Abstract
Lumen formation and maintenance are important for the development and function of essential organs such as the lung, kidney and vasculature. In the Drosophila embryonic trachea, lumena form de novo to connect the different tracheal branches into an interconnected network of tubes. Here, we identify a novel role for the receptor type guanylyl cyclase at 76C (Gyc76C) in de novo lumen formation in the Drosophila trachea. We show that in embryos mutant for gyc76C or its downsteam effector protein kinase G (PKG) 1, tracheal lumena are disconnected. Dorsal trunk (DT) cells of gyc76C mutant embryos migrate to contact each other and complete the initial steps of lumen formation, such as the accumulation of E-cadherin (E-cad) and formation of an actin track at the site of lumen formation. However, the actin track and E-cad contact site of gyc76C mutant embryos did not mature to become a new lumen and DT lumena did not fuse. We also observed failure of the luminal protein Vermiform to be secreted into the site of new lumen formation in gyc76C mutant trachea. These DT lumen formation defects were accompanied by altered localization of the Arf-like 3 GTPase (Arl3), a known regulator of vesicle-vesicle and vesicle-membrane fusion. In addition to the DT lumen defect, lumena of gyc76C mutant terminal cells were shorter compared to wild-type cells. These studies show that Gyc76C and downstream PKG-dependent signaling regulate de novo lumen formation in the tracheal DT and terminal cells, most likely by affecting Arl3-mediated luminal secretion.
Introduction
Many of our essential organs, such as the lung, kidney and vasculature are tube-based structures where gases, nutrients and waste are transported through their respective lumena. For some tubular organs, lumena form de novo whereas for others, lumena form concomitantly with tube formation. Much of our understanding of de novo lumen formation has come from studies in the Drosophila embryonic trachea, a network of interconnected epithelial tubes that transport oxygen and other gases [1, 2]. As tracheal cells migrate out to form the primary branches, blunt-ended tubes with a sealed central lumen are initially formed (Fig 1A). A continuous tubular network is formed when specialized fusion cells at the tips of migrating branches contact each other’s partner in the adjacent segment and mediate de novo lumen formation and lumen fusion (Fig 1B–1D) [3, 4]. Lumen formation in the Drosophila trachea is a complex and highly regulated process involving precise coordination of cytoskeletal proteins, adhesion proteins and components of the vesicular trafficking machinery. During lumen formation fusion cells of opposing tracheal branches, such as the dorsal trunk (DT) contact each other through E-cadherin-mediated adhesion to form actin and microtubule tracks that prefigure the future luminal axis (Fig 1E). This is followed by growth of the pre-existing lumena along the track towards the new lumen site and subsequent expansion to form a lumen of a uniform size. Lumen formation requires targeted exocytosis and plasma membrane remodeling. These processes are mediated by the Arf-like 3 small GTPase (Arl3) which associates with microtubules and vesicles [5, 6], and the COPI coatomer complex that controls vesicular transport [7].
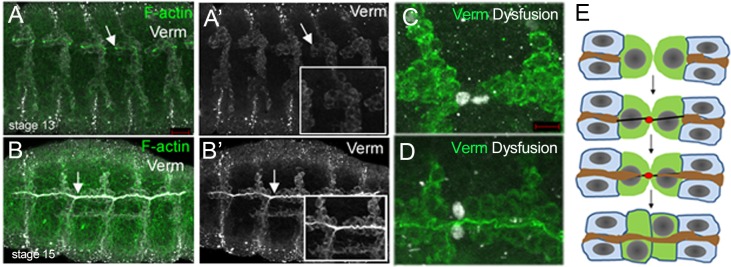
Fig 1. De novo lumen formation in the Drosophila trachea.
In wild-type embryos dorsal trunk (DT) branches are initially blind-ended tubes with a central lumen outlined by F-actin (A and A’, arrows). New lumena form between pre-existing lumena to generate an interconnected network of tubes (B and B’, arrows). DT fusion cells (C and D, white) mediate fusion of the lumen labelled with Vermiform (Verm; green). Schematic diagram of the steps in DT lumen formation (E): fusion cells of opposing DT branches (green) accumulate E-cadherin (red) at the site of contact and form an actin and microtubule cytoskeletal track that spans the fusion cells. Lumena of the DT branches (brown) grow along the track towards the site of contact and the actin/microtubule track matures into a new lumen that connects the pre-existing lumena. Insets in A’ and B’ indicate magnified views of regions in A and B marked by arrows. Embryos in A and B were stained for F-actin (green) and Verm (white). Embryos in C and D were stained for Dysfusion (Dys; white) to label fusion cells and Verm (green) to label the lumen. Scale bars represent 5 μm.
