Synopsis
Metabolomics is an important member of the omics community in that it defines which small molecules may be responsible for disease states. This article reviews the essential principles of metabolomics from specimen preparation, chemical analysis, and advanced statistical methods. Metabolomics in TBI has so far been underutilized. Future metabolomics based studies focused on the diagnoses, prognoses, and treatment effects, need to be conducted across all types of TBI.
Keywords: Small molecules, spectroscopy, metabolism, principal component analysis, biomarkers
Introduction
The explosion of “omics” in various fields of biology has lead to new and exciting areas of research. In addition to genomics and proteomics, metabolism, or the biology of small molecules, was an early adapter to the “omics” mania. The overarching aims of “omics” studies are to characterize large collections of molecules and describe their role(s) in biological systems during normal health and disease. The number of molecules quantified in each sample can easily outnumber the subjects and sophisticated analytical and statistical techniques must be used to exploit the rich information found in the biological specimens. The goal of this chapter is to briefly describe the current state of the art regarding metabolomics and frame it in the context of traumatic brain injury.
Metabolomics is concerned with a diverse set of endogenous, low molecular weight biochemicals that serve as substrates and intermediates of biochemical pathways and/or as signaling molecules. Within the systems biology approach, metabolomics is at the top of the biological continuum and represents a snapshot of the systemic environment created by the genome, transcriptome, and proteome (1). Following TBI, particularly in the acute setting, biochemical homeostasis is disrupted and reflected in the metabolome.
There are two general designs in metabolomics studies (2):
Untargeted, or exploratory, metabolomics studies seek to identify a global metabolite fingerprint by quantifying as many metabolites as possible in the same biological sample.
Targeted metabolomics studies limit the number of metabolites included in analysis to improve data interpretation and answer well defined clinical questions.
Untargeted studies are used to test whether two groups (e.g. healthy subject vs TBI patient) can be discriminated from one another on the basis of biofluid metabolites by including as many metabolites as possible no matter the biochemical pathway involved, independent of molecular identity, and assuming bias free metabolite quantification. Targeted studies will focus on a set of endogenous metabolites and/or may include exogenous isotopically-labeled metabolites to track biochemical pathway activity.
Study of the small molecules that enter biochemical pathways has a long history in TBI research, although application of modern metabolomics techniques to these research questions is sparse. For example, glucose and lactate dynamics in the acute period have been studied with respect to outcome, to disease progression, and to mechanisms.
In the first week post-injury, hyperglycolysis was reported to occur in 56% of patients (3). The hyperglycolytic transient response increases cerebral glucose utilization but, despite adequate oxygenation, is not matched by an increase in the metabolic rate of oxygen. The high energy demands are caused by TBI-induced ionic and neurochemical imbalances (4-6) and increased non-oxidative metabolism of glucose produces excess lactate. High lactate CSF concentrations have been associated with cerebral lactic acidosis and, when prolonged, with poor outcomes (7-9).
Global and regional hyperglycolysis are followed by global cerebral metabolic depression, independent of level of consciousness, that can persist for a month following injury (10-12). Cerebral uptake of both oxygen and glucose are depressed compared to healthy adults and glucose is relatively more depressed.
Throughout the acute post-injury period, TBI causes metabolic crisis that is long lasting and unresolved by standard clinical resuscitation and control of intracranial pressure (ICP) (13,14). Cerebral metabolic crisis is the coincidence of low cerebral glucose concentration and high lactate:pyruvate ratio (LPR). Non-ischemic metabolic crisis results from mitochondrial dysfunction, is exacerbated by and/or increases risk for secondary insults, and prolonged acute metabolic crisis is linked to increased brain atrophy six months following injury (15-18).
In a later publication on blood glucose, lactate and oxygen, the majority of TBI patients showed cerebral lactate uptake (AVD>0) at least once and the favorable outcome patients were associated with cerebral lactate uptake at moderately elevated arterial lactate concentrations (19). The ability of the injured brain to oxidize lactate, and the potential benefits associated with lactate as a cerebral fuel, were recently investigated using the dual isotope tracer technique (20,21).
Based on the prior interest in small molecules in TBI research, metabolomics is well suited to expand our current understanding of dysfunctional cerebral metabolism.
Discussion
Biospecimen sources in TBI
Table 1 summarizes possible biospecimens for use in metabolomics studies in TBI research. Targeted metabolomics analysis of cerebral tissue has been applied to TBI research on animal models (22-28).
