Abstract
Treatment of large bone defects remains an unsolved clinical challenge, despite a wide array of existing bone graft materials and strategies. Local deficiency in osteogenic connective tissue progenitors (CTP-Os) due to tissue loss is one of the central biological barriers to bone regeneration. Density separation (DS) and selective retention (SR) represent two promising methods that can be used intraoperatively to rapidly concentrate cells and potentially select CTP-Os. This project was designed to compare DS and SR using the canine femoral multidefect (CFMD) model. Mineralized cancellous allograft (MCA) was used as a standardized scaffold for cell transplantation. Two experiments were performed using a cohort of six animals in each comparison. In Cohort I, unprocessed bone marrow aspirate (BMA) clot was compared to DS processing. MCA combined with raw BMA or DS processed cells produced a robust and advanced stage of bone regeneration throughout the defect in 4 weeks with reconstitution of hematopoietic marrow. However, the retention of DS processed cells and CTP-Os in the MCA matrix was low compared to BMA clot. In Cohort II, MCA with DS-T cells (addition of calcium chloride thrombin to induce clotting and enhance cell and CTP-O retention) was compared to MCA with SR cells. A mean of 276 ± 86 million nucleated cells and 29,030 ± 10,510 CTP-Os were implanted per defect in the DS-T group. A mean of 76 ± 42 million nucleated cells and 30,266 ± 15,850 CTP-Os were implanted in the SR group. Bone formation was robust and not different between treatments. Histologically, both groups demonstrated regeneration of hematopoietic marrow tissue. However, SR sites contained more hematopoietic vascular tissues, less fibrosis, and less residual allograft, particularly in the intramedullary cavity, suggesting a more advanced stage of remodeling (p = 0.04). These data demonstrate excellent overall performance of DS and SR processing methods. Both methods achieve a bone regeneration response that approaches the limits of performance that can be achieved in the CFMD model. Further advancement and comparison of these intraoperative bone marrow cell processing methods will require use of a larger and more biologically compromised defect site to guide the next steps of preclinical development and optimization.
Introduction
This project was designed to assess and compare density separation (DS) and selective retention (SR), two promising methods for rapid intraoperative processing of bone marrow-derived cells as a means of enhancing bone regeneration.1–7 This study was performed as the second phase of a systematic effort to identify and develop optimal methods for regeneration of bone in large complex bone defects that combine scaffolds, sources of osteogenic cells, and bioactive scaffold modifications. Phase I of this assessment utilized the canine femoral multidefect (CFMD) model to evaluate and compare a promising range of biological scaffold materials designed for use as bone void fillers.8 This first study demonstrated that mineralized cancellous allograft (MCA) was superior to a range of synthetic biodegradable scaffolds. Phase II of this assessment, reported here, focuses on evaluation of the added value of autogenous bone marrow aspirate (BMA) as a source of osteogenic cells and the use of rapid intraoperative processing methods that may be used to enhance the performance of marrow-derived cells.
Intraoperative processing provides a means of increasing the number and concentration of marrow-derived osteogenic connective tissue progenitors (CTP-Os) within a graft site. Intraoperative processing may also provide a means of removing cells that may compete with CTP-Os within the graft site or inhibit the function of CTPs (e.g., nonprogenitor nucleated cells and erythrocytes). Removal of non-CTPs can be measured as an increase in CTP-O prevalence in the graft site (i.e., the number of CTP-Os per million nucleated cells). An associated phase II study evaluating cell sourcing using the CFMD model demonstrated a significant improvement in the histological outcome when BMA was enriched in CTP-Os using magnetic separation (MS) to concentrate and collect CTP-Os based on hyaluronan as a surface marker.9
The current study evaluates two more commonly available and less technically complex methods for marrow processing; DS and SR. DS processing involves the use of a centrifuge to concentrate nucleated cells and CTP-Os by forming a cellular buffy-coat layer, removing serum and erythrocytes (red blood cells [RBCs]). DS is based on the intermediate density of most cells and CTP-Os between serum and RBCs.5,10–13 SR processing involves passing a suspension of cells containing CTP-Os across the surface of a porous material to which CTP-Os are more likely to attach than nonprogenitors or RBCs.4,6,7,14,15 As a result, nonadherent cells and RBCs are discarded and adherent cells that are implanted contain an increased concentration and prevalence of CTP-Os.
