Abstract
Autism spectrum disorder (ASD) is characterized by atypical brain network organization, but findings have been inconsistent. While methodological and maturational factors have been considered, the network specificity of connectivity abnormalities remains incompletely understood. We investigated intrinsic functional connectivity (iFC) for four “core” functional networks—default-mode (DMN), salience (SN), and left (lECN) and right executive control (rECN). Resting-state functional MRI data from 75 children and adolescents (37 ASD, 38 typically developing [TD]) were included. Functional connectivity within and between networks was analyzed for regions of interest (ROIs) and whole brain, compared between groups, and correlated with behavioral scores. ROI analyses showed overconnectivity (ASD > TD), especially between DMN and ECN. Whole-brain results were mixed. While predominant overconnectivity was found for DMN (posterior cingulate seed) and rECN (right inferior parietal seed), predominant underconnectivity was found for SN (right anterior insula seed) and lECN (left inferior parietal seed). In the ASD group, reduced SN integrity was associated with sensory and sociocommunicative symptoms. In conclusion, atypical connectivity in ASD is network-specific, ranging from extensive overconnectivity (DMN, rECN) to extensive underconnectivity (SN, lECN). Links between iFC and behavior differed between groups. Core symptomatology in the ASD group was predominantly related to connectivity within the salience network.
Keywords: autism spectrum disorder, default-mode network, executive control network, functional connectivity, salience network
Introduction
Intrinsic connectivity networks (ICNs) are based on the coupling of spontaneous low-frequency blood oxygen level–dependent (BOLD) signal oscillations in the functional MRI signal between spatially discrete regions (Biswal et al. 1995; Beckmann et al. 2005; Fox and Raichle 2007; Damoiseaux and Greicius 2009). ICNs emerge reliably across participants and scans (Calhoun et al. 2008; Shehzad et al. 2009) and are consistent with structural connectivity (Honey et al. 2007). In development, excitatory and inhibitory functioning largely regulate network sculpting (Wang and Kriegstein 2009), giving rise to two organizing principles: functional integration (high connectivity within networks) and functional segregation (mostly low connectivity between networks) (Friston 2002).
Disruption of network-level intrinsic functional connectivity (iFC) is associated with neurocognitive deficits, including symptomatology in autism spectrum disorders (ASD) (Wass 2011; Vissers et al. 2012). ICNs supporting social, emotional, and language function have been found impaired in ASD, with reports of reduced connectivity within networks (Dinstein et al. 2011; Abrams et al. 2013; von dem Hagen et al. 2013). Across networks, overconnectivity has been found between Theory of Mind (ToM) and mirror neuron systems (Fishman et al. 2014) and between default mode and anteromedial temporal regions (Lynch et al. 2013), potentially reflecting poor network segregation (Rudie et al. 2012). A recent study contrasting task-evoked connectivity and resting-state iFC found reduced discriminability between the two conditions in ASD, associated with restricted and repetitive behaviors (Uddin et al. 2014). Overall however, no consensus as to overarching principles of functional connectivity abnormalities in ASD has been reached. Inconsistencies have been attributed to developmental factors (Uddin, Supekar, and Menon 2013), methodology (Nair et al. 2014), or regional specificity (Lynch et al. 2013). Given the breadth of functional domains affected in ASD (Müller 2007), a parsimonious model for synthesizing results has been proposed. The triple-network approach examines “core” brain networks supporting cognitive, perceptual, affective, and social functions, including the salience (SN), default-mode (DMN), and executive control networks (ECN) thought to be abnormally organized in many psychiatric disorders including ASD (Menon 2011). The current study presents a comprehensive investigation of these networks.
The SN, with nodes in bilateral anterior insulae and dorsal anterior cingulate cortex, is thought to link neocortical processing networks with limbic and autonomic systems related to homeostatic, emotional, and visceral functions (Dosenbach et al. 2007; Seeley et al. 2007). It maintains dense and dynamically variable connectivity with many brain regions (Chang and Glover 2010) and heavily modulates both task-positive and task-negative networks (Sridharan et al. 2008). The SN may thus have a pivotal function in modulating cognitive state (Dosenbach et al. 2007; Seeley et al. 2007) and any compromise may lead to behavioral impairment (Greicius 2008; Uddin, Supekar, and Menon 2013). Decreased activation of SN regions has been shown in ASD during inhibition tasks (Kana et al. 2007; Agam et al. 2010) and during a skin conductance response (Eilam-Stock et al. 2014) possibly reflecting altered autonomic functioning. Furthermore, decreased connectivity has been found within this network (Kana et al. 2007; Ebisch et al. 2011) and between SN nodes and the amygdala (von dem Hagen et al. 2013). However, SN hyperconnectivity has also been reported for children with ASD aged 7–12 years (Uddin, Supekar, Lynch, et al. 2013).
The DMN, or “task-negative” network, is a spatially expansive (Hagmann et al. 2008) system that is relatively inactive during overt task performance. This deactivation is diminished in ASD (Kennedy and Courchesne 2008), particularly during perceptually demanding tasks (Esposito et al. 2006; Ohta et al. 2012). Functional connectivity MRI (fcMRI) studies have shown underconnectivity between posterior and frontal DMN regions in adults and adolescents, associated with social deficits (Monk et al. 2009; Assaf et al. 2010; Weng et al. 2010; von dem Hagen et al. 2013). Findings from younger cohorts, however, have shown DMN overconnectivity (Uddin, Supekar, Lynch, et al. 2013), correlated with symptom severity (Lynch et al. 2013). The participation of multiple DMN regions in one or more subnetworks (Buckner et al. 2009; Smith et al. 2012) may be responsible for divergent findings and nuanced group differences. Dysregulated DMN connectivity may relate to behavioral deficits and concurrent abnormalities in networks supporting externally directed awareness, including attention, salience orienting, affective, visual, and sensorimotor networks (Greicius et al. 2003; Fox et al. 2005).
The executive control network is functionally related with, but separate from the SN (Seeley et al. 2007) and facilitates higher cognitive processes including working memory, set shifting, and inhibition, considered impaired in ASD (Hill 2004; Keehn et al. 2013). During inhibition tasks, the ECN in ASD has been shown to have decreased within-network connectivity (Kana et al. 2007; Solomon et al. 2013), rely more heavily on parietal than frontal subregions (Kana et al. 2013), and recruit atypical brain regions (Solomon et al. 2013). ECN underconnectivity in ASD has been linked to impaired attention (Solomon et al. 2009). While ECN and SN are only weakly correlated with each other, they are both anticorrelated with DMN in TD adults (Seeley et al. 2007). Such anticorrelation between task-positive ECN and task-negative DMN in TD has been found reduced in ASD (Rudie et al. 2012), being a potentially informative classifying feature of the disorder (Anderson et al. 2011).