Table 1.
Biospecimen sources for application of metabolomics in TBI research
| Biospecimen | Pros | Cons |
|---|---|---|
| Tissue | The most direct relevance to the injury, can compare injured hemisphere to uninjured hemisphere | Not applicable to clinical research; rarely would tissue be available and unclear how a metabolomics study could be designed |
| Blood | Easily obtained | Systemic vs. Brain |
| Arteriovenous differences | Systemic minus cerebral metabolites | Jugular catheter is invasive |
| Cerebrospinal fluid | Drained as a treatment for high intracranial pressure, would otherwise be discarded | Commonly available with severe injuries; invasive to obtain otherwise |
| Urine | Easily obtained | Concentration of metabolites a function of systemic dilution; within sample normalization is common |
| Cerebral microdialysate | From the cerebral tissue, as close to the source as possible | Low volumes obtained leading to pooling biofluid over a large periods of time; perfusate and not directly sampling extracellular fluid |
The range of biofluids typically used for metabolomic analysis include, blood (plasma or serum), urine, saliva, cerebral spinal fluid (CSF), and microdialysate. Standard operating procedures for collecting and storing biofluids should be followed (29,30).
Cerebral microdialysate studies based on the hourly metabolite measurements could be considered a type of metabolomics study (31,32). Metabolic crisis is defined as low microdialysis glucose (less than 0.8 mM considered abnormal) coincident with a high lactate:pyruvate ratio (over 40 critical, over 25 abnormal) and has been associated with poor recovery (13-18). Glutamate, glycerol, and urea are also available with the ICSUS Flex Microdialysis Analyzer (M Dialysis AB, Stockholm, Sweden) and microdialysis is considered a valuable tool in the neurological ICU (33). The Cambridge group has published research using pooled cerebral microdialysate (generally over a period of 24 hours) and have introduced tracer metabolites to the tissue through the microdialysis catheter itself (34).
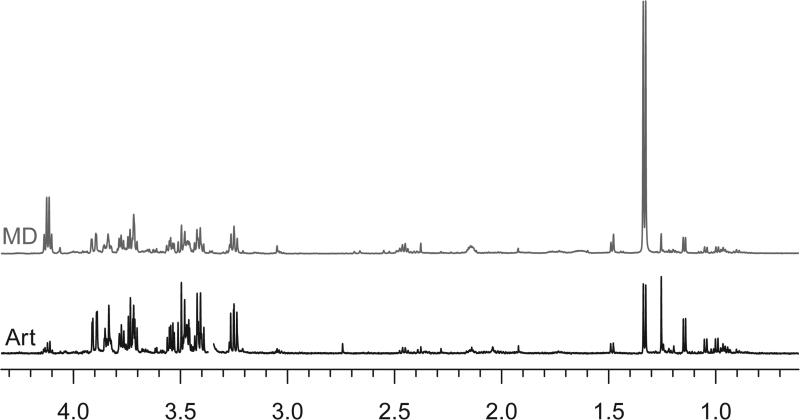
Biospecimen banks may contain multiple biospecimens from the same individual allowing longitudinal metabolomics studies and/or comparison of metabolite profiles between biofluids (35). Figures 1 and 2 present spectra of biofluids collected simultaneously, including arterial blood plasma, jugular venous blood plasma, CSF, and urine (Figure 1). The cerebral microdialysate spectrum is the result of pooling 12 hours of fluid, from midnight to noon, and the corresponding plasma was drawn at 9:30 am (Figure 2).
Figure 1.
Nuclear magnetic resonance (NMR) spectra of arterial blood plasma (Art), jugular venous blood plasma (Jug), cerebrospinal fluid (CSF), and urine. Biospecimens were obtained from a TBI patient within a 5 minute period. The UCLA BIRC research nurse and a research assistant visit the bedside together and, following biofluid draws by the research nurse, samples are immediately placed on ice and processed by the research assistant. Urine and CSF are transferred to Falcon tubes, centrifuged, and frozen on dry ice, and transferred to a −80°C freezer as soon as possible. Blood is transferred to lithium heparin coated tubes, centrifuged, and plasma retained for the freezer.
Figure 2.
Nuclear magnetic resonance (NMR) spectra of arterial blood plasma (Art) and cerebral microdialysate (MD) pooled over a period of 12 hours. Cerebral microdialysis vials are immediately analyzed by the ICSUS flex Microdialysis Analyzer for glucose, lactate, and pyruvate as routine clinical practice and frozen on dry ice and transferred to a −80°C freezer as soon as possible after that.
