.54, 0.96) | 0.65 | -0.16 (-1.45, 1.13) | 0.81 |
|
| |||||||||
| Morphologically normal sperm count (million)b | 8.25 (5.08, 13.1) | 7.90 (4.76, 12.8) | 7.08 (4.18, 11.6) | -3.78% (-25.9%, 24.9%) | 0.77 | -9.14% (-30.8%, 19.4%) | 0.49 | -12.6% (-34.2%, 16.1%) | 0.35 |
We found consistent results from the sensitivity analyses using empirical standard errors in LMEMs and after further adjustment for BMI and smoking (data not shown) and from FEMs which fully adjust for both observed and unobserved factors that do not change within a person across visits51 (Supplementary Table-1).
4. Discussion
In our crossover-crossback study, men who were on non-DBP mesalamine at baseline (B1HB2-arm) and newly exposed for four months (crossover period) to high-DBP had lower semen parameters, primarily % total sperm motility, % progressive sperm motility and motile sperm count. This decrease was more pronounced and became statistically significant even after crossback for four months to their original non-DBP mesalamine. Thus, the effect of high-DBP on sperm motility parameters was not reversible after four months. Among men who were on high-DBP at baseline (H1BH2-arm), there was no statistically significant change in semen parameters after crossover to non-DBP mesalamine or crossback to their original high-DBP mesalamine. Therefore, in both arms, the four month wash-out period was not long enough to reverse the effect of high-DBP exposure.
In the cross-sectional analysis of baseline semen characteristics, unexpectedly, men who were on high-DBP mesalamine at baseline (H1BH2-arm) had non-significantly higher percent sperm motility and normal morphology than men who were on non-DBP mesalamine at baseline (B1HB2-arm). However, among men in the H1BH2-arm, there was a downward trend in all semen parameters associated with increased duration on high-DBP mesalamine. This negative association with years on high-DBP mesalamine among men in H1BH2-arm argues against a ‘protective’ effect of high-DBP and suggests chance sampling variability at baseline due to non-randomization at the baseline and cautions against cross-sectional analyses. These results highlight the power of the study that by design removes subject to subject variability from the comparison of interest, thus avoiding the purely cross-sectional analysis that is likely to suffer from this random variability.
There is an accumulating literature on the association of background low environmental exposure to phthalates with semen quality. A recent meta-analysis that included 14 publications on phthalates and semen quality concluded that higher urinary MBP concentrations were associated with reduced sperm concentration and straight line velocity53. All 14 publications were cross-sectional with 11 conducted in infertility clinics. Two recent US studies on fertile men not included in the meta-analysis29,30 did not find significant associations. Our study differs in many respects from the earlier cross-sectional studies. We used a more powerful crossover-crossback prospective design and therefore had repeated semen samples from men during both high and background DBP exposure. This allowed for within-person comparisons and was by design adjusted for non-time varying covariates. Furthermore, our design allowed us to determine the carryover effect that was not observable in the earlier cross-sectional designs.
Experimental studies, primarily during postnatal exposure19,54, have shown decreased serum testosterone concentration and increased FSH and LH concentrations in male rats exposed to BBzP which suggested tubular atrophy and loss of the germinal epithelium as a result of Sertoli cell injury.
Postnatal exposure to other anti-androgenic phthalates (di(2-ethylhexyl)phthalate) caused germ cell apoptosis with increased membrane localization of Fas55,56 or germ cells detachment from the seminiferous epithelium probably due to cellular adhesion loss between Sertoli cells57. Also, studies suggested that in older animals DBP preferentially targets Sertoli cells 58,59.
Studies during fetal development also have shown that DBP is anti-androgenic. It decreases Leydig cell testosterone production by interfering with steroidogenesis by down-regulating gene and/or protein expression important in the steroidogenic pathway, cholesterol transport and metabolism60-66, while increasing oxidative stress along with reactive oxygen species67.
Several studies have identified numerous medications as a source of phthalate exposure34-36,39,40,43 Phthalate levels in medications may not be openly displayed, due to proprietary formulations36. Medications with phthalates in the coating include mesalamine, didanosine, omeprazole, theophylline34 and other medications including over-the-counter preparations31,35,36. Our research and others have shown that mesalamine medications with DBP contribute to high-DBP exposure as measured by urinary MBP concentrations34,39,40.