The present study examined resting-state iFC within and between SN, DMN, and bilateral ECNs. We hypothesized reduced within-network connectivity in ASD for all four networks. Conversely, given the anticorrelations between the DMN and ECN in typical populations, we expected increased connectivity between these regions in ASD reflecting greater network “cross-talk.” Finally, the SN, as a potential modulator of network organization, was expected to show more iFC correlations with diagnostic and behavioral symptoms than DMN and ECNs. Any iFC anomalies of SN were expected to have specifically heavy impact on behavioral and diagnostic scores, since the SN is a modulator of other networks (including DMN and ECNs) and its anomalous connectivity would therefore broadly affect functional network organization. Most previous functional connectivity studies on ASD have either focused on single regions or networks of interest on the one hand, or have described global connectivity features (e.g., using graph theory). The present study provides a comprehensive view (examining four networks considered to play major roles in cognition), but with focus on the specificity of each network, applying identical analysis pipelines in a single cohort for a comparative investigation.
Materials and Methods
Participants
Forty-five children with ASD and 41 TD children participated in the study. Eight ASD and 3 TD participants were excluded from analysis due to excessive head motion (see below), resulting in a final sample of 37 ASD and 38 TD participants (Table 1). Groups were matched for age, sex, handedness, verbal IQ, nonverbal IQ, and head motion. Autism diagnoses were made using the Autism Diagnostic Observation Schedule (Lord et al. 2000), the Autism Diagnostic Interview-Revised (Lord et al. 1994), and expert clinical judgment according to the DSM-IV-TR (American Psychiatric Association 2000) (Supplementary Table 1). Due to the high level of cooperation required for MRI scanning, only participants with full-scale IQ ≥70 could be included. Medical history was obtained through caregiver report both during initial telephone screening and during formal in-person interview. Participants with medical or neurological conditions, such as Fragile-X syndrome, tuberous sclerosis, epilepsy, and Tourette's syndrome, were excluded. However, participants with ASD who presented with common psychiatric co-morbidities, such as attention-deficit (hyperactivity) disorder (n = 4), anxiety disorder (n = 5), and obsessive-compulsive disorder (n = 1), were not excluded given the prevalence of these disorders in ASD populations (Simonoff et al. 2008). TD participants reported no personal history of ASD or other psychiatric or neurological conditions, and no family history of ASD. Informed assent and consent were obtained from all participants and their caregivers in accordance with the University of California, San Diego and San Diego State University Institutional Review Boards.
Table 1.
Participant characteristics
| Full sample |
Low-motion subsample |
|||||
|---|---|---|---|---|---|---|
| Group |
Group matching | Group |
Group matching | |||
| ASD (N = 37) | TD (N = 38) | P | ASD (N = 32) | TD (N = 33) | P | |
| Age | 13.9 (2.6), 9–17 | 13.0 (2.6), 8–17 | 0.143 | 14.2 (2.5), 9–17 | 13.2 (2.7), 8–17 | 0.114 |
| Sex (male:female) | 32:5 | 30:8 | 0.395 | 29:3 | 28:5 | 0.304 |
| Handedness (right:left) | 32:5 | 31:7 | 0.568 | 27:5 | 26:7 | 0.562 |
| Verbal IQ | 105.5 (19.3), 55–147 | 107.8 (11.8), 73–133 | 0.537 | 106.7 (18.5), 70–147 | 108.8 (10.4), 87–133 | 0.561 |
| Nonverbal IQ | 104.4 (16.9), 62–140 | 107.5 (12.5), 83–137 | 0.375 | 104.9 (15.8), 70–140 | 109.0 (11.8), 88–137 | 0.251 |
| Root mean square of displacement (before censoring) | 0.071 (0.039), 0.019–0.166 | 0.071 (0.048), 0.017–0.246 | 0.950 | 0.062 (0.031), 0.019–0.148 | 0.057 (0.028), 0.017–0.121 | 0.501 |
| Root mean square of displacement (after censoring) | 0.065 (0.031), 0.019–0.122 | 0.064 (0.039), 0.017–0.215 | 0.925 | 0.058 (0.029), 0.019–0.119 | 0.053 (0.022), 0.017–0.096 | 0.373 |
Values shown: mean (SD), range. Significance value, P, for group matching in χ2 tests (age, sex) and t-tests (all others).
ASD, autism spectrum disorder; TD, typically developing.
Data Acquisition
Imaging data were acquired on a GE 3T MR750 scanner with an 8-channel head coil. Head movement was minimized with foam pillows around participants' heads. High-resolution structural images were acquired with a standard fast spoiled gradient echo T1-weighted sequence (repetition time: 11.08 ms; echo time: 4.3 ms; flip angle: 45°; field of view: 256 mm; 256 × 256 matrix; 180 slices; 1 mm3 resolution). Functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence. A 6:10 min resting-state scan was acquired consisting of 185 whole-brain volumes (repetition time: 2000 ms; echo time: 30 ms; 3.4 mm isotropic resolution).
Cognitive assessments were obtained using the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999), and Clinical Evaluation of Language Fundamentals (Semel et al. 2003). Additional behavioral data were acquired through caregiver-report using the Caregiver Sensory Profile (SP; Dunn 1999), Social Responsiveness Scale (SRS; Constantino 2005), and Behaviour Rating Inventory of Executive Function (BRIEF; Gioia 2000).
fMRI Data Preprocessing
Data were preprocessed and analyzed using the Analysis of Functional Neuroimaging suite (AFNI; Cox 1996). The first 5 time points were discarded to allow for T1 equilibration. The remaining 180 time points were motion, slice-time, and field-map corrected. Functional data were aligned to anatomical images, resampled to 3.0-mm isotropic voxels, and warped to the standard MNI152 template in a single transformation step. Data were spatially blurred to a full-width at half-maximum of 6 mm and band-pass filtered using a second-order Butterworth filter (0.008 < f < 0.08 Hz) (Power et al. 2012; Satterthwaite et al. 2012) to isolate frequencies predominated by spontaneous functional correlations (Cordes et al. 2001; Fox and Raichle. 2007). Average time series from trimmed white matter and ventricular compartments (from Freesurfer segmentation) as well as their derivatives were regressed from the data. All nuisance regressors (including motion regressors described below) were band-pass filtered using the same procedures as for BOLD time series (Hallquist et al. 2013).