Integration of metabolomics datasets from different biofluids or integration of metabolomics datasets collected on different platforms are an exciting avenue for TBI researchers, particularly for understanding dysfunction in biochemical pathways and for working toward a systems-level understanding of the cerebral and systemic responses to neurotrauma.
Technologies used in metabolomics
Mass spectrometry (MS) and nuclear magnetic resonance (NMR) are the most common technologies used in metabolomics. Applying these technologies to metabolomics studies requires careful consideration of study goals and the expertise available and detailed reviews are available (36,37).
Table 2 compares MS-based methods and NMR. MS-based methods identify metabolites by the mass-to-charge ratio (m/z) and, when using a chromatography step, the retention time is also required. The intensity of the MS peak at a particular retention time and m/z is proportional to the concentration.
Table 2.
Comparison between mass spectrometry and NMR as it applies to metabolomics research
| Mass Spectrometry | NMR | |
|---|---|---|
| Sensitivity | ≥ pM concentrations | ≥ μM concentrations |
| Sample preparation | Minimal to extensive: deproteinization, derivatization possibly required for targeted studies | Minimal: addition of a buffered deuterium oxide solution, deproteinization optional |
| Sample volume | On the order of 10 μL | On the order of 100 μL or more |
| =Quantification | Relative concentrations are most common, absolute quantification requires internal chemical standard at known concentration for each metabolite | Absolute concentrations are standard; internal chemical standard at known concentration routinely added during sample preparation (44) |
| Chromatography? | Yes, either liquid or gas chromatography used prior to injection into the instrument | Not common |
| Sample recovered? | No: principle relies on fragmentation of molecules and physical interaction with the instrument | Yes: principle relies on nuclear spin, a fundamental property of certain nuclei, and excitation/emission of photons is nondestructive |
| Quality control | Inter- and intra-batch variability requires periodically testing a set of standards and correcting for changes in sensitivity over time after data collection | Not required |
| Global | Yes, but requires quality control and instrument expertise | Yes |
| Targeted: endogenous | Highly sensitive and specific | Depends on the location of the resonance peaks in the spectrum |
| Targeted: exogenous | Yes | Depends, and NMR-active isotopes (13C or 15N) at low natural abundance are helpful |
NMR methods identify metabolites by the spectral peak pattern; all chemicals present in the biofluid containing hydrogen are present in the NMR spectrum. Metabolites with similar chemical structures overlap one another (for example, L-lactate and L-threonine overlap at 1.3 ppm). The integrated area of the spectral peak is proportional to concentration.
Because of the complexity and specialization required for MS-based metabolomics methods, there are companies, such as Metabolon Inc (Durham, NC), that analyze samples and return high quality data, statistical analysis, and chemical identification to their customers. Companies offering fee-for-service analysis generally provide reduced pricing to academic institutions. Additionally, there are NIH funded facilities such as the West Coast Metabolomics Center at UC Davis that can also conduct analysis as a fee-for-service.
Statistical data analysis
With the aim to better understand disease pathology, both implementing and interpreting advanced statistical modeling techniques are required. The large size of the raw data makes all stages of data processing non-trivial.
Prior to statistical exploration of the data, extensive spectral processing is required (38). These steps ensure that (a) high-quality datasets with minimal biases are used, (b) spectral features are assigned to metabolites correctly, and (c) all spectra are aligned prior to metabolite concentration extraction.
Univariate comparisons are helpful in data exploration and may be helpful in discussing an interpretation of the data, but the increasing number of metabolites that modern analytic techniques allow make univariate analyses laborious. Multiple testing corrections, including the Bonferroni correction and the q-value (39), should be employed in order to minimize spurious results in these cases (40).
Multivariate regression methods are more likely to discern statistically significant changes and minimize false positives. Multivariate regression methods include principle components analysis (PCA), partial least squares (PLS), and orthogonal projections to latent structures (OPLS) and while these methods are widely available in statistical computing packages, using the method properly is not trivial and perhaps more important than which method is used (41).
Unsupervised methods, such as principle components analysis (PCA), seek out patterns in the variance of the data without any other prior knowledge about the data. PCA in particular parses out the variance from largest to smallest. Supervised methods identify patterns in the variance associated with a certain trait or variable of interest while down weighting other (perhaps larger) sources of variance.