To identify genes required for tracheal development we previously performed a large scale chemical mutagenesis screen [8]. From this screen we identified a novel allele of the receptor type guanylyl cyclase at 76C (Gyc76C). Guanylyl cyclases (GCs) are a family of soluble and receptor-type enzymes that catalyze the conversion of GTP to cGMP in response to signals, such as nitric oxide (NO), peptide ligands and changes in intracellular calcium [9–12]. In Drosophila, Gyc76C regulates axon guidance by physically associating with the Semaphorin 1a receptor Plexin A [13, 14] whereas Gyc32E is involved in oogenesis and in egg chamber development [15]. Most of the effects of cGMP signaling are mediated by the activation of cGMP-dependent protein kinases (cGKs or PKGs) [9, 10, 16]. The two Drosophila cGMP-dependent kinases, PKG1/DG1 and PKG2/DG2 are encoded by the pkd21D and foraging (for) genes, respectively. for was recently shown to regulate the cytoplasmic-nuclear trafficking of the transcription factor Lola during Drosophila axon guidance [17]. In addition to a role in axon guidance, our previous studies showed that Gyc76C is required for salivary gland and muscle development in the Drosophila embryo. In gyc76C mutants, the salivary gland fails to migrate and the lumen is branched [18]. The accompanying defects in accumulation of the extracellular matrix (ECM) protein laminin and the integrin-adhesion receptor binding protein, talin suggest that the migration and lumen shape defects in gyc76C mutant glands may in part be due to defects in integrin-mediated adhesion to the ECM. We also showed that Gyc76C is required during muscle development for proper localization of integrins at sites of contact between the body wall muscles and tendon cells [19]. Consistent with our demonstration of a role for gyc76C and for in integrin-dependent adhesion, recent studies in the developing wing show that gyc76C and for regulate ECM-remodeling matrix metalloproteinases [20]. Although we reported gyc76C to be expressed in the developing trachea [19], it was previously not known what role gyc76C played in tracheal development. Here, we show that Gyc76C is required for de novo lumen formation in the dorsal trunk (DT) and terminal branches of the embryonic trachea, at least in part by controlling the intracellular localization of Arl3.
Materials and Methods
Drosophila Strains and Genetics
Canton-S flies were used as wild-type controls. gyc76C2388 was obtained by standard EMS mutagenesis as previously described [8]. gyc76Cex173 and UAS-gyc76CWT lines were obtained from A. Kolodkin (Johns Hopkins University School of Medicine, Baltimore, MD). UAS-gyc76CRNAi and UAS-pkg21D RNAi lines were obtained from S. Davies (University of Glasgow, United Kingdom). pkg21Df05504 was obtained from the Exelixis collection at Harvard Medical School and is described in Flybase (http://flybase.bio.indiana.edu/). UAS-mcd8GFP was obtained from the Bloomington Stock Center and is described in FlyBase. Arl3CG6678 was obtained from L. Jiang (Oakland University, Rochester, MI). For tracheal-specific expression of the UAS constructs, we used the breathless (btl)-GAL4 driver.
Immunocytochemistry
Embryo fixation and antibody staining were performed as previously described [21]. The following antisera were used at the indicated dilutions: rabbit Vermiform antiserum at 1:300 (a gift of S. Luschnig); rat Dysfusion antiserum at 1:200 (a gift of S. Crews); mouse 2A12 antiserum at 1:10 and rat E-cadherin antiserum at 1:20 (Developmental Studies Hybridoma Bank, DSHB; Iowa City, IA); guinea pig Arl3 antiserum at 1:200 (a gift of L. Jiang); mouse DSRF antiserum at 1:100 (Active Motif, Carlsbad, CA) and mouse β-galactosidase (β-gal) antiserum at 1:500 (Promega, Madison, WI). Appropriate biotinylated- (Jackson Immunoresearch Laboratories, Westgrove, PA), AlexaFluor 488-, 647- or Rhodamine- (Molecular Probes-Thermofisher Scientific, Waltham, MA) conjugated secondary antibodies were used at a dilution of 1:500. F-actin was detected with phalloidin (1:20; Invitrogen-Thermofisher Scientific) as previously described [22]. Stained embryos were mounted in Aqua Polymount (Polysciences, Inc., Warrington, PA) and thick (1 μm) fluorescence images were acquired on a Zeiss Axioplan microscope (Carl Zeiss) equipped with LSM 510 for laser scanning confocal microscopy at the Weill Cornell Medical College optical core facility (New York, NY).
Quantification of terminal cell lumen length
Terminal cell lumen length was measured from the center of the DSRF-stained nucleus to the tip of the 2A12-stained lumen using Image J software (National Institute of Health, Bethesda, MD). A minimum of 10 lumena were measured for each genotype. Statistical analysis was done using Microsoft Excel.
