Abstract
Dengue has a negative impact in low- and lower middle-income countries, but also affects upper middle- and high-income countries. Despite the efforts at controlling this disease, it is unclear why dengue remains an issue in affluent countries. A better understanding of dengue epidemiology and its burden, and those of chikungunya virus and Zika virus which share vectors with dengue, is required to prevent the emergence of these diseases in high-income countries in the future. The purpose of this review was to assess the relative burden of dengue in four high-income countries and to appraise the similarities and differences in dengue transmission. We searched PubMed, ISI Web of Science, and Google Scholar using specific keywords for articles published up to 05 May 2016. We found that outbreaks rarely occur where only Aedes albopictus is present. The main similarities between countries uncovered by our review are the proximity to dengue-endemic countries, the presence of a competent mosquito vector, a largely nonimmune population, and a lack of citizens’ engagement in control of mosquito breeding. We identified important epidemiological and environmental issues including the increase of local transmission despite control efforts, population growth, difficulty locating larval sites, and increased human mobility from neighboring endemic countries. Budget cuts in health and lack of practical vaccines contribute to an increased risk. To be successful, dengue-control programs for high-income countries must consider the epidemiology of dengue in other countries and use this information to minimize virus importation, improve the control of the cryptic larval habitat, and engage the community in reducing vector breeding. Finally, the presence of a communicable disease center is critical for managing and reducing future disease risks.
Introduction
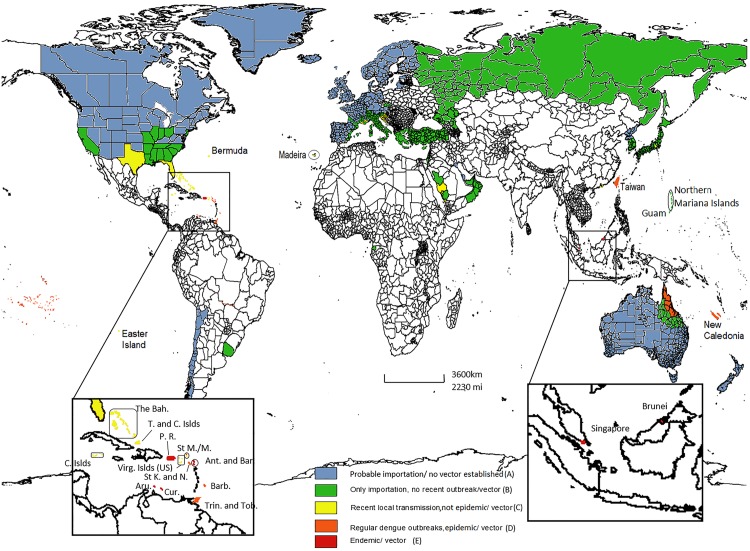
Pathogens and vectors can now be transported rapidly around the world. Consequently, new infections are emerging and some old infections, including dengue, have re-emerged. Dengue virus (DENV), a flavivirus, is transmitted by Aedes mosquitoes and causes large epidemics and endemic transmission in nonimmune populations, particularly in economically disadvantaged tropical and subtropical countries. Some high-income countries (HICs) or states such as Aruba, Brunei Darussalam, Puerto Rico, and Singapore are dengue-endemic. Others, such as the state of Queensland in Australia, French Polynesia, New Caledonia, and Taiwan experience regular dengue outbreaks, whereas still others, such as The Bahamas, Bermuda, France, Japan, and the state of Florida in the United States, experience small outbreaks.
The incidence of dengue has grown markedly throughout the world in recent decades. Dengue is under-reported, but the actual number of dengue infections per year is estimated to be as high as 390 million (95% credible interval, 284–528 million), of which 96 million (67–136 million) manifest clinically [1]. According to the World Health Organization [2], in 2012 the number of deaths caused by dengue was 297, 873, and 159 per million in low-income, lower middle-income and upper middle-income countries, respectively. The number of deaths is lower (38 per million) in HICs [2].
The World Bank Atlas method is used to calculate gross national income (GNI). GNI is a useful and easily available indicator that correlates closely with other, nonmonetary measures of quality of life, such as life expectancy at birth, mortality rates for children, and enrollment rates in school. Economies are defined according to the GNI per capita in 2013 as: low income, US$1,045 or less [3]; middle income, more than US$1,045 but less than US$12,746; and high income, US$12,746 or more [3]. The dengue vector is established in 47 of the 76 HICs listed by the World Bank, and 29 have neither a dengue vector nor local DENV transmission. Nineteen of the 47 HICs have reported only imported cases of dengue, and the other 28 countries have reported recent autochthonous transmission (S1 Table). Of these 28 HICs with local transmission, 14 have reported only small numbers of cases (including Florida), eight have regular large outbreaks (including Queensland and Taiwan), and six are classified as dengue endemic (including Singapore) (Fig 1 and S1 Table).
Fig 1. Occurrence of dengue in HICs as of December 2015.
Dengue poses an economic burden, especially in resource-poor, dengue-endemic countries [4,5]. Despite its lower prevalence in HICs, dengue also has economic costs in these countries [6,7]. Costs per case are generally higher in HICs such as Argentina, Australia, Brunei Darussalam, Singapore, and the US [8]. It is unclear why dengue is transmitted despite intensive surveillance and control programs in HICs [9,10]. The aim of this review is to discuss the relative burden of dengue in four representative HICs. We describe the unique features of dengue and provide a detailed review of the main drivers of dengue transmission in these HICs. We then discuss the public health responses and challenges for public health programs, and we finish with some recommendations for the future.
We restricted our analysis to Singapore, Taiwan, Australia, and the US, with a particular focus on the states of Queensland in Australia and Florida in the US. Queensland and Florida have many similarities [11]. Both have similar weather patterns (tropical to temperate) [12,13] and have experienced population growth because of their favorable climate [11]. East Central Australia and East Central Florida have natural features of ecological significance for mosquito habitats [14]. The environment and ecology of Queensland and Florida are fairly similar and comprise coastal ecosystems, rainforest, freshwater wetlands, floodplain forests, and woodlands [15,16]. Both states contain areas where Aedes (Stegomyia) aegypti (L.), the principal vector of dengue, chikungunya and Zika viruses, is established. Singapore and Taiwan are dengue-endemic and -nonendemic countries, respectively, and are examples of countries with increasing rates of autochthonous dengue and dengue hemorrhagic fever (DHF) despite intensive vector control programs. Lessons learned within these countries provide valuable public health and epidemiological information for other high-income nonendemic countries at risk for the establishment of dengue.
Literature Search
To review this topic, we searched PubMed, ISI Web of Science, and Google Scholar for articles published up to 05 May 2016. The following terms were used: “dengue fever,” “dengue,” “outbreaks,” “transmission,” “burden,” “high-income countries,” and “World Bank income countries” in combination with the name of each of the 76 HICs. Relevant articles referenced in publications were identified by searching the lead author’s personal files in Google Scholar and in the Literature Retrieval System of the Armed Forces Pest Management Board, US. Articles in English, Spanish, and French were reviewed. ProMED-mail [17], Eurosurveillance [18], and DengueMap [19] websites were used to complete the search.
Relative Burden of Dengue in Selected HICs
The number of countries that experienced severe dengue epidemics increased from nine before 1970 to more than 100 in 2015 [20]. DENV now occurs in regions of Africa, the Americas, the eastern Mediterranean, southeast Asia, and the western Pacific [20].
Notwithstanding differences in reporting methods, the economic impacts of dengue on a variety of measures are considerable for HICs. These impacts can be direct, such as the costs of treatment, hospitalization, and prevention, or indirect, such as the loss of productivity related to absence, disability, or death and the effects on tourism [21]. Table 1 presents epidemiological data for dengue infection in Queensland (Australia), Florida (US), Singapore, and Taiwan.
Table 1. The burden of dengue fever, highlighting the number of locally acquired cases in Queensland (Australia), Florida (US), Singapore, and Taiwan.
| Years, location | Serotype(s)* | N dengue cases | N locally acquired cases | N hospitalizations (%) | Incidence per 100,000 | N DHF (%) | N deaths (Case Fatality Rate %) | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Queensland, Australia | 1996–1997, the Torres Strait | DENV-2 | 208 | 202 | 7 (3.48) | 6.49 | 0 | 0 | [22,23] |
| 1997–1999, Cairns; Port Douglas; Mossman | DENV-3 | 498 | 493 | 101 (20.28) | 14.86 | 6 (1.20) | 0 | [24–26] | |
| 2000, Cairns | DENV-2 | 85 | 50 | 0 | 2.44 | 0 | 0 | [25,27] | |
| 2001, Townsville | DENV-2 | 42 | 8 | 1 (11.11) | 1.17 | 4 (9.52) | 0 | [27] | |
| 2002, Kuranda; Townsville; Cairns | DENV-2; -1; -4 | 81 | 25 | 0 | 2.22 | 0 | 0 | [27,28] | |
| 2003–2004, Cairns; Townsville; the Torres Strait | DENV-2 | 999 | 895 | 35 (3.91) | 26.34 | 4 (0.45) | 2 (0.20) | [27,29,30] | |
| 2005, the Torres Strait; Townsville | DENV-4 | 116 | 76 | 0 | 3.04 | 0 | 0 | [27,31] | |
| 2006, Townsville; Cairns | DENV-3; -2 | 76 | 37 | 0 | 1.95 | 0 | 0 | [27,31] | |
| 2007, Townsville | DENV-3 | 119 | 47 | 0 | 2.93 | 0 | 0 | [27,31] | |
| 2008–2009, Port Douglas; Mossman; Townsville; Cairns | DENV-3; -3; -3, -1; -2, -3 | 1,314 | 1,042 | 73 (5.56) | 26.58 | 6 (0.46) | 1 (0.11) | [26,27,31,32] | |
| 2010–2011, Cairns; Townsville | DENV-1, -2, -3, -4; -1, -2, -3 | 474 | 148 | - | 10.94 | 0 | 0 | [31,33] | |
| 2012, Cairns; Townsville | DENV-1, -2; -1, -2 | 244 | 28 | - | 5.30 | 0 | 0 | [31] | |
| 2013, Port Douglas, Mossman; Cairns | DENV-3; -1 | 490 | 222 | - | 10.45 | 0 | 0 | [31,34] | |
| 2014, Cairns; Innisfail; Port Douglas; Charters Towers; Townsville | DENV-1; -1; -3 | 395 | 182 | - | 8.32 | 0 | 0 | [31,34] | |
| 2015, Cairns, Tully, Innisfail, El Arish, Townsville | DENV-1; -2 | 284 | 62 | - | - | 0 | 0 | [35,36] | |
| Florida | 2009, Key West | DENV-1 | 63 | 27 | 0 | 0.3 | 0 | 0 | [37,38] |
| 2010–2011, Key West; Broward; Miami-Dade Counties | DENV-1, -2 | 257 | 65 | 0 | 1 | 0 | 0 | [37–42] | |
| 2011, Miami-Dade, Martin, Hillsborough, Palm Beach Counties | DENV-1, -2 | 68 | 7 | - | - | - | - | [43,44] | |
| 2012, Miami-Dade, Seminale, Osceola Counties | DENV-1, -4, -2, -3 | 139 | 3 | 0 | 0.7 | 0 | 0 | [39,41,45] | |
| 2013, Martin, Miami-Dade Counties | DENV-1, -4, -3, -2 | 149 | 23 | 6 (3.87) | 0.7 | 0 | 0 | [39,41,46,47] | |
| 2014, Miami-Dade County | DENV-1, -2, -3, -4 | 51 | 6 | 0 | 0.2 | 0 | 0 | [39,41,48] | |
| 2015, Broward County | DENV-1, -2 | 85 | 1 | - | - | - | - | [49] | |
| Singapore | 2000, Tampines regional centre, Geylang, Marine Parade | DENV-1, -2, -4, -3 | 673 | 402 | 572 (0.85) | 9.3 | 10 (1.5) | 2 (0.30) | [50,51] |
| 2001, Toa Payoh, Kallang, Hougang, Yishun, Novena | DENV-2, -4, -1 | 2,372 | 2,064 | 1,850 (0.78) | 46.7 | 6 (0.30) | 4 (0.17) | [51,52] | |
| 2002, Bukit Batok; Geylang; Novena; Sengkang, Jurong Ouest; Woodlands | DENV-2, -1, -3, -4 | 3,945 | 3,560 | 3,156 (0.80) | 86.2 | 8 (0.20) | 4 (0.10) | [53,54] | |
| 2003, Ang Mo Kio; Bedok; Hougang; Novena | DENV-2, -1, -3, -4 | 4,788 | 4,542 | 3,734 (0.78) | 108.5 | - | 6 (0.13) | [53] | |
| 2004, Yishun; Ang Mo Kio; Geylang; Bedok | DENV-1, -2, -3, -4 | 9,459 | 9,297 | 7,716 (0.81) | 223.1 | 168 (1.78) | 9 (0.10) | [9,53] | |
| 2005, Yishun; Woodlands; Kallang; Toa Payoh; Jurong; Bukit Batok | DENV-1, -2, -3, -4 | 14,006 | 13,818 | 10,504 (75) | 333.1 | 381 (2.72) | 27 (0.19) | [9,55] | |
| 2006, Bukit Batok, Woodlands, Ang Mo Kio, Pasir Ris, Clementi | DENV-1, -2, -3, -4 | 3,127 | 2,844 | - | 71.0 | 75 (2.40) | 10 (0.32) | [51,56] | |
| 2007, Bukit Batok; Pasir Ris; Woodlands | DENV-2, -1, -3, -4 | 8,826 | 8,637 | - | 192.3 | 189 (2.14) | 24 (0.27) | [57,58] | |
| 2008, Hougang, Jurong Ouest, Serangoon, Woodlands, Ang Mo Kio | DENV-2, -1, -3, -4 | 7,031 | 6,631 | - | 145.3 | 84 (1.19) | 10 (0.14) | [59–61] | |
| 2009, Clementi, Bukit Merah, Hougang, Kallang | DENV-2, -1, -3, -4 | 4,497 | 4,187 | - | 90.2 | 46 (1.02) | 8 (0.18) | [59,60,62] | |
| 2010, Woodlands, Telok Blangah, Newton, Pasir Ris | DENV-2, -1, -3, -4 | 5,363 | 4,978 | - | 105.6 | 34 (0.63) | 6 (0.11) | [59,60,63] | |
| 2011, Paya Lebar, Woodlands, Serangoon, Yishun, Pasir Ris, Tampines | DENV-2, -1, -3, -4 | 5,330 | 5,099 | - | 102.8 | 22 (0.41) | 6 (0.11) | [59,60,64] | |
| 2012, Ang Mo Kio, Bedok, Woodlands, Clementi, Hougang | DENV-2, -1, -3, -4 | 4,632 | 4,369 | - | 87.2 | 30 (0.64) | 2 (0.04) | [60,65] | |
| 2013, Tampines, Yio Chu Kang; Serangoon area, Bedok, Pasir Ris, Jurong, Choa Chu Kang | DENV-1, -2, -3, -4 | 22,170 | 21,863 | - | 410.6 | 93 (0.42) | 8 (0.04) | [59,60] | |
| 2014, Chuan Drive; Bukit Panjang, Choa Chu Kang, Hougang, Bukit Timah, Bedok, Sengkang | DENV-1, -2, -3, -4 | 18,326 | 17,812 | 3,665 (20.00) | 335.0 | 20 (0.11) | 6 (0.03) | [66–68] | |
| 2015, Ang Mo Kio, Bishan North | DENV-2 | 11,298 | - | 2259 (20.00) | - | 12 (0.11) | 4 (0.04) | [67,69] | |
| Taiwan | 2000–2001, Tainan City, Kaohsiung City | DENV-2, -4 | 418 | 340 | - | 0.6 | - | - | [70,71] |
| 2002, Kaohsiung City and Kaohsiung County; Pingtung County | DENV-2 | 5,388 | 5,336 | - | 23.9 | 241 (4.50) | 21 (0.39) | [70,72] | |
| 2003, Kaohsiung City | DENV-2 | 145 | 86 | - | 0.6 | 2 (23.25) | 1 (1.16) | [70,72] | |
| 2004–2005, Pingtung City, Kaohsiung City, Kaohsiung City | DENV-1, -3 | 733 | 538 | - | 1.8 | 8 (1.49) | 0 | [70,72] | |
| 2006, Kaohsiung City; Kaohsiung County; Tainan City; Tainan County; Pingtung County | DENV-3, -1, -2 | 1,074 | 965 | - | 4.7 | 19 (1.97) | 5 (0.47) | [70,72] | |
| 2007, Tainan City; Kaohsiung City | DENV-1 | 2,179 | 2,000 | - | 9.5 | 11 (0.55) | 1 (0.05) | [70,72] | |
| 2008, Kaohsiung City; Kaohsiung County | DENV-1 | 714 | 488 | - | 3.1 | - | - | [45,73] | |
| 2009, Kaohsiung City; Kaohsiung County; Pingtung County | DENV-3 | 1,052 | 848 | - | 4.5 | - | - | [45,73] | |
| 2010, Kaohsiung City; Taipei City; Tainan City; Tainan County | - | 1,896 | 1,592 | - | 8.1 | - | 2 (0.11) | [73] | |
| 2011, Kaohsiung City; Pingtung County; Taipei; Penghu county | - | 1,702 | 1,545 | - | 7.3 | 18 (1.05) | 5 (0.29) | [70] | |
| 2012, Tainan City; Kaohsiung City | DENV-2 | 1,478 | 1,271 | - | 6.3 | 20 (1.35) | 5 (0.34) | [73,74] | |
| 2013, Tainan City; Pingtung County; Taipei; Greater Kaohsiung; New Taipei City | DENV-1 | 860 | 596 | - | 3.6 | 14 (2.35) | 1 (0.17) | [70] | |
| 2014, Kaoshiung City; Chiayi City; Pingtung City | DENV-1 | 15,732 | 15,492 | - | 66.2 | 136 (0.88) | 20 (0.12) | [75,76] | |
| 2015, Tainan City; Kaoshiung City; Pingtung City | - | 42,916 | 42,572 | >44 (0.10) | - | - | 209 (0.49) | [77] |
* serotype known by place is separated by “;”. Dominant serotype, when known, is given in bold font.