For metabolomics to meaningfully impact TBI research, results must be reported numerically, not only as a PC scores plot, and statistical significance reported. Without an attempt at interpreting metabolite loadings within the biological context, metabolomics studies lose scientific impact.
In order for metabolomics techniques to enter the clinic, the discrimination must be superior to the current gold standard. In the case that a metabolomics test does not significantly improve diagnosis accuracy, further research in the area could clarify which biochemical pathways are associated with a disease.
Role of metabolomics in clinical research
Metabolomics has been applied to diagnosis of several diseases, including diabetes and cardiovascular disease (41). Diagnosis of mild TBI or concussions is an area of active research where metabolomics could be applied (42).
This powerful modern research technique, metabolomics, could serve many roles in contemporary TBI clinical research (Box 1). The TBI patient population is heterogeneous for several reasons, both because of the injury itself and because the disease progresses over time differently between individuals. Further, the metabolome is reflective of active biochemical pathways and is also influenced by a subject's environment. This means large patient cohorts will be required to detect small effects.
Box 1.
Possible applications of metabolomics in TBI research
|
Acute injury setting (in the neurological ICU): |
| • Monitoring acute pathophysiology |
| • Predicting onset of secondary injuries, such as high ICP, edema, vasospasm, etc. |
| • Monitoring treatments: identifying candidate treatments and treatment success |
| • Prognosis, long-term outcomes |
|
Chronic injuries: |
| • Monitoring evolving pathophysiology |
| • Rehabilitation suitablility |
| • Association between TBI and later-in-life health issues |
Important for real clinical applications to convert the numerical “significance” to whether this could actually improve things for patients. Not only sensitivity/specificity, but also whether it could improve upon current gold standard.
Example of metabolomics applied to TBI research
We conducted a observational feasibility study aimed at identifying differences in the metabolite profile of TBI patients and healthy control individuals. Six TBI patients (mean age 31; mean GCS: 3.8) and 2 healthy controls were consented for arterial and jugular venous blood sampling. The UCLA institutional review board (IRB) for human research approved this study. TBI subjects were identified, consented by proxy, and enrolled. Eligible patients were mechanically ventilated with moderate or severe head-injured, ages 18 years and older. Normal volunteers were recruited under UCLA IRB approval.
A total of 90 metabolites were identified by liquid-chromatography MS (43), analyzed with an UltiMate 3000RSLC (Thermo Scientific) coupled to a Q Exactive mass spectrometer (Thermo Scientific). The Q Exactive was run with polarity switching (+4.00 kV / −4.00 kV) in full scan mode with an m/z range of 70-1050. Separation was achieved using A) 5 mM NH4AcO (pH 9.9) and B) ACN. The gradient started with 15% A) going to 90% A) over 18 min, followed by an isocratic step for 9 min. and reversal to the initial 15% A) for 7 min. Metabolites were quantified with TraceFinder 3.1 using accurate mass measurements (≤ 3 ppm) and retention times established by running pure standards. PCA was performed using the statistical language R.
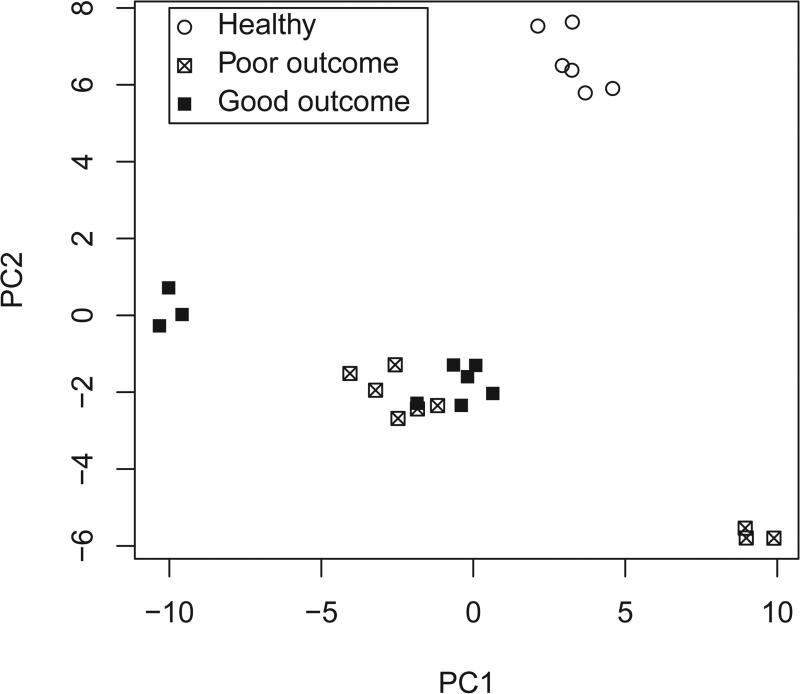
PCA revealed significant separation of the metabolite contributions of TBI versus normal patients in PC2 (p<0.0001, Figure 3). PC1 discriminated between individuals included in the analysis (explaining 26% of the total variance, PC2 explaining 15%). We were unable to discriminate between subjects with 6-month Glasgow Outcome Scale extended (GOSe) scores between 2 and 4 (poor outcome) and those between 5 and 8 (good outcome).
