Abstract
Background:
Exercise is often used in the treatment of chronic neck and shoulder muscle pain. It is likely that psychological aspects have an impact on the results of exercise-based treatments.
Objectives:
(1) To examine the associations between psychological factors and the effect of a home-based physical exercise intervention. (2) To examine differences in psychological factors at baseline between (a) subjects who continued in the trial and those who did not and (b) subjects who completed the intervention and those who did not.
Method:
A total of 57 women with chronic neck and shoulder pain were included in a home-based exercise intervention trial. Pain intensity, disability, and psychological factors (anxiety and depression symptoms, catastrophizing, fear-avoidance beliefs, self-efficacy, and pain acceptance) were measured at baseline, after 4–6 months, and after 1 year of exercise. Associations between the psychological factors and changes in pain intensity and disability were analysed, as well as differences in psychological factors at baseline between subjects who continued in and completed the intervention, and those who did not.
Results:
Associations between positive changes in pain intensity and disability were found for low fear-avoidance beliefs and low-pain self-efficacy at baseline. In addition, fear-avoidance beliefs at baseline were higher in the subjects who dropped out of the intervention than in those who continued. Pain acceptance at baseline was higher in the subjects who completed the intervention at the end of the trial.
Conclusion:
Particularly, fear-avoidance beliefs and pain self-efficacy should be taken into consideration when implementing home-based physical exercise as treatment for chronic neck pain. In addition, high pain acceptance might improve the adherence to prescribed exercise.
Keywords: Exercise, neck pain, psychological factors
Background
In western countries, the prevalence of chronic pain is approximately 20%.1,2 Chronic pain entails major consequences such as physical and psychological disability, decreased quality of life, and a significant socioeconomic burden for society.1–3 Chronic neck and shoulder pain share similar characteristics and consequences as other chronic pain conditions, for example, low back pain and fibromyalgia syndrome. In the general population, the 1-year prevalence of neck pain is approximately 25%.3,4
In the complex chronic pain, neurobiological factors interact with psychological and social factors.2,5,6 Several psychological factors have been linked to the perception of and adjustment to chronic pain. That is, psychological factors are both included in pain perception and give rise to secondary effects that may adversely affect pain perception. Hence, anxiety and depression are negative emotions that may interfere with pain symptoms and increase the severity and complexity of the pain condition.7–10 Catastrophizing related to pain is defined as the mental set during a present or anticipated pain experience that magnifies the severity and impact of the pain. Pain catastrophizing is related to increased pain and disability.8,11 In addition, pain catastrophizing is a central part of the fear-avoidance theory.12–14 Fear avoidance is defined as pain-related fear and anxiety that leads to avoidance of activities, expectations about increased pain, and more self-reported disability.12–14 Self-efficacy is defined as an individual’s own beliefs about the ability to perform tasks and activities, even in the presence of difficulties and adversities.15 General self-efficacy refers to an overall perception of a personal ability to effectively handle a broad range of stressful situations,16 whereas pain self-efficacy is a domain-specific self-efficacy related to performing activities and tasks despite pain.17 The ability to perform desired activities in the presence of pain has also been linked with the concept of acceptance. Acceptance is about not struggling with eliminating pain, but about performing activities that are in line with one’s own goals of daily life. Thus, the purpose of the individuals’ actions is not to decrease pain but to perform activities they value.18,19
Physical exercise has in several systematic reviews been found to be beneficial for chronic neck pain.20–23 However, physical exercise as treatment requires the performance of planned actions in the presence of pain, which links psychological and behavioural factors to the physical exercise treatment. This link is likely to be crucial for the outcome of physical exercise used as treatment.24 Although the optimal dose of exercise is not found yet,23 a clear dose–response relationship between amount of exercise and a decrease in pain intensity has been reported,25 which points at the importance of adherence to prescribed dose of physical exercise. Adherence to prescribed exercise is an essential behavioural factor26,27 related to personal aspects such as having the time for exercise and socioeconomic factors.28,29 In addition, the special characteristics of the physical exercise intervention matters are supervised exercise and self-management techniques.26,30 However, little is known about how psychological factors influence the effects of and adherence to prescribed exercise in chronic pain patients.26
A highly effective management of chronic pain is still not available. The results of the currently best possible chronic pain treatments, such as pharmacological treatment,31,32 multimodal rehabilitation,33 physical exercise,20,34,35 and psychological treatment (primarily cognitive behavioural therapy (CBT)),24,36 have in general been weak to moderate. Thus, optimizing treatment strategies for an effective management of chronic pain are needed. In this respect, more conclusive knowledge on the impact of psychological factors on the outcome of and adherence to physical exercise interventions in chronic pain patients is desirable.
The hypotheses of this study were as follows: (1) There are associations between psychological factors and the effects of an exercise intervention on pain intensity and disability in women with chronic neck and shoulder pain. (2) There are differences in psychological factors that have an impact on adherence to exercise as treatment between (a) subjects who remained in, and those subjects who discontinued the trial before end of the intervention and (b) subjects who adhered to recommended physical exercise, and those subjects who did not.
Objectives
This study has two objectives: (1) to examine associations between psychological variables and the effects on pain intensity and function after 4–6 months and after 1 year of a physical exercise intervention in women with chronic neck and shoulder pain and (2) to examine pain intensity, disability, and psychological variables at baseline (BL) between (a) the subjects who remained in and those who discontinued the trial and (b) between the subjects who adhered to recommended physical exercise intervention and those who did not.
Methods
Study design and setting
This study is an exploratory sub-analysis of data which was collected within a clinical trial that evaluated two different home-based exercise programmes, performed by two groups of subjects.37 That clinical trial focused on pain and function, was registered in ClinicalTrials.gov Id: NCT01876680, and has been published earlier.37 The study was performed at the Pain and Rehabilitation Centre at Linköping University Hospital in Östergötland County, Sweden. The study protocol was approved by the regional ethical committee of Linkoping University, diary number M10-80.
Participants
A total of 57 women from the general population were recruited via advertisements in local newspapers, during a time period of 6 months. Inclusion criteria were being female, aged 20–60 years, and constantly or frequently occurring pain in the neck and shoulder muscles for more than 6 months. In addition, the participants had to exhibit clinically verified symptoms of muscular fatigue or stiffness in the neck, pain spreading from the neck to the back of the head, tightness of neck and shoulder muscles, or tender spots in the muscles38 with a pain intensity of at least 3 on a Numeric Rating Scale (NRS) ranging from 0 to 1039 and/or a decrease in neck function scored as at least a mild degree measured by a questionnaire that addressed function limitations due to neck pain.40,41 Exclusion criteria were widespread pain, major trauma in medical history, clinical signs of cervical radiculopathy, pregnancy, inflammatory and hormonal disorders, neurological causes of the pain, and tendonitis in upper extremities. Thus, women with non-specific neck and shoulder muscle pain, without clinical signs of cervical radiculopathy, tendinitis, or pain due to trauma, were included. After answering the advertisement via telephone or e-mail, interested participants were telephoned to initially check the inclusion and exclusion criteria and to be informed about the trial. Next, eligible participants were sent questionnaires via mail about history of pain, present pain, and function due to the neck pain. Finally, a standardized clinical examination was performed of the neck and upper extremities.42 A flowchart of the inclusion process and details about questionnaires used has been presented previously.37 All subjects signed informed consent before entering the trial.