Head Motion and Global Signal
Given evidence that even micromotion (<1 mm) impacts BOLD correlations (Power et al. 2012; Van Dijk et al. 2012) and the ongoing debate about global signal regression (GSR) (Saad et al. 2012; Power et al. 2014), multiple steps were taken to minimize noise. Motion regressors, including 6 rigid-body motion parameters and their derivatives, were removed from the time series. Time points with excessive head motion (root sum of squares ≥0.5 mm) were censored, including one time point immediately preceding and following motion. Any time point that did not belong to a series of at least 10 consecutive time points remaining after censoring was discarded. Ten participants with <80% surviving time points were excluded from the analysis. The final sample (N = 75) showed no group differences for any of the 6 motion regressors (P values for x, y, z, roll, pitch, and yaw being: 0.67, 0.82, 0.30, 0.46, 0.45, and 0.41, respectively) or the root mean squared deviation (RMSD; P = 0.87) when comparing full-length time series (i.e., prior to time point censoring). As additional precaution, RMSD was entered as a covariate in group-level analyses for any significantly correlated measure. Finally, additional analyses were performed for a low-motion subsample (with censoring at ≥0.25 mm and same exclusionary criteria as described above) of 32 ASD and 33 TD participants (Table 1), with and without GSR.
Regions of Interest in Default-Mode, Salience, and Executive Control Networks
Coordinates from previous studies were used to identify primary seeds within each network: posterior cingulate cortex (PCC; Talairach coordinates: x = −5, y = −49, z = 40) (Weng et al. 2010) for the DMN, right anterior insula (rAI; Talairach: x = 36, y = 16, z = 2) (Ebisch et al. 2011) for the SN, right (rIPL), and left inferior parietal lobules (lIPL) (rIPL; MNI: x = 51, y = −47, z = 42; lIPL; MNI: x = −51, y = −51, z = 36) (Dosenbach et al. 2007) for the ECN. IPL was selected because its location coincided most closely with the strongest bilateral cluster of negative correlation with the PCC seed observed in our TD group, consistent with the concept of anticorrelated task-negative (DMN) and task-positive networks (ECN) (Fox et al. 2005; Uddin et al. 2009).
Additional nodes (regions of interest [ROIs]) for each network were derived using the primary seeds listed above and a data-driven approach (based on Chang and Glover. 2010), given lack of consensus in the literature about precise location and individual variability expected in developing and atypical populations. Following whole-brain connectivity analyses of primary seeds for each network, standardized connectivity maps were entered into one-sample t-tests for both groups pooled together. Connectivity maps were cluster-corrected and thresholds were lifted to identify regions of maximum connectivity (∼40 voxels) with respective network hubs (Supplementary Fig. 1). These were used as search masks. For the DMN, search masks were identified in medial prefrontal cortex (mPFC) and bilateral angular gyri (lAng, rAng); for the SN, in left anterior insula (lAI) and dorsal anterior cingulate cortex (dACC); for rECN, in right dorsolateral prefrontal cortex (rDLPFC) and right ventrolateral prefrontal cortex (rVLPFC); and for lECN, in left dorsolateral prefrontal cortex (lDLPFC) and left ventrolateral prefrontal cortex (lVLPFC). Within each search mask, the peak connectivity (for the respective primary network seed) was identified in each participant, and a 6-mm sphere around the peak was used as a subject-specific ROI.
Data Analysis
Average time series were extracted from each ROI in each participant and correlated with every other voxel in the brain. Correlation coefficients were standardized using Fisher's r-z transform and entered into one- and two-sample t-tests, with cluster correction at P < 0.05 (Forman et al. 1995). Network time series were calculated by averaging across all voxels in all ROIs of a given network. Correlations were computed for all ROI and all network pairings, resulting in a 13 × 13 ROI matrix and a 4 × 4 network matrix (Fig. 1A,C, respectively). A summary “within-network connectivity index” was derived by averaging the z′ values (from Fisher transformation) for all ROI pairings within a network, separately for DMN, SN, and ECNs. Two-sample t-tests were performed for ROI pairs, network pairs, and within-network connectivity indices to detect potential group differences. Connectivity z′ values were compared with diagnostic and behavioral measures (Supplementary Table 1), using Pearson's correlation.
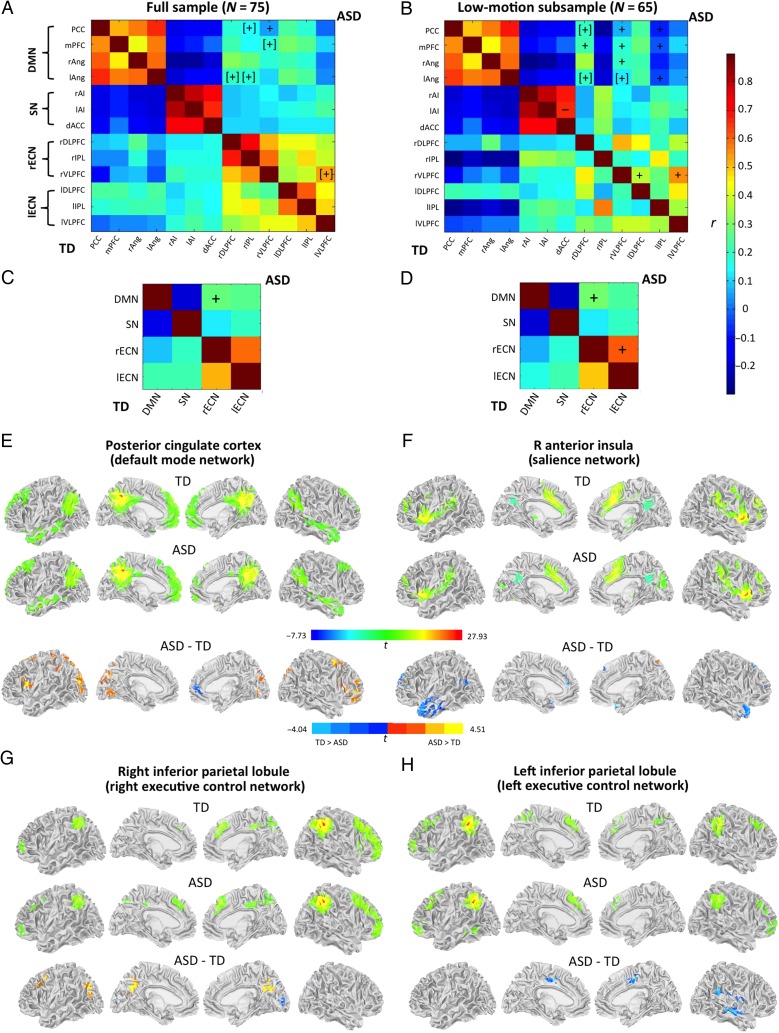
Figure 1.