Figure 3.
Principle components score plot. Eight arterial plasma samples (2 healthy controls) were run in triplicate.
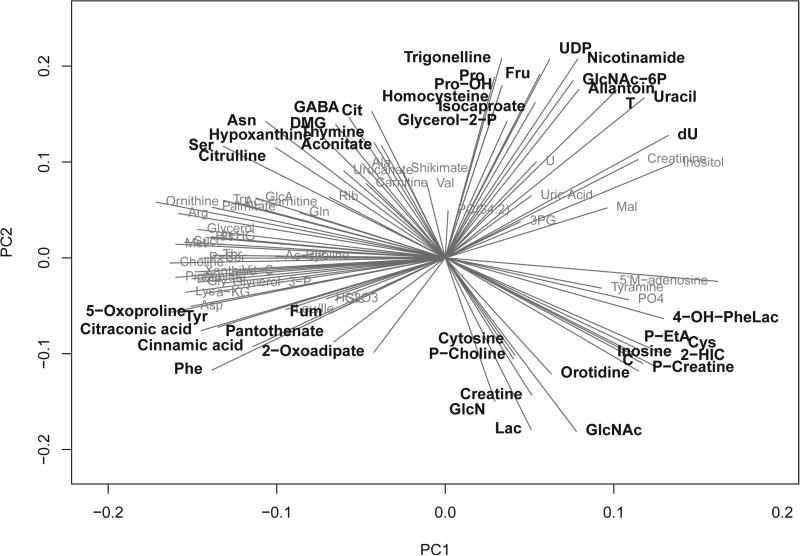
Investigation of the metabolite loadings revealed that metabolites of glycolysis and the TCA cycle, such as fumarate, lactate, and 2-oxoadipate, were the most negative metabolite loadings (Figure 4) and thus make large contributions to the PC scores of brain-injured patients, ranging from approximately −6 to 1 (scores are unit less). In contrast, citrate and aconitate, metabolites in these same pathways, had the largest metabolite loadings and, thus, were decreased in TBI patients compared with normal individuals. Other differences that were identified between these two groups included changes in numerous amino acids (phenylalanine, tyrosine, cysteine), metabolites involved in glycerophospholipid metabolism (phosphoenthanolamine and choline phosphate), and metabolites involved in pyrimidine metabolism (cytosine and orotidine), as presented in Fig 4.
Figure 4.
Principle components loadings plot. Loadings in the top PC2 quartile and bottom PC2 quartile are highlighted.
Summary
There are three major components required for conducting metabolomics studies:
Good specimens collected in an accepted manner.
A strong analytical facility.
Knowledgeable statisticians.
Understanding the metabolomic profile of TBI is an important quest. From the moment of physical insult to rehabilitation and recovery, metabolomics can yield useful information about the pathophysiology of this disease. Metabolomics is an underlying component of precision medicine by allowing the clinician scientist the ability to tailor medical care and treatment. Many more metabolomic studies need to be conducted across the entire spectrum of TBI in order to better understand and treat TBI.
Key Points (3-5).
-
1)
Metabolomics is the study of the small molecules which are reactants, intermediaries, and end products of biological processes. Metabolomics can define healthy and disease states. The genome and proteome, along with environmental factors, contribute to the metabolome. Metabolomic studies can be untargeted or targeted.
-
2)
Metabolomic analytic techniques include nuclear magnetic resonance spectroscopy and mass spectroscopy including gas chromatography or liquid chromatography.