Intervention
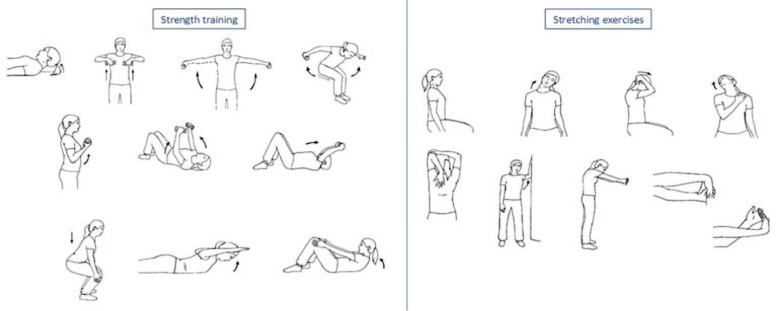
The physical exercise intervention has previously been described in detail.37 In brief, the subjects were initially allocated to two home-based exercise groups for the training of neck and shoulders: one focused on strength and one focused on stretching exercises. The strength training was performed with dumbbells for the upper extremities. For the first 8 weeks of the intervention, the dose of exercise was 2 kg, and three sets of 20 repetitions each. During the remaining intervention period, the dose was an individualized weight adjusted to allow a maximum of 10 repetitions, three sets during 3 weeks, altering with 1 week on the initial dose of exercise. The dose of leg and core muscle training was 20 repetitions, three sets across the whole intervention period. The dose of the stretching exercises was holding each position during 30 s, two repetitions. See Figure 1 for more information. Both groups were expected to perform the exercises at home, three times a week. In addition, both groups were encouraged to perform an optional aerobic activity three times a week and all participants tracked their performed physical exercise in an exercise diary. All participants also received regular support from a physiotherapist about the physical exercise via e-mail and phone every 4–8 weeks, more frequently at the beginning of the trial. The length of the intervention was 1 year with follow-up measurements after 4–6 months. However, at the end of the intervention period, no differences were found in pain intensity and function between the two intervention groups, and not either in numbers of completers and non-completers.37 Thus, the two intervention groups were merged in the present analysis. There was no control group, that is, a group with neck and shoulder pain without intervention in this trial.
Figure 1.
The exercise intervention consisted of home-based exercise, three times a week, during 1 year. Focus was on either strength training or stretching exercises. The strengthening exercises addressed neck flexion muscles, muscles in the upper extremities (the trapezius, deltoids, rhomboids, pectoralis, latissimus, and biceps), core muscles (back and abdominals), and the leg muscles (the hamstrings, quadriceps, and gluteal muscles). The stretching exercises addressed muscles in the neck, shoulders, and upper extremities (the trapezius, neck extensors, neck flexors, pectoralis, triceps, rhomboids, wrist extensors, and wrist flexors).
Subgroups
In this study, two subgroups were defined: (1) subjects who discontinued their participation in the trial were classified as drop-outs (these subjects stated explicitly that they, for various reasons, did not want to continue in the trial) and (2) the subjects who remained in the trial were classified as completers or non-completers depending on their adherence to the physical exercise intervention. Based on earlier studies on dose–response results,25,43 a completer was defined as a participant who performed the physical exercise at least 1.5 times a week for eight continuous weeks just before outcome measurements.
Outcomes
Outcome measurements were conducted using valid and reliable self-rating scales at BL, at follow-up after 4–6 months of physical exercise (FU4–6), and at the end of the trial after 1 year of physical exercise (END12).
Pain intensity was measured with a written 11 grade NRS39 (0–10). Zero indicated no pain at all, and 10 indicated worst pain possible.
Disability was measured with the Swedish version of the Neck Disability Index (NDI),40,41 which examines the disability due to neck pain. This index consists of 10 items reflecting daily life activities that can be affected by neck pain. The scores range from 0 (no limitations) to 5 (major limitations). The scores are added to create a total sum reflecting the degree of disability: 0–4 = none; 5–14 = mild; 15–24 = moderate; 25–34 = severe; and over 34 = complete.41 For the NDI, a Cronbach’s alpha ranging between 0.74 and 0.93 has been reported.41 Internal consistency for the NDI in this study was considered and Cronbach’s alpha was calculated for BL, FU4–6, and END12, as 0.78, 0.83, and 0.76, respectively.
Anxiety and depression symptoms were measured using the Hospital Anxiety and Depression Scale (HADS).44,45 This scale consists of 14 items covering two subscales, one for anxiety and one for depression. The HADS detects anxiety and depressive symptoms in a general medical setting. A higher score represents a higher symptom severity. The HADS makes use of two cut-off scores for each subscale; 8 or more indicates the possible existence of a disorder and 11 or more indicates the probable existence of a disorder.46 Theoretical range for each subscale is 0–21. In this study, the two subscales are used separately in the analyses. Cronbach’s alpha has in a review been reported as 0.83 (mean) for HADS-anxiety and 0.82 (mean) for HADS-depression.44 Internal consistency for the two subscales for anxiety and depression in this study was considered and Cronbach’s alpha was calculated for anxiety measured at BL, FU4–6, and END12, as 0.77, 0.82, and 0.72, respectively, and for depression measured at BL, FU4–6, and END12, as 0.62, 0.80, and 0.81, respectively.
Pain catastrophizing was measured using the Pain Catastrophizing Scale (PCS).47,48 This scale is widely used and assesses catastrophizing in clinical and non-clinical settings. The scale consists of 13 items, including three aspects of catastrophizing – rumination, magnification, and helplessness. Each score is rated from 0 (not at all) to 4 (all the time). The possible range is 0–52 point, with lower scores indicating less catastrophizing. The total score is used in the analyses in this study. Cronbach’s alpha in an outpatient pain setting has earlier been reported to be 0.92 for the total scale.47 Internal consistency for the PCS in this study was considered and Cronbach’s alpha was calculated for the total score at BL, FU4–6, and END12, as 0.85, 0.91, and 0.91, respectively.
Fear-avoidance beliefs were measured using the Fear-Avoidance Beliefs Questionnaire (FABQ).49 The FABQ assesses fear-avoidance beliefs about the pain related to two subscales – physical activity and work. The questionnaire consists of in total 16 items about physical activity and work that may cause pain. Each item is scored from 0 (not agree at all) to 6 (completely agree). Total score can range between 0 and 66, with lower scores representing lower levels of fear avoidance. Cronbach’s alpha can be considered moderate to high, for example, 0.88 for FABQ – work, and 0.77 for FABQ – physical activity.49,50 The total score is used in the analyses in this study. Internal consistency for the FABQ in this study was considered and Cronbach’s alpha was calculated for the total score at BL, FU4–6, and END12, as 0.85, 0.87, and 0.83, respectively.