Correlation matrices for all ROIs in ASD (upper right triangles) and TD groups (lower left) using (A) full sample and (B) low-motion subsample. Correlation matrices for networks (averaged across ROIs) using (C) full sample and (D) low-motion subsample. DMN, default-mode network; SN, salience network; rECN, right executive control network; lECN, left executive control network. +/−, significantly greater/weaker FC in ASD compared with TD group (P < 0.05, FDR-corrected); [+]/[−], analogous group differences at P < 0.05, uncorrected. For detailed statistical listing, see Supplementary Table 2. (E–H) Surface renderings of within-group functional connectivity (top) and between-group difference (bottom) for primary seeds of (E) default mode network, (F) salience network, as well as (G) right and (H) left executive control networks (all clusters P < 0.05, cluster-corrected).
Results
Network and ROI Connectivity
Several pairings between DMN regions and rECN regions showed overconnectivity in the ASD group. Specifically, overconnectivity was found for the PCC with rVLPFC and rIPL, for the mPFC with the rVLPFC, for the lAng with the rDLPFC and rIPL, for the right VLPFC with the left VLPFC (Supplementary Table 2 for statistics). However, after correcting for multiple comparisons using false discovery rate (FDR), only overconnectivity for PCC–rVLPFC remained significant (Fig. 1A). Pairings between the networks showed significant overconnectivity in ASD for DMN–rECN (Fig. 1C). No significant group differences were found for within-network connectivity indices.
Whole-Brain Connectivity Analysis
Whole-brain analyses for network seed regions in PCC, rAI, rIPL, and lIPL showed expected connectivity with other regions of respective networks in both groups (Fig. 1E–H). Between-group findings, however, revealed overconnectivity in the ASD group for PCC with numerous regions in frontal, parietal, and occipital lobes bilaterally, accompanied by underconnectivity in right medial frontal cortex (Fig. 1E; cluster listings in Supplementary Table 3). For rIPL, overconnectivity was seen in left premotor cortex and bilateral precuneus, accompanied by small underconnectivity clusters in right parieto-occipital regions, including primary visual cortex (Fig. 1G). Extensive underconnectivity in the ASD group was found for the rAI seed with anterior temporal and dorsolateral prefrontal regions bilaterally and left posterior superior temporal sulcus (Fig. 1F), and for the lIPL seed with bilateral dorsal cingulate cortex and right superior temporal regions extending into the insula (Fig. 1H).
Low-Motion and GSR Analyses
For the low-motion subsample (with censoring at ≥0.25 mm, but no GSR; Table 1), we found a similar pattern of overconnectivity in the ASD group between DMN and bilateral ECN regions (Fig. 1B,D). Despite reduced power in the subsample, the effects were more robust, reaching FDR-corrected significance between several DMN regions and rDLPFC, rVLPFC, and lIPL (Fig. 1B). At the network level, rECN was found overconnected with DMN and lECN in the ASD group (Fig. 1D). After partialling out the global signal and its derivative at the 0.25 mm censoring threshold, findings at the network level were identical, whereas those for ROI pairs were similar, but overall weaker (Supplementary Fig. 2).
In whole-brain analyses for the low-motion subsample (without GSR), connectivity results were consistent with the full-sample analysis (Supplementary Fig. 3A), with additional overconnectivity effects for rAI in right parietal, and left orbitofrontal and posterior thalamic regions, and for lIPL in right orbitofrontal cortex and supplementary motor area. Inclusion of GSR resulted in an overall similar pattern, but with additional underconnectivity clusters in midcingulate regions for the PCC seed, in PCC for rAI, and in pericalcarine visual cortex for rIPL (Supplementary Fig. 3B).
Demographic Variables
Effects of demographic variables on network connectivity measures were tested and partialled out if significant (P < 0.05). For age, a negative linear correlation was found with DMN–lECN connectivity in the ASD group (r = −0.42, P = 0.010), but not the TD group (r = 0.20, P = 0.24), with a significant group by age interaction (t = 2.46, P = 0.017; Fig. 2A). Age was marginally correlated with SN-lECN connectivity in the ASD group (r = 0.32 P = 0.052), with a quadratic effect in the TD group (t = 2.52 P = 0.013), and a group by age interaction (t = 2.52 P = 0.014). Additionally, a quadratic age effect was also found for SN-rECN connectivity in the TD group (t = 2.90 P = 0.006), with no group by age interaction.