Epidemiological Burden of Dengue
Queensland, Australia
DENV is the third most common mosquito-borne virus infecting Australians after the alphaviruses Ross River and Barmah Forest viruses. The first reference to dengue in Australia appeared in The Australian Medical Journal in 1873 and highlighted the importation of eight cases from Mauritius via the ship Charles Auguste [25]. From 1896–1897, in Charters Towers in North Queensland, Hare published the first reports of dengue fever based on clinical data collected from a number of practitioners in Queensland and observed that comorbidity (diabetes, alcoholism) contributed to the severity of dengue in old patients [78]. Since the early 1990s, DENV outbreaks have increased in severity, frequency, and length in northeast Australia despite increased public health control efforts [22,79].
The risk of local transmission is currently restricted to northeast of Queensland (specifically, the northern half) where Ae. aegypti is present and where viremic travelers arriving from dengue-endemic, mainly low-income, countries in the Asian-Pacific region regularly introduce the virus [22,80,81].
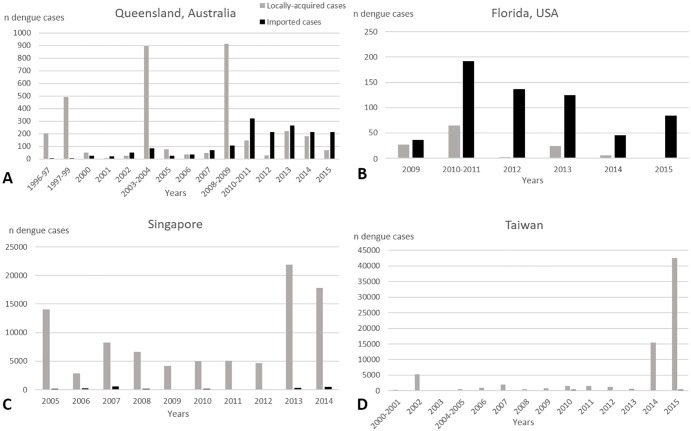
Imported cases are reported throughout Australia, and the number and frequency of overseas-acquired dengue cases is increasing [33,82,83] (Fig 2A). The largest number of locally acquired dengue cases (over 1,000 cases) recorded in Queensland occurred from 2008–2009 [32]. Severe DHF and dengue shock syndrome are rare in North Queensland [84]. There have been 20 DHF cases since 1996 in Australia, and three fatalities related to dengue fever nationwide in more than a century [32].
Fig 2. Number of locally acquired and imported dengue cases.
Florida, US
Dengue is now the leading cause of acute febrile illness in US travelers returning from the Caribbean, South America, and Asia [85]. Early reports of dengue in Florida include outbreaks in the Dry Tortugas in 1903 and Miami in 1904 and 1908 [86]. Outbreaks of dengue were reported later in 1922 and 1934 in Florida [87]. Since 1934 in Florida, and since 1945 in the rest of continental US (except for the Texas–Mexico border), no autochthonous dengue cases were reported until 2009. However, in 2009–2010, an outbreak of dengue was identified in Key West, southern Florida, where there were 22 cases of locally acquired dengue in the summer and autumn of 2009 and 66 cases in 2010. No dengue cases have been reported in Key West since November 2010 [88]. Between November 2010 and December 2014, there were 2,463 cases for the entire continental US; the two states reporting most of the imported dengue cases were New York (n = 574) and Florida (n = 495) [40]. Florida reported the largest number of locally acquired dengue cases (n = 97) with peaks in 2010 (60%) and 2013 (25%) [41] (Fig 2B). In 2011 and 2012, only isolated cases of autochthonous DENV transmission were reported in Florida, and none were reported in Key West. In mid-August 2013, local transmission was reported from Martin County in east-central Florida. By late September, a total of 22 locally acquired dengue cases were reported from Rio and nearby Jensen Beach [89]. An additional dengue case was reported in 2013 from Miami-Dade County. During the outbreak in 2013, which involved the DENV-1 serotype, 24 of 28 cases were symptomatic, and six of the 24 patients were hospitalized [90]. No DHF- or dengue-associated deaths have been reported in Florida.
Singapore
Singapore is hyperendemic for dengue, and all four serotypes (DENV-1 to -4) circulate [91]. The number of dengue cases has surged since the 1990s. Until 2013, epidemic cycles were occurring every 5–6 years with peaks in July and August. Infection and mortality in Singapore are reported principally among adults [92]. A retrospective case-control study was performed for the period 01 January to 31 September 2004 and included all cases of dengue-associated mortality treated in Tan Tock Seng Hospital, Singapore [93]. Of the seven patients who died, five (71.4%) were male [93]. In 2005, 14,006 cases were reported, and about 80% were among adults [9]. After small numbers of cases in 2006, Singapore experienced another large DENV outbreak in 2007, with 8,826 cases [94] (Fig 2C). That same year, the serotype DENV-2 re-emerged with a clade replacement and overtook DENV-1, which had been circulating since 2004, and remained the predominant serotype until 2013 [57]. A change in the predominant DENV serotype may have triggered a spike in dengue cases because of a lack of serotype-specific immunity [9]. In 2013, there was a record 22,248 reported dengue cases, with 842 dengue cases in a single week [95]. The risk of acquired dengue for nonimmune visitors ranged from 42/100,000 during a low season of a nonepidemic year to 1,700/100,000 during the high season of an epidemic year in Singapore [96].
Taiwan
In Taiwan, several DENV outbreaks occurred before 1945, and then there were no outbreaks until 1981 [97]. Before 2014, the largest and most severe outbreak happened in 2002 (5,388 and 241 DHF cases) [72]. In 2002, there were fewer imported cases in the southern (n = 13) compared with the northern (n = 28) parts of Taiwan probably because of fewer visits from travelers and fewer workers from endemic countries in the south. However, 98% of locally acquired cases occurred in the south where Ae. aegypti is most abundant [98]. Since 2011, the Centers of Disease Control, ROC (Taiwan), has reported more than 800 imported cases and 18,500 locally acquired dengue cases, with a significant increase in 2014 [99,100] (Fig 2D). For the first time since formal records were first kept in 1981, the largest DENV outbreak occurred in 2015 with 42,916 reported cases (42,572 locally-acquired, 209 deaths) [75,77]. Most of these cases (53.4%) occurred in Tainan city and 44.5% in Kaohsiung city located in southern Taiwan [75,77].
Outbreaks of dengue usually peak in the summer and autumn [101]. Despite repeated dengue outbreaks caused by importation to the southern part of the island, Taiwan is not currently considered dengue-endemic [72]. Based on a study of 9,939 cases of dengue fever and DHF from 2000 to 2007, the duration of illness for dengue fever and DHF (excluding fatalities) was 8.4 and 10.8 days, respectively [102]. Active surveillance from 2004–2007 reported a DENV seroprevalence of 1.1% (of 42,150 total cases) and 77% of cases as primary dengue [72].
Economic Burden of Dengue
Estimates of the economic burden of dengue are calculated by adding the direct and indirect costs. The direct costs consist of health care costs such as those related to the provision of health care, non-health care costs such as those related to consumption of resources like transport, and household expenditures. The indirect costs are those associated with the loss in productivity from illness and death [103]. A robust assessment of the economic burden helps to i) identify information gaps, research needs, and refinements to the national statistical reporting systems; ii) argue that policies on dengue control and prevention should be given a high priority on the public health policy agenda; and iii) provide a baseline measure to determine the cost effectiveness of dengue policies and programs [104]. However, few studies have assessed the economic burden of dengue [105]. The estimation of the true extent of dengue, its incidence, and its associated costs have substantial uncertainty, partly because of unreported and unrecognised apparent DENV infections [8].
In 2013, there were an estimated total of 58.40 million symptomatic DENV infections globally, including 13,586 fatal cases [8]. The annual global cost of dengue illness averaged US$8,900 million. Globally, 18% of cases are admitted to the hospital, 48% remain ambulatory, and 34% do not seek medical care. Costs per case are generally higher in HICs, especially in Australia and the US [8].
Queensland, Australia
The average work time lost was 10.5 days among people who recalled a dengue-like illness during the 1993 dengue epidemic in Charters Towers, Australia [106]. From 1990 to 2008, the cost of lost work and the control cost averaged an estimated US$36.18 million (2.01 million per annum) [107]. However, this calculation does not include physical and psychological suffering. A delayed response to dengue outbreaks of 4–6 weeks would result in 86 times (or US$13 million in 2003) and 346 times (or US$382 million in 2009) higher dengue illness costs versus a scenario with active surveillance and response within 2 weeks, respectively [108]. Therefore, effective dengue surveillance and response lowers costs associated with dengue.
Florida, US
Cost has not been estimated in Florida specifically, but estimates have been made for the US and many central and south American countries [109]. Shepard et al. (2011) estimated the aggregate annual total cost of dengue in the Americas to be about US$2.1 billion for the period 2000–2007 [109]. The US had the highest estimated average total cost per dengue case (ambulatory cost added to hospitalized cost = US$19,413) among the selected countries.
Singapore
Among the 12 countries analysed by Shepard et al. [4] (Bhutan, Brunei, Cambodia, East Timor, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam), which did not include the US, Australia, or Taiwan, the predicted values of direct and indirect unit costs per dengue case were the highest in Singapore (direct costs: $2,455, indirect costs: $1,821 in 2010 US dollars). In Singapore, Carrasco et al. [6] investigated the cost-effectiveness of future vaccination programs and estimated that, from 2000 to 2009, the average economic impact of dengue illness in constant 2010 US dollars ranged from $0.85 billion to $1.15 billion, of which control costs constituted 42%–59% [6].
Taiwan
In Taiwan, it cost about US$227 (2011) to treat a patient with dengue fever [110]. Tseng et al. (2009) estimated the economic impacts of climate change on dengue in Taiwan. The probability of being infected by DENV due to climate change ranges from 12% to 43% to 87%, which represents low, mid, and high probabilities when the temperature increased by 1.8°C [111]. People would pay US$22.8, US$102.3, and US$162.28 (in 2008), respectively, per year in order to avoid the increased probabilities of being infected with DENV. Visitor numbers for September 2015 were 50% lower than the same period in 2014, due at least in part to dengue [112].
Drivers of Dengue Transmission
Nonclimatic factors include the epidemiology, ecology, and distribution of the vector, socioeconomic, and environmental factors; climatic factors include weather variability, climate change, and extreme events. These factors are discussed in detail in the following sections.
Vector Distribution
Ae. aegypti is most prevalent in highly urbanized areas, whereas Ae. albopictus is present mainly in rural, suburban, and vegetated urban areas [113–115]. Abiotic factors including urban landscape, temperature, humidity, and rainfall are strongly associated with the abundance of both species [116]. Displacement of Ae. aegypti by Ae. albopictus because of resource competition among larvae and crossmating between Ae. albopictus males and Ae. aegypti females might reduce the overall risk of dengue transmission [115,117,118]. Continued vector surveillance is crucial in susceptible areas currently free of Ae. aegypti and Ae. albopictus.
Ae. albopictus and Ae. aegypti are present in Queensland, Florida, Singapore, and Taiwan. The former is not yet established in Monroe County, Florida [119] or in mainland Queensland, where Ae. aegypti is prevalent. In Singapore, Ae. aegypti predominates [120]. In Taiwan, Ae. aegypti only occurs south of the Tropic of Cancer (23°26′N 120°8′E, Chiayi and Hualien Counties), and Ae. albopictus is distributed throughout the country and below an elevation of 1,500 m above sea level [98].