Results
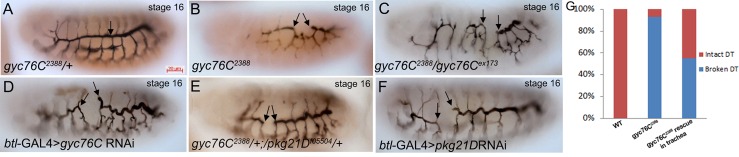
To determine gyc76C function in tracheal development we analyzed embryos mutant for gyc76C2388 [18] and gyc76Cex173[13]. In embryos heterozygous for a null allele of gyc76C, gyc76C2388, the DT lumen is a continuous structure (Fig 2A). By contrast, in gyc76C2388 homozygous embryos the lumen was disconnected at various points along the length of the DT (Fig 2B). Approximately 93% of gyc76C2388 homozygous embryos showed DT lumen defects compared to wild-type and heterozygous siblings that showed no defects (Fig 2G). Similarly, DT lumen defects were observed in embryos homozygous for gyc76Cex173, trans-heterozygous for gyc76ex173 and gyc76C2388 and embryos expressing gyc76C RNAi specifically in the trachea with the breathless (btl)-GAL4 driver (Fig 2C and 2D and data not shown). Similar to gyc76C mutant embryos, expression of RNAi to pkg21D, encoding the Drosophila cGMP-dependent protein kinase 1 (PKG1) resulted in a DT lumen defect (Fig 2F). Embryos trans-heterozygous for gyc76C2388 and a loss-of-function allele of PKG1, pkg21Df05504 also showed DT lumen defects, suggesting a strong genetic interaction between gyc76C2388 and pkg21D (Fig 2E).
Fig 2. DT lumen defects in gyc76C and pkg21D mutant embryos.
In gyc76C2388 heterozygous embryos (A) the DT lumen is continuous (A, arrow) whereas in homozygous siblings (B), gyc76C2388 gyc76Cex173 trans-heterozygous embryos (C), embryos expressing gyc76C RNAi in the trachea with btl-GAL4 (D), gyc76C2388 pkg21Df05504 trans-heterozygous embryos (E) and embryos expressing pkg21D RNAi in the trachea (F), the DT lumen is disconnected (B-F, arrows). Graph depicting percentage of intact (G, red) and broken (G, blue) DT lumena in wild-type embryos, gyc76C2388 homozygous embryos and gyc76C2388 homozygous embryos expressing wild-type gyc76C (gyc76CWT) in the trachea with btl-GAL4 (gyc76CWT rescue in trachea). All embryos shown were stained for 2A12 to mark the tracheal lumen (dark brown) and β-galactosidase (β-gal) (brown) to distinguish heterozygous from homozygous embryos.
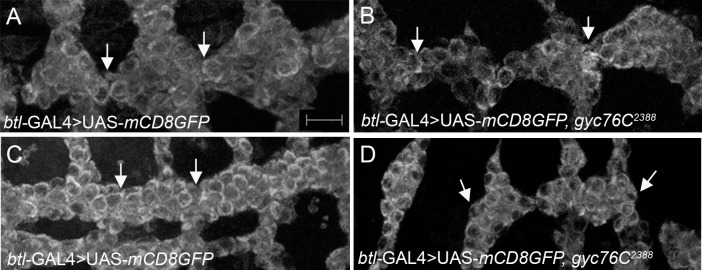
To test if the discontinuous tracheal lumen observed in gyc76C mutant embryos is due to a cell migration or a lumen fusion defect, we analyzed embryos expressing cytoplasmic mCD8-GFP specifically in the trachea of gyc76C2388 heterozygous and homozygous embryos. In gyc76C2388 heterozygous and homozygous embryos, DT cells of all embryos analyzed migrated normally to contact their counterparts in the neighboring tracheal metameres at stage 12 (Fig 3A and 3B). However, as embryogenesis progressed DT branches of gyc76C2388 homozygous embryos did not remain connected with many of them disconnected by stage 15, unlike their heterozygous siblings (Fig 3C and 3D). Thus, DT cells of gyc76C2388 mutant embryos migrated normally; however, because the lumena did not fuse, some branches remained separated and an interconnected tracheal network did not form.
Fig 3. gyc76C mutant DT cells migrate but do not remain connected.
DT cells of wild-type embryos expressing mCD8-GFP in the trachea with btl-GAL4 (A and C, arrows) migrate towards each other at stage 12 and remained connected by stage 15 (C). DT cells of gyc76C2388 homozygous embryos expressing mCD8GFP migrate towards each other at stage 12 (B, arrows) but do not remain connected to each other by stage 15 (D, arrows). All embryos shown were stained for GFP with those in B and D being also stained for β-gal (not shown). Scale bar in A represents 5 μm.
We previously showed that gyc76C mRNA is expressed in the trachea from the onset of primary branch migration until the end of embryogenesis [19]. To test if gyc76C is required cell-autonomously in the trachea we expressed wild-type gyc76C (gyc76CWT) in the trachea of gyc76C2388 homozygous embryos with btl-GAL4. Expression of gyc76CWT reduced the percentage of embryos with DT lumen fusion defects from 95% to 55% (Fig 2G). It is possible that lumen fusion defects persist because of an insufficient amount and/or temporal requirement of wild-type gyc76C expression. These data demonstrate that gyc76C acts in the trachea to regulate tracheal lumen formation.