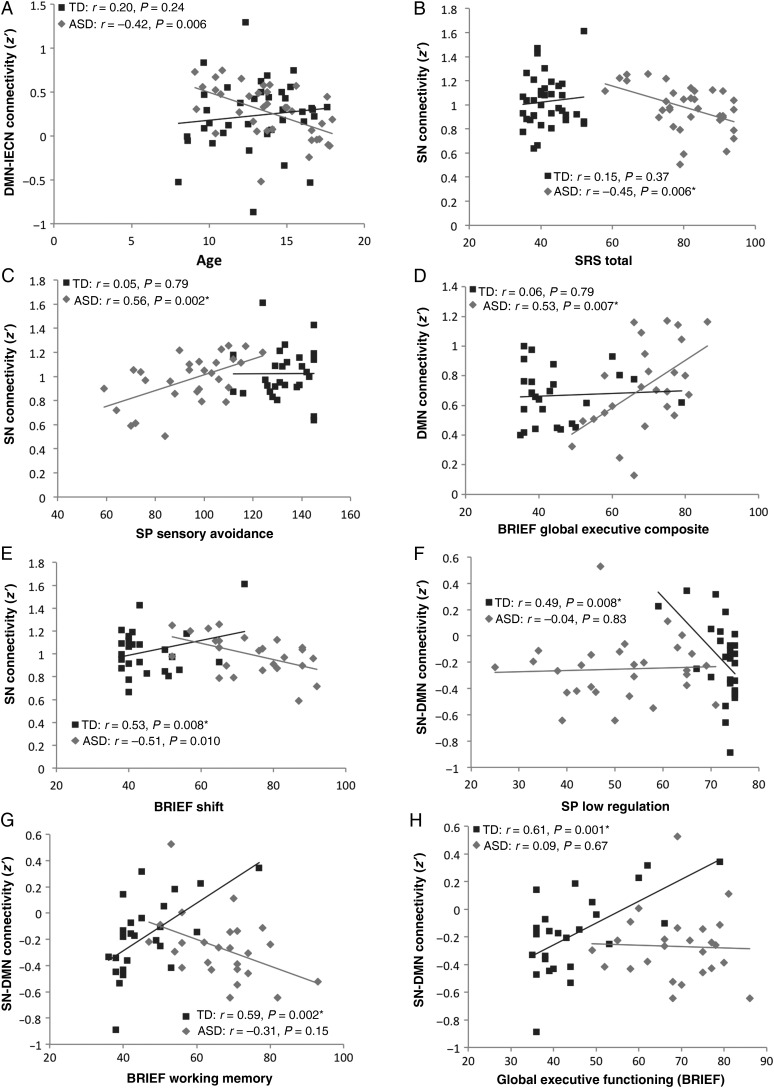
Figure 2.
Correlations of demographic and behavioral measures with connectivity for TD (blue) and ASD groups (red): (A) DMN–lECN within-network connectivity by age, (B) SN within-network connectivity index by SRS Total, (C) SN within-network connectivity index by SP Sensory Avoidance, (D) DMN within-network connectivity index by BRIEF Global Executive Composite, (E) SN within-network connectivity index by BRIEF Shift, (F) SN–DMN connectivity index by SP Low Regulation, (G) SN-DMN connectivity index by BRIEF Working Memory, and (H) SN–DMN connectivity by BRIEF Global Executive Functioning. *P < 0.05, FDR-corrected.
Handedness significantly affected DMN–SN connectivity in the ASD group (t = 2.18, P = 0.036), and SN–rECN (t = 2.08, P = 0.045) and lECN–rECN connectivity (t = 4.09, P < 0.001) in the TD group, with right-handedness predicting greater iFC. Furthermore in the ASD group, positive correlations were found for full-scale IQ with SN–rECN connectivity (r = 0.38, P = 0.027), for verbal IQ with SN–lECN connectivity (r = 0.36, P = 0.036), and for RMSD with DMN–lECN connectivity (r = 0.40, P = 0.016). In the TD group, full-scale IQ was positively correlated with SN connectivity (r = 0.36, P = 0.027). Significant variables, as well as motion (RMSD), were partialled out in behavioral analyses reported in the following section.
Behavioral Correlations
Pearson's correlations were run separately for each group and corrected using FDR (Supplementary Table 4–5). In the ASD group, reduced SN within-network connectivity was associated with more symptoms on SRS Total (Fig. 2B) and Mannerisms scores and on SP Sensory Avoidance scores (Fig. 2C). Higher DMN within-network connectivity was associated with more executive impairment on the BRIEF Initiate and Working memory Subscales, the Metacognition Index, and the Global Executive Composite (Fig. 2D). No correlations were found between connectivity and diagnostic measures. In the TD group, higher SN within-network connectivity was associated with higher scores (reflecting lower abilities) on the BRIEF Inhibit and Shift Subscales (Fig. 2E) as well as the Behavior Regulation Index and Global Executive Composite.
For between-network connectivity, robust correlations that survived FDR correction were seen only in the TD group. Specifically, higher SN-DMN connectivity was associated with greater impairment on the SRS Awareness, Cognition, and Total scores, on SP Low Regulation scores, and on the BRIEF Inhibit, Shift, Working Memory, Planning/Organizing, Monitor subscores, Behavior Regulation Index, Metacognition Index, and Global Executive Composite (Fig. 2F–H). One TD participant had several very high BRIEF scores (>2 SD from the mean for the TD group), but did not meet any exclusionary criteria (as listed above), with a full-scale IQ of 93. After removing this outlier from correlations with BRIEF scores, all effects described above remained significant, except the one shown in Figure 2E.
Discussion
Functional connectivity research in ASD has been plagued by inconsistent findings, with proposals and reported effects ranging from predominant underconnectivity (Schipul et al. 2011) to predominant overconnectivity (Supekar et al. 2013). Aside from methodological (Nair et al. 2014) or developmental (Uddin, Supekar and Menon 2013) factors, it is not well understood how network-specific patterns may affect differential findings. Our study examined four of the most prominent networks (default mode, salience, left [lECN] and right executive control [rECN]), with two main findings: 1) While limited focus on network nodes yielded moderate evidence of overconnectivity in ASD (especially between DMN and ECNs; Fig. 1), whole-brain analyses showed a distinct pattern with representative seeds of DMN (PCC) and right ECN (rIPL) characterized by predominant overconnectivity in ASD across multiple brain regions, but those of the SN (rAI) and left ECN (lIPL) by predominant underconnectivity. 2) Links between network-specific iFC and behavioral indices (sociocommunicative, sensory, executive) differed substantially between ASD and TD groups (i.e., of the 23 correlations that reached FDR-corrected significance, not a single one was shared between the 2 groups; cf. Fig. 2).