The movement of Ae. albopictus from the Torres Strait Islands, Queensland, to the mainland, with subsequent potential expansion southwards, could increase the area that is receptive to DENV epidemics [121,122] and also expand the area receptive to transmission of other viruses including chikungunya and Zika. This is also the case in central, northern, and eastern parts of Taiwan, where only Ae. albopictus exists and outbreaks occur rarely. Ae. aegypti is thus the main vector, but Ae. albopictus can transmit dengue in the absence of Ae. aegypti [71]. The number of locally acquired cases is higher in the southern than in other parts of Taiwan, despite a lower number of imported cases. Therefore, the presence of Ae. aegypti may be essential to outbreak initiation. In Southern Florida, [116,119] Ae. aegypti is more abundant in the early dry season than in the late wet season and can spread during the early wet season in the absence of Ae. albopictus [116].
Epidemiology and Ecology of Transmission
High DENV virus replication rates are associated with a high vertical transmission rate in mosquitoes [123]. Fewer Ae. aegypti than Ae. albopictus progeny are infected vertically, although the latter species may sustain DENV during interepidemic periods [123,124]. Vertical transmission of DENV-1 from infected Ae. aegypti female mosquitoes to their eggs may have served as an interepidemic reservoir between outbreaks in successive years in Key West, Florida [125], as could low-level horizontal transmission, especially if not detected. In Taiwan, although continuation of outbreaks through winter is rare, three overwinter outbreaks occurred from1987–2010: 100,000 cases from 1987–1988; more than 5,000 cases from 2001–2002; and more than 1,400 cases from 2009–2010 [101].
Many dengue importations into Australia are presumably undetected or unreported [120]. In Charters Towers, Queensland, subterranean breeding (e.g., in septic tanks, sump pits, telecommunication pits, mine shafts, and wells) contributed significantly to Ae. aegypti populations and therefore DENV-2 transmission during the 1993 outbreak [126]. Many larval sites are cryptic and difficult to locate, particularly subterranean sites and elevated sites such as rainwater tanks and roof gutters [127]. Subterranean sump pits and wells can support large populations of Ae. aegypti, especially during the winter season when surface containers are dry [127–129]. A reduction in the number of large water storage containers likely reduced Ae. aegypti distribution in the past [130].
In Singapore, refuse is commonly an Aedes oviposition site [131]. The main breeding habitats in homes are domestic containers, flower pot plates, ornamental containers, and plants, and in public areas discarded receptacles, closed perimeter drains, gully traps, Housing and Development Board (HDB) corridor drain/gullies, and plants [132].
Increased Movement and Proximity to Endemic Regions
With increased transport of materials and people, the potential for vector transportation and virus transmission and spread has increased substantially [133]. In Queensland, Florida, and Taiwan, the arrival of viremic travelers is currently necessary for local transmission. In Australia, dengue is now more often diagnosed than malaria in ill travelers who have returned from tropical regions (except Africa) [134]. International arrivals to Townsville and Cairns airports commenced in 1980 and 1984, respectively, and consequently the number of travelers from dengue-endemic countries has increased significantly [135]. Because Queensland, Florida, Singapore, and Taiwan are popular tourist destinations, this increases the likelihood of virus introduction and subsequent local transmission.
During the 1997–1999 epidemic in North Queensland, the movement of viremic individuals facilitated multiple initiation foci [23]. Today in the US, Miami, Florida is the most used airport in terms of the number of international passengers boarding American carriers [136]. Key West is a tourist destination with more than 2 million visitors annually [137]. In 2013, Changi Airport, the main airport in Singapore, handled more than 53.7 million passengers with an annual growth rate of 4.7% [138,139]. About 203 million travelers and 68 million conveyances were reported to cross the border between the southern Malaysian state of Johor and Singapore in 2013 [140]. During the second largest DENV outbreak in Taiwan in 2014, a total of 44.8 million passengers (an increase of 12.6% over 2013) were traveling on international, cross-strait, and transit flights [141]. As a travel hub, Singapore [56] and Taiwan experience continuous importation of DENV.
Proximity to endemic regions and the increased number of dengue cases in neighboring countries presents a significant risk for countries with endemic vectors [142]. In Australia, among cases with a known source, 23% originated overseas from 1991 to 1999 and 64% from 2000 to 2012. Between 1995 and 2011, the main sources of dengue importation in Australia were from neighboring dengue-endemic countries such as Indonesia (24.6%), Papua New Guinea (23.2%), Thailand (13.4%), East Timor (8.9%), and the Philippines (6.7%) [33]. Florida is also very close to dengue-endemic countries in the Caribbean (e.g., Puerto Rico), Central America (Honduras), and South America (Brazil) and is the top US destination for Puerto Rican migrants [143]. In Singapore from 2000 to 2014, the neighboring dengue-endemic countries Indonesia and Malaysia were the two main sources of dengue importations, contributing 38% and 26%, respectively, of the total imported cases in Singapore [50–53,55,58,60–65,68]. Virus importation by tourists and migrant workers (e.g., from China in 2002 [144]) from dengue-endemic countries and decreasing herd immunity have contributed significantly to the failure of dengue control in Singapore [145]. Over the past two decades in Taiwan, imported cases have played an important role in the major outbreaks of dengue [146]. Between 1981 and 2010, imported dengue cases primarily involved travellers from the Philippines, Vietnam, Thailand, and Indonesia [101].
Housing Structure, Urbanization and Population Growth
In many tropical regions, urbanization, lack of dependable water systems, overcrowding, and poor housing contribute to large dengue epidemics [80]. This is less of a problem in HICs. However, in North Queensland and Key West, Florida, dengue transmission has been associated with old unscreened housing, allowing access to Ae. aegypti [147,148]. Typical “Queenslander” houses, designed with architectural characteristics suitable to a tropical or subtropical climate such as large sprawling timber structure on stumps, and an extensive deep shaded verandah, are being replaced by new apartment blocks designed with screening and air conditioning in Cairns and other Far North Queensland centers. These should limit dengue transmission [127] because avoidance of mosquito bites effectively prevents transmission [149]. In Queensland, sites such as backpacker hostels are identified as “ignition” or starting points, whereas centers where communities congregate, such as schools and churches, are identified as “dissemination” points [23] from which the virus may spread to multiple geographic foci [23].
Between 2006 and 2011, Australia, particularly Queensland, experienced rapid population growth [150,151], with a total of 121,000 new Queensland residents over the five years [152]. The Queensland average annual population growth was 2%, [150,151] primarily driven by overseas immigration [153]. The 2006 Census shows that the main countries of origins of new Cairns residents were the United Kingdom (UK), Japan, Philippines, Korea, and Papua New Guinea, whereas other regional areas, such as Townsville and Mackay, feature migrants prominently from the UK, India, the Philippines, and South Africa [152].
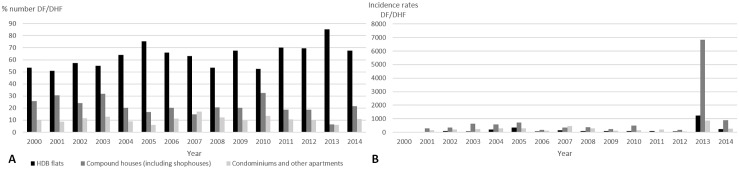
Singapore is highly urbanized with a population of 5.47 million in 2014, an increase of 1.3% from 2013, and a population density of 7,615 persons per km2 [154]; high population density increases dengue risk. In Singapore, about 80% of the houses are managed by the HDB [155]. Before the mid-2000s, most houses had bamboo pole holders outside the kitchen windows that were used to dry laundry, but also provided good breeding grounds for mosquitoes [156]. Now, the bamboo poles can be covered [157], supported from both ends, or replaced by a laundry rack. Although these changes should help prevent the breeding of Aedes mosquitoes, no studies have confirmed this. Over the period 2002–2014, residents in HDB compound houses, free standing properties (including shops and houses), and condominiums constituted 70%, 19%, and 11%, respectively, of the dengue cases. Incidence rate was higher in those living in free standing properties [51,53,55,58,60–65,68] (Fig 3).
Fig 3. Percentage and incidence rate of reported indigenous dengue fever and DHF cases by housing type for Singapore residents from 2000–2014.
In Taiwan, from 1998 to 2002, a higher level of urbanization was also associated with increased risk of dengue fever at the township level [158]. Poor environmental hygiene usually goes with rapid urbanization, which tends to increase vector breeding. It is unclear why the 2015 outbreak clustered intensively in Tainan city, but a persistent problem of illegal dumping of garbage in the local parks possibly contributed through pooling of water and consequent mosquito oviposition [159].
Weather Variability, Climate Change, and Extreme Events
Climate change may contribute to the emergence and re-emergence of dengue through its interactions with biotic and abiotic factors. However, due to the complex interaction of climate and other factors, the magnitude and direction of changes in incidence are difficult to predict.
Australian annual average daily mean temperatures have increased progressively by 0.9°C since 1910, and the frequency of extreme weather has changed, with more heat and fewer cool extremes [160]. Substantial warming has occurred in the three oceans (Pacific, Indian, Southern) surrounding Australia [161]. By 2070, Australian temperatures are projected to rise by 1–5°C, depending on global CO2 emissions. Consequently, climate sensitive diseases are expected to increase in incidence [162].
In southern Florida, climatic factors may be the cause of competition between Ae. albopictus and Ae. aegypti [163]. The presence of Ae. albopictus was significantly lower after the dry season than during the wet season, whereas the Ae. aegypti presence was consistent [163]. However, local coexistence of these species is possible when a warm dry climate favors Ae. aegypti and reduces the competition from Ae. albopictus by increasing mortality of Ae. albopictus eggs [163]. In the laboratory, dry periods cause disproportionately greater mortality of Ae. albopictus eggs compared to Ae. aegypti eggs [163].
In Singapore, from 1972 to 2014, the annual mean temperature has increased from 26.6°C to 27.7°C, and rainfall has become more intense [164]. Temperature, relative humidity, and El Niño Southern Oscillation were significantly and independently associated with dengue cases in Singapore [165]. Moreover, in Singapore and Taiwan, rainfall, temperature, and dengue fever incidence are positively related [166,167]. Health officials in Singapore reported a surge in dengue cases in December 2015, which coincided with warmer weather associated with the El Niño phenomenon [69], although no study has confirmed this.
In Taiwan, dengue transmission typically begins with importation in the summer and ends in the winter. The combination of its subtropical climate, neighboring hyperendemic countries, the hot rainy summer (average temperature, 28°C) and dry cold winter (average 20°C), and the coexistence of two vectors facilitates dengue transmission in the summer and autumn [72]. In 2007, after an initial wave of dengue infections in Taiwan, the temperature was reduced by two typhoons, and fewer cases were reported. However, in early autumn, the subsequent increase in moisture and temperature caused a second wave of dengue fever [168]. The exact causes of the large outbreak in Kaohsiung, Taiwan, in 2014 are not known, but it is believed that higher mean temperatures than in previous years (+0.5°C–1.3°C from June–September 2014) contributed [75]. The causes of the intensive and clustered 2015 outbreak in Tainan are also poorly understood. Large areas of standing water accumulated in some parks after flooding and only drained for two to three weeks may have been the primary breeding areas for mosquitoes that sustained the epidemic [159].
Drought together with population and economic growth have caused anthropogenic environmental changes. These include limited water supply relative to demand in Queensland cities, despite flooding, which has led to an increase in rainwater tank usage. Regulations and guidelines mandate or recommend measures to prevent mosquito breeding in tanks, including flap valves and screens with mesh size of no more than 1 mm [169]. Nonetheless, an increase in domestic water storage could increase future mosquito populations and consequently dengue risk [170]. In North Queensland, dengue transmission is also associated with unscreened rainwater tanks. Drought and consequent changes in water storage practices are projected to increase the Ae. aegypti range [170]. Under climate projections, shade-dependent evaporation interacts with container size and biological characteristics, such as resistance to cold and egg dessication, to determine geographic range for Ae. aegypti [171]. Simulation modelling indicates that climate warming may lead to increases or decreases in dengue vector abundance, depending on the level of carbon emissions [172].
Responses and Challenges
Public Health Responses
Queensland, Australia
Since the 1992–1993 dengue epidemic, public health authorities in north Queensland have intensified surveillance, control, and education programs (e.g., the “Tip it, Store it, Throw it” message), and the development and use of predictive tools [27]. Strategies are applied at three different activity levels: ongoing prevention; response to sporadic cases; and outbreak response [127]. When a case is notified, officials interview the patient and complete a case report form [27]. Control activities include larval and adult control within a 200 m radius of the case residence and high-risk contact areas [27]. The use of indoor residual spraying targeting resting sites of Ae. aegypti has been shown to significantly decrease dengue transmission in Cairns [173] and has become the primary adulticiding method used. Regulation of water storage to minimize mosquito breeding is particularly important for detecting and eliminating the most productive sites [174].
Florida, US
Following the introduction of West Nile virus in the northeastern US in the summer and autumn of 1999, the US Centers for Disease Control and Prevention (CDC) and the Department of Agriculture cosponsored a meeting of experts to develop guidelines for the surveillance, prevention, and control of West Nile virus and other arboviruses [175]. Dengue became a nationally notifiable disease in the US in 2009 because of concerns about the increasing number of importations and the consequent risk for local transmission and transfusion transmission [176]. During the Key West outbreaks, the Florida Keys Mosquito Control District (FKMCD) used all means available to reduce the populations of Ae. aegypti. Response strategies in Key West included public meetings and communication, education at schools, and tourism council press releases [42]. Tourism, public health, and vector control officials collaborated in response. Health department personnel conducted outreach visits to clinicians. Vector control activities include spraying adulticides within a 200 m radius around homes of case-patients, outdoor residual and spatial insecticide treatments, door-to-door campaigns to educate the public, and finding and eliminating mosquito breeding sites [42]. Florida law mandates inspections and the elimination of breeding sites; however, fines are not usually imposed and violations are frequent [42]. Since the implementation of response strategies, autochthonous dengue infections still occur in Key West, and a 2012 survey of the decision makers involved in the control of the outbreak revealed the need to focus prevention strategies on educational campaigns [177].
Singapore
The vector-control programs instigated in the late 1960s have progressively reduced dengue transmission and thus herd immunity. As a consequence, the incidence of dengue has surged since the 1990s, mainly in young adults [178]. Low herd immunity and a shift from domestic to nondomestic transmission (e.g., schools, hospitals, and workplaces) are the main issues in Singapore [179]. The number of potential breeding sites in nondomestic settings is large, and these are poorly identified and localized. The National Environment Agency (NEA) adopts a multipronged approach to control dengue comprising preventive surveillance and control, public education, and community involvement, enforcement, and research. The strategies include active surveillance in areas prone to dengue or where mosquito populations are high, and source reduction when cases are reported [180].