The human daily DBP intake from the maximum recommended dose of Asacol® is 21 mg42,68. Therefore, the estimated maximum daily DBP intake is 350 μg/kg/day based upon 60 kg body weight. Similarly, the human DBP daily intake from the maximum recommended dose of Asacol®HD is 48 mg69 with the estimated maximum daily DBP intake of 800 μg/kg/day. These intakes are orders of magnitude higher than the estimated average DBP human intake (0.84-5.22 μg/kg/day) 70, several times higher than the US Environmental Protection Agency (EPA) reference dose (100 μg/kg/day)39 and orders of magnitude higher than the European tolerable daily intake (10 μg/kg/day)71.
We considered measuring urinary MBP concentrations in the urine sample collected at each study visit. However, due to the unique crossover-crossback design and the very high exposure from Asacol® (1,000 fold higher than urinary levels when not taking Asacol®), we concluded that urinary MBP concentrations would not add significant information beyond using medication as an indicator of high and low DBP exposure. We offer three primary justifications for not measuring urinary MBP concentrations. First, clear documentation that Asacol® contributes to very high exposure to DBP 31,34-36,39,40,42,68,69. Second, the study is considered interventional in which we assigned participants to the different medications. Therefore, the data was appropriately analyzed based on the assigned medication (as an intention to treat analysis) for our interventional design. Third, from a statistical perspective, continuous urinary data would essentially dichotomize exposure given the very large differences in urinary MBP when taking Asacol® and we would use the same modeling approach. Specifically, the estimates of exposure response, even if we had the continuous urinary MBP concentrations, would have been completely dominated by the medication type (1,000 times difference when taking Asacol®) with no overlap in urinary MBP concentrations between those visits when taking Asacol® and those visits when not taking Asacol®.
Our study had several potential limitations including non-randomization of mesalamine at baseline which may have resulted in chance sampling variability at the baseline. However, there is no reason to believe that the prescribing practices of physicians were associated with the presence or absence of DBP enteric coating. To assign high-DBP exposure, we relied on self-reported use of mesalamine medications as prescribed over the study period72 rather than measuring urinary MBP concentrations which only represent recent exposure (i.e., past several hours) due to the short DBP half-life. Although the sample size for this study was not very large due to the innovative study and length of participation, the power of the study came from the unique design that removes subject to subject variability from the comparison of interest avoiding the purely cross-sectional analyses that likely to suffer from this random variability. We also conducted a post-hoc power analysis and the study had a sufficient number of participants to provide 80% power for detecting at least 23% decrease in sperm concentration and an absolute mean difference of at least 0.4 ml of ejaculate volume, 1% fewer morphologically normal sperm, 6% fewer motile sperm and 4% fewer progressive motile sperm, which is reasonable for a study of this design. Finally, although semen quality is an imperfect predictor of fertility73, semen analysis is considered the cornerstone of the laboratory evaluation for infertile men74.
Our study had several important strengths. We implemented a unique innovative study design that is rarely performed in environmental epidemiology. We were able to compare, within the same men, their semen parameters during periods of high-DBP to background-DBP exposure and vice versa accounting for confounding by measured and unmeasured non-time-varying characteristics75. This is a major strength as compared to previous cross-sectional studies. We were able to explore whether there was a carryover effect of high-DBP exposure. Our study was not restricted to infertile men as most of the previous literature. The means of all semen parameters were comparable to those for fertile US adult men4. Although there may be a concern of generalizing results from men with IBD, which has a significantly increasing temporal trend in incidence worldwide especially in industrialized countries76, these results raise concern about high-DBP exposure and poor semen quality. In addition, there is no evidence that IBD or mesalamine is linked to male infertility77-81. Asacol® and its mesalamine alternatives for treating IBD have the same active ingredients, therefore confounding by indication was unlikely.
Compared to environmental background exposure, we were able to explore high-DBP exposure (1,000 times background). A recent gastroenterology consensus report has recommended switching to non-DBP mesalamine in pregnant women or who may contemplate pregnancy33, without any recommendations for men of reproductive age. Therefore, our results will be informative for future recommendations of use of non-DBP mesalamine formulations among men. To our knowledge, no previous human studies investigated effects of such high-DBP exposure on semen parameters.