The feasibility and value of density gradient centrifugation bone marrow technologies in the treatment of bone defect have been demonstrated clinically in a prospective study by Jager et al., which showed that concentrate marrow can be an alternative to autogenous bone graft and help reduce donor-site morbidity.16,17 Brodke et al. demonstrated that in a large canine critical defect model, graft materials could be enriched with osteoprogenitor cells using SR technologies and that the SR-enriched grafts were a viable alternative to autologous bone for the repair of large critical-sized defects.4 Lee and Goodman reported that they achieved a clinically therapeutic effect in treating secondary osteonecrosis of the femoral condyles using demineralized cancellous bone chip mixtures mixed with SR cells.18 There are strong theoretical reasons to consider using one or both of these rapid methods for intraoperative processing when designing cell therapy strategies. An increase in concentration allows more CTP-Os to be placed within the defect sites.5 An increased prevalence of CTP-Os means that the implanted CTP-Os will have fewer cells to compete with for limited supply of oxygen in the defect site.14,19–21 Removal of RBCs limits the debris that is placed into the defect site and the associated inflammatory response needed to clear the debris from the site where the bone is desired.22–24 This study provides the first attempt to objectively evaluate and compare these methods for processing marrow-derived cells, using a biologically relevant large animal model. Our two specific hypotheses are as follows: (1) the number of marrow-derived cells and CTPs that are delivered into a defect site will be dependent upon methods that are used for processing and transplantation and (2) the concentration of cells and CTPs within the defect will influence the outcome of tissue regeneration in a defect site (amount of bone formed and the quality of vascularity and other nonbone tissue in the defect site).
Materials and Methods
Animals
This study was conducted with approval from the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC) under protocols numbers 2012-0685 and 2012-0788 and the Animal Care and Use Review Office (ACURO) of US Army Medical Research and Materiel Command (MRMC) under protocol number 08288003.67. Study animals were cared for in accordance with the principles of the Guide for the Care and Use of Laboratory Animals.25
Twelve adult purposely bred male coonhounds (34.4 ± 2.3 kg, age 1.1 ± 0.2 years [range 1.0–1.6 years]) were used. These were divided into two 6-animal cohorts, Cohort I and Cohort II, as described below.
CFMD model
The CFMD model has been well described.8,9,26,27 In brief, the CFMD model provides four 10-mm diameter by15-mm-long cylindrical defects for assessment in each subject. These defects are placed in the lateral cortex of the proximal femur. Each defect site is separated by a minimum of 1.5 cm of normal bone and marrow, so that the sites do not interact. The availability of data from four sites in each subject enables comparison of two materials, while controlling for variation between implant sites and subjects. The defects are designed to be of sufficient size to create a biological environment in which the interior of the defect is characterized by profound hypoxia, a key feature of large clinical defects that is not modeled in small animal defects.27 Bone formation and revascularization within the defect occur through a process of ingrowth that has a radially oriented “outside in” pattern, which can be readily measured and characterized using microcomputed tomography and histological methods. As a result, the extent to which a bone healing response progresses into the center of these defects provides an objective comparison of the efficacy of the methods used at each site.
Implant materials
MCA bone matrix (canine) was prepared and packaged by the Musculoskeletal Transplant Foundation (MTF) (Edison, NJ) using canine bones from left and right humeri and right femur. Donor bone was sterilely harvested following euthanasia of canine subjects used in prior experiments. Harvest, shipping, and processing were performed using the standardized methods for preparation of commercially available mineralized cancellous bone matrix, consistent with clinical guidelines established by the American Association of Tissue Banks. Cuboidal chips were prepared with a dimension of roughly 3 × 3 × 3 mm to enable uniform packing of ∼22 chips to fill each femoral defect site. Sterile processing was maintained throughout with standard confirmatory cultures. No terminal sterilization was used. One donor animal (humeri and femur) provides enough MCA for 6–8 recipients. Therefore, one donor provided all of the MCA for Cohort I and one donor provided all of the MCA for Cohort II. This minimized the potential confounding effect of allograft source when making comparisons between cell processing methods.