Overall Pattern of Findings and Implications for Triple-Network Model
Our selection of networks of interest was informed by the triple-network hypothesis (Menon 2011), according to which many psychiatric and neurologic conditions are characterized by disorder-specific patterns of increased or reduced function and connectivity in SN, DMN, and ECNs. The overall pattern observed in our study, showing an underconnected SN accompanied by complex patterns of over- and underconnectivity of DMN and left and right ECNs, was relevant to this hypothesis in several respects. First, it showed that all of the networks implicated by this hypothesis were indeed affected by atypical iFC in the ASD group. Second, it generally supported the expectation of network-specific connectivity aberrations, i.e., underconnectivity for some, but overconnectivity for other network nodes. However, since our study did not include participants with other types of psychiatric or developmental disorders, we could not test the hypothesis of disorder-specific anomalies. Third and more specifically, our findings support an impaired hub function of the SN, which was underconnected internally and with many brain regions outside the network. Such underconnectivity in ASD is likely to affect the SN's typical role of switching between DMN and ECNs, both of which were characterized by atypical iFC with numerous other brain regions. Fourth, although we found that iFC within the SN was correlated with sociocommunicative abilities (SRS Total score) in the ASD group, many other connectivity measures (e.g., iFC between SN and other networks) did not show robust behavioral correlations surviving FDR correction. Specifically in regard to symptom measures derived from parental ratings (such as the SRS), tight correlations with imaging metrics may, however, not be a realistic expectation. Aside from a potentially crucial role of networks such as the SN in ASD, many other factors will affect symptomatology, including history of interventions and other individually variable experiential and environmental factors. In this context, our finding of SN within-network iFC accounting for over 20% of the variability of SRS Total scores was remarkably robust, especially given that the SN and other networks of interest in the present study do not belong to the social cognition domain proper.
Default Mode Network
As a whole, the DMN was overconnected with the right ECN in the ASD group. In TD adults, DMN and task-positive networks, such as the ECNs, are functionally segregated and anticorrelated (Uddin et al. 2009; Carbonell et al. 2014). However, robust anticorrelations are not yet present in children (Fair et al. 2009; Dosenbach et al. 2010), consistent with our findings (Fig. 1). Anticorrelations in the TD group were only found between DMN nodes and IPL bilaterally in analyses with stricter motion censoring. Overconnectivity in the ASD group observed for multiple DMN–rECN ROI pairings thus partly reflected reduced anticorrelations (rather than robust positive correlations), indicating a reduction in the typical segregation between networks, consistent with some previous reports (Shih et al. 2011; Rudie et al. 2012). However, iFC between DMN and lECN decreased with age, only in the ASD group (Fig. 2A), possibly suggesting compensatory or late maturational effects in network segregation.
Whole-brain analyses for the primary DMN seed in PCC showed a distinct pattern, with a large underconnectivity cluster in mPFC, but extensive overconnectivity clusters across multiple occipital, parietal, and frontal regions bilaterally. Underconnectivity between PCC and mPFC (another major node of the DMN) has been observed in previous studies (Kennedy and Courchesne 2008; Monk et al. 2009; Assaf et al. 2010; Murdaugh et al. 2012; Starck et al. 2013; von dem Hagen et al. 2013; Washington et al. 2014; Doyle-Thomas et al. 2015), being one of the most replicated findings in the ASD iFC literature (although it was not seen in our low-motion subsample, without GSR; Supplementary Fig. 3). Connectivity within the DMN was not related to sociocommunicative measures. However, greater iFC within this network was associated with “reduced” executive abilities (as detected on several BRIEF scores) in the ASD group—links that were entirely absent in the TD group (Fig. 2D). This finding may relate to impaired downregulation of DMN during cognitive processing in ASD (Kennedy et al. 2006). Studies in neurotypical adults have shown an association between greater DMN activity and reduced executive task performance (Anticevic et al. 2010) and vigilance (Hinds et al. 2013). An analogous association has been found tying strength of anticorrelations between DMN and task-positive network to executive task performance (Kelly et al. 2008). Reduction of these neurotypical anticorrelations was reflected in our finding of overconnectivity between PCC and bilateral ventral DLPFC in the ASD group (Fig. 1E). IFC between PCC and DLPFC has been found to be negatively related to task performance relying on selective attention (Prado and Weissman. 2011), suggesting that this finding may relate to attentional abnormalities in ASD (Allen and Courchesne 2001; Keehn et al. 2013).
While most previous studies of the DMN in ASD (as cited above) focused on underconnectivity for this network, few have reported overconnectivity outside the DMN (Monk et al. 2009; Lynch et al. 2013; Doyle-Thomas et al. 2015). The extensive and robust overconnectivity effects observed in our study suggest that reduced differentiation of the DMN and atypical “cross-talk” with exogenous brain regions (Fishman et al. 2014) may be the most prominent feature of atypical DMN organization in ASD.
Salience Network
Results for the SN stood out because measures of sociocommunicative impairment and sensory abnormalities were exclusively linked to connectivity within this one network. Greater iFC within the salience network was associated with reduced social impairment on the SRS and fewer sensory anomalies on the SP (Fig. 2B,C). Interestingly, such correlations were absent in the TD group, indicating ASD-specific links. ROI analyses showed extremely high BOLD correlations among nodes of this network in both groups (Fig. 1A); however, at a more conservative motion threshold, iFC between left AI and dorsal ACC was significantly reduced in the ASD group (Fig. 1B). This finding is consistent with an impaired hub function of the AI, as hypothesized by Menon and colleagues (Uddin and Menon 2009; Menon 2011), which would reduce the ability of people with ASD to flexibly engage and disengage ECNs and DMN by detecting and filtering affective, interoceptive, and autonomic inputs. Reduced iFC between AI and ACC in ASD may furthermore relate to impaired goal-directed action (Menon 2011).
Whole-brain analyses for the primary SN seed in right AI furthermore showed extensive underconnectivity in the ASD group with bilateral anterior temporal lobes, which are putative regions of the SN (Seeley et al. 2007) and reportedly involved in social cognition (Simmons et al. 2009) and regulation of visceral states based on heteromodal sensory input (Olson et al. 2007). Additional smaller underconnectivity clusters were detected in medial superior frontal gyri bilaterally. On the left, this cluster occurred in a medial prefrontal region known for its role in social cognition (ToM) (Gallagher et al. 2000). Reduced activation in this region during ToM tasks has been observed in several ASD studies (Happé et al. 1996; Castelli et al. 2002; Kana et al. 2009). A meta-analysis of 39 autism fMRI studies by Di Martino et al. (2009) identified rAI as one of the sites of consistently reduced activation during performance on social (compared with nonsocial) tasks in ASD.
Our findings differ from two previous studies. Testing adolescents with ASD, Ebisch et al. (2011) reported only a single effect for a seed in rAI, which was underconnectivity with the right amygdala (not replicated in our study). Conversely, Uddin et al. (2013) reported overconnectivity within a salience network derived from independent component analysis in children with ASD. However, participants were mostly younger (7.5–11.9 years) and whole-brain connectivity of the SN was not examined in this study. In addition, sample sizes were limited in both and the treatment of head motion was less conservative than in the present study, which likely affected observed group differences (Power et al. 2012; Van Dijk et al. 2012; Satterthwaite et al. 2013).