5. Conclusions
High-DBP exposure from Asacol® (mesalamine) had adverse effects on semen parameters in adult men, with the most robust effects on sperm motility. Most importantly, the effect of DBP carried-over even after removing the exposure suggesting that high-DBP had longer term effects on spermatogenesis. Further studies are needed to explore whether the carryover effect is potentially reversible after a wash-out period longer than four months. Finally, studies of the effect of high-DBP exposure on sperm epigenetics and reproductive and non-reproductive hormones would add additional insights. Attention should be given to such high-exposure sources and our study and further research will provide more guidance on further regulation of DBP coating of medications.
Supplementary Material
Table-3.B. Adjusted effect (95%CI) of crossover, crossback and carryover on semen parameters among men starting on mesalamine medication containing dibutyl phthalate (H1BH2-arm)a.
| H1BH2-arm[High (H1)-Background (B) - High (H2)] dibutyl phthalate exposure]: 47 men, 199 semen samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fitted Means (95%CI) | Comparisons | ||||||||
| Semen quality parameter |
|
|
|||||||
| Baseline (H1) |
Crossover (B) |
Crossback (H2) |
Crossover Effect (95%CI) B-H1 |
P value |
Crossback Effect (95%CI) H2-B |
P value |
Carryover Effect (95%CI) H2-H1 |
P value |
|
| Ejaculate volume (mL) | 2.47 (2.11, 2.84) | 2.51 (2.11, 2.91) | 2.44 (2.02, 2.86) | 0.04 (-0.22, 0.29) | 0.77 | -0.07 (-0.35, 0.21) | 0.62 | -0.03 (-0.32, 0.25) | 0.81 |
|
| |||||||||
| Sperm concentration (million/mL)b | 57.5 (45, 73.5) | 55.4 (42.5, 72.1) | 60.6 (46, 79.9) | -3.7% (-18.4%, 13.6%) | 0.65 | 9.49% (-8.77%, 31.4%) | 0.33 | 5.43% (-12.1%, 26.5%) | 0.57 |
|
| |||||||||
| Total sperm count (million)b | 124 (92.1, 167) | 124 (89.6, 171) | 130 (92.6, 182) | -0.31% (-18.9%, 22.5%) | 0.98 | 4.86% (-16.5%, 31.6%) | 0.68 | 4.54% (-16.7%, 31.1%) | 0.70 |
|
| |||||||||
| %Total sperm motility | 52.8 (46.6, 59.1) | 49.6 (42.8, 56.3) | 51.6 (44.6, 58.6) | -3.28 (-7.54, 0.97) | 0.13 | 2.02 (-2.68, 6.72) | 0.40 | -1.26 (-5.95, 3.42) | 0.60 |
|
| |||||||||
| %progressive sperm motility | 32.6 (28.2, 36.9) | 31.1 (26.4, 35.8) | 32.3 (27.4, 37.2) | -1.49 (-4.46, 1.49) | 0.33 | 1.2 (-2.09, 4.49) | 0.47 | -0.29 (-3.56, 2.99) | 0.86 |
|
| |||||||||
| Motile sperm count (million)b | 62.7 (42, 93.4) | 54.7 (35.5, 83.9) | 63.3 (40.5, 98.6) | -12.6% (-31.8%, 12.1%) | 0.29 | 15.4%(-12.2%, 51.8%) | 0.30 | 0.91% (-23.2%, 32.7%) | 0.95 |
|
| |||||||||
| %Normal sperm morphology | 7.75 (6.67, 8.83) | 6.74 (5.52, 7.96) | 7.64 (6.33, 8.95) | -1.01 (-2.0, -0.02) | 0.04 | 0.9 (-0.21, 2.01) | 0.11 | -0.11(-1.19, 0.97) | 0.84 |
|
| |||||||||
| Morphologically normal sperm count (millionb)b | 10.3 (7.23, 14.5) | 8.52 (5.76, 12.4) | 10.6 (7.12, 15.6) | -15.6% (-32.2%, 5.05%) | 0.13 | 22.0% (-4.23%, 55.3%) | 0.11 | 2.95% (-19.1%, 31%) | 0.81 |
:Adjusted for abstinence time (categorical: < 2 days, 2≤days<4 and ≥ 4 days), age (continuous), season (warm (April through September) vs cold) and period (# years on DBP-containing mesalamine medication at baseline) in the mixed effect model.
:Sperm concentration, total sperm count, motile sperm count and morphologically normal sperm count are presented as percentage change by exponentiating the log-transformed Beta coefficient. The sum of the percent changes for crossover and cross back do not equal the total carryover effect for the log-transformed variables because they are on the multiplicative scale. For the variables that are not log-transformed, the sum of the percent changes for crossover and cross back equal the total carryover effect as they represent the absolute difference on the additive scale
Motile sperm count (million) and morphologically normal sperm count (million) were log-transformed after adding 1 due to few zero values, they were back transformed and 1 was subtracted from the means.