General self-efficacy was measured using the General Self-Efficacy Scale (GSES).16,51 The GSES assesses people’s beliefs in their own ability to handle novel or difficult situations in general and to cope with adversities. The scale consists of 10 items, each item is scored from 1 (not at all true) to 4 (exactly true), range 10–40, with higher scores showing more self-efficacy. Cronbach’s alpha 0.92 has recently been reported for the Swedish version.52 Internal consistency for the GSES in this study was considered and Cronbach’s alpha was calculated for at BL, FU4–6, and END12, as 0.46, 0.91, and 0.93, respectively.
Pain self-efficacy was measured using the Pain Self-Efficacy Questionnaire (PSEQ).17 The PSEQ assesses people’s confidence in performing a particular behaviour irrespective of their pain. The questionnaire consists of 10 items that reflect a wide variety of tasks that can be affected by pain. Each item is scored from 0 (not at all confident) to 6 (completely confident) and the total score ranges between 0 and 60, with higher score showing more pain self-efficacy. Cronbach’s alpha has earlier been calculated as 0.92.17 A translation of the English version of the PSEQ, from English to Swedish and back again, was performed by authorized translators on account of this study. Internal consistency for the PSEQ in this study was considered and Cronbach’s alpha was calculated at BL, FU4–6, and END12, as 0.92, 0.93, and 0.94, respectively.
Pain acceptance was measured using the Chronic Pain Acceptance Questionnaire (CPAQ).53–55 The CPAQ assesses acceptance of chronic pain focusing on behavioural aspects of acceptance. The questionnaire consists of two subscales – pain willingness and engagement in activities. The CPAQ includes 20 items, each scored from 0 (never true) to 6 (always true). Possible range is 0–120, with higher scores indicating more pain acceptance. The total score is used in the analyses in this study. Cronbach’s alpha has been reported for the Swedish version as 0.91 for the total score.53 Internal consistency for the CPAQ in this study was considered and Cronbach’s alpha was calculated for the total score at BL, FU4–6, and END12, as 0.81, 0.85, and 0.86, respectively.
Statistics
We analysed descriptive data, differences between different time points and between the subgroups, correlations, and linear regressions using the statistical package IBM SPSS Statistics (version 22.0; IBM Corporation, New York, USA). When the data were ordinal and not normally distributed, non-parametric analyses were chosen and descriptive data are presented with median values and interquartile ranges (25th–75th percentiles). Differences between different time points were analysed using Wilcoxon signed-rank test. Spearman’s rho correlation analysis was used to examine the covariation between the psychological variables and pain intensity and function. Linear regression analyses were used to examine psychological influences on function. Differences in the subgroups were analysed using Mann–Whitney U test. For all statistical analyses, a probability of <0.05 (two-tailed) was set as criteria for statistical significance. Missing data in the questionnaires were not replaced with any value, so subjects with missing data are not included in the statistical analyses.
Multivariate data analysis
Multiple linear regression assumes that the regressor (X) variables are independent (i.e. multi-collinearity is not present). It was obvious that several variables were correlated in this study. To handle this correlation, partial least squares regression (PLSR) was employed using SIMCA-P+ for the multivariate regression analyses.56 Changes in disability were regressed at FU and at END using the BL variables under investigation. The importance of the variables is measured as a variable influence on projection (VIP) value. This value indicates the relevance of each X variable pooled over all dimensions and Y variables – the group of variables that best explains Y. VIP 1.0 was considered significant. Coefficients (partial least squared scaled and centred regression coefficients) were used to note the direction of the relationship (positive or negative). If necessary, the variables were transformed. R2 describes the goodness of fit – the fraction of sum of squares of all the variables explained by a principal component.57 Q2 describes the goodness of prediction – the fraction of the total variation of the variables that can be predicted by a principal component using cross-validation methods.
Prior to PLSR, principal components analysis (PCA) was applied. PCA extracts and displays systematic variation in a data matrix. Variables loading on the same component are correlated and variables with high loadings but with different signs are negatively correlated. Significant variables with high loadings (positive or negative) are more important for the component under consideration than variables with lower absolute loadings.57 The obtained components are per definition not correlated and are arranged in decreasing order with respect to explained variation. The purpose of applying PCA in this study was to identify multivariate outliers using the two powerful methods available in SIMCA-P+: (1) score plots in combination with Hotelling’s T2 (identifies strong outliers) and (2) distance to model in X-space (identifies moderate outliers). No multivariate outliers were identified in this study.
Results
Descriptive data
The median age of the 57 subjects was 43 years (standard deviation (SD): 8.5 years). They had experienced pain for a median of 8.5 years (25th–75th percentiles; 5–14 years). Of these 57 subjects, 45 remained in the trial until the FU4–6 and 41 remained until the END12. At FU4–6, 37 subjects were defined as completers and 8 as non-completers. At END12, 22 subjects were defined as completers and 19 as non-completers.
Pain intensity, function, and psychological variables at BL, FU4–6, and at the END12
Pain intensity ratings in the neck and shoulders decreased significantly between BL and END12 (Table 1). Disability decreased significantly between BL and FU4–6 as well as between BL and END12. General self-efficacy, pain self-efficacy, and pain acceptance increased significantly and fear avoidance decreased significantly between BL and FU4–6 as well as between BL and END12. Furthermore, anxiety symptoms, depression symptoms, and pain catastrophizing decreased significantly between BL and END12. In fact, all variables showed improvements at END12 (Table 1).
Table 1.
Descriptive data for the pain intensity ratings, disability scores, and psychological factors at baseline (BL), follow-up after 4–6 months (FU4–6), and end of the trial after 1 year (END12).