Actin and E-cadherin maturation defects in gyc76C and pkg21D mutant trachea
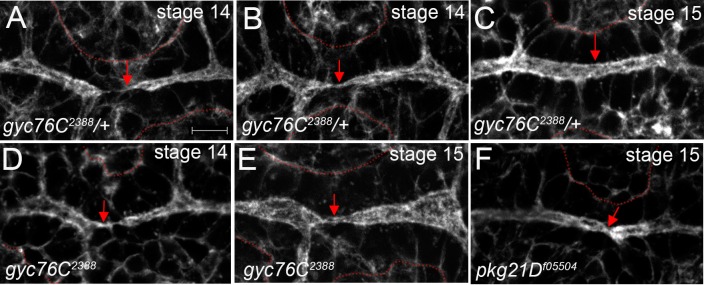
During DT lumen formation an actin-rich track assembled between the fusion cells of two adjacent DT branches in gyc76C2388 heterozygous embryos as in wild-type embryos (Fig 4A and data not shown). This is followed by growth of the pre-existing lumena of opposing DT branches towards the site of contact between the fusion cells (Fig 4B). The actin track then matured into a new lumen that connected the pre-existing lumena (Fig 4C). In gyc76C2388 homozygous embryos, the actin track was assembled between the fusion cells in the same temporal manner as in heterozygous siblings (Fig 4D). However, the actin track of gyc76C2388 homozygous embryos did not mature into a new lumen at the stage when in heterozygous siblings the new lumen had already expanded to the same diameter as the pre-existing lumena (Fig 4E). We observed a similar defect in pkg21Df05504 mutant embryos where the lumen remained constricted at the site of fusion (Fig 4F).
Fig 4. Actin track does not mature in gyc76C and pkg21D mutant trachea.
In DT branches of gyc76C2388 heterozygous embryos (A-C), an actin track forms between fusion cells of opposing branches (A, arrow) followed by the maturation of the track (B, arrow) into a new lumen that is continuous with and of equal diameter as the pre-existing lumena (C, arrow). In gyc76C2388 homozygous embryos the actin track forms (D, arrow) but does not mature into a new lumen (E, arrow). In pkg21Df05504 mutant embryos the site of lumen fusion is constricted (F, arrow). All embryos were labeled for F-actin with phalloidin and β-gal (not shown). Dotted red line outlines the DT cells. Scale bar represents 5 μm.
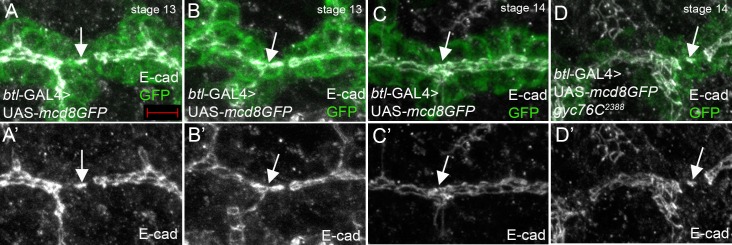
In DT cells of wild-type embryos expressing mCD8-GFP in the trachea, the cell-cell adhesion protein, E-cadherin (E-cad) accumulated at the site of contact between the fusion cells at the onset of lumen formation (Fig 5A). E-cad then expanded to become continuous between the fusion cells and the adjacent DT cells (Fig 5B and 5C). gyc76C2388 mutant DT cells accumulated E-cad at the site of contact between the fusion cells; however, E-cad did not expand and remained at the initial contact site (Fig 5D). These data together demonstrate that Gyc76C is not required for the initial formation of the actin track or the accumulation of E-cad at the site of new lumen formation but is required for the maturation and expansion of the actin track and E-cad.
Fig 5. E-cad contact site failed to expand in gyc76C mutant embryos.
In wild-type embryos expressing mcd8-GFP in the trachea with btl-GAL4 (A-C), E-cad initially accumulates as a patch at the site of contact between the two fusion cells (A and A’, arrows) and then becomes continuous with the pre-existing lumena (B, B’, C and C’, arrows). In gyc76C2388 homozygous embryos expressing tracheal mcd8-GFP (D) E-cad accumulates at the fusion cell contact site but does not expand to connect with the pre-existing lumena (D and D’, arrows). Embryos were stained for E-cad (white), GFP (green) and β-gal (not shown). Scale bar represents 5 μm.