Experimental design
Surgery and sample collection were performed between August 2012 and February 2013. In each experiment, the two processing methods were randomly assigned within each animal to either an “ABBA” configuration (three dogs) or a “BAAB” configuration (three dogs) to control for possible site or proximity effects, where “A” is one method (e.g., DS) and “B” is the other (e.g., SR) (Fig. 1). Euthanasia was performed 4 weeks after implantation using Beuthanasia™ solution (5 mL/5 kg IV) (Merck Animal Health, Summit, NJ). The femur was explanted and cleared of adhering tissue. Individual defect sites were separated using a band saw and fixed in 10% neutral-buffered formalin. After 48 h, the solution was replaced with 70% ethanol to prevent demineralization.
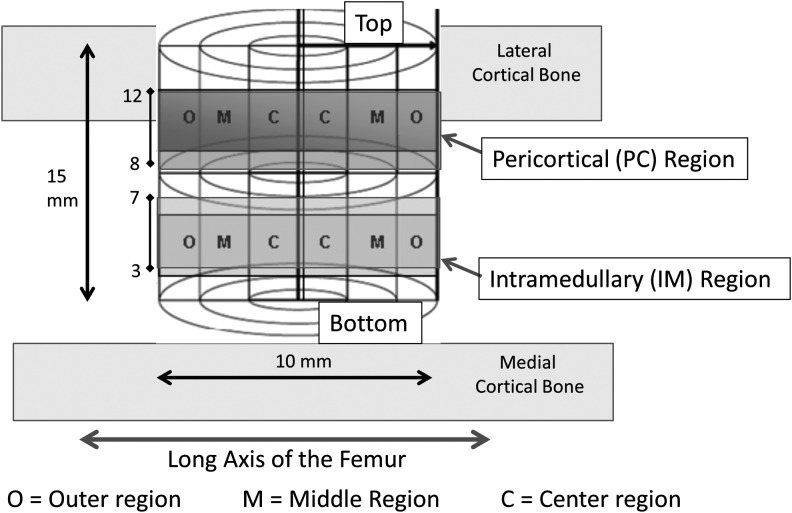
FIG. 1.
Region of interests within a defect site. The pericortical (PC) region represents the region between 8 and 12 mm from the bottom of the grafted site, which is adjacent to the cortex. The intramedullary (IM) region is defined as the region between 3 and 7 mm from the bottom of the grafted site, which is separated by several millimeters from the endosteal source of osteogenic cells and bound only by cells from the marrow cavity.
An outcome assessment 4 weeks after implantation was selected for two reasons: (1) prior experience in this model has shown that autogenous cancellous bone (unpublished) and MCA9 can achieve robust bone formation and even an advanced state of intramedullary (IM) remodeling in the CFMD model as early as 4 weeks and (2) those methods that are most effective in initiating a rapid onset of bone formation and ingrowth are, we feel, the methods that are most likely to improve upon existing therapies when advanced into more rigorous preclinical models and ultimately into clinical practice. Therefore, assessment at later time points might be too late to detect clinically important differences in the rate of onset and extent of new bone formation and remodeling events when effective scaffolds and cell processing methods are used.