-
3)
The following three major components are required for conducting metabolomics studies: good specimens collected in an accepted manner; a strong analytical facility; and knowledgeable statisticians.
-
4)
Common data elements (CDE), biobanks, and collaborations/multidisciplinary teams will be necessary to produce meaningful results.
-
5)
Heterogeneity of disease and progressive nature mean that applying metabolomics to predicting secondary events and/or long-term risks could be highly impactful.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illness. American Journal of Respiratory and Critical Care Medicine. 2011;184:647–655. doi: 10.1164/rccm.201103-0474CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowling FG, Thomas M. Analyzing the metabolome. In: Trent R, editor. Clinical Bioinformatics. second edition. Humana Press; 2014. pp. 31–45. chapter 3. [Google Scholar]
- 3.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Becker DP, Tamura T, Hovda D. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 5.Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Lactate accumulation following concussive brain injury: the role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;20:196–2042. doi: 10.1016/0006-8993(94)01444-m. [DOI] [PubMed] [Google Scholar]
- 6.Sunami K, Nakamura T, Ozawa Y, Kubota M, Namba H, Yamaura A. Hypermetabolic state following experimental head injury. Neurosurg Rev. 1989;12(Suppl 1):400–415. doi: 10.1007/BF01790682. [DOI] [PubMed] [Google Scholar]
- 7.De Salles AAF, Kontos HA, Becker DP, Yang MS, Ward JD, Moulton R, Gruemer HD, Lutz H, Maset AL, Jenkins L, Marmarou A, Muizelaar P. Prognostic significance of ventricular CSF lactic acidosis in severe head injury. J Neurosurg. 1986;65:615–624. doi: 10.3171/jns.1986.65.5.0615. [DOI] [PubMed] [Google Scholar]
- 8.De Salles AAF, Muizelaar JP, Young HF. Hyperglycemia, cerebrospinal fluid lactic acidosis, and cerebral blood flow in severely head-injured patients. Neurosurg. 1987;21:45–50. doi: 10.1227/00006123-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Inao S, Marmarou A, Clarke GD, Andersen BJ, Fatouros PP, Young HF. Production and clearance of lactate from brain tissue, cerebrospinal fluid, and serum following experimental brain injury. J Neurosurg. 1988;69:736–744. doi: 10.3171/jns.1988.69.5.0736. [DOI] [PubMed] [Google Scholar]
- 10.Bergsneider M, Hovda D, McArthur D, Etchepare M, Huang S-C, Sehati N, Satz P, Phelps M, Becker D. Metabolic recovery following human traumatic brain injury based on FDG-PET: Time course and relationship to neurological disability. J Head Trauma Rehabil. 2001;16:135–148. doi: 10.1097/00001199-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bergsneider M, Hovda DA, Lee SM, Kelly DF, McArthur DL, Vespa PM, Lee JH, Huang SC, Martin NA, Phelps ME, Becker DP. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000;17:389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- 12.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 13.Stein NR, McArthur DL, Etchepare M, Vespa PM. Early cerebral metabolic crisis after TBI influences outcome despite adequate hemodynamic resuscitation. Neurocrit Care. 2012;17:49–57. doi: 10.1007/s12028-012-9708-y. [DOI] [PubMed] [Google Scholar]
- 14.Vespa PM, Bergsneider M, Hattori N, Wu H-M, Huang S-C, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J Cerebr Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, Hovda DA, Alger JR, Vespa PM. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36:2871–2877. doi: 10.1097/CCM.0b013e318186a4a0. [DOI] [PubMed] [Google Scholar]
- 16.Vespa PM, McArthur DL, O'Phelan K, Glenn TC, Etchepare M, Kelly DF, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: A microdialysis study. J Cerebr Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- 17.Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, McArthur DL, Alger JR, Etchepare M, Hovda DA, Glenn TC, Huang S, Dinov I, Vespa PM. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cerebr Blood Flow Metab. 2010;30:883–894. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: Indices of oxygen, glucose, and lactate metabolism. J Cerebr Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 20.Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda DA, Vespa PM, Brooks GA. Lactate: Brain fuel in human traumatic brain injury. a comparison to normal healthy control subjects. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3483. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn TC, Martin NA, McArthur DL, Vespa PM, Horning MA, Johnson ML, Brooks GA. Endogenous nutritive support following traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3482. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartnik BL, Hovda DA, Lee PWN. Glucose metabolism after traumatic brain injury: Estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J Neurotrauma. 2007;24:181–194. doi: 10.1089/neu.2006.0038. [DOI] [PubMed] [Google Scholar]