Executive Control Networks
ECNs were overconnected with DMN for multiple ROI pairings, as discussed above. Whole-brain FC patterns for primary ECN seeds in IPL differed greatly between hemispheres. Right IPL was overconnected with several fronto-parietal regions, whereas left IPL was underconnected with right temporal and bilateral cingulate regions. While left hemisphere executive control regions have been implicated in motor attention (Rushworth et al. 2001) and verbal decision making (Stephan et al. 2003), right-hemisphere regions are more active during visuospatial orientation and attention (Kinsbourne 1987; Stephan et al. 2003) and have been shown to engage more than the left executive control regions during difficult goal-oriented planning (Newman et al. 2003). IFC between left and right ECNs, as a whole, was also increased in the ASD group on low-motion analyses (Fig. 1D). The asymmetric findings for ECNs may relate to pervasive right-hemisphere shifts of functional networks (including fronto-parietal networks), as recently reported (Cardinale et al. 2013).
Age-Related Effects
Although the fcMRI literature encompasses findings from infancy into adulthood, it is not well understood how iFC changes in ASD relate to age. Maturational changes are likely to affect atypical iFC patterns observed in ASD (Uddin, Supekar and Menon 2013) and several cross-sectional fcMRI studies have indeed examined age-related effects (Shih et al. 2011; Padmanabhan et al. 2013; Bos et al. 2014; Doyle-Thomas et al. 2015; Nomi and Uddin 2015), although not always with clear-cut findings (Alaerts et al. 2015). In our study, we found two atypical age-related effects in the ASD group that both concerned lECN. First, iFC with the DMN decreased with age (Fig. 2A), suggesting overconnectivity between normally segregated task-positive and task-negative networks in young children, but not adolescents with ASD. This pattern may indicate delayed network segregation (Rudie et al. 2012) and is consistent with a general pattern of age-related change from early overconnectivity to normal iFC levels in adulthood (Nomi and Uddin 2015). The second atypical finding was increasing iFC between lECN and SN with age—a finding not easily compatible with a transition from child overconnectivity to adult underconnectivity in ASD, as proposed by Uddin et al. (2013).
Technical Aspects and Limitations
Groups were tightly motion-matched and our censoring cutoff (0.5 mm in the full sample) was relatively strict for a pediatric study. Between-group findings were largely confirmed for a low-motion subsample (censored at 0.25 mm), although with a slight shift towards lower signal correlations. Addition of a global signal regressor did not enhance this shift towards anticorrelations (contrary to theoretical expectation (Murphy et al. 2009)), presumably because residual global noise inflating signal correlations was already minimized in our low-motion subsample. However, addition of GSR appeared to distort some of the group effects, as previously predicted based on simulated data (Saad et al. 2012) and observed in actual ASD data (Gotts et al. 2013). Since the overall distribution of BOLD correlations will be centered around zero within each group (and participant) with GSR on statistical grounds (rather than as a true reflection of iFC), the distribution of between-group differences will correspondingly be balanced between over- and underconnectivity effects. The distinct pattern of predominantly overconnected (PCC, rIPL) and underconnected (rAI, lIPL) seeds observed in our primary analyses was thus lost in analyses with GSR. However, the co-occurrence of effects with opposite polarity (over vs. underconnectivity) for different networks in the same dataset suggests that this pattern (observed in the absence of GSR) was not an artifact of residual global noise, but a true effect.
Compared with extensive whole-brain findings showing network-specific over- and underconnectivity, results from a connectivity matrix including only 3–4 ROIs from each network were comparably modest (Fig. 1A,C). This highlights the conclusion from an earlier meta-analysis (Müller et al. 2011), which suggested that fcMRI analyses limited to presumptive network ROIs risk missing the “big picture” of connectivity anomalies in ASD.
In contrast to an earlier study (Fishman et al. 2014) that found overconnectivity between 2 networks (mirror neuron and ToM) to be linked to diagnostic severity in ASD, we did not observe such correlations for between-network iFC in the present study. Several links of between-network iFC (SN–DMN) with behavioral measures detected in the TD group were absent in the ASD group, but group differences (interaction effects) were significant only for the BRIEF Working Memory measure (Supplementary Table 5). Other correlations for between-network iFC and SRS (and other) scores (with r > 0.3) in the ASD group and various interaction effects did not remain significant after FDR correction. Sample size limitations and variability within our ASD cohort may have contributed to these null findings.