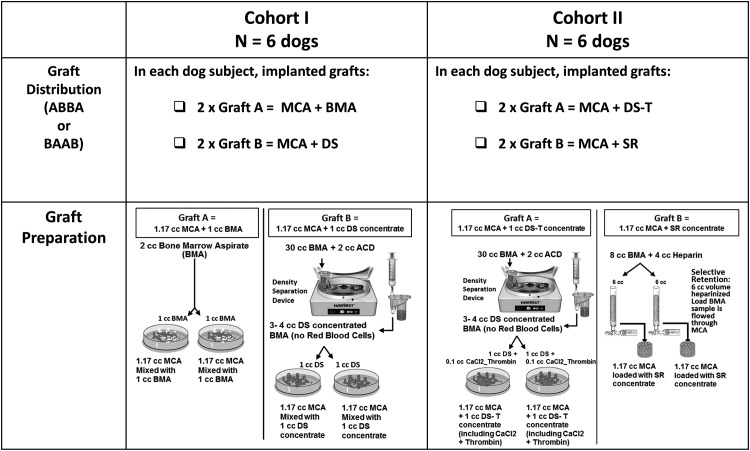
Cohort I: It compared the performance of MCA soaked with an unprocessed BMA to MCA soaked in a BMA concentrate prepared using the DS method. Both preparations used BMA, which was harvested from the proximal humerus following the induction of anesthesia (Fig. 2).
FIG. 2.
Experimental design description showing Cohort I: bone marrow aspirate (BMA) versus density separation (DS) processing and Cohort II: DS-T versus selective retention (SR) processing.
For preparation of BMA grafts, a single 2 cc aspirate of bone marrow was harvested using standard clinical technique using a Lee-Lok needle (Lee Medical, Plainsboro, NJ) without an anticoagulant. This was allowed to clot naturally. MCA (1.17 cc) was prewetted with normal saline. Excess fluid was poured off. The BMA clot (1 cc) was then mechanically mixed with MCA and allowed to sit for 20 min in a humidified container before implantation.7,14,28 A separate 1 cc bone marrow was aspirated for cell analysis.
To prepare DS grafts, 15 separate 2 cc aspirates were collected as previously described28 and rapidly mixed in a pooled bag containing 2 cc of acid-citrate-dextrose solution A (ACD-A) anticoagulant, U.S.P. (Citra Labs, Braintree, MA), providing a 32 cc suspension of marrow-derived cells. Two cubic centimeters of this sample was assayed for cells and CTP-Os, and 30 cc of this anticoagulated sample was processed on the Harvest SmartPrep 2® BMAC system (Harvest Technologies, Plymouth, MA). The Harvest system separates nucleated cells using a 1.060 g/mL density floating shelf, which captures the cellular buffy-coat layer, and concentrates nucleated cells into a 3–4 cc volume.10 The graft for each implant site was then prepared using 1.17 cc of prewetted MCA by dripping on 1 cc of the marrow concentrate and allowing this to sit in a humidified container for 20 min before use, to allow cells and CTP-Os to attach.
Samples of both, the original anticoagulated BMA sample and the concentrated sample, were analyzed to measure total cells and CTP-Os using established methods for cell counting and colony formation by CTP-Os29 (see section below “Assay of Osteoblastic Progenitors using a Colony-Forming Unit Assay for Connective Tissue Progenitors”). In addition, any residual fluid that was not retained and implanted with the MCA graft was also analyzed to measure total cells and CTP-Os. This analysis allowed sequential assessment of the yield of cells and CTP-Os in the initial harvest, the step of DS processing, the efficiency of attachment of cells and CTP-Os to the matrix, and the total number of cells and CTP-Os that were implanted at each site. Six coonhounds and a total of 24 sites, 12 for each method were used in this comparison.
Cohort II: The preparation of DS samples was identical as in Cohort I with one exception. Clotting was induced in DS samples in Cohort II by dripping 0.1 cc of calcium chloride and thrombin (1 cc CaCl2 and 1000 IU thrombin [King Pharmaceuticals, Bristol, TN]) on the MCA after addition of the 1 cc of marrow concentrate. This composition is abbreviated below as DS-T to distinguish it from the nonclotted DS preparation used in Cohort I (Fig. 2).