Vermiform is not transported into the new lumen site in gyc76C mutant trachea
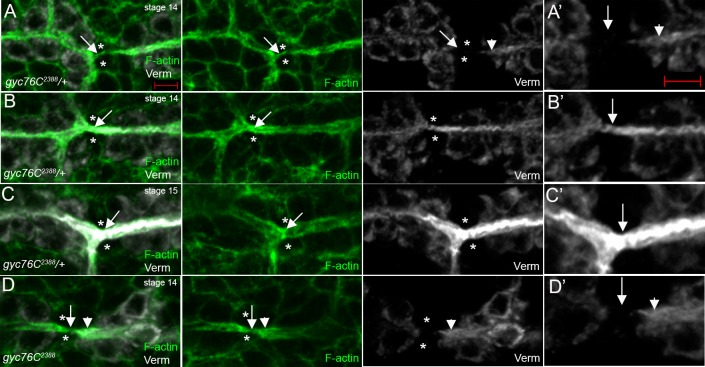
To test if gyc76C is required for membrane transport into the site of de novo lumen formation we analyzed the localization of Vermiform, a chitin-modifying enzyme [23]. In gyc76C2388 heterozygous embryos, as in wild-type embryos, Verm was initially found in the cytoplasm and pre-existing lumena of DT cells but not in the fusion cells (Fig 6A). As the actin track matured into a new lumen and the pre-existing lumena fused, Verm accumulated in the newly formed lumen but was absent from the cytoplasm of the fusion cells (Fig 6B). Even when the newly formed lumen expanded to the same diameter as the pre-existing lumena, Verm was not detected in the fusion cells (Fig 6C). These data show that luminal proteins such as Verm, that are not secreted by the fusion cells but instead by the neighboring DT cells are transported into the site of new lumen formation. In gyc76C2388 homozygous embryos, Verm was synthesized and secreted into the pre-existing lumena by the DT cells, albeit at a reduced level; however, Verm was not detected at the site where the new lumen should have formed (Fig 6D). Thus, gyc76C is required for delivery of the luminal protein Verm into the site of new lumen formation.
Fig 6. Verm is not transported into the lumen fusion site in gyc76C mutant trachea.
In gyc76C2388 heterozygous embryos (A-C), Verm (A and A’, white) is initially absent from the site of new lumen formation (A and A’, arrow) marked by the actin track (A, green) that forms between the fusion cells (A, asterisks). As the new lumen forms (B and B’), and expands to the same diameter as the pre-existing lumena (C and C’, arrow), Verm is now present in the new lumen (B’ and C’, white). In gyc76C2388 homozygous embryos (D), Verm (D and D’, white) is absent from the site of lumen fusion (D and D’, arrows) although it is present at reduced levels in the pre-exisiting lumena (D and D’, arrowheads). Panels A’-D’ are magnified views of the fusion sites indicated by arrows in A-D, respectively. All embryos were stained for F-actin with phalloidin (green), Verm (white) and β-gal (not shown). Embryos in A, B and D are at stage 14 whereas the embryo in C is at stage 15. Scale bars represent 5 μm.
Gyc76C genetically interacts with Arl3 and controls Arl3 localization in DT fusion cells
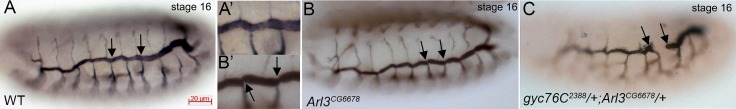
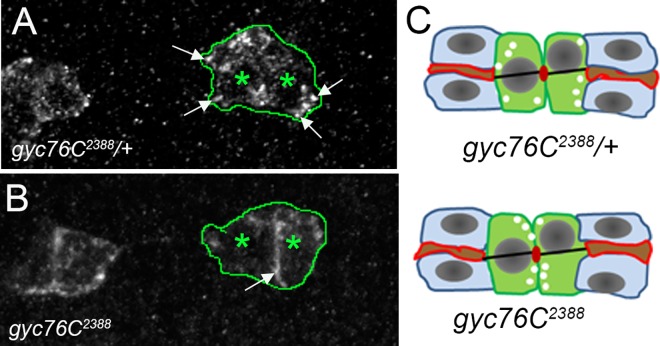
In embryos mutant for an Arf-like 3 GTPase (Arl3), DT lumena failed to fuse [5, 6]. This is thought to be due to a defect in vesicle-vesicle and vesicle-plasma membrane fusion. In embryos homozygous for a hypomorphic allele of Arl3, Arl3CG6678 [5] the DT lumen was continuous; however, the lumena were constricted at sites of fusion, unlike in wild-type embryos (Fig 7A and 7B). In embryos trans- heterozygous for gyc76C2388 and Arl3CG6678 DT lumena failed to fuse, like in gyc76C2388 mutant embryos (Fig 7C). In wild-type tracheal fusion cells endogenous Arl3 is found as cytoplasmic puncta and at the contact site between fusion cells [5, 6]. In gyc76C2388 heterozygous embryos, endogenous Arl3 was found as cytoplasmic puncta whereas in gyc76C2388 homozygous embryos, Arl3 was enriched at the contact sites between the fusion cells (Fig 8A–8C). Thus, loss of gyc76C altered the subcellular localization of Arl3 in DT fusion cells.
