Abstract
There are growing concerns that cumulative repetitive head impact exposure through routine participation in contact and collision sports is associated with increased risk of long-term problems in memory and cognition, including the development of chronic traumatic encephalopathy (CTE). CTE is a distinctive neurodegenerative disease that occurs as a result of repetitive head impacts (RHI) including concussion and subconcussion. Like most neurodegenerative diseases, CTE can only be diagnosed by postmortem neuropathologic examination of brain tissue. Recently a panel of exerts concluded that CTE is a unique disorder with a pathognomonic lesion that can be reliably distinguished from other neurodegenerative diseases, such as Alzheimer’s disease and frontotemporal lobar degeneration. The pathognomonic lesion of CTE consists of a perivascular accumulation of hyperphosphorylated tau protein in neurons and astrocytes in an irregular pattern, and is typically most prominent at the depths of the cerebral sulci. Clinically CTE is associated with violent behaviors, explosivity, a loss of control, depression, suicide, memory loss and cognitive changes. While the exact incidence and prevalence of CTE remain unknown, there is increasing evidence that CTE affects amateur atheletes as well as professional athletes and military veterans. Given the millions of contact sport athletes and military service members who are exposed to RHI each year, CTE has become a major public health concern. There is a critical need for identification of CTE during life, improved understanding of the epidemiology and pathobiology, and the development of effective prevention and treatment strategies for CTE.
Keywords: Chronic traumatic encephalopathy, repetitive head impacts, traumatic brain injury, neurodegenerative disease, tau protein, subconcussion, concussion
INTRODUCTION
There are growing concerns that cumulative repetitive head impact exposure through routine participation in contact and collision sports is associated with increased risk of chronic neurological and neuropsychiatric problems.1, 2 Among the issues associated with cumulative repetitive mild traumatic brain injury are persistent postconcussive symptoms and long-term problems in memory and cognition, including the development of chronic traumatic encephalopathy (CTE).2–7
CTE is a unique neurodegenerative disorder that occurs as a latent consequence of cumulative repetitive head impacts (RHI), including concussion and subconcussion. CTE was first associated with the sport of boxing in 1928, when Harrison Stanford Martland described the clinical features of a neuropsychiatric syndrome that affected pugilists, a condition then known as “punch drunk” or “dementia pugilistica”.8 Over the following decades, it was gradually recognized that the condition affected men and women with a broad range of exposure to repetitive brain trauma, including physical abuse,9 head-banging,10, 11 poorly controlled epilepsy, “dwarf-throwing”,12 and rugby.11 The term “Chronic Traumatic Encephalopathy” or “CTE” was introduced by Critchley in his 1949 monograph “ “Punch drunk syndromes: the chronic traumatic encephalopathy of boxers”13 and has subsequently become the preferred designation. Recently, CTE has been described in athletes playing popular modern contact sports including American football, soccer, baseball, wrestling, ice hockey, as well as military personnel exposed to RHI during military service, including explosive blast.1, 2, 6, 14–16 Currently, one of the great concerns to public heath is the identification of CTE in teens and amateur athletes at the high school and collegiate levels.2, 6, 17 Although this past decade has seen a dramatic increase in public awareness of CTE and an equally dramatic rise in scientific research focused on the long-term effects of RHI, the science to identify the precise risks of RHI exposure and the development of CTE in amateur and professional athletes and military veterans lags behind. Like many neurodegenerative diseases, currently CTE can only be diagnosed after death by neuropathologic examination, and the precise incidence and prevalence of CTE remain unknown. Large scale, longitudinal prospective studies are critically needed to directly address these public concerns and close the existing gaps in the basic and clinical science related to the natural history, evaluation and management, and longterm effects of RHI exposure.
NEUROPATHOLOGY OF CTE
Microscopic Pathology
The neuropathology of CTE is increasingly well-defined. In 2013, in the largest case series study to date, McKee and colleagues reported the spectrum of p-tau pathology in 68 male subjects with a history of exposure to RHI with neuropathological evidence of CTE, ranging in age from 17 to 98 years (mean 59.5 years). In young subjects with the mildest forms of CTE, focal perivascular epicenters of hyperphosphorylated tau (p-tau) immunoreactive NFTs and astrocytic inclusions were found clustered at the depths of the cortical sulci; in subjects with severe disease, a profound tauopathy involved widespread brain regions.2 Other abnormalities encountered in advanced disease included abnormal deposits of phosphorylated TAR DNA-binding protein of 43 kDa (TDP-43) protein, neuroinflammation, varying amounts of beta amyloid plaques, neuronal loss and white matter degeneration. Based on these findings, preliminary criteria for the neuropathological diagnosis of CTE and a 4-tiered staging system for grading pathological severity were proposed (Figure 1).