In Singapore, pyrethroids were commonly used for fogging in the past and are still commonly used as insecticide by the pest control industry. Consequently, Ae. aegypti has become pyrethroid-resistant in Singapore [181]. Crossresistance between dichlorodiphenyltrichloroethane and pyrethroids occurs [182]. Therefore, viable alternatives including biological control are urgently needed. However, biocontrol approaches might impose evolutionary selective pressures on the virus and vectors. The viral diversity resulting from the increase in dengue transmission over recent years and reduced mosquito population because of vector control may also have contributed to selection pressure and to the evolution of a virus lineage with better fitness; i.e., DENV that would spread better through Ae. aegypti population [91]. Although new environmentally friendly biocontrol strategies show promise, an improved understanding of the biology and ecology of the vectors is crucial [130].
The Singapore government introduced a fine in 2005, which doubled in 2008, for all homes found to be breeding mosquitoes [183]. For example, in 2012, 900 mosquito-breeding offenses were detected on construction sites, with 626 being first-time offenders [184]. To implement an effective vector-control program, more officers were recruited to conduct routine checks of homes within dengue-active zones in 2013. When residents did not respond to notices, the NEA used its legislative powers to gain entry to homes [185]. The NEA fined a town council US$160 (in 2013 US dollars) for letting mosquitoes breed in water tanks. Town councils which break the law twice could be fined up to US$1,500. As of August 2014, the NEA has inspected 1.9 million houses or premises and has deployed more than 1,000 gravitraps in dengue clusters for mosquito-control purposes, in addition to regular fogging and space spraying in certain hotspots. The NEA has prosecuted 14 contractors and issued 62 stop-work orders [186]. Since March 14, 2016, the owners of homes found to be breeding mosquitoes are fined US$148 (in 2016 US dollars), regardless of whether the home is within a dengue cluster [187].
Since 2015, the NEA has also increased its epidemiological investigation to identify potential sources of infection other than registered residential addresses, such as workplaces and areas of congregation. NEA improved its dengue control by i) increasing inspections by creating dedicated construction site teams, ii) publishing the list of sites issued with stop-work orders on the dengue microsite to serve as a deterrent to contractors, iii) requesting contractors to put in place a temperature-screening regime to identify cases earlier, iv) encouraging application of insect repellent and, v) for dengue-infected workers, to sleep under bed nets or in air-conditioned sick bays to prevent further transmission [188].
Taiwan
Before 2009, the Taiwan Centers for Disease Control (TCDC) used only a clinical case definition for reporting dengue, which may have led to over-reporting. The TCDC’s current dengue case definition, established in 2009 and in use since, requires laboratory confirmation for reported cases [189]. Dengue must be reported to TCDC within 24 hours [72]. A central command center was established to provide resources and personnel from various ministries (Education, Interior, National Defense) and agencies (department of health, environmental protection administration, government information office). The aim was to foster close cooperation with local government units and public education to reduce vector breeding. These efforts have been associated with reduced case numbers from 2010 to 2012 [70]. However, it is difficult to confirm this trend because of the short period.
When Aedes larvae are found or when an individual refuses control measures, a significant fine is imposed [190]. Taiwan had also established airport fever screening [191]. Questionnaire screening was used from 1998 to 2002, but following the 2003 severe acute respiratory syndrome outbreak, fever screening at the airport was added. Fever screening at the airport has been successful in identifying 45% of 542 known imported dengue cases with fever [191]. However, the intensity of local transmission is not proportional to the number of imported cases [191]. Pyrethroids are also often used to control Ae. aegypti and thereby suppress epidemics in Taiwan. However, Ae. aegypti has also become pyrethroid-resistant in Taiwan [192].
Community Involvement
Singapore has in place community-based programs for the removal of potential breeding habitats [193], which involve the community in mosquito control through environmental management, health education, and community ownership (“bottom-up” approach) (e.g., “Do the Mozzie Wipeout” Campaign, “Do the 5-Step Mozzie Wipeout”) [194]. This is essential for avoiding community complacency. However, the community-based approach can be slow or demotivating and, as a consequence, unsustainable [195]. Bottom-up and top-down approaches should be combined [195].
In addition to the proactive measures against mosquito-breeding sites, efforts have been made to increase opportunities for people to interact through social media focused on dengue transmission. Examples include “Stop Dengue Now” and “Amy Khor” in Singapore and “Follow the Moz” or “Eliminate Dengue” Facebook pages in Queensland. Unfortunately, the latter two are no longer updated. Although the use of these forms of online social media cannot provide a solution for reducing dengue transmission, it empowers citizens by giving them easy access to knowledge and by proposing a form of participatory surveillance involving mutuality and sharing.
Singapore citizens actively voice opinions on social media about dengue. For example: “(…) Each time an NEA officer knocks on my door, I cooperate with them but at the same time, I wonder whether the door-to-door checks aren't a waste of time when my HDB neighbourhood is strewn with litter such as empty cigarette packs, plastic bags, and dirty food containers. Each piece of litter is a breeding ground (…)” [196], “Residents must take greater responsibility to rid their homes of mosquitoes breeding. I have often witnessed households not responding to NEA officers knock (…)” [197]. Local residents in Singapore also have the opportunity to volunteer in efforts to control dengue. Dressed in yellow vests and red shirts, they go from door to door encouraging their neighbors to assist in controlling dengue [198].
A smartphone application designed to encourage citizens to identify and report potential breeding sites of Aedes mosquitoes is also available (e.g., TrashWatch, http://www.vectoranalytica.com/inc/products/trashwatch/). However, some countries, including Australia, do not have access to this application.
Challenges
From 2012–2013, the Queensland Government budget cuts impacted hospital and health services in Queensland, mainly in Metro North (–22.5 million Australian Dollars) and Metro South (–18.8 million) areas in Brisbane, Gold Coast (–9.2 million), Townsville (–7.8 million), Sunshine Coast (–7 million), and Cairns (–6.5 million) [199]. These cuts have impaired dengue prevention and control programs [200]. Moreover, unlike the US, Singapore, and Taiwan, Australia lacks a single center, such as the CDC, for dissemination of information to the public, relevant organizations, and government agencies. In the US, public health budget cuts have also impaired vector surveillance and control programs [201]. In 2011, the Florida Department of Health Infectious Disease Control branch budget was reduced by US$3.8 million with consequent loss of 172 full-time positions in health departments [202]. In early 2016, the President of the US asked Congress to allocate US$1,900 million to surveillance and control of the Zika virus, Ae. Aegypti, and Ae. albopictus, but, to date, lawmakers had not acted [203]. In Florida, the mosquito control budget varies greatly between counties. Lee County, home to Fort Myers and 700,000 people [204], funded by local property taxes, spends $16 million a year and has a staff of 88, ten helicopters, and four propeller planes for spraying [205]. In contrast, Vasquez, an entomologist and director of the mosquito-control district of Florida’s Miami Dade County, Florida’s biggest county with 2.6 million people, spends a $1.6 million budget a year, enough for 15 employees. Moreover, his inspectors are not always welcomed by the community and have to deal with denial, angry homeowners, and, sometimes, local politics [203].
Early recognition and notification of dengue cases [33] and timely initiation of appropriate supportive care is critical to reduce medical complications and mortality among patients with severe forms of dengue [206]. In Florida, at least until 2010, medical and public health practitioners tended to have limited experience with dengue, and the failure of diagnosis and reporting led to delayed detection of DENV introduction [7]. In the Key West 2009–2010 outbreak, initial cases were attributed by local physicians to nonspecific viral illness without consideration of dengue [207]. Although Singapore generally has an efficient clinical service, hospitals are sometimes crowded [208].
Because mosquitoes sufficient to infect an entire neighborhood can breed in a small number of negligently maintained properties, citizen cooperation is indispensable [209]. However, cooperation between citizens and public officials is often lacking, and there may be outright obstruction of mosquito-control efforts. Unfortunately, control is often seen as the exclusive responsibility of local and state mosquito-control agencies [89].
Transfusion-transmitted dengue is rare [210], but no licensed donor-screening test for DENV is available worldwide. Consequently, many blood donations are unable to be collected during epidemics [211]. Rigorous selection of donors offers protection but is labour intensive, costly, may affect sufficiency of supply, and may not be possible.
Lessons and Recommendations
What Do These Areas Have in Common?
The countries and states presented in this review have in common i) a competent mosquito vector, ii) introduction of DENV by travelers, iii) increased local transmission of dengue coinciding with population growth and increased mobility, iv) recent budget cuts impacting public health services, and v) a largely nonimmune population.
The four countries and states have areas where Ae. aegypti is established. Outbreaks rarely occur where only Ae. albopictus is present (e.g., central, northern, and eastern parts of Taiwan and the Torres Straits Islands in Australia). The four countries and states are surrounded by dengue-endemic countries and have air connections with dengue-endemic countries. In Queensland, Florida, and Taiwan, the arrival of viremic travelers is a prerequisite for local transmission. Both Taiwan and Singapore are highly populated and urbanized, increasing dengue transmission risk. Both Florida and Queensland have experienced past budget cuts that affected public health services. Florida and Queensland are particularly at risk for autochthonous dengue outbreaks due to its largely nonimmune populations.
What Is Unique?
The countries and states presented in this review also display unique features, such as i) their dengue epidemic/hyperendemic status, and consequent mortality rate, ii) the frequency, amplitude, and duration of outbreaks, iii) their vector control program and community engagement.
Dengue is hyperendemic in Singapore and Taiwan, but epidemic in Queensland and Florida, and the rarity or absence of serious outcomes in Australia and Florida may be due to the infrequency of consecutive infections with different serotypes. DHF and deaths are rare in Australia, and absent in Florida, but occur often in Singapore and Taiwan. Mortality is usually linked to delayed provision of supportive treatment or presence of comorbidities [212].
The recent epidemiology of dengue in Singapore was characterized by a 5–6-year cycle [9,56]. However, since 2013 in Singapore, the incidence rates have markedly increased, exceeding 10,000 cases each year. Dengue transmission in Florida is still reasonably limited, and the majority of dengue cases reported are travel-associated cases [88].
In Queensland, the “Tip it, Store it, Throw it” message is now being heard by the local community according to Queensland Health’s Cairns acting director of tropical public health services [35]. In Far North Queensland, the number of dengue fever cases in 2015 was 33% lower than the total dengue cases in 2014 [36]. Compare to the other three countries and states, Singapore citizens have more opportunities to engage on social media about dengue, to be aware of the problem, and thus to be proactive. However, despite the hard work of contractors engaged by the NEA to clean out certain private estates and public areas throughout Singapore, littering remains an environmental and social problem linked to economic and health issues [131].
Management Options in the Near Future
This review summarises key determinants for dengue transmission, which could be better managed to help reduce transmission (Table 2). Some are relevant for many countries and states (e.g., proximity to endemic countries, population growth and movement, lack of awareness, and engagement of residents/tourists) and some are country/region specific (e.g., high level of urbanization, budget cuts, housing structure).
Table 2. Key determinants for ongoing dengue burden in Queensland (Australia), Singapore, Taiwan, and Florida (US) and recommendations.
| Key determinants | Key recommendations | |
|---|---|---|
| Queensland, Australia |
|
|
| Florida, US |
|
|
| Singapore |
|
|
| Taiwan |
|
Vaccine
The world’s first dengue vaccine, Dengvaxia (CYD-TDV) by Sanofi Pasteur, first registered in Mexico early December 2015, and later approved by the Philippines and Brazilian authorities, has provided some hope [213]. CYD-TDV is a live recombinant tetravalent dengue vaccine that has been evaluated as a three-dose series on a 0/6/12 month schedule in Phase III clinical studies, and is for use in individuals aged 9–45 years living in endemic areas [213]. This vaccine is currently being reviewed by around 20 countries in Asia and Latin America [214]. The Phase III trials showed a variation in vaccine efficacy according to the age at vaccination and serostatus before vaccination [213,215]. Five additional vaccine candidates are under evaluation in clinical trials [213]. Recommendations concerning the CYD-TDV vaccine will be published by the World Health Organization in July 2016 [213].
Vector and Virus Control
In 2012, the FKMCD announced that it had partnered with the British firm Oxitec to release large numbers of genetically modified Ae. aegypti males in Key West, Florida [89]. This project was considered but rejected by some residents, and the use of genetically modified male mosquitoes in Key West is still in negotiations and is awaiting the US Food and Drug Administration approval [89,216]. The re-emergence of dengue in Florida as well as the threat posed to the US from other emerging mosquito-borne arboviruses (e.g., chikungunya, Zika viruses) emphasizes the need for strong vector-borne disease surveillance and mosquito-control infrastructure to rapidly identify and control outbreaks of dengue or other mosquito-borne diseases.
Transinfection of Ae. aegypti with the endosymbiotic bacterium Wolbachia pipientis is undergoing field trials in Australia, Indonesia, Vietnam, Brazil, and Columbia [217]. Some strains of Wolbachia can reduce Ae. aegypti lifespan and reproduction and thus interfere with DENV replication and transmission [218,219]. The virulent Wolbachia strain wMelPop has been shown to cause widespread degeneration of tissues (brain, retina, muscle) and early death in Ae. aegypti adults [220] and eggs [221] but failed to establish following field releases. Releases of the more benign wMel strain led to establishment in seven Cairns suburbs (infection frequency > 90%), and this strain has persisted for at least five years [222,223]. A field trial was later launched in Townsville, North Queensland, in October 2014 [224]. In Singapore, the NEA’s Environmental Health Institute has tested the use of Wolbachia bacteria in the laboratory, but there have not been field trials. Reliance on insecticides for dengue control has been excessive [225], and these compounds are costly and sometimes ineffective in urban areas.
Modeling and Geographic Information Systems
Such deficiencies and limitations of current vector-control strategies have necessitated the development of predictive mathematical, statistical, or spatial models for dengue early warning systems [225–228]. The development of a climate-driven spatiotemporal prediction model and the use of geographic information system in dengue surveillance are essential to inform disease prevention and control interventions. Yu et al. (2011) [166] proposed a spatiotemporal climate-based model of early dengue fever warning in southern Taiwan, based on stochastic Bayesian Maximum Entropy analysis. The analysis provides the required “one-week-ahead” outbreak warnings based on spatiotemporal predictions of dengue fever distributions, that can be used by the Taiwan Disease Control Agency to timely identify, control, and prevent dengue fever transmission [166]. Hii et al. (2012) [229] developed a weather-based dengue forecasting model that allows warning 16 weeks in advance of dengue epidemics in Singapore with high sensitivity and specificity. The authors demonstrate that models using temperature and rainfall could be simple, precise, and low cost tools for dengue forecasting and could be used to enhance decision making on the timing and scale of vector control programs [229]. However, these tools also have limitations because the factors used for prediction may interact nonlinearly [230]. Models may also under- or overestimate the projected incidence, and they are often regionally specific and consequently not suitable for global projections. Similarly, global models [231] are not necessarily appropriate for small-scale projections. Geographic information system technology is a useful tool for creating a user-friendly map to show the physical location of dengue cases from authoritative sources such as the NEA (e.g., Outbreak, http://outbreak.sgcharts.com/). Finally, traveller behaviour, vector evolution, biocontrol impacts, and other important risks are unpredictable.