Fig 7. Arl3 genetically interacts with gyc76C to control DT lumen fusion.
In wild-type embryos (A) the DT lumen is continuous by stage 16 (A and A’, arrows). In embryos homozygous for Arl3CG6678 (B) the DT lumen is constricted at sites of fusion (B and B’, arrows) and in embryos trans-heterozygous for gyc76C2388 and Arl3CG6678 (C) DT lumena failed to fuse (C, arrow). All embryos shown are at stage 16 and were stained for 2A12 and β-gal (not shown). Panels A’ and B’ are magnified views of regions in A and B marked by arrows.
Fig 8. Arl3 is mislocalized in gyc76C2388 mutant fusion cells.
In gyc76C2388 heterozygous embryos (A), Arl3 is found in a punctate pattern in the cytoplasm of the fusion cells (A, arrows). In gyc76C2388 homozygous embryos (B), Arl3 is predominantly found at contact sites between the two adjacent fusion cells (B, arrow). Diagram depicting mislocalization of Arl3-positive puncta in gyc76C2388 heterozygous embryos compared to homozygous siblings (C). Red outlines in C indicate E-cadherin-mediated cell-cell contact sites, the actin and microtubule track in black and fusion cells in green. Asterisks in A and B indicate fusion cells. Embryos in A and B were stained for Arl3 (white) and β-gal (not shown).
Gyc76C is required for de novo lumen formation in terminal cells
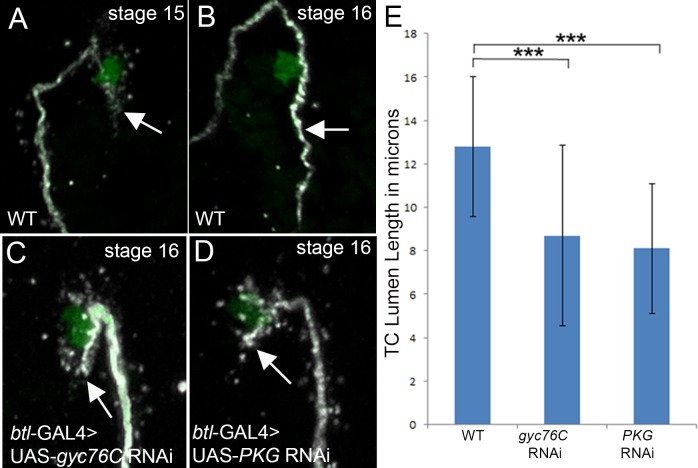
To test if gyc76C is required for de novo lumen formation in other tracheal branches we analyzed lumen formation in the terminal cells (TCs) which form a seamless intracellular lumen. Unlike the DT where new lumena form and connect with pre-existing lumena, TC lumen formation involves the inward growth of an intracellular lumen [24, 25]. We analyzed the dorsal terminal branches of wild-type embryos and embryos expressing RNAi to gyc76C or pkdg21D specifically in the trachea with the btl-GAL4 driver. In wild-type embryos the TC lumen elongated between stages 15 and 16 (Fig 9A and 9B). By contrast, in TCs where gyc76C or pkg21D have been knocked down with RNAi, the lumen was not elongated and an increased number of punctate structures were observed in the cytoplasm of the TCs (Fig 9C and 9D). Quantification of the TC lumen length showed significant reduction in gyc76C RNAi- and pkdg21D RNAi-expressing trachea compared to WT (Fig 9E). Thus, gyc76C and pkg21D are required for de novo lumen formation in the terminal branches.
Fig 9. Gyc76C and PKG1 are required for terminal cell lumen elongation.
In terminal cells of wild-type embryos (A and B) the TC lumen begins to form at stage 15 (A) and is elongated by stage 16 (B). In embryos expressing gyc76C RNAi (C) or PKG1 RNAi (D) specifically in the trachea with btl-GAL4, TC lumena do not elongate (C and D, arrows). Quantification of TC lumen length shows that the lumena of trachea expressing gyc76C RNAi or PKG1 RNAi are significantly shorter than those of wild-type trachea (E). Embryos in A-D were stained for DSRF (green) to label TC nuclei and 2A12 (white) to label the lumena. *** = p<0.01.