| BL values Median (25th, 75th percentiles), n |
FU4–6 values Median (25th, 75th percentiles), n |
p value (BL–FU4–6) | Changes BL–FU4–6
Median (25th, 75th percentiles), n |
END12 values Median (25th, 75th percentiles), n |
p value (BL–END12) | Changes BL–END12
Median (25th, 75th percentiles), n |
|
|---|---|---|---|---|---|---|---|
| Pain in the neck | 5 (4, 7), 56 | 5 (2, 7), 44 | 0.13 | 1 (−1, 2), 43 | 3 (2, 7), 39 | 0.003* | 2 (0, 4), 38 |
| Pain in the shoulders | 4 (3, 6), 56 | 4 (0, 7), 44 | 0.15 | 1 (−1, 3), 43 | 3 (0, 6), 39 | 0.016* | 1 (0, 2), 38 |
| Disability | 13 (10, 18), 55 | 11 (7, 15), 43 | 0.021* | 2 (−1, 4), 42 | 10 (4, 12), 37 | <0.001* | 4 (1, 9), 36 |
| Anxiety | 5 (3, 8), 55 | 5 (3, 9), 44 | 0.85 | 0 (−1, 2), 42 | 4 (2, 6), 38 | 0.027* | 2 (−1, 3), 36 |
| Depression | 4 (1, 6), 56 | 3 (1, 7), 45 | 0.21 | 0 (−1, 2), 44 | 2 (1, 5), 39 | 0.007* | 1 (−1, 2), 38 |
| Pain catastrophizing | 12 (7, 20), 54 | 8 (6, 16), 45 | 0.16 | 0 (−2, 5), 42 | 11 (5, 17), 39 | 0.014* | 3 (−2, 6), 36 |
| Fear avoidance | 21 (15, 28), 56 | 15 (8, 26), 42 | 0.010* | 2 (−1, 10), 42 | 13 (8, 21), 37 | <0.001* | 4 (0, 10), 36 |
| General self-efficacy | 31 (28, 33), 55 | 31 (29, 35), 45 | 0.022* | 1 (−1, 4), 43 | 33 (28, 36), 38 | 0.009* | 2 (−1, 5), 36 |
| Pain self-efficacy | 49 (42, 55) 56 | 51 (47, 58), 44 | 0.005* | 3 (0, 6), 43 | 53 (46, 59), 38 | 0.011* | 4 (−1, 8), 37 |
| Pain acceptance | 77 (67, 87), 52 | 85 (77, 94), 43 | <0.001* | 7 (3, 16), 39 | 88 (76, 97), 39 | 0.001* | 9 (−1, 23), 35 |
Statistically significant differences between BL and FU4–6, and BL and END12 were analysed with Wilcoxon signed-rank test.
Statistical change. The median change for pain intensity ratings, disability scores, and psychological factors at FU4–6 and END12 are also shown. Positive values in the changes columns imply improvement.
After descriptive data, the following results are analysed and presented as follows: correlation analyses for the whole group (psychological variables and pain intensity and disability), regression analyses for the whole group (i.e. multivariable, bivariate linear regression analyses – psychological variables and disability – and multivariate regression analyses), and differences within the two subgroups.
Correlation analyses
Associations of psychological variables at BL with pain and disability and their changes
Pain acceptance at BL was negatively correlated with pain intensity in the neck at BL (Table 2). Fear-avoidance beliefs at BL were negatively correlated with changes in pain intensity in the shoulders at FU4–6. Pain self-efficacy at BL was negatively correlated with changes in pain intensity in the neck at END12. Fear-avoidance beliefs at BL were positively correlated with disability at BL, and pain self-efficacy and pain acceptance at BL were negatively correlated with disability at BL (Table 3).
Table 2.
Correlations (Spearman’s rho) between pain intensity in the neck and shoulders and psychological variables at baseline (BL) together with correlations of changes for these variables from BL to follow-up after 4–6 months (FU4–6) and correlations of changes for these variables from BL to end of the intervention after 1 year (END12).
| Psychological factors BL | Pain in the neck |
Pain in the shoulders |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL |
Changes BL − FU4–6 |
Changes BL − END12 |
BL |
Changes BL − FU4–6 |
Changes BL − END12 |
|||||||||||||
| Rho | p value | n | Rho | p value | n | Rho | p value | n | Rho | p value | n | Rho | p value | n | Rho | p value | n | |
| Anxiety | −0.08 | 0.57 | 55 | −0.25 | 0.11 | 42 | −0.16 | 0.35 | 37 | 0.16 | 0.26 | 55 | −0.18 | 0.22 | 42 | −0.26 | 0.13 | 37 |
| Depression | −0.05 | 0.74 | 56 | −0.19 | 0.22 | 43 | 0.10 | 0.57 | 38 | 0.14 | 0.29 | 55 | −0.19 | 0.22 | 43 | −0.11 | 0.95 | 38 |
| Pain catastrophizing | 0.17 | 0.23 | 54 | −0.15 | 0.36 | 41 | −0.06 | 0.72 | 36 | 0.02 | 0.88 | 54 | −0.20 | 0.21 | 41 | −0.06 | 0.73 | 36 |
| Fear avoidance | 0.10 | 0.45 | 56 | −0.24 | 0.12 | 43 | −0.21 | 0.21 | 38 | 0.04 | 0.75 | 56 | −0.33 | 0.029* | 43 | −0.29 | 0.08 | 38 |
| General self-efficacy | −0.09 | 0.52 | 55 | 0.10 | 0.54 | 42 | 0.14 | 0.40 | 37 | 0.12 | 0.37 | 55 | 0.21 | 0.18 | 42 | 0.19 | 0.27 | 37 |
| Pain self-efficacy | −0.25 | 0.06 | 56 | −0.10 | 0.54 | 43 | −0.38 | 0.019* | 38 | −0.10 | 0.45 | 56 | 0.10 | 0.51 | 43 | −0.20 | 0.23 | 38 |
| Pain acceptance | −0.30 | 0.033* | 52 | −0.05 | 0.74 | 40 | −0.09 | 0.62 | 35 | −0.14 | 0.32 | 52 | 0.14 | 0.40 | 40 | −0.00 | 0.99 | 35 |
A significant correlation.
Table 3.
Correlations (Spearman’s rho) between disability and psychological factors at baseline (BL), correlations between psychological variables at BL and changes in disability from BL to follow-up after 4–6 months (FU4–6), and correlations between psychological variables at BL and changes in disability from BL to end of the intervention after 1 year (END12).
| Psychological factors BL | Disability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| BL |
Changes BL − FU4–6 |
Changes BL − END12 |
|||||||
| Rho | p value | n | Rho | p value | n | Rho | p value | N | |
| Anxiety | 0.08 | 0.55 | 54 | −0.08 | 0.62 | 41 | −0.22 | 0.20 | 35 |
| Depression | 0.12 | 0.37 | 55 | −0.20 | 0.21 | 42 | −0.12 | 0.48 | 36 |
| Pain catastrophizing | 0.20 | 0.16 | 53 | −0.04 | 0.82 | 41 | 0.17 | 0.35 | 34 |
| Fear avoidance | 0.39 | 0.003* | 55 | 0.03 | 0.84 | 42 | −0.16 | 0.34 | 36 |
| General self-efficacy | 0.04 | 0.78 | 54 | −0.11 | 0.49 | 41 | −0.02 | 0.90 | 35 |
| Pain self-efficacy | −0.39 | 0.003* | 55 | −0.06 | 0.71 | 42 | −0.30 | 0.08 | 36 |
| Pain acceptance | −0.46 | 0.001* | 51 | −0.06 | 0.71 | 40 | −0.20 | 0.27 | 33 |
A significant correlation.
Associations of changes in psychological variables and changes in pain intensity and disability
Changes in depression symptoms were positively correlated with changes in pain intensity in the neck (rho = 0.39, p = 0.009) at FU4–6 (data not in table). In addition, changes in depression symptoms were positively correlated with changes in pain intensity in the neck (rho = 0.47, p = 0.003,) and shoulders (rho = 0.60, p < 0.001) at END12. Changes in depression symptoms (rho = 0.43, p = 0.008) and changes in pain self-efficacy (rho = 0.39, p = 0.019) were positively correlated with changes in disability at END12.