Perspectives
Our study goes beyond numerous and often inconsistent reports of underconnectivity or overconnectivity in ASD for single seeds or networks of interest (as reviewed in Müller et al. 2011; Vissers et al. 2012). Although 4 major functional networks (default, salience, lECN and rECN) all showed atypical connectivity, the precise iFC patterns differed dramatically, within each network, between the networks, and between each network and other brain regions. The significance of these differential patterns of iFC abnormality was underscored by links with sociocommunicative, sensory, and executive behaviors. These links differed substantially between ASD and TD groups, highlighting the potentially crucial role of the salience network in core sociocommunicative domains of autistic symptomatology and sensory anomalies.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This study was supported by the National Institutes of Health R01-MH081023 (R.A.M.), K01 MH097972 (I.F.), with additional funding from Autism Speaks Dennis Weatherstone Predoctoral Fellowship #7850 (A.N.) and for MRI scanning in 12 participants from the Congressionally Directed Medical Research Programs (CDMRP AR093335, PI: J. Pineda). Special thanks to the children and families who participated.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, Uddin LQ, Menon V. 2013. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci USA. 110:12060–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y, Joseph RM, Barton JJ, Manoach DS. 2010. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 52:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Nayar K, Kelly C, Raithel J, Milham MP, Di Martino A. 2015. Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Soc Cogn Affect Neurosci. doi:10.1093/scan/nsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Courchesne E. 2001. Attention function and dysfunction in autism. Front Biosci. 6:D105–D119. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2000. Diagnostic and statistical manual of mental disorders-IV-TR. Washington: (DC: ): American Psychiatric Association. [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, Cooperrider JR, Zielinski BA, Ravichandran C, Fletcher PT. 2011. Functional connectivity magnetic resonance imaging classification of autism. Brain. 134:3742–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. 2010. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 49:2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 53:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bos DJ, van Raalten TR, Oranje B, Smits AR, Kobussen NA, van Belle J, Rombouts SA, Durston S. 2014. Developmental differences in higher-order resting-state networks in autism spectrum disorder. Neuroimage. 4:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. 2008. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 29:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell F, Bellec P, Shmuel A. 2014. Quantification of the impact of a confounding variable on functional connectivity confirms anti-correlated networks in the resting-state. Neuroimage. 86:343–353. [DOI] [PubMed] [Google Scholar]
- Cardinale RC, Shih P, Fishman I, Ford LM, Müller R-A. 2013. Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder: an fMRI study using independent component analysis. JAMA Psychiatry. 70:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. 2002. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 125:1839–1849. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 50:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. 2005. Social Responsiveness Scale (SRS). Los Angeles: (CA: ): Western Psychological Services. [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. 2001. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. 2009. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 213:525–533. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. 2009. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. 2011. Disrupted neural synchronization in toddlers with autism. Neuron. 70:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN et al. . 2010. Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Thomas KA, Lee W, Foster NE, Tryfon A, Ouimet T, Hyde KL, Evans AC, Lewis J, Zwaigenbaum L, Anagnostou E. 2015. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann Neurol. 77:866–876. [DOI] [PubMed] [Google Scholar]
- Dunn W. 1999. The sensory profile manual. San Antonio: (TX: ): The Psychological Corporation. [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. 2011. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 32:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam-Stock T, Xu P, Cao M, Gu X, Van Dam NT, Anagnostou E, Kolevzon A, Soorya L, Park Y, Siller M. 2014. Abnormal autonomic and associated brain activities during rest in autism spectrum disorder. Brain. 137:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F. 2006. Independent component model of the default-mode brain function: assessing the impact of active thinking. Brain Res Bull. 70:263–269. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, Müller R-A. 2014. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry. 71:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. 2002. Beyond phrenology: what can neuroimaging tell us about distributed circuitry. Annu Rev Neurosci. 25:221–250. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happae F, Brunswick N, Fletcher PC, Frith U, Frith CD. 2000. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 38:11–21. [DOI] [PubMed] [Google Scholar]
- Gioia GA. 2000. BRIEF: behavior rating inventory of executive function: professional manual: Psychological Assessment Resources.
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. 2013. The perils of global signal regression for group comparisons: a case study of autism spectrum disorders. Front Hum Neurosci. 7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. 2008. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 21:424–430. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher PC, Frith U, Johansson M, Gillberg C, Dolan RJ, Frackowiak RSJ, Frith CD. 1996. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 8:197–201. [DOI] [PubMed] [Google Scholar]
- Hill EL. 2004. Executive dysfunction in autism. Trends Cogn Sci. 8:26–32. [DOI] [PubMed] [Google Scholar]
- Hinds O, Thompson TW, Ghosh S, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. 2013. Roles of default-mode network and supplementary motor area in human vigilance performance: evidence from real-time fMRI. J Neurophysiol. 109:1250–1258. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O. 2007. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 104:10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Adam Just M. 2009. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 4:135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. 2007. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 62:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Liu Y, Williams DL, Keller TA, Schipul SE, Minshew NJ, Just MA. 2013. The local, global, and neural aspects of visuospatial processing in autism spectrum disorders. Neuropsychologia. 51:2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Müller R-A, Townsend J. 2013. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 37:164–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2008. Competition between functional brain networks mediates behavioral variability. Neuroimage. 39:527–537. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. 2008. Functional abnormalities of the default network during self-and other-reflection in autism. Soc Cogn Affect Neurosci. 3:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. 2006. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA. 103:8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M. 1987. Mechanisms of unilateral neglect. Adv Psychol. 45:69–86. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. 2013. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 74:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. 2009. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 47:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA. 2007. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 13:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. 2011. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 21:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Shinkareva SV, Deshpande HR, Wang J, Pennick MR, Kana RK. 2012. Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One. 7:e50064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced. Neuroimage. 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, Müller RA. 2014. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum Brain Mapp. 35:4035–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. 2003. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 41:1668–1682. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. 2015. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yamada T, Watanabe H, Kanai C, Tanaka E, Ohno T, Takayama Y, Iwanami A, Kato N, Hashimoto R-I. 2012. An fMRI study of reduced perceptual load-dependent modulation of task-irrelevant activity in adults with autism spectrum conditions. Neuroimage. 61:1176–1187. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. 2007. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 130:1718–1731. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O'Hearn K. 2013. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 7:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Weissman DH. 2011. Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 56:2276–2282. [DOI] [PubMed] [Google Scholar]
- Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, Dapretto M. 2012. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 22:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. 2001. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 13:698–710. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE et al. . 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. 2011. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. 2003. Clinical evaluation of language fundamentals. 4th ed San Antonio: (TX: ): The Psychological Corporation. [Google Scholar]
- Shehzad Z, Kelly AC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB. 2009. The resting brain: unconstrained yet reliable. Cereb Cortex. 19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller R-A. 2011. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry. 70:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. 2009. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 49:2705–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. 2008. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 47:921–929. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF. 2012. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci USA. 109:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. 2009. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 47:2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Yoon JH, Ragland JD, Niendam TA, Lesh TA, Fairbrother W, Carter CS. 2013. The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biol Psychiatry. 76:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck T, Nikkinen J, Rahko J, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila M-L, Jansson-Verkasalo E. 2013. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Front Hum Neurosci. 7:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR. 2003. Lateralized cognitive processes and lateralized task control in the human brain. Science. 301:384–386. [DOI] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, Yerys BE, Vaidya CJ, Menon V. 2013. Brain hyperconnectivity in children with autism and its links with social deficits. Cell Rep. 5:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. 2009. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 30:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. 2009. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 33:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, Phillips J, Feinstein C, Abrams DA, Menon V. 2014. Brain state differentiation and behavioral inflexibility in autism. Cereb Cortex. doi:10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. 2013. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 70:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2013. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. 2012. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 36:604–625. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. 2013. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. 2009. Defining the role of GABA in cortical development. J Physiol (Lond). 587:1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease-Ference ER, Girton L, Hailu A, Mbwana J. 2014. Dysmaturation of the default mode network in autism. Hum Brain Mapp. 35:1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S. 2011. Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn. 75:18–28. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler abbreviated scale of intelligence. San Antonio: (TX: ): Psychological Corporation. [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. 2010. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 1313:202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.