SR processing involved collection of five separate 2 cc aspirates, each using a 10-cc syringe containing 1 cc of heparinized saline (1000 U/mL), which was pooled to provide a 15 cc heparinized suspension of marrow-derived cells.6,7,9,28 MCA (1.17 cc) was placed in a porous flow-through chamber, as previously described.9 A 6 cc volume of the heparinized suspension “loading sample” was flowed through the MCA at a linear flow rate of 25 mm/min, allowing cells and CTP-Os that attached to the MCA to remain with the MCA in the chamber and allowing other cells, RBCs and any CTP-Os, which did not attach to the MCA to be retrieved in an “effluent sample.” Again, analysis of the initial sample and the effluent sample in each matrix allowed assessment of the efficiency of cell and CTP-O retention, concentration, yield, and fold change in concentration for each MCA implant. Six coonhounds and a total of 24 sites, 12 for each method, were used in this comparison.
MicroCT processing and analysis
Microcomputed tomography (MicroCT) was used as the primary outcome measure to evaluate the amount and distribution of mineralized tissue (i.e., the combination of new bone formation plus any residual unresorbed MCA scaffold). MicroCT scans were obtained of each graft site before processing for histological assessment using a calibrated GE eXplore Locus MicroCT scanner (GE Healthcare, Milwaukee, WI) as previously described.8 Each specimen was visualized as a three-dimensional volume using MicroView software (GE Healthcare). Each defect site was defined using a cylindrical “defect template” volume of 10 mm in diameter and 15 mm in length size, which was manually positioned using the circular introitus and marks from the flat finishing drill on the opposite cortex as fiduciary guides. New bone formation within the defect site was identified based on voxel density above the bone threshold defined in Hounsfield Units (HU). A bone threshold (900 HU) was defined and applied in the analysis of all samples. This threshold was selected by a skilled operator who was blinded with respect to the materials being assessed. This threshold was slightly below the density of native trabecular bone and any periosteal new bone formation evident in the samples, but excluded native soft tissues.
Histology analysis
Histology was used to assess three key outcome measures that cannot be evaluated based on microCT: (1) screening for evidence of local inflammation that indicates infection within a defect site or reaction to implanted materials, (2) the relative extent of remodeling of the implanted MCA scaffold (microCT cannot differentiate between mineralization associated with new bone formation and mineralization that is associated with residual allograft matrix), and (3) assessment of quantitative differences in the nature of the tissue forms (bone and nonbone) that reflect the quality of bone and soft tissue that was regenerated as a result of each treatment.9,30
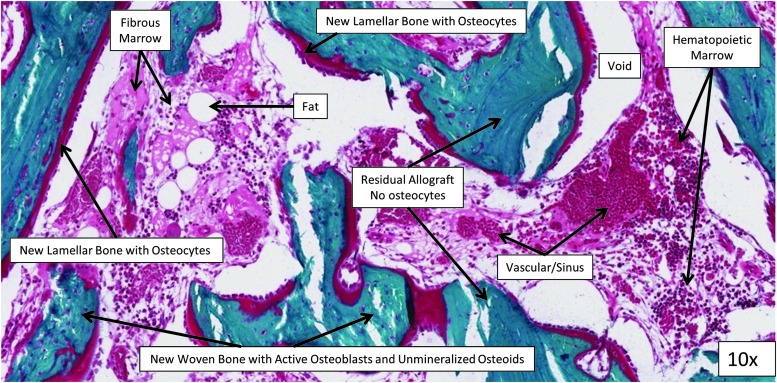
Each sample was processed and analyzed in the Bone Histomorphometry Laboratory at the Mayo Clinic (Rochester, MN) using undecalcified processing. After dehydration in a graded series of alcohols, the bone segment containing each bone defect was embedded in methyl methacrylate without decalcification. A Leica RM 2265 microtome was used to cut 5-μm-thick sections parallel to the long bone axis, through the middle axis of the defect site. Sections were stained with modified Gomori's trichrome, scanned using a NanoZoomer Digital Pathology System (Hamamatsu, Bridgewater, NJ), and analyzed using manual analysis with the OsteoMeasure system (OsteoMetrics, Decatur, GA). To provide systematic and representative sampling, a contiguous series of 20× magnification fields were examined, extending in a transaxial plane across the defect site through the midportion of the pericortical (PC) and IM regions (across the entire defect width at 10 and 5 mm, respectively, from the bottom of the defect) similar to the microCT analysis.9 The center, middle, and outer regions are compared (Fig. 1). Regions of new lamellar bone with osteocytes, new woven bone with unmineralized osteoids on its surface, acellular residual MCA, fibrous marrow tissue, sinus/vascular space, hematopoietic marrow, and void space were identified and manually traced by a skilled technician (Fig. 3). The relative area for each of these parameters was expressed as a percentage of the total tissue area in the defect site.
