Abstract
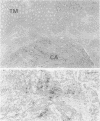
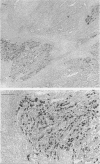
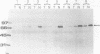
AIMS--Immunostaining of chromogranin identifies gastrointestinal mucosal endocrine cells. The detailed distribution and significance of chromogranin positive cells in colorectal carcinomas and in transitional mucosa remain unclear. The aim of this study was to clarify these aspects. METHODS--The distribution of chromogranin positive cells was studied by immunohistochemical methods in normal epithelium remote from carcinoma, in transitional mucosa, and in carcinomas of the colorectum. In selected cases northern or western blot analyses were performed. RESULTS--Chromogranin positive cells were seen in the lower third of the normal crypts and less frequently in transitional mucosa. Thirty five per cent (n = 38) of colorectal carcinomas showed immunohistochemically positive carcinoma cells in the tumour tissue. Northern and western blot analyses showed similar results. There was no difference in clinicopathological factors, including prognosis, between chromogranin positive cases of colorectal carcinoma (n = 38) and chromogranin negative cases (n = 70). CONCLUSIONS--Neuroendocrine cell differentiation is controlled in transitional mucosa and the presence of chromogranin positive cells in carcinoma tissue does not influence the patient's prognosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J. W., Wiggers T., Verstijnen K., Bosman F. T. The occurrence and clinicopathological significance of serotonin immunoreactive cells in large bowel carcinoma. J Pathol. 1986 Jun;149(2):97–102. doi: 10.1002/path.1711490204. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Murray S. S., Burton D. W., Parmer R. J., O'Connor D. T., Delegeane A. M., Mellon P. L. A cloned chromogranin A (CgA) cDNA detects a 2.3Kb mRNA in diverse neuroendocrine tissues. Biochem Biophys Res Commun. 1986 May 29;137(1):418–423. doi: 10.1016/0006-291x(86)91226-x. [DOI] [PubMed] [Google Scholar]
- Facer P., Bishop A. E., Lloyd R. V., Wilson B. S., Hennessy R. J., Polak J. M. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985 Dec;89(6):1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Grimelius L. A silver nitrate stain for alpha-2 cells in human pancreatic islets. Acta Soc Med Ups. 1968;73(5-6):243–270. [PubMed] [Google Scholar]
- Habib N. A., Dawson P. M., Blount M. A., Cox S., Krausz T., Wood C. B. Study of the histochemical changes in mucus from normal and tumour bearing mucosa in patients with colorectal cancer. Eur J Surg Oncol. 1985 Sep;11(3):243–245. [PubMed] [Google Scholar]
- Hamada Y., Oishi A., Shoji T., Takada H., Yamamura M., Hioki K., Yamamoto M. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer. 1992 Jun 1;69(11):2641–2646. doi: 10.1002/1097-0142(19920601)69:11<2641::aid-cncr2820691104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Helman L. J., Ahn T. G., Levine M. A., Allison A., Cohen P. S., Cooper M. J., Cohn D. V., Israel M. A. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem. 1988 Aug 15;263(23):11559–11563. [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Iwashita A. [Argyrophil and argentaffin cells in carcinomas of the colon and rectum (author's transl)]. Fukuoka Igaku Zasshi. 1979 Jul;70(7):370–390. [PubMed] [Google Scholar]
- Kyzer S., Mitmaker B., Gordon P. H., Schipper H., Wang E. Proliferative activity of colonic mucosa at different distances from primary adenocarcinoma as determined by the presence of statin: a nonproliferation-specific nuclear protein. Dis Colon Rectum. 1992 Sep;35(9):879–883. doi: 10.1007/BF02047877. [DOI] [PubMed] [Google Scholar]
- Lawson M. J., White L. M., Coyle P., Butler R. N., Roberts-Thomson I. C., Conyers R. A. An assessment of proliferative and enzyme activity in transitional mucosa adjacent to colonic cancer. Cancer. 1989 Sep 1;64(5):1061–1066. doi: 10.1002/1097-0142(19890901)64:5<1061::aid-cncr2820640517>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Listinsky C. M., Riddell R. H. Patterns of mucin secretion in neoplastic and non-neoplastic diseases of the colon. Hum Pathol. 1981 Oct;12(10):923–929. doi: 10.1016/s0046-8177(81)80198-0. [DOI] [PubMed] [Google Scholar]
- Mori M., Shimono R., Adachi Y., Matsuda H., Kuwano H., Sugimachi K., Ikeda M., Saku M. Transitional mucosa in human colorectal lesions. Dis Colon Rectum. 1990 Jun;33(6):498–501. doi: 10.1007/BF02052146. [DOI] [PubMed] [Google Scholar]
- Mori M., Staniunas R. J., Barnard G. F., Jessup J. M., Steele G. D., Jr, Chen L. B. The significance of carbonic anhydrase expression in human colorectal cancer. Gastroenterology. 1993 Sep;105(3):820–826. doi: 10.1016/0016-5085(93)90900-w. [DOI] [PubMed] [Google Scholar]
- Murray S. S., Deaven L. L., Burton D. W., O'Connor D. I., Mellon P. L., Deftos L. J. The gene for human chromogranin A (CgA) is located on chromosome 14. Biochem Biophys Res Commun. 1987 Jan 15;142(1):141–146. doi: 10.1016/0006-291x(87)90462-1. [DOI] [PubMed] [Google Scholar]
- Park J. G., Choe G. Y., Helman L. J., Gazdar A. F., Yang H. K., Kim J. P., Park S. H., Kim Y. I. Chromogranin-A expression in gastric and colon cancer tissues. Int J Cancer. 1992 May 8;51(2):189–194. doi: 10.1002/ijc.2910510205. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Rindi G., Buffa R., Sessa F., Tortora O., Solcia E. Chromogranin A, B and C immunoreactivities of mammalian endocrine cells. Distribution, distinction from costored hormones/prohormones and relationship with the argyrophil component of secretory granules. Histochemistry. 1986;85(1):19–28. doi: 10.1007/BF00508649. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J. 1967 May;103(2):483–492. doi: 10.1042/bj1030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Jr, Haggitt R. C. The prevalence and prognostic significance of argyrophil cells in colorectal carcinomas. Am J Surg Pathol. 1984 Feb;8(2):123–128. doi: 10.1097/00000478-198402000-00006. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Gao H., Chen Y., Wang Y., He J., Jin C. Biopathologic characteristics of DNA content in crypt cells of transitional mucosa adjacent to carcinomas of the rectum and rectosigmoid. Dis Colon Rectum. 1992 Jul;35(7):670–675. doi: 10.1007/BF02053758. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]