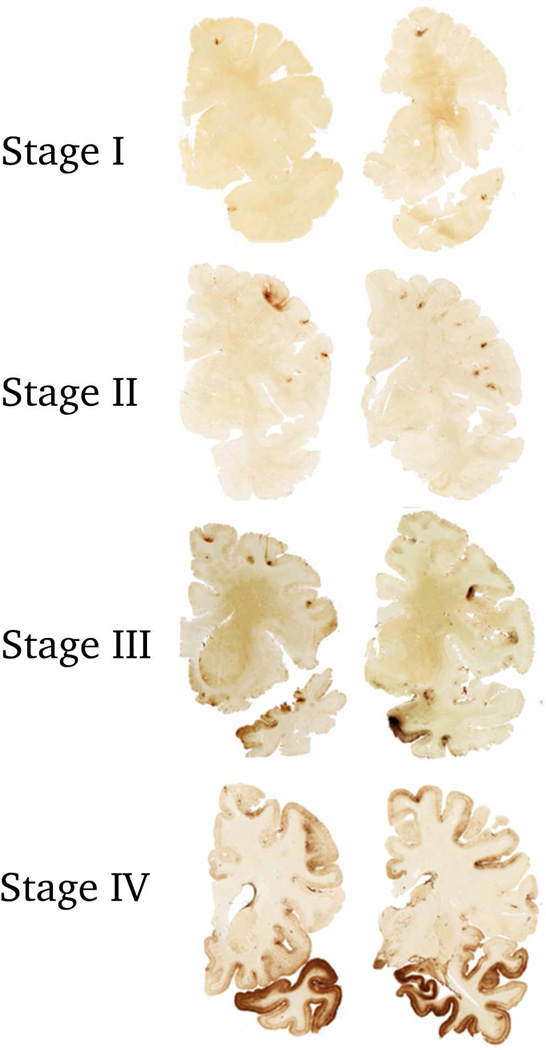
Figure 1. Stages of Hyperphosphorylated Tau Pathology in CTE.
In stage I CTE, p-tau pathology is restricted to isolated foci in the cerebral cortex, the focal lesions consist of perivascular accumulation of p-tau as neuronal and astrocytic inclusions, with NFTs and dot-like structures.
In stage II CTE, there are multiple p-tau lesions typically found at the depths of the cerebral sulci In stage III CTE, p-tau pathology is widespread in the cortex and the amygdala, hippocampus and entorhinal cortex show neurofibrillary pathology.
In stage IV CTE, there is widespread severe p-tau pathology affecting most regions of the cerebral cortex and the medial temporal lobe, with sparing of the calcarine crtex. All images, CP-13 immunostained 50 μ tissue sections.
In 2015, as the first part of a series of consensus panels funded by the NINDS/NIBIB to define the neuropathological criteria for CTE, these preliminary neuropathological criteria were used by 7 expert neuropathologists to blindly evaluate 25 cases of various tauopathies, including CTE, Alzheimer’s disease, progressive supranuclear palsy, argyrophilic grain disease, corticobasal degeneration, primary age-related tauopathy, and parkinsonism dementia complex of Guam without any knowledge of the subjects age, sex, athletic history, clinical symptoms or gross neuropathological findings. The results demonstrated that there was good agreement among the neuropathologists who reviewed the cases (Cohen’s kappa: 0.67) and even better agreement between reviewers and the diagnosis of CTE (Cohen’s kappa: 0.78) using the preliminary criteria. In addition, the panel refined the preliminary criteria and defined CTE as a distinctive disease with a pathognomonic lesion.18 The pathognomonic lesion of CTE was defined as an accumulation of abnormal tau in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern (Figure 2). The group also defined supportive but non-specific features of CTE (Table 1 (adapted from18) and determined that the diagnostic features of CTE were distinct from the age-related astrocytic p-tau pathology (ARTAG) commonly found in the white matter of the temporal lobe and basal regions of the brain.19 ARTAG is non-specific and non-diagnostic, and may be found in a variety of conditions, including aging, CTE and many others.