FIG. 3.
Histology area definitions (magnification at 10×).
Assay of osteoblastic progenitors using a colony-forming unit assay for connective tissue progenitors
The number of osteoblastic connective tissue progenitors in each sample was assayed using an established colony-forming assay. Samples from each fraction were counted in a Beckman Coulter ViCell XR Cell Counter (Model No. 731050; Beckman Coulter). Cells were plated at a density of 250,000 cells/chamber (4.2 cm2), and cultured at 37°C at 5% CO2 with medium changes on days 2 and 3. The osteogenic medium consisted of alpha-minimum essential medium Eagle (α-MEM) with 10% fetal bovine serum, 1 U/mL penicillin, 0.1 mg/mL streptomycin, 10−8 M dexamethasone, and 50 μg/mL ascorbate. Day 6 cultures were fixed with 1:1 acetone:methanol for 10 min and stained for nuclei (4′,6-diamidino-2-phenylindole [DAPI]) and alkaline phosphatase (AP) as a marker for the preosteoblastic activity. Chambers were scanned and analyzed using Colonyze™ software, identifying colonies containing eight or more cells in a cluster. CTP-O prevalence (PCTP-0) was defined as the number of CTP-Os per 106 cells plated.29,30
Characterization of the effects of cell processing steps
The volume, cell concentration ([Cells]), and CTP-O Prevalence (PCTP-O) were directly measured in the initial marrow aspirate sample and in the cell populations resulting from processing. Using these metrics, the effect of processing was characterized based on the following definitions:
NCell = number of cells (in millions)
PCTP-O = Prevalence CTP-Os = number of CTP-O-derived colonies formed per 106 cells plated
CTP-Os = Number of CTPs in the initial sample, where CTP-Os = Cells × PCTP-O
[Cell] = Cells per mL
[CTP-O] = CTP-Os per mL
Fold Δ[Cell] (Fold change in [Cell]) = [Cell] in graft/[Cell] for starting sample
Fold Δ[CTP-O] (Fold change in [CTP]) = [CTP-O] in graft/[CTP-O] in starting sample
RECell (cell retention efficiency cells) = Cells in graft/Cells in initial sample × 100
RECTP-O (CTP-O retention efficiency) = CTP-Os in graft/CTP-Os in initial sample × 100
Selection Ratio (relatively likelihood of CTP-O vs. cell retention) = RECTP-O/RECell
Statistical analysis
Continuous measures (cell data) were described using means, standard deviations, and percentiles. For microCT Data (Percent bone volume) and histology data, an ANOVA model was used to test for differences between four bone grafting treatments (BMA, DS, DS-T, and SR), surgical sites (proximal or distal sites), depth regions within a defect site (PC region or IM region), and radial distance within a defect site (Center, Middle, Outer). The effect of canine subject was included as a random factor. All two-way interactions were included as factors in the model. The results for %BV, histology, are expressed as mean ± standard error (SE) of pooled estimate. All tests were performed at a significance level of 0.05. SAS® software 9.2 (SAS®, Cary, NC) and JMP® 9.0.0 software (SAS) were used for all analyses.
Results
Animal care
One of the 12 animals, a subject in Cohort II, developed a fracture of the femur 2 weeks after surgery. The animal was immediately euthanized and excluded from further analysis. There were no other complications.