Conclusion
Changes in climatic conditions, patterns of human settlement, movement, and population density, distribution of the two main mosquito vectors, and water-management technologies have all influenced the occurrence of dengue outbreaks since the mid-20th century. The competitive relationship between the two main vectors, Ae. aegypti and Ae. Albopictus, may lead to future changes in the epidemiology of the disease. This review has considered dengue transmission in Queensland in Australia, Singapore, Taiwan, and Florida in the US. We have identified important epidemiological issues in these regions, such as population growth in the four settings, increases in local transmission despite control efforts in Singapore and Taiwan, increased human mobility from neighboring endemic countries (especially in Taiwan and Singapore), lack of citizen engagement in the four settings, and highly populated urban areas (in Taiwan and Singapore). Budget cuts in health and the current absence of a practical vaccine contribute to an increased risk.
In the HICs discussed, dengue has become an increasing challenge, despite active vector control programs. However, it would be naïve to assume a single cause, or even a small set of causes, is adequate to explain the change in dengue epidemiology in any or all of the countries discussed. Host, agent, and environmental factors all interact. These include demography, travel, strain variation, and changes in housing and the global environment. Because the factors affecting dengue incidence and transmission are diverse, a single model for disease control is unlikely to be applicable in all settings. Assuming ongoing global economic growth, other countries where Ae. aegypti and/or Ae. albopictus are established (e.g., France and Japan) may, in future experience, regulate dengue transmission [232,233]. The epidemiology of dengue will continue to change. Although Ae. aegypti is the dominant urban vector, Ae. albopictus can also cause outbreaks, especially in less tropical areas. Flexibility, ingenuity, and imagination will be required to control dengue in the face of these challenges.
Key Learning Points
Increased human mobility from neighboring endemic countries.
Lack of awareness and engagement of residents/tourists/migrant workers (excess of litter is a recurrent issue).
Resistance to pyrethroids insecticides.
Budget cuts in health.
Top Five Papers
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature- Letter Research 2013:5. doi: doi:10.1038/nature12060.http://dx.doi.org/10.1038/nature12060
Adalja AA, Sell TK, Bouri N, Franco C. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg Infect Dis. 2012 http://dx.doi.org/10.3201/eid1804.110968 1.
Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–3. doi: http://dx.doi.org/10.1016/S0966-842X(01)02288-0.
Hayden MH, Cavanaugh JL, Tittel C, Butterworth M, Haenchen S, Dickinson K, et al. Post outbreak review: Dengue preparedness and response in Key West, Florida. Am J Trop Med Hyg. 2015;93(2):397–400.http://www.ajtmh.org/content/93/2/397.abstract
Ooi E-E, Goh K-T, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–93.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3373041/
Supporting Information
(DOCX)
Acknowledgments
We are indebted to the private and public health laboratories and health departments in the states and territories of Australia that collect the data provided by the National Notifiable Diseases Surveillance System, Australian Government, Department of Health, and Queensland Health. We thank the Ministry of Health, Singapore for generously providing dengue surveillance data. Australian governments fund the Australian Red Cross Blood Service to provide blood, blood products, and services to the Australian community. We also thank Alexandra Raulli, Tropical Public Health Unit, Queensland Health. We acknowledge the late Susan Butler for her constructive editing of the early versions of the manuscript, the late Emeritus Professor Tony J. McMichael for his inspirational advice and Emeritus Professor Adrian Sleigh for his valuable support and advice. We acknowledge copyediting of the manuscript by Laurel T. Mackinnon, PhD, ELS.
Funding Statement
This work was financially supported by the National Health and Medical Research Council [Project Grant APP1003371] https://www.nhmrc.gov.au/ Principal Investigator D.Harley. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature- Letter Research 2013:5 10.1038/nature12060. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Health estimates 2014 summary tables: Deaths by cause, age and sex, by World Bank income group category, 2000–2012 [Internet]. World Health Organization, Geneva, Switzerland, http://www.who.int/healthinfo/global_burden_disease/en/. June 2014 [Accessed: 04 May 2016]; [Google Scholar]
- 3.The World Bank. Updated Income Classifications. 2014 [Accessed: 19/09/2014]. Available from: http://data.worldbank.org/news/2015-country-classifications.
- 4.Shepard D, Undurraga E, Halasa Y. Economic and disease burden of dengue in southeast Asia. PLoS Negl Trop Dis. 2013;7(2):e2055 10.1371/journal.pntd.0002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clinical Epidemiology. 2013. 5(1):299–309. 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco L, Lee L, Lee V, Ooi E, Shepard D, Thein T. Economic impact of dengue illness and the cost-effectiveness of future vaccination programs in Singapore. PLoS Negl Trop Dis. 2011;5(12):e1426 10.1371/journal.pntd.0001426.http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0001426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Shea J, Niekus MR. Potential social, economic, and health impacts of dengue on Florida. Adv Trop Med Pub Health Int. 2013;3(2):32–64.http://www.scopemed.org/?mno=36744 [Google Scholar]
- 8.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016. 10.1016/S1473-3099(16)00146-8 [DOI] [PubMed] [Google Scholar]
- 9.Koh BKW, Ng LC, Kita Y, Tang CS, Ang LW, Wong KY, et al. The 2005 dengue epidemic in Singapore: Epidemiology, prevention and control. Ann Acad Med Sing. 2008;37:538–45.http://www.annals.edu.sg/pdf/37VolNo7Jul2008/V37N7p538.pdf [PubMed] [Google Scholar]
- 10.Goh K. Dengue—a re-emerging infectious disease in Singapore. Annals of the Academy of Medicine, Singapore. 1997;26:664–70. [PubMed] [Google Scholar]
- 11.Sipe NG, Mayere-Donehue S, Dedekorkut-Howes A. Measuring urban form: a comparative analysis of South East Queensland and South Florida. The 3rd World Planning Schools Congress; 4–7 July 2011; Perth, WA2011.
- 12.Administration NOaA. NSTA INTERACTIVE: Climate Zones.http://oceanservice.noaa.gov/education/pd/oceans_weather_climate/media/climate_zones.swf
- 13.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World Map of Köppen-Geiger Climate Classification updated. Meteorol Z. 2006;15:259–63. [Google Scholar]
- 14.Dale PE, Carlson DB, Easton CS. Four degrees of latitude: mosquito control on the "right" coast of Australia and Florida, USA? J Am Mosq Control Assoc. 2008;24(3):11. [DOI] [PubMed] [Google Scholar]
- 15.USGS. National Gap Analysis Program (GAP) | Land Cover Data Viewer 2016 [Accessed: 16 May 216]. Available from: http://gis1.usgs.gov/csas/gap/viewer/land_cover/Map.aspx.
- 16.GBRMPA. Coastal ecosystems management—case study: urban planning. Townsville: 2013. [Accessed: 16 May 2016];Available from: http://elibrary.gbrmpa.gov.au/jspui/bitstream/11017/2900/2/Case_study_urban_planning_Mt_Peter.pdf
- 17.International Society for Infectious Diseases. ProMED-mail [Internet]. [Accessed: 28 November 2014]; Available from: http://www.promedmail.org/
- 18.European Centre for Disease Prevention and Control. Eurosurveillance [Internet]. [Accessed: 28 November 2014]; Available from: http://www.eurosurveillance.org/Public/Search/SearchResults.aspx
- 19.Centre for Diseases Control. DengueMap [Internet]. [Accessed: 28 November 2014]; Available from: http://www.healthmap.org/dengue/en/
- 20.Media Centre_Dengue and severe dengue [Internet]. 2016; April 2016 [Accessed: 20 April 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/index.html
- 21.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention, and control. 2nd ed Geneva: World Health Organization; 1997. viii, 84 p. p. [Google Scholar]
- 22.Hanna JN, Ritchie SA, Merritt AD, van den Hurk AF, Phillips DA, Serafin IL, et al. Two contiguous outbreaks of dengue type 2 in north Queensland. Med J Aust. 1998;168(5):221–5. ISI:000072647700006; [DOI] [PubMed] [Google Scholar]
- 23.Ritchie SA, Hanna JN, Hills SL, Piispanen JP, McBride WJH, Pyke A, et al. Dengue control in north Queensland, Australia: Case recognition and selective indoor residual spraying. Dengue Bulletin. 2002;26:7–13.http://apps.who.int/iris/bitstream/10665/163762/1/dbv26p7.pdf [Google Scholar]
- 24.Hanna JN, Ritchie SA, Phillips DA, Serafin IL, Hills SL, van den Hurk AF, et al. An epidemic of dengue 3 in far north Queensland, 1997–1999. Med J Aust. 2001;174(4):178–82. ISI:000167135900007.http://europepmc.org/abstract/MED/11270758 [DOI] [PubMed] [Google Scholar]
- 25.Medical Society of Victoria. The Australian Medical Journal. 117 Collins-street east, Wednesday, May 7, 1873
- 26.Dillon J, McBride WJH. Dengue fever in Queensland, 2008–9. Official Journal of The Australasian College of Tropical Medicine. 2014;15(2). [Google Scholar]
- 27.Health Queensland. Queensland. Dengue fever management plan (DMP) 2010–2015 2011;Available from: http://www.health.qld.gov.au/dengue/documents/dengue-mgt-plan.pdf
- 28.Hanna JN, Ritchie SA, Hills SL, Pyke AT, Montgomery BL, Richards AR, et al. Dengue in north Queensland, 2002. Commun Dis Intell. 2003;27: 384–9.http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-cdi-2003-cdi2703-htm-cdi2703k.htm [PubMed] [Google Scholar]
- 29.Hanna JN, Ritchie SA, Richards AR, Taylor CT, Pyke AT, Montgomery BL, et al. Multiple outbreaks of dengue serotype 2 in north Queensland, 2003/04. Aust N Z J Public Health. 2006;30(3):220–5. 10.1111/j.1467-842X.2006.tb00861.x. ISI:000241262700004. 10.1111/j.1467-842X.2006.tb00861.x [DOI] [PubMed] [Google Scholar]
- 30.McBride WJH. Deaths associated with dengue haemorrhagic fever: the first in Australia in over a century. Med J Aust. 2005;183(1):35–7. ISI:000230951600015.https://www.mja.com.au/journal/2005/183/1/deaths-associated-dengue-haemorrhagic-fever-first-australia-over-century?0=ip_login_no_cache%3Da350eda06e11bfb664626a74214684a1 [DOI] [PubMed] [Google Scholar]
- 31.State of Queensland (Queensland Health). Queensland dengue management plan 2015–2020.July 2015 03/05/2016. Available from: https://www.health.qld.gov.au/publications/clinical-practice/guidelines-procedures/diseases-infection/governance/dengue-mgt-plan.pdf.