Regression analyses
Multivariable bivariate linear regression analyses on psychological variables and changes in disability and on changes in psychological variables and changes in disability
BL scores on fear-avoidance beliefs, pain self-efficacy, and pain acceptance significantly influenced disability at BL (Table 4). Furthermore, BL scores on pain self-efficacy significantly influenced changes in disability at END12.
Table 4.
Linear regression analyses of disability measured by the Neck Disability Index at baseline (BL), of changes in disability from BL to follow-up after 4–6 months (FU4–6) and of changes in disability from BL to end of the intervention after 1 year (END12) as dependent variables and the psychological factors at BL as separate independent variables.
| Psychological factors BL | Disability BL |
Changes in disability BL − FU4–6 |
Changes in disability BL − END12 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | Confidence interval | p value | Parameter estimate | Confidence interval | p value | Parameter estimate | Confidence interval | p value | |
| Anxiety | 0.036 | −0.454; 0.527 | 0.88 | 0.024 | −0.425; 0.474 | 0.91 | −0.156 | −0.636; 0.324 | 0.51 |
| Depression | 0.297 | −0.234; 0.791 | 0.28 | −0.298 | −0.866; 0.271 | 0.30 | −0.197 | −0.876; 0.481 | 0.56 |
| Pain catastrophizing | 0.141 | −0.078; 0.359 | 0.20 | −0.010 | −0.219; 0.199 | 0.92 | 0.147 | −0.112; 0.405 | 0.26 |
| Fear avoidance | 0.232 | 0.102; 0.363 | 0.001* | 0.047 | −0.087; 0.181 | 0.48 | 0.002 | −0.182; 0.185 | 0.98 |
| General self-efficacy | −0.014 | −0.302; 0.274 | 0.92 | −0.015 | −0.340; 0.390 | 0.92 | 0.051 | −0.323; 0.424 | 0.78 |
| Pain self-efficacy | −0.307 | −0.441; −0.172 | <0.001* | −0.044 | −0.202; 0.113 | 0.57 | −0.268 | −0.482; −0.053 | 0.016* |
| Pain acceptance | −0.218 | −0.323; −0.114 | <0.001* | −0.005 | −0.114; 0.105 | 0.93 | −0.071 | −0.210; 0.067 | 0.30 |
Unadjusted data.
Significance.
Changes in fear-avoidance beliefs scores at FU4–6 as well as at END12 significantly influenced changes in disability at the corresponding times (Table 5). In addition, changes in pain self-efficacy significantly influenced changes in disability at END12.
Table 5.
Linear regression analyses of changes in disability measured by the Neck Disability Index from baseline (BL) to follow-up after 4–6 months (FU4–6) and of changes in disability from BL to end of the intervention after 1 year (END12) as dependent variables and changes in the psychological variables for corresponding periods as separate independent variables.
| Changes in psychological factors BL − FU4–6 | Changes in disability BL − FU4–6 |
Changes in psychological factors BL − END12 | Changes in disability BL − END12 |
||||
|---|---|---|---|---|---|---|---|
| Parameter estimate | Confidence interval | p value | Parameter estimate | Confidence interval | p value | ||
| Anxiety | 0.422 | −0.170; 1.015 | 0.16 | Anxiety | 0.371 | −0.267; 1.010 | 0.25 |
| Depression | 0.056 | −0.630; 0.743 | 0.89 | Depression | 0.885 | 0.207; 1.563 | 0.012* |
| Pain catastrophizing | 0.111 | −0.147; 0.369 | 0.39 | Pain catastrophizing | 0.227 | −0.074; 0.528 | 0.13 |
| Fear avoidance | 0.285 | 0.084; 0.486 | 0.007* | Fear avoidance | 0.200 | 0.000; 0.401 | 0.05 |
| General self-efficacy | −0.086 | −0.541; 0.368 | 0.70 | General self-efficacy | 0.010 | −0.440; 0.460 | 0.96 |
| Pain self-efficacy | 0.156 | −0.038; 0.350 | 0.11 | Pain self-efficacy | 0.236 | 0.018; 0.454 | 0.035* |
| Pain acceptance | 0.009 | −0.146; 0.164 | 0.91 | Pain acceptance | 0.073 | −0.049; 0.196 | 0.23 |
Unadjusted data.
Significance.
Multivariate regression analyses
It was not possible to significantly regress the changes in pain intensities using BL variables.
PLSR was used to investigate which BL variables best predicted changes in disability at FU4–6 and END12. The regression of changes in disability (data not in table) at END12 (R2 = 0.24, Q2 = 0.09) revealed that the following BL variables were significant regressors: pain self-efficacy (VIP = 2.09 (−) and disability (VIP = 1.72 (+)). Hence, low-pain self-efficacy and high disability at BL were associated with positive changes in disability at END12. It was not possible to significantly regress the changes in disability at FU4–6.
Differences in pain intensity, disability, and psychological variables within the two subgroups at BL
Subjects who discontinued versus subjects who remained in the trial
BL values of disability (p = 0.037) and fear-avoidance beliefs (p = 0.048) were higher among the subjects who discontinued the trial within 4–6 months. In the discontinued group, the median (25th–75th percentiles) value for disability was 17 (13–22) and the median value for fear-avoidance beliefs was 24 (21–31). Among the subjects who remained in the trial, the median values for disability and fear avoidance were 13 (10–17) and 19 (11–28), respectively.
Completers versus non-completers
BL values of pain acceptance were higher (p = 0.018) among those who had completed the training until END12 (pain acceptance median: 81; 25th–75th percentiles: 72–92), compared to the respective values of the non-completers (67; 58–84).
Discussion
The hypotheses of this study could in part be confirmed. There were associations between some of the psychological factors and the effects of the exercise intervention on pain intensity and disability in women with chronic neck and shoulder pain. In addition, we found a few differences in psychological factors between (a) subjects who remained in, and those subjects who discontinued the trial before end of the intervention, and (b) subjects who adhered to recommended physical exercise, and those subjects who did not. Low fear-avoidance beliefs and low-pain self-efficacy at BL showed more apparent associations to positive effects of the exercise intervention than did the other psychological factors measured. In the subgroup analyses, higher fear-avoidance beliefs at BL differentiated the subjects who dropped out of the trial from those subjects who continued. Higher pain acceptance at BL was found for subjects who were defined as completers at the end of the trial.