Discussion
We demonstrate in this study that Gyc76C and its downsteam effector PKG1 are required for de novo lumen formation in the Drosophila embryonic trachea. During DT lumen fusion, the actin track and the initial site of E-cad-mediated contact between fusion cells formed normally; however, a new lumen did not form and the pre-existing lumena of adjacent DT branches did not fuse. The presence of the secreted protein Verm in the DT cells and lumena but not at the lumen fusion site suggests that the gyc76C lumen defect is due at least in part to defects in the transport and/or fusion of vesicles at the lumen fusion site. Gyc76C may control vesicle transport and/or fusion through Arl3 GTPase which is known to be required for vesicle-vesicle and vesicle-plasma membrane fusion [5, 6]. The altered localization of Arl3 in gyc76C mutant fusion cells may prevent proper routing, delivery or fusion of vesicles necessary for the formation of a new lumen. Arl3 is known to regulate the localization of the exocyst subunit Sec5 at contact points between fusion cells and along the actin track, and has been shown to interact with microtubules [5, 6]. Thus, Gyc76C-dependent regulation of Arl3 localization in tracheal fusion cells maybe important for exocyst-mediated vesicle transport along actin and/or microtubule tracks and membrane fusion.
Our finding that gyc76C and pkg21D regulate de novo lumen formation in both the DT and TCs suggests a common mechanism for de novo lumen formation in different branches of the Drosophila trachea. Despite the distinct morphology of the DT lumen and the TC lumen, there are conserved features of lumen formation between the two tubular structures. For example, similar to the actin and microtubule tracks that form during DT lumen fusion, actin and microtubules are organized along the elongating lumen of the TC [25, 26]. Moreover, the exocyst complex is required for membrane trafficking events during lumen formation in both the DT and the TC [5, 6, 27]. Thus, gyc76C and pkg21D likely regulate de novo lumen formation in both the DT and TCs through a common mechanism.
We previously reported that gyc76C is required for proper localization of the βPS integrin subunit at the myotendinous junctions of developing somatic muscle, and for laminin localization around the migrating salivary gland [18, 19]. Since newly synthesized integrin and laminin proteins are transported through the endomembranous system, our data on Gyc76C function in the developing trachea, somatic muscle and salivary gland indicate a conserved role in vesicle transport and/or fusion. This is consistent with studies in mammalian cells where cGMP dependent protein kinases are shown to regulate a number of different membrane trafficking events, such as phagocytosis [28] and synaptic vesicle trafficking [29–31]. Moreover, loss of cGKII, the mammalian homolog of PKG2, results in intestinal secretory defects (Pfeifer et al., 1996). Interestingly, Arf and Arf-like GTPases, like guanylyl cyclases and cGMP dependent protein kinases, are requried for exocytosis in neuronal cells [32, 33]. Thus, cGMP signaling through Arl GTPases may be a conserved mechanism for regulating membrane transport in a number of distinct cell types.
Acknowledgments
We are grateful to Stefan Luschnig, Lan Jiang, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for providing fly lines and antisera. We also thank the Weill Cornell Medical College optical core facility.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by NIH grant GM082996 to M.M.M. There was no additional external funding received for this study.
References
- 1.Baer MM, Chanut-Delalande H, Affolter M. Cellular and Molecular Mechanisms Underlying the Formation of Biological Tubes. Current Topics in Developmental Biology. 2009;89:137–62. 10.1016/S0070-2153(09)89006-6 [DOI] [PubMed] [Google Scholar]
- 2.Schottenfeld J, Song Y, Ghabrial A. Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol. 2010;22:633–9. 10.1016/j.ceb.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caviglia S, Luschnig S. Tube fusion: Making connections in branched tubular networks. Seminars in Cell & Developmental Biology. 2014;31:82–90. [DOI] [PubMed] [Google Scholar]
- 4.Sigurbjornsdottir S, Mathew R, Leptin M. Molecular mechanisms of de novo lumen formation. Nature Reviews. 2014;15:665–76. 10.1038/nrm3871 [DOI] [PubMed] [Google Scholar]
- 5.Jiang L, Rogers SL, Crews ST. The Drosophila dead end Arf-like 3 GTPase controls vesicle trafficking during tracheal fusion cell morphogenesis. Dev Biol. 2007;311:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakihara K, Shinmyozu K, Kato K, Wada H, Hayashi S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 GTPase in Drosophila. Mechanisms of Development. 2008;125:325–36. [DOI] [PubMed] [Google Scholar]
- 7.Grieder NC, Caussinus E, Parker DS, Cadigan K, Affolter M. γCOP is required for apical secretion and epithelial morphogenesis in Drosophila melanogaster. PLoS One. 2008;3:e3241 10.1371/journal.pone.0003241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myat M, Lightfoot H, Wang P, Andrew D. A molecular link between FGF and Dpp signaling in branch-specific migration of the Drosophila trachea. Developmental Biology. 2005;281:38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies SA. Signalling via cGMP: Lessons from Drosophila. Cellular Signalling. 2006;18:409–21. [DOI] [PubMed] [Google Scholar]