- 23.Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: A 13C NMR study. J Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- 24.Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- 25.Bentzer P, Davidsson H, Grande P. Microdialysis-based long-term measurement of energy-related metabolites in the rat brain following a fluid percussion trauma. J Neurotrauma. 2000;17:441–447. doi: 10.1089/neu.2000.17.441. [DOI] [PubMed] [Google Scholar]
- 26.Clausen F, Hillered L, Gustafsson J. Cerebral glucose metabolism after traumatic brain injury in the rat studied by 13C-glucose and microdialysis. Acta Neurochir. 2011;153:653–658. doi: 10.1007/s00701-010-0871-7. [DOI] [PubMed] [Google Scholar]
- 27.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, Marmarou A, Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurg. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- 28.Viant MR, Lyeth BG, Miller MG, Berman RF. An NMR metabolomic investigation of early metabolic disturbances following TBI in a mammalian model. NMR Biomed. 2005;18:507–516. doi: 10.1002/nbm.980. [DOI] [PubMed] [Google Scholar]
- 29.Manley GT, Diaz-Arrastia R, Brophy M, Engel D, Goodman C, Gwinn K, Veenstra TD, Ling G, Ottens AK, Tortella F, Hayes RL. Common data elements for traumatic brain injury: Recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AIR, Mukherjee P, Yuh EL, Puccio AM, Schnyer DM, Manley GT, TRACK-TBI Investigators including. Casey SS, Cheong M, Dams-O'Connor K, Hricik AJ, Knight EE, Kulubya ES, Menon DK, Morabito DJ, Pacheco JL, Sinha TK. Transforming research and clinical knowledge in traumatic brain injury pilot: Multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30:1831–1844. doi: 10.1089/neu.2013.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalloh I, Helmy A, Shannon RJ, Gallagher CN, Menon DK, Carpenter KLH, Hutchinson PJ. Lactate uptake by the injured human brain: Evidence from an arteriovenous gradient and cerebral microdialysis study. J Neurotrauma. 2013;30:2031–2037. doi: 10.1089/neu.2013.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timofeev I, Carpenter K, Nortje J, Al-Rawi P, O'Connell M, Czosnyka M, Smielewski P, Pickard J, Menon D, Kirkpatrick P, Gupta A, Hutchinson P. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 233 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson P, O'Phelan K, the Participants of the International Multi-disciplinary Consensus Conference on Multimodality Monitoring International multidisciplinary consensus conference on multimodality monitoring: cerebral metabolism. Neurocrit Care. 2014;21:S148–S158. doi: 10.1007/s12028-014-0035-3. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher CN, Carpenter KLH, Grice P, Howe DJ, Mason A, Timofeev I, Menon DK, Kirkpatrick PJ, Pickard JD, Sutherland GR, Hutchinson PJ. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–2849. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- 35.Robinette SL, Lindon JC, Nicholson JK. Statistical spectroscopic tools for biomarker discovery and systems medicine. Anal Chem. 2013;85:5297–5303. doi: 10.1021/ac4007254. [DOI] [PubMed] [Google Scholar]
- 36.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Gowda GAN, Ye T, Raftery D. Advances in NMR-based biofluid analysis and metabolite profiling. Analyst. 2010;135:1490–1498. doi: 10.1039/c000091d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso A, Marsal S, Julia A. Analytical methods in untargeted metabolomics: state of the art in 2015. Frontiers in Bioengineering and Biotechnology. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.A Dabney JD. Storey, and with the assistance from GR Warnes. qvalue: Q-value estimation for false discovery rate control. R package version 1.36.0 [Google Scholar]
- 40.Broadhurst DI, Kell DB. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2006;2:171–196. [Google Scholar]
- 41.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics - a review in human disease diagnosis. Analytica Chimica Acta. 2010;659:23–33. doi: 10.1016/j.aca.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 42.Jeter CB, Hergenroeder GW, Ward NH, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acid and their metabolite levels. J Neurotrauma. 2013;30:671–679. doi: 10.1089/neu.2012.2491. [DOI] [PubMed] [Google Scholar]
- 43.Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK, McCormick F, Graeber TG, Christofk HR. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolahan SM, Hirt D, Glenn TC. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis; 2015. p. 25. chapter Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.