- 32.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, et al. An explosive epidemic of DENV-3 in Cairns, Australia. Plos One. 2013;8(7):e68137 10.1371/journal.pone.0068137.http://dx.doi.org/10.1371%2Fjournal.pone.0068137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viennet E, Ritchie S, Faddy H, Williams C, Harley D. Epidemiology of dengue in a high-income country: a case study in Queensland, Australia. Parasit Vectors. 2014;7(1):379 10.1186/1756-3305-7-379.http://www.parasitesandvectors.com/content/7/1/379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tropical public health news. [Internet]. 2015. Tropical Public Health Services (Cairns) quarterly newsletter; [3]. Available from: https://www.health.qld.gov.au/cairns_hinterland/docs/tph-news-mar15.pdf
- 35.Dengue season draws to a close in Far North [Internet]. 2015 [Accessed: 05 May 2016]. Available from: http://www.cairnspost.com.au/lifestyle/dengue-season-draws-to-a-close/news-story/b5786cff2409d148ec865ff0ab95dd1c
- 36.National Notifiable Diseases Surveillance System. Number of notifications of Dengue virus infection*, received from State and Territory health authorities in the period of 1991 to 2015 and year-to-date notifications for 2016 [Internet]. Australian Government- Department of Health. 2016 [Accessed: 15 May 2016]; Available from: http://www9.health.gov.au/cda/source/rpt_4.cfm
- 37.Añez G, Rios M. Dengue in the United States of America: a worsening scenario? BioMed Research International. 2013;2013:13 10.1155/2013/678645. 10.1155/2013/678645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radke EG, Gregory CJ, Kintziger KW, Sauber-Schatz EK, Hunsperger EA, Gallagher GR, et al. Dengue Outbreak in Key West, Florida, USA, 2009. Emerg Infect Dis. 2012;18(1):135–7. 10.3201/eid1801.110130. PMC3310087.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3310087/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Health Florida. Archive press releases [Internet]. [Accessed: 24 December 2014]; Available from: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/archive-press-releases.html
- 40.U.S. Department of the Interior, U.S. Geological Survey. Dengue fever (imported) Human [Internet]. CDC. 2014 [Accessed: 10 December 2015]; Available from: http://diseasemaps.usgs.gov/dep_us_human.html
- 41.U.S. Department of the Interior, U.S. Geological Survey. Dengue fever (locally acquired) Human [Internet]. CDC. 2014 [Accessed: 10 December 2015]; Available from: http://diseasemaps.usgs.gov/del_us_human.html
- 42.Adalja AA, Sell TK, Bouri N, Franco C. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg Infect Dis. 2012. 10.3201/eid1804.110968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teets FD, Ramgopal MN, Sweeney KD, Graham AS, Michael SF, Isern S. Origin of the dengue virus outbreak in Martin County, Florida, USA 2013. Virology Reports. 2014;1–2:2–8. 10.1016/j.virep.2014.05.001.http://www.sciencedirect.com/science/article/pii/S2214669514000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florida reports 1st locally transmitted dengue fever case in 2015 in Broward County [Internet]. 26 December 2015 [Accessed: 05 May 2016]. Available from: http://outbreaknewstoday.com/florida-reports-1st-locally-transmitted-dengue-fever-case-in-2015-in-broward-county-67432/
- 45.Hsieh C-J, Chen M-J. Epidemiological trends of dengue infection in Taiwan. European Journal of Health. 2013;5http://www.scik.org/index.php/ejh/article/view/1139/519 [Google Scholar]
- 46.Health Florida. Florida Arbovirus Surveillance—Week 1: December 29, 2013—January 4, 2014. 2014. [Accessed: 04 May 2016];Available from: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/2014/week1arbovirusreport-1-4-14.pdf
- 47.Health Florida. Florida Arbovirus Surveillance—December 30, 2012 –January 5, 2013 2013. [Accessed: 04 May 2016];Available from: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/2013/week1arbovirusreport-1-5-2013_corrected.pdf
- 48.Health Florida. Florida Arbovirus Surveillance- Week 53: December 28, 2014 –January 3, 2015. 2015. [Accessed: 04 May 2016];Available from: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/2014/week53ArbovirusReport-1-3-15.pdf
- 49.Health Florida. Florida Arbovirus Surveillance Week 2: January 10–16, 2016—Arbovirus surveillance in Florida includes endemic mosquito-borne viruses 2016.http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/2016/week2arbovirusreport-1-16-16.pdf
- 50.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2000. 2001 01 Nov 2011. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2001/2/chap2_00.pdf
- 51.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2006. 2007 01 Jan 2007. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2007/2/MOH%20Annual%20Report%2006_full%20version.pdf
- 52.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2001. 2002 01 Jan 2007. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2002/3/chap2_01.pdf
- 53.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2004. 2005 01 Jan 2005. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2005/the_communicablediseasesurveillanceinsingapore2004.html
- 54.Publication of the Committee on Epidemic Diseases. Dengue control in Singapore. Epidemiological News Bulletin 2003;29(5).https://www.moh.gov.sg/content/dam/moh_web/Statistics/Epidemiological_News_Bulletin/2003/enb05_03w.pdf [Google Scholar]
- 55.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2005. 2006 01 Jan 2006. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2006/2/MOH_annual_06_full_version.pdf
- 56.Lee KS, Lai YL, Lo S, Barkham T, Aw P, Ooi PL, et al. Dengue virus surveillance for early warning, Singapore. Emerg Infect Dis. 2010;16(5):847–9. ISI:000277209900018.<Go to ISI>://000277209900018 10.3201/eid1605.091006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ler TS, Ang LW, Yap GSL, Ng LC, Tai JC, James L, et al. Epidemiological characteristics of the 2005 and 2007 dengue epidemics in Singapore—similarities and distinctions. Western Pacific Surveillance and Response Journal. 2011;2(2):24–9. 10.5365/wpsar.2010.1.1.011.http://www.wpro.who.int/wpsar/volumes/02/2/2011_OR_Ler_etal/en/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2007. 2008 01 Jan 2008. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2008/2/full%20version%20report%202007.pdf
- 59.Thein TL, Ooi EE, Low JGH, Leo YS. Differentiating early adult dengue from acute viral respiratory infections—A comparative analysis. Dengue Bulletin. 2011;35:76–83. [Google Scholar]
- 60.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2013 [Internet]. 2014 [Accessed: 18 Dec 2014]; Available from: https://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2014/communicable-diseases-surveillance-in-singapore-2013.html
- 61.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2008. 2009 02 Jan 2009. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2009/Full%20version%20of%20the%20report%202008.pdf
- 62.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2009. 2010 01 Oct 2010. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2010/2/Full%20Version%20of%20the%20Report%202009.pdf
- 63.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2010. 2011 01 Nov 2011. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2011/Communicable%20Diseases%20Surveillance%20in%20Singapore%202010/Full%20Version%20of%20the%20Report%202010.pdf
- 64.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2011. 2012 15 Nov 2012. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2012/Communicable%20Diseases%20Surveillance%20in%20Singapore%202011/Full%20Version%20of%20the%20Report%202011.pdf
- 65.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2012. 2013 22 Nov 2013. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2013/Full%20Version.pdf
- 66.National Environment Agency. Latest dengue data [Internet]. 2014. [Accessed: 02 January 2015]; Available from: http://www.dengue.gov.sg/subject.asp?id=73
- 67.Ministry of Health Singapore. Weekly infectious disease bulletin—epidemiological week 52, 27 Dec-2 Jan 2016. 2016. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Statistics/Infectious_Diseases_Bulletin/2015/December/2015_week_52.pdf
- 68.Ministry of Health Singapore. Communicable Diseases Surveillance in Singapore 2014. 2015 11 Dec 2015. [Accessed: 04 May 2016];Available from: https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2015/Full%20Version.pdf
- 69.Singapore reports December surge in dengue fever with warmer weather [Internet]. 01/01/2016 [Accessed: 04 May 2016]. Available from: http://outbreaknewstoday.com/singapore-reports-december-surge-in-dengue-fever-with-warmer-weather-34915/
- 70.Liu YT, Wang JH, Wu JW, Lee CC, Lin YC, Liu CC, et al. Operation status and experience of running central epidemic command center for dengue fever in 2010 Taiwan Epidemiol Bull. 2012;23(13):193–207. [Google Scholar]
- 71.Lei H-Y, Huang J-H, Huang K-J, Chang C. Status of dengue control programme in Taiwan—2001. Dengue Bulletin. 2002;26:14–23.http://apps.searo.who.int/PDS_DOCS/B0222.pdf [Google Scholar]
- 72.Lin C-C, Huang Y-H, Shu P-Y, Wu H-S, Lin Y-S, Yeh T-M, et al. Characteristic of dengue disease in Taiwan: 2002–2007. Am J Trop Med Hyg. 2010;82(4):731–9. 10.4269/ajtmh.2010.09-0549.http://www.ajtmh.org/content/82/4/731.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Diseases Control, Ministry of HEALTH and Welfare. 2013–2014 Centers for Disease Control Annual Report Taiwan: 2014;Available from: http://www.cdc.gov.tw/uploads/files/201408/533702a2-eedd-40da-8570-c2e15369825d.pdf
- 74.Press releases—As number of new dengue fever cases confirmed last week reaches this year’s new record high, Taiwan CDC urges public to clean up and remove vector-breeding sites to prevent dengue fever transmission [Internet]. 29/10/2013; 21/12/2014. Available from: http://www.cdc.gov.tw/english/info.aspx?treeid=bc2d4e89b154059b&nowtreeid=ee0a2987cfba3222&tid=263A8C7B0103C30B
- 75.Wang S-F, Chang K, Lu R-W, Wang W-H, Chen Y-H, Chen M, et al. Large Dengue virus type 1 outbreak in Taiwan. Emerg Microbes Infect. 2015;4:e46 10.1038/emi.2015.46. 10.1038/emi.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang SF, Wang WH, Chang K, Chen YH, Tseng SP, Yen CH, et al. Severe Dengue Fever Outbreak in Taiwan. Am J Trop Med Hyg. 2016;94(1):193–7. Epub 2015/11/18. 10.4269/ajtmh.15-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.CECC for Dengue Outbreak announces new deaths associated with dengue and urges public to reinforce cleaning and emptying of vector breeding sites to lower risk of transmission [Internet]. 2015; 2015-12-23 [Accessed: 04 May 2016]. Available from: http://www.cdc.gov.tw/english/info.aspx?treeid=bc2d4e89b154059b&nowtreeid=ee0a2987cfba3222&tid=C294BB0FF01A2E5C
- 78.Hare FE. The 1897 epidemic of dengue in north Queensland. The Australasian Medical Gazette. 1898;17:98–107. [Google Scholar]
- 79.Hanna JN, Ritchie SA. Outbreaks of dengue in north Queensland, 1990–2008. Commun Dis Intell. 2009;33(1):1–88.http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3301e.htm [PubMed] [Google Scholar]
- 80.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–3. 10.1016/S0966-842X(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 81.Leggat PA. Dengue in northern Queensland, Australia: Risk from travellers or risk to travellers? Trav Med Infect Dis. 2009;7(4):212–4. ISI:000207943000007; [DOI] [PubMed] [Google Scholar]
- 82.Warrilow D, Northill JA, Pyke AT. Sources of dengue viruses imported into Queensland, Australia, 2002–2010. Emerg Infect Dis. 2012. 18(11):1850–7. 10.3201/eid1811.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.NNDSS Annual Report Writing Group. Australia's notifiable disease status, 2010: Annual report of the National Notifiable Diseases Surveillance System Commonwealth of Australia 2012, March 2012 [PubMed]
- 84.McElroy KL, Santiago GA, Lennon NJ, Birren BW, Henn MR, Munoz-Jordan JL. Endurance, refuge, and reemergence of dengue virus type 2, Puerto Rico, 1986–2007. Emerg Infect Dis. 2011;17(1):64–71. ISI:000285904600010.http://wwwnc.cdc.gov/eid/article/17/1/pdfs/10-0961.pdf 10.3201/eid1701.100961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, Sonnenburg FV, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. The New England Journal of Medicine. 2006;354(2):119–30. [DOI] [PubMed] [Google Scholar]
- 86.Hribar LG. Influence and impact of mosquito-borne diseases on the history of Florida, USA. Life Excit Biol 2013;1(53–68). [Google Scholar]
- 87.Brathwaite Dick O, San Martín JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg. 2012;87(4):584–93. 10.4269/ajtmh.2012.11-0770.http://www.ajtmh.org/content/87/4/584.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Health Florida. Dengue fever.Available from: http://www.floridahealth.gov/diseases-and-conditions/dengue/
- 89.Rey JR. Dengue in Florida (USA). Insects. 2014;5:991–1000. 10.3390/insects5040991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Health Florida. Martin County dengue outbreak and serosurvey. Martin County, Florida [Internet]. 2014 Available from: http://www.floridahealth.gov/%5C/diseases-and-conditions/dengue/_documents/mc-dengue-survey-summary-june2014.pdf
- 91.Lee K-S, Lo S, Tan SS-Y, Chua R, Tan L-K, Xu H, et al. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infection, Genetics and Evolution. 2012;12(1):77–85. 10.1016/j.meegid.2011.10.012 10.1016/j.meegid.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 92.Yap G, Li C, Mutalib A, Lai Y-L, Ng L-C. High rates of inapparent dengue in older adults in Singapore. Am J Trop Med Hyg. 2013;88(6):1065–9. 10.4269/ajtmh.12-0150.http://www.ajtmh.org/content/88/6/1065.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ong A, Sandar M, Chen MI, Sin LY. Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. International Journal of Infectious Diseases. 2007;11(3):263–7. ISI:000246398800016.<Go to ISI>://000246398800016 [DOI] [PubMed] [Google Scholar]
- 94.Arima Y, Edelstein ZR, Han KK, Maytsui T, on behalf of the Emerging Disease Surveillance and Response Team DoHSaE, World Health Organization Regional Office for the Western Pacific. Epidemiologic update on the dengue situation in the Western Pacific Region, 2011. Western Pacific Surveillance and Response Journal. 2013;4(2): 10.5365/wpsar.2012.3.4.019.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3762964/pdf/WPSAR.2013.4.2-047.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ministry of Health Singapore. Weekly Infectious Diseases Bulletin 2013. Available from: https://www.moh.gov.sg/content/moh_web/home/statistics/infectiousDiseasesStatistics/weekly_infectiousdiseasesbulletin.html?year=2013.