B1HB2-arm: B1 represents background low-DBP exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background low-exposure after crossback.
H1BH2-arm: H1 represents high-DBP at baseline, B represents background low-DBP exposure after crossover and H2 represents high-DBP after crossback.
Note: 2 samples missing morphology; negative sign means a decrease or % decrease for the log-transformed variables compared to the measure in the previous period.
Abbreviations: DBP, dibutyl phthalate; B1HB2: Background1-High-Background2 DBP exposure; H1BH2; High1-Background-High2 DBP exposure; 95%CI, 95% Confidence Interval.
Highlights.
Mesalamine with coating containing dibutyl phthalate(DBP) impart high-DBP exposure.
Men newly exposed to DBP containing mesalamine had a reduction in semen quality.
The most robust effect of high-DBP exposure from mesalamine was on sperm motility.
High-DBP effect on sperm motility persisted for 4 months after removing exposure.
Acknowledgments
The authors gratefully acknowledge the study participants, all members of the MARS study team and the clinical staff.
Funding: This work was supported by National Institute of Environmental Health Sciences (NIEHS) [grants R01ES017285 and P30ES000002] and support for Feiby L. Nassan during her doctoral studies from the Leslie Silverman Industrial Hygiene Fund, Benjamin Greely Ferris Jr. Fellowship in Environmental Epidemiology, and Cyprus Endowment for the Environment and Public Health at the Harvard T.H. Chan School of Public Health.
Abbreviations
- DBP
dibutyl phthalate
- FDA
Food and Drug Administration
- 5-ASA
5-aminosalicylic acid
- IBD
Inflammatory Bowel Disease
- UC
ulcerative colitis
- CD
Crohn's disease
- MBP
monobutyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- MARS
Mesalamine And Reproductive health Study
- BIMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women's Hospital
- MGH
Massachusetts General Hospital
- IRB
institutional review board
- B1HB2
Background1-High-Background2 DBP exposure
- H1BH2
High1-Background-High2 DBP exposure
- QC
quality control
- BMI
body mass index
- Kg
Kilogram
- m
meter
- SD
standard deviation
- LMEM
mixed effects models
- FEM
fixed effect models
- N
number of men
- WHO
World Health Organization
- EPA
Environmental Protection Agency
Footnotes
Potential Conflict of interest: Joshua Korzenik, research support from Abbvie, Transparency Life Sciences and Takeda, consulting with Abbvie, a founder of a company called ColonaryConcepts; Alan C. Moss, prior research grants from Shire, Salix and Aptalis, manufacturers of mesalamine medications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ (Clinical research ed) 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centola GM, Blanchard A, Demick J, Li S, Eisenberg ML. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U.S. Sperm Bank. Andrology. 2016 Jan 20; doi: 10.1111/andr.12149. [DOI] [PubMed] [Google Scholar]
- 3.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiological reviews. 2016 Jan;96(1):55–97. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swan SH, Brazil C, Drobnis EZ, et al. Geographic differences in semen quality of fertile U.S. males. Environmental health perspectives. 2003 Apr;111(4):414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 6.Congress PL-t. CONSUMER PRODUCT SAFETY IMPROVEMENT ACT OF 2008. 2008 [Google Scholar]
- 7.Cater BR, Cook MW, Gangolli SD, Grasso P. Studies on dibutyl phthalate-induced testicular atrophy in the rat: effect on zinc metabolism. Toxicol Appl Pharmacol. 1977 Sep;41(3):609–618. doi: 10.1016/s0041-008x(77)80014-8. [DOI] [PubMed] [Google Scholar]
- 8.Park JD, Habeebu SS, Klaassen CD. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology. 2002 Feb 28;171(2-3):105–115. doi: 10.1016/s0300-483x(01)00567-4. [DOI] [PubMed] [Google Scholar]
- 9.Shono T, Taguchi T. Short-time exposure to mono-n-butyl phthalate (MBP)-induced oxidative stress associated with DNA damage and the atrophy of the testis in pubertal rats. Environmental science and pollution research international. 2014 Feb;21(4):3187–3190. doi: 10.1007/s11356-013-2332-3. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Bu T, Su H, et al. Inutero exposure to diisononyl phthalate caused testicular dysgenesis of rat fetal testis. Toxicology letters. 2014 Nov 22;232(2):466–474. doi: 10.1016/j.toxlet.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 11.van den Driesche S, McKinnell C, Calarrao A, et al. Comparative Effects of Di(-Butyl) Phthalate Exposure on Fetal Germ Cell Development in the Rat and in Human Fetal Testis Xenografts. Environmental health perspectives. 2014 Dec 16; doi: 10.1289/ehp.1408248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International journal of andrology. 2006 Feb;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-145. [DOI] [PubMed] [Google Scholar]