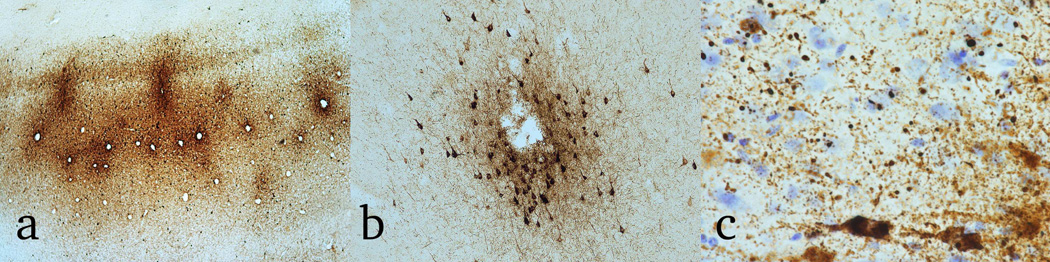
Figure 2. The Pathognomonic Lesion of CTE.
The pathognomonic lesion of CTE consists of an accumulation of abnormally phosphorylated tau in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern. There are typically neurofibrillary tangles, ptau inclusions in thorned astrocytes as well as dot-like structures in the neuropil. a. Magnification ×40, b magnification × 200, c. magnification × 600; all images, CP-13 immunostained 50 μ tissue sections, (c) counterstained with cresyl violet.
Table 1.
Preliminary NINDS criteria for the pathological diagnosis of CTE (adapted from18)
| REQUIRED FOR DIAGNOSIS OF CTE | |
| 1. | The pathognomonic lesion consists of p-tau inclusions in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci. |
| SUPPORTIVE NEUROPATHOLOGICAL FEATURES OF CTE | |
| P-tau-related pathologies: | |
| 1. | Abnormal p-tau immunoreactive pretangles and NFTs preferentially affecting superficial layers (layers II–III). |
| 2. | In the hippocampus, NFTs found preferentially in affecting CA2, CA4 and CA1 |
| 3. | NFTs in subcortical nuclei, including the mammillary bodies, hypothalamus, amygdala, thalamus, nucleus basalis of Meynert, raphe nuclei, substantia nigra and locus coeruleus. |
| 4. | P-tau immunoreactive astrocytic inclusions in the subpial and periventricular regions. |
| 5. | P-tau immunoreactive large dot-like structures (in addition to some threadlike neurites) |
| Non-ptau-related pathologies: | |
| 1. | Macroscopic features: Disproportionate dilatation of the third ventricle, septal abnormalities, mammillary body atrophy, and contusions or other signs of previous traumatic injury. |
| 2. | TDP-43 immunoreactive neuronal cytoplasmic inclusions and dot-like structures in the hippocampus, anteromedial temporal cortex and amygdala |
Using the NINDS criteria for CTE, Bieniek and colleagues reviewed the clinical records and brains of 1,721 cases donated to the Mayo Clinic Brain Bank over the past 18 years, and found 32% of contact sport athletes had evidence of CTE pathology.17 No cases of CTE were found in 162 control brains without a history of brain trauma or in 33 cases with a history of a single traumatic brain injury. Of the 21 with CTE pathology, 19 had participated in football or boxing, and many were multiple sport athletes including rugby, wrestling, basketball, and baseball. One athlete played only baseball, and another athlete only played basketball. Similarly, Ling and colleagues screened 268 cases of neurodegenerative disease and controls in the Queen Square Brain Bank for Neurological Disorders using the preliminary McKee criteria2 and found changes of CTE in 11.9% of neurodegenerative disorders and 12.8% of elderly controls. Of the cases with changes of CTE, 93.8% had a history of TBIs, 34% had participated in high-risk sports including rugby, soccer, cricket, lacrosse, judo and squash, and 18.8% were military veterans.20
Beta amyloid pathology in CTE
Beta-amyloid (Aβ) plaques are found in 52% of individuals with CTE; Aβ plaques are significantly associated with age in CTE and have not been found before the age of 50 years.21 Aβ plaques in CTE, when they occur, are typically less dense than in Alzheimer’s disease and predominantly diffuse.4 Aβ plaques are also significantly associated with accelerated tauopathy, Lewy body formation, dementia, Parkinsonism and inheritance of the ApoE4 allele.21
Gross Pathology
Gross macroscopic alterations are usually found only in moderate to severe CTE. Common gross neuropathological changes include:
Cavum septum pellucidum and septal fenestrations
Reduced brain weight and cerebral atrophy; the atrophy is typically bilateral and most severe in the frontal and medial temporal lobes, including the hippocampus, amygdala and entorhinal cortex
Thalamic and hypothalamic atrophy, including the mammillary bodies
Thinning of the corpus callosum, particularly in the posterior isthmus
Ventricular dilation with disproportionate dilation of the IIIrd ventricle
Depigmentation of the locus coeruleus and substantia nigra
CLINICAL ASPECTS OF CTE
Clinical Presentation
The clinical presentation of CTE characteristically begins in one or more of four distinct domains: mood, behavior, cognitive and motor. Early behavioral symptoms include explosivity, verbal and physical violence, loss of control, impulsivity, paranoia and rage behaviors.22–24 Cognitively, the most prominent deficits are memory, executive functioning and impaired attention. Approximately 45% of subjects with CTE develop dementia; of subjects over the age of 60 years, 66% develop dementia. Complaints of chronic headaches occur in 30%;22 motor symptoms, including dysarthria, dysphagia, coordination problems, and Parkinsonism may also develop.