- 96.Massad E, Wilder-Smith A. Risk estimates of dengue in travelers to dengue endemic areas using mathematical models. J Travel Med. 2009;16(3):191–3. ISI:000266029700006.http://onlinelibrary.wiley.com/doi/10.1111/j.1708-8305.2009.00310.x/abstract 10.1111/j.1708-8305.2009.00310.x [DOI] [PubMed] [Google Scholar]
- 97.Lin HM, Chen CS, Hsu CC, Chung CL. Dengue vector density survey in Liuchiu, pingtung, Taiwan. (in Chinese with English summary). Chinese J Microbiol Immunol. 1986;19:218–23.http://www.unboundmedicine.com/medline/citation/2880696/[Dengue_vector_density_survey_in_Liuchiu_Pintung_Taiwan]_ [PubMed] [Google Scholar]
- 98.Yang C-F, Hou J-N, Chen T-H, Chen W-J. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014;130:17–23. 10.1016/j.actatropica.2013.10.013.http://www.sciencedirect.com/science/article/pii/S0001706X13002908 10.1016/j.actatropica.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control ROCT. National Notifiable Disease Surveillance System [Internet]. 2014 Available from: http://www.cdc.gov.tw/english/surveillance.aspx?treeid=00ed75d6c887bb27&nowtreeid=f0131176aa46d5db
- 100.Centers for Disease Control ROCT. Press releases. As dengue cases occurred in eastern Taiwan. [Internet]. 23/12/2014 [Accessed: 24 December 2014]; Available from: http://www.cdc.gov.tw/english/info.aspx?treeid=bc2d4e89b154059b&nowtreeid=ee0a2987cfba3222&tid=EF8884C94C8FF4BD
- 101.Chang S-F, Huang J-H, Shu P-Y. Characteristics of dengue epidemics in Taiwan. Journal of the Formosan Medical Association. 2012;111(6):297–9. 10.1016/j.jfma.2011.12.001.http://www.sciencedirect.com/science/article/pii/S0929664612000940 10.1016/j.jfma.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 102.Chuang JH. Research Data Archive, Center for Disease Control, The Executive Yuan, R.O.C. Available from: http://www.cdc.gov.tw/uploads/files/7185353c-beef-4444-9dca-145cced702d6.pdf
- 103.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clinical and Molecular Hepatology. 2014;20(4):327–37. 10.3350/cmh.2014.20.4.327. PMC4278062.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4278062/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Constenla D, Armien B, Arredondo J, Carabali M, Carrasquilla G, Castro R, et al. Costing Dengue Fever Cases and Outbreaks: Recommendations from a Costing Dengue Working Group in the Americas. Value in Health Regional Issues. 2015;8:80–91. 10.1016/j.vhri.2015.06.001.http://www.sciencedirect.com/science/article/pii/S2212109915000473 [DOI] [PubMed] [Google Scholar]
- 105.Stahl H-C, Butenschoen VM, Tran HT, Gozzer E, Skewes R, Mahendradhata Y, et al. Cost of dengue outbreaks: literature review and country case studies. BMC Public Health. 2013;13(1):1–11. 10.1186/1471-2458-13-1048. 10.1186/1471-2458-13-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McBride WJH, Mullner H, LaBrooy JT, Wronski I. The 1993 dengue 2 epidemic in Charters Towers, north Queensland: clinical features and public health impact. Epidemiol Infect. 1998;121(1):151–6. ISI:000075871700016.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2809485/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canyon DV. Historical analysis of the economic cost of dengue in Australia. Journal of Vector Borne Diseases. 2008;45:245–8.http://www.mrcindia.org/journal/issues/453245.pdf [PubMed] [Google Scholar]
- 108.Vazquez-Prokopec GM, Chaves LF, Ritchie SA, Davis J, Kitron U. Unforeseen costs of cutting mosquito surveillance budgets. PLoS Negl Trop Dis. 2010;4(10):e858 10.1371/journal.pntd.0000858. http://dx.doi.org/10.1371%2Fjournal.pntd.0000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic Impact of Dengue Illness in the Americas. Am J Trop Med Hyg. 2011;84(2):200–7. 10.4269/ajtmh.2011.10-0503.http://www.ajtmh.org/content/84/2/200.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiueh T-S. Transfusion transmission risk of dengue viruses in an endemic area. ISBT Science Series. 2011;6(2):313–5. 10.1111/j.1751-2824.2011.01535.x. 10.1111/j.1751-2824.2011.01535.x [DOI] [Google Scholar]
- 111.Tseng WC, Chen CC, Chang CC, Chu YH. Estimating the economic impacts of climate change on infectious diseases: a case study on dengue fever in Taiwan. Climatic Change. 2009;92(1–2):123–40. ISI:000262392900009.<Go to ISI>://000262392900009 [Google Scholar]
- 112.Cases of dengue fever multiply in Southern Taiwan [Internet]. September 15, 2015 [Accessed: 03 May 2016]. Available from: http://sinosphere.blogs.nytimes.com/2015/09/15/taiwan-dengue-fever-tourism-cases/?_r=0
- 113.Cox J, Grillet ME, Ramos OM, Amador M, Barrera R. Habitat segregation of dengue vectors along an urban environmental gradient. Am J Trop Med Hyg. 2007;76(5):820–6. ISI:000246326300008; [PubMed] [Google Scholar]
- 114.Braks MAH, Honorio NA, Lourenco-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J Med Entomol. 2003;40(6):785–94. [DOI] [PubMed] [Google Scholar]
- 115.Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc Suppl. 1988;4:1–39. [PubMed] [Google Scholar]
- 116.Reiskind MH, Lounibos LP. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med Vet Entomol. 2013;27(4):421–9. 10.1111/mve.12000 [DOI] [PubMed] [Google Scholar]
- 117.Lima-Camara TN, Torres Codeço C, Alves Honório N, Vieira Bruno R, Afranio Peixoto A, Lounibos LP. Male accessory gland substances from Aedes albopictus affect the locomotor activity of Aedes aegypti females. Mem Inst Oswaldo Cruz. 2013;108(1):18–25. 10.1590/0074-0276130381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg. 2011;85(2):265–70.http://www.ajtmh.org/content/85/2/265.long 10.4269/ajtmh.2011.10-0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richards SL, Anderson SL, Alto BW. Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in the Florida Keys. J Med Entomol. 2012;49(4):942–6. 10.1603/ME11293 [DOI] [PubMed] [Google Scholar]
- 120.Gubler DJ. Prevention and control of Aedes aegypti-borne diseases: lesson learned from past successes and failures. As Pac J Mol Biol Biotechnol 2011;19(3):111–4.http://www.msmbb.org.my/apjmbb/html193/193f.pdf [Google Scholar]
- 121.Russell RC, Currie BJ, Lindsay MD, Mackenzie JS, Ritchie SA, Whelan PI. Dengue and climate change in Australia: predictions for the future should incorporate knowledge from the past. Med J Aust. 2009;190(5):265–8. ISI:000265400000015.https://www.mja.com.au/journal/2009/190/5/dengue-and-climate-change-australia-predictions-future-should-incorporate [DOI] [PubMed] [Google Scholar]
- 122.Williams CR. The asian tiger mosquito (Aedes albopictus) invasion into Australia: a review of likely geographic range and changes to vector-borne disease risk. Trans R Soc S Aust. 2012;136(2):128–36.http://www.tandfonline.com/doi/abs/10.1080/03721426.2012.10887167#.VKnzKCuUd8E [Google Scholar]
- 123.Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. Trans-ovarial transmission of dengue viruses by mosquitos Aedes albopictus and Aedes aegypti. Am J Trop Med Hyg. 1983;32(5):1108–19. ISI:A1983RM77500035.http://www.ajtmh.org/content/32/5/1108.long [DOI] [PubMed] [Google Scholar]
- 124.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in Central Africa. Vector-Borne and Zoonotic Diseases. 2009;10(3):259–66. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- 125.Buckner EA, Alto BW, Lounibos LP. Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. J Med Entomol. 2013;50(6):1291–7. 10.1603/ME13047.http://www.bioone.org/doi/abs/10.1603/ME13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Russell BM, McBride WJH, Mullner H, Kay BH. Epidemiological significance of subterranean Aedes aegypti (Diptera: Culicidae) breeding sites to dengue virus infection in charters towers, 1993. J Med Entomol. 2002;39(1):143–5. ISI:000178371600022; [DOI] [PubMed] [Google Scholar]
- 127.Ritchie SA. Dengue vector bionomics: Why Aedes aegypti is such a good vector.Ch.24 In: Gubler D F J, Ooi EE, Vasudeban S, editor. Dengue and Dengue Hemorrhagic Fever: CAB International; 2014. [Google Scholar]
- 128.Montgomery BL, Ritchie SA, Hart AJ, Long SA, Walsh ID. Subsoil drain sumps are a key container for Aedes aegypti in Cairns, Australia. J Am Mosq Control Assoc. 2004;20(4):365–9. [PubMed] [Google Scholar]
- 129.Kay BH, Barkerhudson P, Stallman ND, Wiemers MA, Marks EN, Holt PJ, et al. Dengue fever—reappearance in northern Queensland after 26 Years. Med J Aust. 1984;140(5):264–8. ISI:A1984SF19100006; [DOI] [PubMed] [Google Scholar]
- 130.Williams CR, Bader CA, Kearney MR, Ritchie SA, Russell RC. The extinction of dengue through natural vulnerability of its vectors. PLoS Negl Trop Dis. 2010;4(12). ISI:000285547000021.http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.National Environment Agency. Towards a cleaner Singapore—Sociological study on littering in Singapore. 2011 [Google Scholar]
- 132.National Environment Agency. Know the Potential Breeding Sites 2015 [Accessed: 15 May 2016]. Available from: http://www.dengue.gov.sg/subject.asp?id=100.
- 133.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17(1):136–73.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC321469/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schwartz E, Weld LH, Wilder-Smith A, von Sonnenburg F, Keystone JS, Kain KC, et al. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis. 2008;14(7):1081–8. ISI:000257294700012.http://wwwnc.cdc.gov/eid/article/14/7/07-1412_article 10.3201/eid1407.071412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Knope K, The National Arbovirus and Malaria Advisory Committee, Giele C. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Australian Government-Department of Health., 2013;Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/cdi3701f [PubMed]
- 136.Office of the Assistant Secretary for Research and Technology, Bureau of Transportation Statistics. January 2014 U.S. Airline Systemwide Passengers Up 0.8 Percent from January 2013. In: Transportation USDo, editor. Washington.April 2014.http://www.rita.dot.gov/bts/press_releases/bts018_14
- 137.Key West Chamber of Commerce. Key West statistics on tourism [Internet]. 2009 Available from: http://www.keywestchamber.org/statistics-on-tourism.html
- 138.Changi Airport Singapore. Facts & statistics [Internet]. August 2014 Available from: http://www.changiairport.com/our-business/about-changi-airport/facts-statistics
- 139.Changi Airport Group. A record 51 million passengers for Changi Airport in 2012 Singapore2013. Available from: http://www.changiairportgroup.com/export/sites/caas/assets/media_release_2013/Media_Release_-_A_record_51_million_passengers_for_Changi_Airport_in_2012_xwebx.pdf.
- 140.Corporate Communications Division. Immigration & checkpoints authority. Annual report 2013. 2013. [Accessed: 14 May 2016];Available from: https://www.ica.gov.sg/data/resources/docs/ICA%20Annual%20Report%202013%20v3.pdf
- 141.Ministry of Transportation ROC. Bolstering cross-strait and international exchanges, providing travelers with safe and outstanding service. [Internet]. October 2014 [Accessed: 06 May 2016]; Available from: http://www.motc.gov.tw/en/home.jsp?id=258&parentpath=0,150,250
- 142.Gardner LM, Fajardo SD, Waller T, Wang O, Sarkar S. A predictive spatial model to quantify the risk of air-travel-associated dengue importation into the United States and Europe. Journal of Tropical Medicine 2012:11 10.1155/2012/103679.http://www.hindawi.com/journals/jtm/2012/103679/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garcia-Ellin JC. Internal Migration of Puerto Ricans in the United States. New York: Center for Puerto Rican Studies, Hunter College; 2013. [Accessed: 05 May 2016];Available from: http://centropr.hunter.cuny.edu/sites/default/files/Data_Briefs/Centro_RB2013-04_Internal_Migration.pdf [Google Scholar]
- 144.Seet RCS, Ooi EE, Wong HB, Paton NI. An outbreak of primary dengue infection among migrant Chinese workers in Singapore characterized by prominent gastrointestinal symptoms and a high proportion of symptomatic cases. J Clin Virol. 2005;33(4):336–40. 10.1016/j.jcv.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 145.Goh K. Changing epidemiology of dengue in Singapore. Lancet. 1995;346:1098 10.1016/S0140-6736(95)91771-3.http://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2895%2991771-3/abstract [DOI] [PubMed] [Google Scholar]
- 146.King C-C, Wu Y-C, Chao D-Y, Lin T-H, Chow L, Wang H-T, et al. Major epidemics of dengue in Taiwan in 1981–2000: Related to intensive virus activities in Asia. Dengue Bulletin. 2000;24:1–10.http://apps.searo.who.int/PDS_DOCS/B0639.pdf [Google Scholar]
- 147.Ritchie SA, Long S, Smith G, Pyke A, Knox TB. Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J Med Entomol. 2004;41(1):1–4. ISI:000188709100001.http://www.bioone.org/doi/pdf/10.1603/0022-2585-41.1.1 [DOI] [PubMed] [Google Scholar]
- 148.Allen G. Tropical disease buzzes back into U.S. [Internet]. 2011 [Accessed: 22 December 2014]; Available from: http://www.npr.org/2011/06/10/137056759/tropical-disease-buzzes-back-into-u-s
- 149.Koopman JS, Prevots DR, Marin MAV, Dantes HG, Aquino MLZ, Longini IM, et al. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133(11):1168–78. ISI:A1991FP76200010.http://aje.oxfordjournals.org/content/133/11/1168 [DOI] [PubMed] [Google Scholar]
- 150.Australian Bureau of Statistics. 2006 Census QuickStats: Australia [Internet]. 2007 Available from: http://www.multiculturalaustralia.edu.au/doc/2006census-quickstats-australia.pdf
- 151.Australian Bureau of Statistics. QuickStats-2011 Census of Population and Housing [Internet]. 2013 Available from: http://www.censusdata.abs.gov.au/census_services/getproduct/census/2011/quickstat/0
- 152.Author. Immigration in the last 5 years—focus on Queensland. January 6,2012 Date [cited Access Date].Available from:http://blog.id.com.au/2012/population/australian-demographic-trends/immigration-in-queensland/. [Accessed: 04/05/2016]
- 153.HIA Economics Group Research Note. Australia's Population Growth—Current Trends. Campbell: February 2013
- 154.Singapore DoS. Population and Population Structure_ Key demographic indicators, 1970–2014. 2015.http://www.singstat.gov.sg/statistics/browse-by-theme/population-and-population-structure
- 155.Department of Statistics Singapore. Latest data [Internet]. Singapore Government. 31 Oct 2014 Available from: http://www.singstat.gov.sg/statistics/latest-data
- 156.Misra Y. The economic penalty of dengue. North Carolina: Duke University Durham; 2011. [Google Scholar]
- 157.Do the Mozzie Wipeout [Internet]. Available from: http://www.dengue.gov.sg/images/materials/NEA_MozzieWipeoutToolkit_for_Schools.pdf
- 158.Wu P, Lay J, Guo HR H, Lin C, Lung S, Su H. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci Total Environ. 2009;407:2224–33. 10.1016/j.scitotenv.2008.11.034.http://www.sciencedirect.com/science/article/pii/S0048969708011509 [DOI] [PubMed] [Google Scholar]
- 159.Responses to “How Taiwan Can Prepare for the Next Dengue Fever Crisis” [Internet]. January 29, 2016 [Accessed: 02 May 2016]. Available from: http://thinking-taiwan.com/taiwan-prepare-dengue-schwartz/#sthash.2er5q9S9.dpuf
- 160.CSIRO and the Australian Bureau of Meteorology. State of the climate 2014;Available from: http://www.bom.gov.au/state-of-the-climate/
- 161.Church JA, Hunter JR, McInnes KL, White N. Sea-level rise around the Australian coastline and the changing frequency of extreme sea-level events. Australian Meteorological Magazine. 2006;55(4):253–60.http://staff.acecrc.org.au/~johunter/AMM_Church_et_al_2006.pdf [Google Scholar]
- 162.Patz JA, Epstein PR, Burke TA, Balbus JM. Global climate change and emerging infectious diseases. The Journal of the American Medical Association. 1996;275:217–23.http://jama.jamanetwork.com/article.aspx?articleid=394508 [PubMed] [Google Scholar]
- 163.Juliano SA, O'Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130(3):458–69.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2944657/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.National Climate Change Secretariat Prime Minister's Office Singapore. Impact of Climate Change on Singapore 2016 [Accessed: 04 May 2016]. Available from: https://www.nccs.gov.sg/climate-change-and-singapore/national-circumstances/impact-climate-change-singapore.