- 13.Kim TS, Jung KK, Kim SS, et al. Effects of in utero exposure to DI(n-Butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. Journal of toxicology and environmental health Part A. 2010;73(21-22):1544–1559. doi: 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- 14.Motohashi M, Wempe MF, Mutou T, et al. Male rats exposed in utero to di(n-butyl) phthalate: Age-related changes in Leydig cell smooth endoplasmic reticulum and testicular testosterone-biosynthesis enzymes/proteins. Reproductive toxicology (Elmsford, N Y) 2015 Dec 17;59:139–146. doi: 10.1016/j.reprotox.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Bao AM, Man XM, Guo XJ, et al. Effects of di-n-butyl phthalate on male rat reproduction following pubertal exposure. Asian journal of andrology. 2011;13(5):702–709. doi: 10.1038/aja.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013 Sep;154(9):3460–3475. doi: 10.1210/en.2012-2227. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Kim HJ, Im JY, et al. Hypothyroidism protects di(n-butyl) phthalate-induced reproductive organs damage in Sprague-Dawley male rats. J Toxicol Sci. 2008;33(3):299–306. doi: 10.2131/jts.33.299. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi T, Ichihara T, Kawabe M, et al. Renal toxicity induced by folic acid is associated with the enhancement of male reproductive toxicity of di(n-butyl)phthalate in rats. Reproductive Toxicology. 2004;18(1):35–42. doi: 10.1016/j.reprotox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Nagao T, Ohta R, Marumo H, Shindo T, Yoshimura S, Ono H. Effect of butyl benzyl phthalate in Sprague-Dawley rats after gavage administration: a two-generation reproductive study. Reproductive toxicology (Elmsford, N Y) 2000 Nov-Dec;14(6):513–532. doi: 10.1016/s0890-6238(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 20.Kranvogl R, Knez J, Miuc A, Voncina E, Voncina DB, Vlaisavljevic V. Simultaneous determination of phthalates, their metabolites, alkylphenols and bisphenol A using GC-MS in urine of men with fertility problems. Acta Chim Slov. 2014;61(1):110–120. [PubMed] [Google Scholar]
- 21.Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environmental science and pollution research international. 2014 Sep;21(18):11066–11074. doi: 10.1007/s11356-014-2986-5. [DOI] [PubMed] [Google Scholar]
- 22.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology Nov. 2006;17(6):682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Bao H, Liu F, Zhang J, Shen H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environment international. 2012 Jul;42:78–83. doi: 10.1016/j.envint.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Tranfo G, Caporossi L, Paci E, et al. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicology letters. 2012 Aug 13;213(1):15–20. doi: 10.1016/j.toxlet.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Wirth JJ, Rossano MG, Potter R, et al. A pilot study associating urinary concentrations of phthalate metabolites and semen quality. Systems biology in reproductive medicine. 2008 May-Jun;54(3):143–154. doi: 10.1080/19396360802055921. [DOI] [PubMed] [Google Scholar]
- 26.Toshima H, Suzuki Y, Imai K, et al. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: a pilot study on exposure and semen quality. Int J Hyg Environ Health. 2012 Sep;215(5):502–506. doi: 10.1016/j.ijheh.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang YX, You L, Zeng Q, et al. Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environmental research. 2015 Oct;142:1–9. doi: 10.1016/j.envres.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson J, Rylander L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Giwercman A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environment international. 2015 Dec;85:54–60. doi: 10.1016/j.envint.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Human reproduction (Oxford, England) 2015 Nov;30(11):2645–2657. doi: 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston SW, Mendiola J, Bellamy AR, et al. Phthalate exposure and semen quality in fertile US men. Andrology. 2015 Nov 24; doi: 10.1111/andr.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Molecular nutrition & food research. 2011 Jan;55(1):7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 32.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environmental health perspectives. 2004 Mar;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology. 2016 Mar;150(3):734–757.e731. doi: 10.1053/j.gastro.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environmental health perspectives. 2009 Feb;117(2):185–189. doi: 10.1289/ehp.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seckin E, Fromme H, Volkel W. Determination of total and free mono-n-butyl phthalate in human urine samples after medication of a di-n-butyl phthalate containing capsule. Toxicology letters. 2009 Jul 10;188(1):33–37. doi: 10.1016/j.toxlet.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environmental health perspectives. 2012 Mar;120(3):379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA. Food and Drug Administration. [Accessed March 2016];Limiting the use of certain phthalates as excipients in CDER-regulated products. 2012 http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm330792.htm.