The age of symptom onset varies from as early as 19 to over 65 years of age. The precise factors that modulate clinical expression of disease are not known; cognitive reserve and lifestyle choices might play important roles. Typically, the clinical symptoms of the disease begin after a latency period of approximately 15 years. Most subjects have a history of concussions, however, 16% of CTE subjects with neuropathologically confrmed CTE have no history of concussion suggesting that subconcussive hits and cumulative exposure to trauma are sufficient to lead to CTE. Overall, the number of years of RHI exposure, not the number of concussions, is significantly associated with hyperphosphorylated tau (p-tau) pathology in CTE.2
Stern and colleagues22 reported that there are two distinct clinical presentations of CTE:
Younger age of onset (mean age of approximately 35 years) with initial behavioral (predominantly explosivity, impulsivity, and physical and verbal violence) and mood changes (depression, hopelessness, suicidality) that later progressed to deficits in cognition.
Older age of onset (mean age of approximately 60 years), with initial cognitive impairment (most prevalent impairments in episodic memory, executive function, and attention). Very subjects in this subgroup exhibit behavioral/mood changes throughout the course of the illness.
The older subgroup that presents with cognitive symptoms is also more likely to exhibit more advanced neuropathology relative to the behavioral/mood subgroup (54.5% versus 27.3% stage IV CTE). Dementia is also most prevalent in the cognitive subgroup, with an average interval of 8 years between dementia diagnosis and death.
Proposed diagnostic research criteria
Recently, Montenigro and colleagues23 conducted a systematic literature review of >200 published cases to develop diagnostic research criteria for the clinical manifestations of CTE, or Traumatic Encephalopathy Syndrome (TES). TES refers to the clinical diagnosis of CTE, whereas CTE is used for cases with neuropathological verification. The diagnosis of TES requires five general criteria, three core clinical features, and nine supportive features.
The general criteria for TES include:
A history of multiple impacts to the head (e.g., via contact sports, military service, domestic violence, head banging, among others), including concussion and subconcussion.
No other neurological disorder that accounts for all of the clinical features; although it can be comorbid with other psychiatric and neurodegenerative conditions.
Presence of clinical features for at least 12 months.
At least one core clinical feature that is a change from baseline.
A minimum of two of supportive features.
The three core clinical features include:
Behavioral symptoms primarily characterized by aggressive and explosive behaviors
Mood dysfunction characterized by depression and related symptoms (e.g., hopelessness, depression)
Cognitive difficulties that involve cognitive decline and impaired cognitive test performance (i.e., 1.5 SD below the normative mean) in attention, executive function, and/or episodic memory.
Supportive features involve impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, documented decline, and delayed symptom onset (at least two years after RHI exposure). These core and supportive features are used to classify individuals into one of the four distinct diagnostic TES variants: TES behavioral/mood variant (TES-BMv), TES cognitive variant (TES-COGv), TES mixed variant (TES-MIXv), and TES dementia (TES-D) (Table 2).
Table 2.