Fear avoidance
This study found associations between low fear-avoidance beliefs at BL and a decrease in shoulder pain after 4–6 months of exercise. Furthermore, this study found that a decrease in fear-avoidance beliefs after exercise was associated with a decrease in disability. These results could reflect a behaviour related to the concept of low fear avoidance that is characterized by confrontation, which might have facilitated improvement in pain and disability. The present findings imply that lower fear-avoidance beliefs are advantageous for a positive outcome of a home-based exercise intervention, which is in line with the fear-avoidance model.13,14 Namely, the fear-avoidance model consists of two parts, one with negative consequences and the other with positive consequences. In the negative part, a pain experience is followed by catastrophizing, which in turn leads to avoidance and more pain and disability. In the positive part, a pain experience is followed by no fear, which in turn leads to confrontation and a possibility to recovery. High levels of fear avoidance have earlier been associated with more disability, more pain, and poorer prognosis of chronic pain conditions.58–60 However, the clinical importance of fear avoidance and its role for prognosis and treatment outcome in chronic pain is still not conclusive.61–65
Another finding in this study was a decrease in fear-avoidance beliefs after the exercise intervention although the intervention did not directly target the psychological factors. Similar results of a decrease in fear-avoidance beliefs as a result of physical-exercise-based interventions have been reported earlier,66,67 and also for workplace exercise in subjects reporting pain in the neck and shoulders.68 Altogether, these findings indicate that a specific treatment with the explicit purpose to reduce fear avoidance is not always required. Instead, physical exercise interventions per se might lead to a change in fear avoidance. This possibility is interesting, as high fear-avoidance beliefs may influence disability.12,69 Considering that it is still unclear why physical exercise is beneficial for chronic pain,70,71 it is possible to speculate that one part of the explanation might be a decrease in fear-avoidance beliefs.
Participants in this study who discontinued the trial before FU4–6 had higher fear-avoidance beliefs at BL compared to those who continued in the trial. Previous research has shown that high fear-avoidance beliefs in the presence of pain impair the ability to perform physical activities,72,73 which also is in line with the results of this study and with the theoretical concept of fear avoidance.13,14 Our results further strengthen the relevance of fear-avoidance beliefs for the outcome of exercise as treatment. This understanding may indicate that individuals with high fear-avoidance beliefs do not benefit from home-based exercise interventions, but instead require an exercise intervention with another design.
The fear-avoidance model assumes that avoidance is preceded by catastrophizing and fear.13,14,64 Catastrophizing has been seen as a psychological factor that affects chronic pain conditions regarding severity of pain and disability.11,74,75 However, reviews of previous research indicate also that there are in part conflicting evidence about the association between catastrophizing and pain and disability, and that the level of catastrophizing might influence the association.74,76 The latter view is in line with the results of this study: the levels of catastrophizing were rather low, and no associations were found between catastrophizing and the outcome of the exercise intervention. In addition, the fear-avoidance model has been criticized because it assumes a stepwise reasoning on catastrophizing (fear) exists before the avoidance behaviour is exhibited.64,65 The results of our study strengthen the arguments of the fear-avoidance model as a multidimensional model in which avoidance of activities can be present without excessive catastrophizing.64,65
Pain self-efficacy
In this study, we found associations between low-pain self-efficacy at BL and positive changes in pain intensity and disability at the end of the trial, and we also found associations between high pain-self-efficacy at BL and low disability at BL. Several previous studies have addressed relations between pain self-efficacy and pain or disability at defined time point(s),77 and other aspects of self-efficacy such as exercise self-efficacy related to adherence to exercise in subjects with neck and shoulder pain have also been studied earlier.78,79 High self-efficacy has often been considered to be associated with low pain intensity and disability,6,77 which is in line with our measures on self-efficacy and disability at BL. However, associations between BL pain self-efficacy and changes in pain intensity and disability as a result of a specific treatment, as in our study, are rarely investigated.77 That makes our results of the negative association between pain self-efficacy at BL and improvements in pain intensity and disability at the end of the trial particularly interesting. A possible explanation of this result is that the well-defined, structured, and professionally supported exercise intervention might have served as an effective support for the subjects to perform the exercise intervention. Thus, the design of the physical exercise intervention might have compensated for uncertainty about the subjects’ own ability to perform desired actions in spite of pain (i.e. the ability to perform the physical exercise in spite of pain). That is, the subjects with lower pain self-efficacy at BL benefited as well from the structured home-based exercise intervention. In addition, this study found that pain self-efficacy increased during the exercise intervention period. Furthermore, the increase in pain self-efficacy was found to be associated with a decrease in disability at the end of the trial. These results are in line with the theoretical assumptions of pain self-efficacy,6,15,17 previous research,80,81 and the arguments presented earlier in this study about the effects of a structured, long-term home-based intervention that reduce uncertainty of performance in the presence of pain.
Pain acceptance
In this study, subjects who were defined as completers at the end of the trial had higher pain acceptance at BL compared to those who were defined as non-completers at the end of the trial. The higher initial pain acceptance among the end-completers implies that higher acceptance of the pain is beneficial for long-term adherence to a home-based exercise intervention. This result can be well understood in view of the theoretical explanation of pain acceptance, which basically is about not struggling to get rid of the pain, but instead focusing on behaviours that lead to desired goals for daily living.18,19 Thus, higher pain acceptance improves the likelihood that a patient will adhere to prescribed exercises. Adherence to prescribed exercise is one crucial factor when applying exercise as treatment in chronic pain conditions. The results of this study indicate that it might be valuable to address pain acceptance to enhance adherence to home-based exercise.
General considerations
The above results were found although the subjects only had modest impairments with respect to the psychological factors measured. In addition, improvements were found for all outcomes (psychological factors, pain intensity, and disability) after 1 year of physical exercise. However, the associations between the psychological factors and the effects of physical exercise were rather small. It is noteworthy that catastrophizing at BL was not associated with either pain intensity or disability at BL or the outcomes of the exercise intervention.
Limitations
A major limitation in this study is its small sample size and this might have influenced the results and at the minimum make the results less interpretable. Low adherence to the exercise intervention is also a troublesome aspect; that is, the number of subjects who discontinued (n = 16) or were defined as non-completers (n = 8) at the end of the trial was rather high. In addition, the subjects were all women, so it remains open whether men would show similar characteristics.
Conclusion and clinical implications
Low fear-avoidance beliefs and low-pain self-efficacy at BL showed associations with positive effects of the physical exercise intervention. The overall results of this study indicate that there are associations between psychological factors and the effects of exercise as treatment, even in populations with generally modest impairments regarding psychological factors. Clinical implications of the results of this study are that fear-avoidance beliefs and pain self-efficacy should be taken into consideration when implementing a home-based physical exercise as treatment for chronic neck pain. In addition, awareness of pain acceptance might be valuable for adherence to prescribed exercise.
Footnotes
Author contribution: LK participated in the design of the study, implemented the intervention, and drafted the article. EPT and GA participated in the design of the study and drafted the manuscript. BG conceived of the study, participated in the design of the study, and performed some of the statistical analyses. BL conceived of the study, participated in the design of the study, participated in some of the statistical analyses, and drafted the manuscript. All authors participated in the revisions of different versions of the manuscript and approved the final manuscript. Data can be accessed from the corresponding author.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the regional ethical committee of Linkoping University, diary number M10-80. This original article is a sub-analysis of data collected within a clinical trial, registration number; ClinicalTrials.gov Id: NCT01876680. That clinical trial37 was published in 2014.