- 38.Song Y, Zhang J, Yu S, et al. Effects of chronic chromium(vi) exposure on blood element homeostasis: an epidemiological study. Metallomics : integrated biometal science. 2012 May;4(5):463–472. doi: 10.1039/c2mt20051a. [DOI] [PubMed] [Google Scholar]
- 39.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environmental health perspectives. 2004 May;112(6):751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hait EJ, Calafat AM, Hauser R. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin) 2014 Oct 20;1(1) doi: 10.4161/endo.25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC. Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. [Accessed February 2016];2015 Feb; 2015; http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf.
- 42.FDA. Summary Review for Regulatory Action, Division Director Review NDA 204412, Division of Gastroenterology and Inborn Errors Products. [Accessed January, 2016];CENTER FOR DRUG EVALUATION AND RESEARCH. 2012 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204412Orig1s000SumR.pdf.
- 43.Gallinger ZR, Nguyen GC. Presence of phthalates in gastrointestinal medications: is there a hidden danger? World J Gastroenterol. 2013 Nov 7;19(41):7042–7047. doi: 10.3748/wjg.v19.i41.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan N, Abbas AM, Koleva YN, Bazzano LA. Long-term mesalamine maintenance in ulcerative colitis: which is more important? Adherence or daily dose. Inflamm Bowel Dis. 2013 May;19(6):1123–1129. doi: 10.1097/MIB.0b013e318280b1b8. [DOI] [PubMed] [Google Scholar]
- 45.Walmsley RS, Ayres RCS, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998 Jul 1;43(1):29–32. doi: 10.1136/gut.43.1.29. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980 Mar 8;1(8167):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 47.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science (New York, N Y) 1963 Apr 12;140(3563):184–186. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- 48.Perry MJ, Chen X, McAuliffe ME, Maity A, Deloid GM. Semi-automated scoring of triple-probe FISH in human sperm: methods and further validation. Cytometry A. 2011 Aug;79(8):661–666. doi: 10.1002/cyto.a.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO. WHO laboratory manual for the Examination and processing of human semen. 5th. Geneva, Switzerland: World Health Organization Department of Reproductive Health and Research; 2010. [Google Scholar]
- 50.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertility and sterility. 1988 Jan;49(1):112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 51.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. (2nd) 2011 [Google Scholar]
- 52.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series. 1995;854:1–452. [PubMed] [Google Scholar]
- 53.Cai H, Zheng W, Zheng P, et al. Human urinary/seminal phthalates or their metabolite levels and semen quality: A meta-analysis. Environmental research. 2015 Oct;142:486–494. doi: 10.1016/j.envres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal DK, Maronpot RR, Lamb JCt, Kluwe WM. Adverse effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male rats. Toxicology. 1985 Jun 14;35(3):189–206. doi: 10.1016/0300-483x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- 55.Ichimura T, Kawamura M, Mitani A. Co-localized expression of FasL, Fas, Caspase-3 and apoptotic DNA fragmentation in mouse testis after oral exposure to di(2-ethylhexyl)phthalate. Toxicology. 2003 Dec 15;194(1-2):35–42. doi: 10.1016/j.tox.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Richburg JH, Nanez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1999 Nov 1;160(3):271–278. doi: 10.1006/taap.1999.8786. [DOI] [PubMed] [Google Scholar]
- 57.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biology of reproduction. 2010 Mar;82(3):516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Human reproduction update. 2001 May-Jun;7(3):231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 59.Foster PM, Foster JR, Cook MW, Thomas LV, Gangolli SD. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of di-n-pentyl phthalate. Toxicology and applied pharmacology. 1982 Mar 30;63(1):120–132. doi: 10.1016/0041-008x(82)90031-x. [DOI] [PubMed] [Google Scholar]