- 10.Lucas K, Pitari G, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacological Reviews. 2000;52:375–413. [PubMed] [Google Scholar]
- 11.Morton D. Invertebrates yield a plethora of atypical guanylyl cyclases. Mol Neurobiol. 2004;2:97–116. [DOI] [PubMed] [Google Scholar]
- 12.Overend G, Cabrero P, Guo A, Sebastian S, Cundall M, Armstrong H, et al. The receptor guanylate cyclase Gyc76C and a peptide ligand, NPLP1-VQQ, modulate the innate immune IMD pathway in respose to salt stress. Peptides. 2011;34:209–18. Epub Aug 27. 10.1016/j.peptides.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 13.Ayoob J, Yu H, Terman J, Kolodkin A. The Drosophila receptor Guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24:6639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chak K, Kolodkin A. Function of the Drosophila receptor guanylyl cylcase Gyc76C in PlexA-mediated motor axon guidance. Development. 2014;141:136–47. 10.1242/dev.095968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gigliotti S, Cavaliere V, Manzi A, Tino A, Graziani F, Malva C. A membrane guanylate cyclase Drosophila homolog gene exhibits maternal and zygotic expression. Dev Biol. 1993;159:450–61. [DOI] [PubMed] [Google Scholar]
- 16.Kalderon D, Rubin G. cGMP-dependent protein kinase genes in Drosophila. Journal of Biological Chemistry. 1989;264:10738–48. [PubMed] [Google Scholar]
- 17.Peng Q, Wang Y, Li M, Yuan D, Xu M, Li C, et al. cGMP-dependent protein kinase encoded by foraging regulates motor axon guidance in Drosophila by suppressing Lola function. Journal of Neuroscience. 2016;36:4635–46. 10.1523/JNEUROSCI.3726-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel U, Myat M. Receptor guanylyl cyclase Gyc76C is required for invagination, collective migration and lumen shape in the Drosophila embryonic salivary glan. Biology Open. 2013;000:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel U, Davies S, Myat MM. Receptor-type guanylyl cyclase Gyc76C is required for development of the Drosophila embryonic somatic muscle. Biology Open. 2012;1:507–15. 10.1242/bio.2012943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleede J, Blair S. The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of Drosophila Melanogaster. PLoS Genet. 2015;11:e1005576 10.1371/journal.pgen.1005576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu N, Bagumian G, Galiano M, Myat MM. Rho GTPase controls Drosophila salivary gland lumen size through regulation of the actin cytoskeleton and Moesin. Development. 2011;138:5415–27. 10.1242/dev.069831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirraglia C, Walters J, Ahn N, Myat M. Rac1 GTPase acts downstream of αPS1βPS integrin to control collective migration and lumen size in the Drosophila salivary gland. Dev Biol. 2013;377:21–32. 10.1016/j.ydbio.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermehren-Schmaedick A, Ainsley J, Johnson W, Davies S, Morton D. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–96. 10.1534/genetics.110.118166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samakovlis C, Manning G, Steneberg P, Hacohen N, Cantera R, Krasnow M. Genetic control of epithelial tube fusion during Drosophila tracheal development. Development. 1996;122:3531–6. [DOI] [PubMed] [Google Scholar]
- 25.Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Current Biology. 2010;20:359–66. 10.1016/j.cub.2009.12.043 [DOI] [PubMed] [Google Scholar]
- 26.Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, et al. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Current Biology. 2006;16:1531–7. [DOI] [PubMed] [Google Scholar]
- 27.Jones A, Nikolova L, Schjelderup A, Metzstein M. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Developmental Biology. 2014;390:41–50. 10.1016/j.ydbio.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao T, You H, Li C, Chang J, Chang S, Chen C. Cyclic GMP-dependent protein kinase II is necessary for macrophage M1 polarization and phagocytosis via toll-like receptor 2. J Mol Med. 2015;93:523–33. 10.1007/s00109-014-1236-0 [DOI] [PubMed] [Google Scholar]
- 29.Petrov A, Giniatullin A, Sitkikova G, Zefirov A. The Role of cGMP-Dependent Signaling Pathway in Synaptic Vesicle Cycle at the Frog Motor Nerve Termini. The Journal of Neuroscience. 2008;28:13216–22. 10.1523/JNEUROSCI.2947-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eguchi K, Nakanishi S, Takagi H, Taoufiq Z, Takahashi T. Maturation of a PKG-dependent retrograde mechanism for exoendocytic coupling of synaptic vesicles. Neuron. 2012;74:517–29. 10.1016/j.neuron.2012.03.028 [DOI] [PubMed] [Google Scholar]
- 31.Micheva K, Buchanan J, Holz R, Smith S. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nature Neuroscience. 2003;6:925–32. [DOI] [PubMed] [Google Scholar]
- 32.Klassen M, Wu Y, Maeder C, Nakae I, Cueva J, Lehrman E, et al. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron. 2010;66:710–23. 10.1016/j.neuron.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bader M, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S. Coupling actin and membrane dynamcs during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742:37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.