Traumatic Encephalopathy Syndrome (TES) Diagnostic Variants23
| TES Variant | Symptoms |
|---|---|
| Behavioral/Mood | Only behavioral or mood core features (or both):
|
| Cognitive | Only cognitive core features
|
| Mixed | Both cognitive core features and behavioral or mood core features (or both) |
| Dementia | Progressive decline in cognitive core features with or without behavioral or mood core features, in addition to evidence of functional impairment |
TES = Traumatic Encephalopathy Syndrome
Motor features are added as a modifier to the variant, if present, and the clinical course should also be described (e.g., ‘stable course,’ ‘progressive course,’ or ‘unknown/inconsistent’), with the exception of TES-D in which a progressive course is a requirement. Taken together, the development of TES has provided initial criteria to aid in the investigation of the pathophysiological mechanisms of CTE and assist in the designation of “Unlikely CTE,” “Possible CTE,” or “Probable CTE.”
In vivo biomarkers
TES diagnostic criteria are meant to be used in conjunction with future biomarkers for in vivo diagnoses of Possible or Probable CTE. That is, an individual who meets criteria for TES and exhibits biomarker evidence of CTE (when they become available) would be diagnosed with Probable CTE. Currently, there are no validated biomarkers for CTE, but there are a number of potential future biomarkers for Probable CTE that remain to be validated:
Normal amyloid beta levels and elevated p-tau/tau ratios in the cerebrospinal fluid23
Structural MRI-measured abnormalities of the septum pellucidum, and volumetric determination of cortical thinning, white matter reduction and cortical atrophy23
Advanced structural and functional neuroimaging modalities (e.g., diffusion tensor imaging, magnetic resonance spectroscopy, functional magnetic resonance imaging, susceptibility-weight imaging, positron emission tomography; PET).
PET imaging has enabled the study of cerebral protein pathology in Alzheimer’s disease and Frontotemporal Lobar Degeneration and may become useful one day for the detection of p-tau pathology in subjects with suspected CTE. Young subjects with CTE would be expected to be Aβ negative using (11)C-Pittsburgh compound B ((11)C-PiB) or (18)F-florbetapir amyloid-β (Aβ) PET radioligands;25 whereas novel PHF-tau radioligands such as [F-18]-T80726 would be expected to show uptake in the gray-white matter cortical junctions. In addition, PET radiolabelled probes to detect neuroinflammatory activity (e.g., activated microglia) may potentially be useful to show neuroinflammatory changes characteristic of early and advanced CTE.27
Risk factors
Among former football players, duration of football career, age at death, and years since football retirement all correlate with the pathological severity of CTE.2 Age of first exposure to RHI may also play a role in modulating the response to RHI. Former professional NFL players who played football before the age of 12 years had more severe cognitive loss on neuropsychiatric testing and increased microstructural abnormalities in the corpus callosum on diffusion tensor imaging compared to age-matched subjects who did not play football until after the age of 12 years.28, 29 Genes, most likely multiple genes, are also likely to modify CTE risk, including the inheritance of the apolipoprotein e4 (APoE e4) allele, MAPT H1 haplotype and TMEM106B.17 Lifestyle factors (e.g., alcohol, substance abuse, performance enhancing drugs, obesity) might also influence susceptibility or disease progression, but have not yet been tested empirically.
CONCLUSIONS
The past decade has seen a marked rise in scientific research on the long-term effects of RHI and the development of CTE, yet there remain many knowledge gaps that limit our understanding of this disease. While there is overwhelming evidence that CTE affects some professional football players, the risk for CTE in amateur contact sport athletes at the high school and collegiate levels remains to be determined. Larger, prospective studies are critically needed to address CTE risk for the general population and military veterans to define the parameters of RHI exposure, gender and genetics that influence susceptibility. Furthermore, prospective studies are needed to precisely define the clinical manifestations of the disease and the role of factors such as cognitive reserve, lifestyle choice and comorbid medical conditions in the clinical expression of the disease. In vivo biomarkers to facilitate the clinical diagnosis of CTE and monitor potential therapies are urgently needed. Given the millions of Americans involved in contact sports, as well as military personnel who experience RHI, CTE is clearly a public health concern. Although there is intense public pressure to address these concerns immediately and reduce the dangers of contact sports among amateur and professional athletes as well as to protect and improve care for military veterans, these solutions will require large scale, longitudinal prospective studies as well as diligence and patience.
KEY POINTS.