- 60.Akingbemi BT, Youker RT, Sottas CM, et al. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol Reprod. 2001 Oct;65(4):1252–1259. doi: 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- 61.Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicological sciences : an official journal of the Society of Toxicology. 2003 Jun;73(2):431–441. doi: 10.1093/toxsci/kfg087. [DOI] [PubMed] [Google Scholar]
- 62.Borch J, Axelstad M, Vinggaard AM, Dalgaard M. Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicology letters. 2006 Jun 1;163(3):183–190. doi: 10.1016/j.toxlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Chauvigne F, Plummer S, Lesne L, et al. Mono-(2-ethylhexyl) phthalate directly alters the expression of Leydig cell genes and CYP17 lyase activity in cultured rat fetal testis. PloS one. 2011;6(11):e27172. doi: 10.1371/journal.pone.0027172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Euling SY, White LD, Kim AS, et al. Use of genomic data in risk assessment case study: II. Evaluation of the dibutyl phthalate toxicogenomic data set. Toxicol Appl Pharmacol. 2013 Sep 15;271(3):349–362. doi: 10.1016/j.taap.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Ovacik MA, Sen B, Euling SY, Gaido KW, Ierapetritou MG, Androulakis IP. Pathway modeling of microarray data: a case study of pathway activity changes in the testis following in utero exposure to dibutyl phthalate (DBP) Toxicol Appl Pharmacol. 2013 Sep 15;271(3):386–394. doi: 10.1016/j.taap.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006 Jun 1;223(1-2):144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Ao H, Chen L, et al. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicology in vitro : an international journal published in association with BIBRA. 2012 Sep;26(6):950–955. doi: 10.1016/j.tiv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 68.FDA. ASACOL® (mesalamine) delayed-release tablets, for oral use - HIGHLIGHTS OF PRESCRIBING INFORMATION. [Accessed January, 2016];2015 http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm215476.htm, http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/019651s025lbl.pdf.
- 69.FDA. Asacol® HD (mesalamine) delayed-release tablet for oral administration -HIGHLIGHTS OF PRESCRIBING INFORMATION. [Accessed January, 2016];2010 http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021830s005lbl.pdf.
- 70.Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009 Jul 27;364(1526):2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.EFSA. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Di-Butylphthalate (DBP) for use in food contact materials. EFSA J 242:1-17 European Food Safety Authority. 2005 Question No. EFSAQ-2003-192. [Google Scholar]
- 72.Gifford AE, Berg AH, Lahiff C, Cheifetz AS, Horowitz G, Moss AC. A random urine test can identify patients at risk of mesalamine non-adherence: a prospective study. Am J Gastroenterol. 2013 Feb;108(2):249–255. doi: 10.1038/ajg.2012.419. [DOI] [PubMed] [Google Scholar]
- 73.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. New England Journal of Medicine. 2001;345(19):1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 74.Diagnostic evaluation of the infertile female: a committee opinion. Fertility and sterility. 2015 Jun;103(6):e44–50. doi: 10.1016/j.fertnstert.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014 Oct;43(5):1645–1655. doi: 10.1093/ije/dyu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 77.Palomba S, Sereni G, Falbo A, et al. Inflammatory bowel diseases and human reproduction: a comprehensive evidence-based review. World journal of gastroenterology : WJG. 2014 Jun 21;20(23):7123–7136. doi: 10.3748/wjg.v20.i23.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Paolo MC, Paoluzi OA, Pica R, et al. Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001 Oct;33(7):563–569. doi: 10.1016/s1590-8658(01)80108-0. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nature clinical practice Gastroenterology & hepatology. 2007 Mar;4(3):160–170. doi: 10.1038/ncpgasthep0696. [DOI] [PubMed] [Google Scholar]
- 80.Teruel C, Lopez-San Roman A, Bermejo F, et al. Outcomes of pregnancies fathered by inflammatory bowel disease patients exposed to thiopurines. The American journal of gastroenterology. 2010 Sep;105(9):2003–2008. doi: 10.1038/ajg.2010.138. [DOI] [PubMed] [Google Scholar]
- 81.Sato A, Naganuma M, Asakura K, et al. Conception outcomes and opinions about pregnancy for men with inflammatory bowel disease. Journal of Crohn's & colitis. 2010 Jun;4(2):183–188. doi: 10.1016/j.crohns.2009.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.