Funding: This study was supported by grants from Swedish council for Working Life and Social Research (2007-0760); AFA Insurance (140341); Swedish Research Council (2014-2979), and County Council of Östergötland (LiO-536211). The funding bodies had no role with respect to the study design and the collection, analysis, and interpretation of data. In addition, the funding bodies also had no input in the report writing and the final decision to submit the paper for publication.
Informed consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Leadley RM, Armstrong N, Lee YC, et al. Chronic diseases in the European Union: the prevalence and health cost implications of chronic pain. J Pain Palliat Care Pharmacother 2012; 26: 310–325. [DOI] [PubMed] [Google Scholar]
- 2. Van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth 2013; 111: 13–18. [DOI] [PubMed] [Google Scholar]
- 3. Hoy DG, Protani M, De R, et al. The epidemiology of neck pain. Best Pract Res Clin Rheumatol 2010; 24: 783–792. [DOI] [PubMed] [Google Scholar]
- 4. Hogg-Johnson S, Van Der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008; 33: S39–S51. [DOI] [PubMed] [Google Scholar]
- 5. Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007; 133: 581–624. [DOI] [PubMed] [Google Scholar]
- 6. Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol 2002; 70: 678–690. [DOI] [PubMed] [Google Scholar]
- 7. Linton SJ. A transdiagnostic approach to pain and emotion. J Appl Biobehav Res 2013; 18: 82–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keefe FJ, Rumble ME, Scipio CD, et al. Psychological aspects of persistent pain: current state of the science. J Pain 2004; 5: 195–211. [DOI] [PubMed] [Google Scholar]
- 9. Lucchetti G, Oliveira AB, Mercante JP, et al. Anxiety and fear-avoidance in musculoskeletal pain. Curr Pain Headache Rep 2012; 16: 399–406. [DOI] [PubMed] [Google Scholar]
- 10. Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011; 67: 942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flink IL, Boersma K, Linton SJ. Pain catastrophizing as repetitive negative thinking: a development of the conceptualization. Cogn Behav Ther 2013; 42: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zale EL, Lange KL, Fields SA, et al. The relation between pain-related fear and disability: a meta-analysis. J Pain 2013; 14: 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leeuw M, Goossens ME, Linton SJ, et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 2007; 30: 77–94. [DOI] [PubMed] [Google Scholar]
- 14. Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012; 153: 1144–1147. [DOI] [PubMed] [Google Scholar]
- 15. Bandura A, Adams NE, Beyer J. Cognitive processes mediating behavioral change. J Pers Soc Psychol 1977; 35: 125–139. [DOI] [PubMed] [Google Scholar]
- 16. Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol 2005; 139: 439–457. [DOI] [PubMed] [Google Scholar]
- 17. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007; 11: 153–163. [DOI] [PubMed] [Google Scholar]
- 18. McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain 2014; 15: 221–234.24581630 [Google Scholar]
- 19. Hayes SC, Levin ME, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther 2013; 44: 180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kay TM, Gross A, Goldsmith CH, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev 2012; 8: CD004250. [DOI] [PubMed] [Google Scholar]
- 21. O’Riordan C, Clifford A, Van De Ven P, et al. Chronic neck pain and exercise interventions: frequency, intensity, time, and type principle. Arch Phys Med Rehabil 2014; 95: 770–783. [DOI] [PubMed] [Google Scholar]
- 22. Bertozzi L, Gardenghi I, Turoni F, et al. Effect of therapeutic exercise on pain and disability in the management of chronic nonspecific neck pain: systematic review and meta-analysis of randomized trials. Phys Ther 2013; 93: 1026–1036. [DOI] [PubMed] [Google Scholar]
- 23. Gross A, Kay TM, Paquin JP, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev 2015; 1: Cd004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morley S, Williams A, Eccleston C. Examining the evidence about psychological treatments for chronic pain: time for a paradigm shift? Pain 2013; 154: 1929–1931. [DOI] [PubMed] [Google Scholar]
- 25. Nikander R, Mälkiä E, Parkkari J, et al. Dose-response relationship of specific training to reduce chronic neck pain and disability. Med Sci Sports Exerc 2006; 38: 2068–2074. [DOI] [PubMed] [Google Scholar]
- 26. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2010; 1: CD005956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leijon ME, Faskunger J, Bendtsen P, et al. Who is not adhering to physical activity referrals, and why? Scand J Prim Health Care 2011; 29: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leijon ME, Bendtsen P, Stahle A, et al. Factors associated with patients self-reported adherence to prescribed physical activity in routine primary health care. BMC Fam Pract 2010; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rhodes RE, Warburton DE, Murray H. Characteristics of physical activity guidelines and their effect on adherence: a review of randomized trials. Sports Med 2009; 39: 355–375. [DOI] [PubMed] [Google Scholar]
- 30. Beinart NA, Goodchild CE, Weinman JA, et al. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: a systematic review. Spine J 2013; 13: 1940–1950. [DOI] [PubMed] [Google Scholar]
- 31. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011; 27: 449–462. [DOI] [PubMed] [Google Scholar]
- 32. Moore A, Derry S, Eccleston C, et al. Expect analgesic failure; pursue analgesic success. BMJ 2013; 346: f2690. [DOI] [PubMed] [Google Scholar]
- 33. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015; 350: h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saragiotto BT, Maher CG, Yamato TP, et al. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev 2016; 1: CD012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 2013; 12: CD010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012; 11: CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karlsson L, Takala EP, Gerdle B, et al. Evaluation of pain and function after two home exercise programs in a clinical trial on women with chronic neck pain – with special emphasises on completers and responders. BMC Musculoskelet Disord 2014; 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol 2007; 21: 447–463. [DOI] [PubMed] [Google Scholar]
- 39. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011; 152: 2399–2404. [DOI] [PubMed] [Google Scholar]
- 40. Ackelman BH, Lindgren U. Validity and reliability of a modified version of the neck disability index. J Rehabil Med 2002; 34: 284–287. [DOI] [PubMed] [Google Scholar]
- 41. Vernon H. The neck disability index: state-of-the-art, 1991-2008. J Manipulative Physiol Ther 2008; 31: 491–502. [DOI] [PubMed] [Google Scholar]
- 42. Ohlsson K, Attewell RG, Johnsson B, et al. An assessment of neck and upper extremity disorders by questionnaire and clinical examination. Ergonomics 1994; 37: 891–897. [DOI] [PubMed] [Google Scholar]