A panel of expert neuropathologists recently defined chronic traumatic encephalopathy (CTE) as a unique neurodegenerative tauopathy characterized by a pathognomonic lesion. The pathognomonic lesion consists of a perivascular accumulation of abnormally hyperphosphorylated tau in neurons and astrocytes distributed in an irregular fashion with a propensity for sulcal depths of the cerebral cortex.
The development of research criteria for the clinical diagnosis of CTE, known as Traumatic Encephalopathy Syndrome (TES), will facilitate clinical research in CTE.
The number of years of exposure to contact sports, not the number of concussions, is significantly associated with more severe tau pathology in CTE, suggesting that repetitive head trauma, including subconcussive injury, is the primary stimulus for disease.
Recent studies in neurodegenerative disease brain bank cohorts suggest that among amateur athletes, changes of CTE are more common than previously recognized.
The development of in vivo biomarkers for CTE to facilitate the diagnosis of CTE during life as well as therapeutic strategies to help individuals with suspected CTE are critically needed.
Acknowledgments
FUNDING
The authors gratefully acknowledge the use of the resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA, USA), the Boston VA Healthcare System, and the Boston University School of Medicine. We also gratefully acknowledge the help of all members of the Chronic Traumatic Encephalopathy Program at Boston University School of Medicine, the Boston VA, as well as the individuals and families whose participation and contributions made this work possible. This work was supported by the Department of Veterans Affairs, the Veterans Affairs Biorepository (CSP 501), National Institute of Neurological Disorders and Stroke (1U01NS086659-01), the National Institute of Aging Boston University Alzheimer’s Disease Center (P30AG13846; supplement 0572063345–5), Department of Defense (W81XWH-13-2-0064, CENC award WXWH-13-2-0095), the National Operating Committee on Standards for Athletic Equipment and the Concussion Legacy Foundation. This work was also supported by unrestricted gifts from the Andlinger Foundation, the World Wrestling Entertainment and the National Football League. Michael Alosco is also supported by the T32-AG06697 post-doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
All authors report no financial conflicts.
REFERENCES
- 1.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAllister T, Flashman L, Maerlender A, et al. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78(22):1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy After Repetitive Head Injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee AC, Daneshvar DH, Alvarez VE, et al. The neuropathology of sport. Acta Neuropathol. 2014;127(1):29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee AC, Stein TD, Kiernan PT, et al. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25(3):350–364. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martland HS. Punch drunk. J Am Med Assoc. 1928;91(15):1103–1107. [Google Scholar]
- 9.Roberts G, Whitwell H, Acland PR, et al. Dementia in a punch-drunk wife. The Lancet. 1990;335(8694):918–919. doi: 10.1016/0140-6736(90)90520-f. [DOI] [PubMed] [Google Scholar]
- 10.Hof P, Knabe R, Bovier P, et al. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol. 1991;82(4):321–326. doi: 10.1007/BF00308819. [DOI] [PubMed] [Google Scholar]
- 11.Geddes J, Vowles G, Nicoll J, et al. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98(2):171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 12.Williams DJ, Tannenberg AE. Dementia pugilistica in an alcoholic achondroplastic dwarf. Pathology. 1996;28(1):102–104. doi: 10.1080/00313029600169653. [DOI] [PubMed] [Google Scholar]
- 13.Critchley M. In: Punch-drunk syndromes: the chronic traumatic encephalopathy of boxers. Hommage a Clovis Vincent, editor. Paris: Maloine; 1949. [Google Scholar]
- 14.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 15.Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1093. doi: 10.1227/01.NEU.0000245601.69451.27. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta neuropathologica. 2015 doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta neuropathologica. :1–16. doi: 10.1007/s00401-015-1509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling H, Holton JL, Shaw K, et al. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1496-y. [DOI] [PubMed] [Google Scholar]
- 21.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130(1):21–34. doi: 10.1007/s00401-015-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5–8):1–17. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mez J, Solomon T, Daneshvar D, et al. Pathologically confirmed chronic traumatic encephalopathy in a 25 year old former college football player. JAMA Neurology. doi: 10.1001/jamaneurol.2015.3998. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Breault C, Joshi AD, et al. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. Journal of Nuclear Medicine. 2013;54(1):70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 27.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70(3):374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 28.Stamm JM, Bourlas AP, Baugh CM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;84(11):1114–1120. doi: 10.1212/WNL.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamm JM, Koerte IK, Muehlmann M, et al. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]