- 165.Earnest A, Tan SB, Wilder-Smith A. Meteorological factors and El Nino Southern Oscillation are independently associated with dengue infections. Epidemiol Infect. 2012;140(7):1244–51. Epub 2011/09/13. 10.1017/s095026881100183x ; [DOI] [PubMed] [Google Scholar]
- 166.Yu H-L, Yang S-J, Yen H-J, Christakos G. A spatio-temporal climate-based model of early dengue fever warning in southern Taiwan. Stochastic Environmental Research and Risk Assessment. 2011;25(4):485–94. 10.1007/s00477-010-0417-9.http://link.springer.com/article/10.1007%2Fs00477-010-0417-9 [DOI] [Google Scholar]
- 167.Hii YL, Rocklöv J, Ng N, Tang CS, Pang FY, Sauerborn R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Global Health Action. 2009;2http://europepmc.org/abstract/MED/20052380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morin CW, Comrie AC, Ernst K. Climate and dengue transmission: evidence and implications. Environ Health Perspect. 2013;121(11–12):1264–72.http://ehp.niehs.nih.gov/1306556/ 10.1289/ehp.1306556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.State of Queensland. Public Health Regulation 2005 [Internet]. 25 November 2012 Available from: https://www.legislation.qld.gov.au/LEGISLTN/SUPERSED/P/PubHealR05_121125.pdf
- 170.Beebe NW, Cooper RD, Mottram P, Sweeney AW. Australia's dengue risk driven by human adaptation to climate change. PLoS Negl Trop Dis. 2009;3(5):9 ISI:000267268000003.http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: the dengue mosquito Aedes aegypti in Australia. Funct Ecol. 2009;23(3):528–38. ISI:000266024900009.http://www.ral.ucar.edu/staff/steinhoff/Exp_summer2013/Papers/Experiments/Kearney_et_al_2009.pdf [Google Scholar]
- 172.Williams C, Mincham G, Ritchie S, Viennet E, Harley D. Bionomic response of Aedes aegypti to two future climate change scenarios in far north Queensland, Australia: implications for dengue outbreaks. Parasit Vectors. 2014;7(1):447 10.1186/1756-3305-7-447.http://www.parasitesandvectors.com/content/7/1/447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vazquez-Prokopec G, Kitron U, Montgomery B, Horne P, Ritchie S. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Williams N. Modified vector attack on dengue. Curr Biol. 2010;20(23):R1004–R5. ISI:000285213500005.http://www.sciencedirect.com/science/article/pii/S0960982210015137 [DOI] [PubMed] [Google Scholar]
- 175.CDC. Guidelines for surveillance, prevention, and control of West Nile virus infection—United States. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4902a1.htm. [PubMed] [Google Scholar]
- 176.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report_ Locally acquired dengue _ Key West, Florida, 2009–2010. 2010
- 177.Hayden MH, Cavanaugh JL, Tittel C, Butterworth M, Haenchen S, Dickinson K, et al. Post outbreak review: Dengue preparedness and response in Key West, Florida. Am J Trop Med Hyg. 2015;93(2):397–400.http://www.ajtmh.org/content/93/2/397.abstract 10.4269/ajtmh.15-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N Engl J Med. 2015;372(2):113–23. 10.1056/NEJMoa1411037. 25365753.http://www.nejm.org/doi/full/10.1056/NEJMoa1411037 [DOI] [PubMed] [Google Scholar]
- 179.Ooi E-E, Goh K-T, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–93.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3373041/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.National Environment Agency. Operations strategy 2016. Available from: http://www.dengue.gov.sg/subject.asp?id=34.
- 181.Koou SY, Chong CS, Vythilingam I, Ng LC, Lee CY. Pyrethroid resistance in Aedes aegypti larvae (Diptera: Culicidae) from Singapore. J Med Entomol. 2014;51(1):170–81. [DOI] [PubMed] [Google Scholar]
- 182.Marcombe S, Farajollahi A, Healy SP, Clark GG, Fonseca DM. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. Plos One. 2014;9(7):e101992 10.1371/journal.pone.0101992.http://dx.doi.org/10.1371%2Fjournal.pone.0101992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.S$200 fine for all homes found breeding mosquitoes [Internet]. 2016. Today Online; 28 February 2016 [Accessed: 19/05/2016]. Available from: http://www.todayonline.com/singapore/mozzie-wipeout-campaign-launched-will-run-next-2-weeks?singlepage=true
- 184.Environment Issues brought out by Workers’ Party MPs [Internet]. 2013 [Accessed: 05 May 2016]. Available from: http://www.theonlinecitizen.com/2013/03/environment-issues-brought-workers-party-mps/
- 185.Khalik S. Dengue cases expected to surpass 1,000 a week. Asiaone_ Your Health. 2013-06-14.
- 186.Russell RC, Dwyer DE. Arboviruses associated with human diseasein Australia. Microbes and Infection. 2000;2(14):1693–704. 10.1016/S1286-4579(00)01324-1.http://www.sciencedirect.com/science/article/pii/S1286457900013241 [DOI] [PubMed] [Google Scholar]
- 187.S$200 fine for homes found breeding mosquitoes from Mar 14 [Internet]. 28 Feb 2016 [Accessed: 05 May 2016]. Available from: http://www.channelnewsasia.com/news/singapore/s-200-fine-for-homes/2556000.html
- 188.Dengue Cases May Exceed 30,000 In 2016 [Internet]. 18 February 2016 [Accessed: 04 May 2016]. Available from: https://www.moh.gov.sg/content/moh_web/home/pressRoom/pressRoomItemRelease/2016/dengue-cases-may-exceed-30-000-in-2016.html
- 189.McKerr C, Lo Y-C, Edeghere O, Bracebridge S. Evaluation of the National Notifiable Diseases Surveillance System for Dengue Fever in Taiwan, 2010–2012. PLoS Negl Trop Dis. 2015;9(3):e0003639 10.1371/journal.pntd.0003639. PMC4368052.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4368052/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teng H, Chen T, Tsai S, Lin C, Chiou H, Lin M, et al. Emergency vector control in a DENV-2 outbreak in 2002 in Pingtung City, Pingtung County, Taiwan. Jpn J Infect Dis. 2007;60(5):271–9.http://www0.nih.go.jp/JJID/60/271.html [PubMed] [Google Scholar]
- 191.Kuan MM, Lin T, Chuang JH, Wu HS. Epidemiological trends and the effect of airport fever screening on prevention of domestic dengue fever outbreaks in Taiwan, 1998–2007. International Journal of Infectious Diseases. 2010;14(8):E693–E7. ISI:000282662100008.http://www.sciencedirect.com/science/article/pii/S1201971210023416 10.1016/j.ijid.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang Y-Y, Liu Y, Teng H-J, Sauman I, Sehnal F, Lee H-J. Circadian control of permethrin-resistance in the mosquito Aedes aegypti. Journal of Insect Physiology. 2010;56(9):1219–23. 10.1016/j.jinsphys.2010.03.028.http://www.sciencedirect.com/science/article/pii/S0022191010001034 [DOI] [PubMed] [Google Scholar]
- 193.Lee AS. NEA looking to infect Aedes mosquitoes in dengue fight 2014 [Accessed: June 16, 2014]. Available from: http://www.todayonline.com/singapore/nea-looking-infect-aedes-mosquitoes-dengue-fight
- 194.Community urged to stay vigilant despite drop in dengue cases [Internet]. 2015; 19 April 2015 [Accessed: 10 May 2016]. Available from: http://www.nea.gov.sg/corporate-functions/newsroom/news-releases/community-urged-to-stay-vigilant-despite-drop-in-dengue-cases
- 195.Gubler DJ. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Am J Trop Med Hyg. 1989;40(6):571–8.http://www.ajtmh.org/content/40/6/571.long [DOI] [PubMed] [Google Scholar]
- 196.Ong SGJ. Each time an NEA officer knocks on my door, I cooperate with them but at the same time, I wonder whether the door-to-door checks aren't a waste of time when my HDB neighbourhood is strewn with litter such as empty cigarette packs, plastic bags, and dirty food containers. Each piece of litter is a breeding ground. 2015, December 31 [Accessed: 10 May 2016]. Available from: https://www.facebook.com/notes/amy-khor/protecting-ourselves-against-dengue/1203990529611386.
- 197.Chan R. Residents must take greater responsibility to rid their homes of mosquitoes breeding. I have often witnessed households not responding to NEA officers knock. 2015, December 29 [Accessed: 10 May 2016]. Available from: https://www.facebook.com/notes/amy-khor/protecting-ourselves-against-dengue/1203990529611386.
- 198.National Environment Agency. Volunteer With Us. Available from: http://www.nea.gov.sg/events-programmes/volunteer-with-us.
- 199.Forty-six jobs axed as federal government cuts health budget [Internet]. 2013 [Accessed: 07 May 2016]. Available from: http://www.brisbanetimes.com.au/queensland/fortysix-jobs-axed-as-federal-government-cuts-health-budget-20130117-2cv7a.html
- 200.Australia Independent. Cumulative List of Funding and staffing cuts to services, staff, funding and programs by Campbell Newman LNP Government 2012. Campbell Newman & LNP cuts2013.
- 201.American Society for Microbiology. Centers for disease control and prevention—FY 2011 testimony 2010. Available from: http://www.asm.org/index.php/component/content/article?id=8018.
- 202.Health News Florida. DOH Layoffs Hit Some Harder [Internet]. 24 August 2011 [Accessed: 01 February 2015]; Available from: http://health.wusf.usf.edu/post/layoffs-hit-some-county-health-units-harder-others
- 203.Only a dozen inspectors stand between Zika and Miami [Internet]. 1 June 2016 [Accessed: 4 June 2016]. Available from: http://www.bloomberg.com/news/articles/2016-06-01/swat-team-miami-s-mosquito-man-faces-zika-with-bare-bones-crew
- 204.United States Census Bureau. QuickFacts _ Lee County, Florida [Internet]. 2016 [Accessed: 10 May 2016]; Available from: http://www.census.gov/quickfacts/table/PST045215/12071
- 205.Florida’s Mosquito Control Forces Mobilize Against Zika Threat [Internet]. June 17, 2016 [Accessed: 20 June 2016]. Available from: http://khn.org/news/floridas-mosquito-control-forces-mobilize-against-zika-threat/
- 206.Mayurasakorn S, Suttipun N. The Impact of a Program for Strengthening Dengue Hemorrhagic Fever Case Management on the Clinical Outcome of Dengue Hemorrhagic Fever Patients. Southeast Asian Journal of Tropical Medicine and Public Health. 2010;41(4):858–63. ISI:000280521900010.<Go to ISI>://000280521900010 [PubMed] [Google Scholar]
- 207.Graham AS, Pruszynski CA, Hribar LJ, Demay DJ, Tambasco AN, Hartley AE, et al. Mosquito-associated Dengue Virus, Key West, Florida, USA, 2010. Emerg Infect Dis. 2011;17(11):2074–5. ISI:000296670300019.<Go to ISI>://000296670300019 10.3201/eid1711.110419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.The Singapore health system—achieving positive health outcomes with low expenditure [Internet]. 19 April 2010. Watson Wyatt Healthcare Market Review, October 2004 [Accessed: 16 May 2016]. Available from: https://web.archive.org/web/20100419231253/ http://www.watsonwyatt.com/europe/pubs/healthcare/render2.asp?ID=13850
- 209.Chadee DD. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. Bull Entomol Res. 2004;94(3):201–7. Epub 2004/06/12. [DOI] [PubMed] [Google Scholar]
- 210.Punzel M, Korukluoğlu G, Caglayik DY, Menemenlioglu D, Bozdag SC, Tekgündüz E, et al. Dengue virus transmission by blood stem cell donor after travel to Sri Lanka; Germany, 2013. Emerg Infect Dis. 2014;20(8):1366–69.http://wwwnc.cdc.gov/eid/article/20/8/14-0508 10.3201/eid2008.140508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT, et al. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013;19(5):3. 10.3201/eid1905.121664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Organization WH. Dengue and dengue haemorrhagic fever: Diagnosis, Treatment, Prevention and control. 1997:84. Epub Second. [Google Scholar]
- 213.Immunization Vaccines and Biologicals [Internet]. 2016. Dengue vaccine research; 15 April 2016. Available from: http://www.who.int/immunization/research/development/dengue_vaccines/en/
- 214.Immunization, vaccines and biologicals [Internet]. 14 December 2015. Dengue vaccine research. Available from: http://www.who.int/immunization/research/development/dengue_vaccines/en/
- 215.Vannice KS, Durbin A, Hombach J. Status of vaccine research and development of vaccines for dengue. Vaccine. 10.1016/j.vaccine.2015.12.073.http://www.sciencedirect.com/science/article/pii/S0264410X16002930 [DOI] [PubMed] [Google Scholar]
- 216.Storr KA. When science bites back. The release of genetically modified mosquitoes to control dengue is still raising opposition from Key West residents 2014. Available from: http://scienceline.org/2014/03/when-science-bites-back/
- 217.Eliminate dengue our challenge. Eliminate dengue program 2016 [Accessed: 20 May 2016]. Available from: http://www.eliminatedengue.com/project.
- 218.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–U107. ISI:000294209400037.http://www.nature.com/nature/journal/v476/n7361/full/nature10356.html 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 219.Brownstein JS, Hett E, O'Neill SL. The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebr Pathol. 2003;84:24–9.http://www.sciencedirect.com/science/article/pii/S002220110300082X [DOI] [PubMed] [Google Scholar]
- 220.Min K-T, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA. 1997;94(20):10792–6. 10.1073/pnas.94.20.10792.http://www.pnas.org/content/94/20/10792.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.McMeniman CJ, O'Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis. 2010;4(7):6 ISI:000280412300017.http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8(2):e2688 10.1371/journal.pntd.0002688.http://dx.doi.org/10.1371%2Fjournal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Eliminate dengue our challenge. Cairns field trial update September 2013 [Accessed: 1st October 2013]. Available from: http://www.eliminatedengue.com/library/publication/document/field_trial_update/20130919_ba_mb_trial_update_handout_combined.pdf.
- 224.Eliminate dengue our challenge. Eliminate Dengue Townsville 2014. Available from: http://www.eliminatedengue.com/townsville.
- 225.Focks D, Daniels E, Haile D, Keesling J. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53(5):489–506.http://www.ajtmh.org/content/53/5/489.long [DOI] [PubMed] [Google Scholar]
- 226.Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, et al. Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis. 2009;3(9):e508 10.1371/journal.pntd.0000508 http://www.ncbi.nlm.nih.gov/pubmed/19721700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): Analysis of the literature and model development. J Med Entomol. 1993;30(6):1003–17. [DOI] [PubMed] [Google Scholar]
- 228.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): Simulation results and validation. J Med Entomol. 1993;30(6):1018–28. [DOI] [PubMed] [Google Scholar]
- 229.Hii YL, Zhu H, Ng N, Ng LC, Rocklöv J. Forecast of Dengue Incidence Using Temperature and Rainfall. PLoS Negl Trop Dis. 2012;6(11):e1908 10.1371/journal.pntd.0001908.http://dx.doi.org/10.1371%2Fjournal.pntd.0001908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bannister-Tyrrell M, Williams C, Ritchie SA, Rau G, Lindesay J, Mercer G, et al. Weather-driven variation in dengue activity in Australia examined using a process-based modeling approach. Am J Trop Med Hyg. 2013;88(1):65–72. 10.4269/ajtmh.2012.11-0451.http://www.ajtmh.org/content/88/1/65.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Patz JA, Martens WJM, Focks DA, Jetten TH. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ Health Perspect. 1998;106(3):147–53. ISI:000075378600019.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1533051/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kutsuna S, Hayakawa K, Kato Y, Fujiya Y, Mawatari M, Takeshita N, et al. Comparison of clinical characteristics and laboratory findings of malaria, dengue, and enteric fever in returning travelers: 8-year experience at a referral center in Tokyo, Japan. Kansenshogaku Zasshi. 2015;Suppl 13:34–8. http://www.ncbi.nlm.nih.gov/pubmed/26529984 [PubMed] [Google Scholar]
- 233.Septfons A, Deniau J, Poujol I, Sauthier N, Servas V, Cochet A. Bilan de la surveillance épidémiologique renforcée du chikungunya et de la dengue. Saison 2013. France métropolitaine. Revue d'Épidémiologie et de Santé Publique. 2014;62, Supplement 5(0):S234 10.1016/j.respe.2014.06.202.http://www.sciencedirect.com/science/article/pii/S0398762014005537 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)