- 43. Pedersen MT, Andersen LL, Jorgensen MB, et al. Effect of specific resistance training on musculoskeletal pain symptoms: dose-response relationship. J Strength Cond Res 2013; 27: 229–235. [DOI] [PubMed] [Google Scholar]
- 44. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 45. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 46. Terluin B, Brouwers EP, van Marwijk HW, et al. Detecting depressive and anxiety disorders in distressed patients in primary care; comparative diagnostic accuracy of the Four-Dimensional Symptom Questionnaire (4DSQ) and the Hospital Anxiety and Depression Scale (HADS). BMC Fam Pract 2009; 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osman A, Barrios F, Gutierrez P, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 2000; 23: 351–365. [DOI] [PubMed] [Google Scholar]
- 48. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assessment 1995; 7: 524–532. [Google Scholar]
- 49. Waddell G, Newton M, Henderson I, et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993; 52: 157–168. [DOI] [PubMed] [Google Scholar]
- 50. Lundberg M, Grimby-Ekman A, Verbunt J, et al. Pain-related fear: a critical review of the related measures. Pain Res Treatment 2011; 2011: 494196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Love J, Moore CD, Hensing G. Validation of the Swedish translation of the general self-efficacy scale. Qual Life Res 2012; 21: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 52. Carlstedt E, Lexell EM, Pessah-Rasmussen H, et al. Psychometric properties of the Swedish version of the General Self-Efficacy Scale in stroke survivors. Int J Rehabil Res 2015; 38: 333–337. [DOI] [PubMed] [Google Scholar]
- 53. Wicksell RK, Olsson GL, Melin L. The Chronic Pain Acceptance Questionnaire (CPAQ)-further validation including a confirmatory factor analysis and a comparison with the Tampa Scale of Kinesiophobia. Eur J Pain 2009; 13: 760–768. [DOI] [PubMed] [Google Scholar]
- 54. McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004; 107: 159–166. [DOI] [PubMed] [Google Scholar]
- 55. Reneman MF, Dijkstra A, Geertzen JHB, et al. Psychometric properties of Chronic Pain Acceptance Questionnaires: a systematic review. Eur J Pain 2010; 14: 457–465. [DOI] [PubMed] [Google Scholar]
- 56. Eriksson L, Kettaneh-Wold N, Wold S, et al. Introduction to multi-and megavariate data analysis using projection methods (PCA & PLS). Umeå: Umetrics, 1999. [Google Scholar]
- 57. Eriksson L, Johansson E, Kettaneh-Wold N, et al. Multi- and megavariate data analysis; part I and II. 2 ed. Umeå: Umetrics, 2006. [Google Scholar]
- 58. Woby SR, Watson PJ, Roach NK, et al. Adjustment to chronic low back pain – the relative influence of fear-avoidance beliefs, catastrophizing, and appraisals of control. Behav Res Ther 2004; 42: 761–774. [DOI] [PubMed] [Google Scholar]
- 59. Elfving B, Andersson T, Grooten WJ. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int 2007; 12: 14–24. [DOI] [PubMed] [Google Scholar]
- 60. Lee K-C, Chiu TTW, Lam T-H. The role of fear-avoidance beliefs in patients with neck pain: relationships with current and future disability and work capacity. Clin Rehabil 2007; 21: 812–821. [DOI] [PubMed] [Google Scholar]
- 61. Pincus T, Vogel S, Burton AK, et al. Fear avoidance and prognosis in back pain: a systematic review and synthesis of current evidence. Arthritis Rheum 2006; 54: 3999–4010. [DOI] [PubMed] [Google Scholar]
- 62. Wertli MM, Rasmussen-Barr E, Weiser S, et al. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J 2014; 14: 816.e4–836.e4. [DOI] [PubMed] [Google Scholar]
- 63. Wertli MM, Rasmussen-Barr E, Held U, et al. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J 2014; 14: 2658–2678. [DOI] [PubMed] [Google Scholar]
- 64. Wideman TH, Asmundson GG, Smeets RJ, et al. Rethinking the fear avoidance model: toward a multidimensional framework of pain-related disability. Pain 2013; 154: 2262–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pincus T, Smeets RJ, Simmonds MJ, et al. The fear avoidance model disentangled: improving the clinical utility of the fear avoidance model. Clin J Pain 2010; 26: 739–746. [DOI] [PubMed] [Google Scholar]
- 66. Kernan T, Rainville J. Observed outcomes associated with a quota-based exercise approach on measures of kinesiophobia in patients with chronic low back pain. J Orthop Sports Phys Ther 2007; 37: 679–687. [DOI] [PubMed] [Google Scholar]
- 67. Rolving N, Christiansen DH, Andersen LL, et al. Effect of strength training in addition to general exercise in the rehabilitation of patients with non-specific neck pain. A randomized clinical trial. Eur J Phys Rehabil Med 2014; 50: 617–626. [PubMed] [Google Scholar]
- 68. Jakobsen MD, Sundstrup E, Brandt M, et al. Effect of workplace- versus home-based physical exercise on muscle response to sudden trunk perturbation among healthcare workers: a cluster randomized controlled trial. Biomed Res Int 2015; 2015: 902896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rainville J, Smeets RJEM, Bendix T, et al. Fear-avoidance beliefs and pain avoidance in low back pain – translating research into clinical practice. Spine J 2011; 11: 895–903. [DOI] [PubMed] [Google Scholar]
- 70. Nijs J, Kosek E, Van Oosterwijck J, et al. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15: ES205–ES213. [PubMed] [Google Scholar]
- 71. Steiger F, Wirth B, De Bruin ED, et al. Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur Spine J 2012; 21: 575–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, et al. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res 2003; 26: 101–108. [DOI] [PubMed] [Google Scholar]
- 73. Lindstroem R, Graven-Nielsen T, Falla D. Current pain and fear of pain contribute to reduced maximum voluntary contraction of neck muscles in patients with chronic neck pain. Arch Phys Med Rehabil 2012; 93: 2042–2048. [DOI] [PubMed] [Google Scholar]
- 74. Wertli MM, Burgstaller JM, Weiser S, et al. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine 2014; 39: 263–273. [DOI] [PubMed] [Google Scholar]
- 75. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009; 9: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J 2014; 14: 2639–2657. [DOI] [PubMed] [Google Scholar]
- 77. Jackson T, Wang Y, Wang Y, et al. Self-efficacy and chronic pain outcomes: a meta-analytic review. J Pain 2014; 15: 800–814. [DOI] [PubMed] [Google Scholar]
- 78. Andersen LL. Influence of psychosocial work environment on adherence to workplace exercise. J Occup Environ Med 2011; 53: 182–184. [DOI] [PubMed] [Google Scholar]
- 79. Pedersen MM, Zebis MK, Langberg H, et al. Influence of self-efficacy on compliance to workplace exercise. Int J Behav Med 2013; 20: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Costa LDCM, Maher CG, McAuley JH, et al. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain 2011; 15: 213–219. [DOI] [PubMed] [Google Scholar]
- 81. Dobkin PL, Liu A, Abrahamowicz M, et al. Predictors of disability and pain six months after the end of treatment for fibromyalgia. Clin J Pain 2010; 26: 23–29. [DOI] [PubMed] [Google Scholar]