MicroCT percent bone volume data
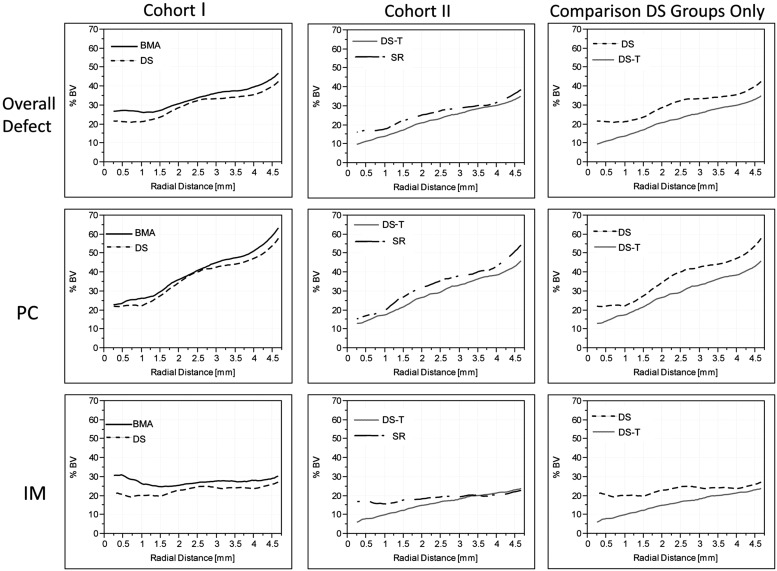
To illustrate the distribution of voxels above bone density within the defects in each cohort and group, Figure 4 presents the mean percent of bone volume (%BV) data plotted against radial distance from the center of each defect in nine two-dimensional (2D) line plots. The three plots in the left column, from top to bottom, present data from the overall defect, the PC region only, and the IM region only, for Cohort I. The plots in the center column present data from Cohort II. The plots on the right provide a secondary comparison of the DS processing methods used in Cohort I and II.
FIG. 4.
Mean percent bone volume (%BV) plots versus the radial position in the defect. PC: The PC region represents the region between 8 and 12 mm from the bottom of the grafted site, which is adjacent to the cortex. IM: The IM region is defined as the region between 3 and 7 mm from the bottom of the grafted site, which is separated by several millimeters from the endosteal source of osteogenic cells and bound only by cells from the marrow cavity, as illustrated in Figure 1. The highest %BV was found near the periphery of the defect, with generally lower %BV in the middle and central regions. Mean %BV was highest in the PC region of the defect and lower in the IM region (p < 0.001). The graphs in the right column provide a secondary comparison of the DS processing methods used in Cohort I and II, showing. There was no statistical difference between DS samples processed without coagulation, and DS-T samples processed with calcium thrombin was added to the DS concentrate.
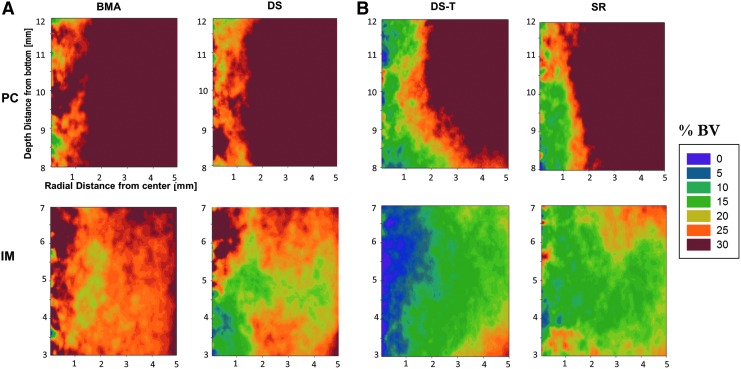
Figure 5A and 5B represent the same data as in Figure 4 as 2D contour plots, illustrating the variation in mineral density with respect to both depth and vertical positioning within the defects in each group.
FIG. 5.
Two-dimensional contour plots of percent bone volume using color map ranging from 0% (purple) to 30% (red) for (A) Cohort I and (B) Cohort II. All treatment groups demonstrated excellent overall performance of both DS and SR processing methods. In Cohort I, mean %BV for BMA group was 33.7% versus 30.1% for DS, but not statistically significant (p = 0.63). In Cohort II, mean %BV for SR group was 26.3% versus 22.5% for the DS-T sites, but not statistically significant (p = 0.36).
Overall, these data demonstrated that the highest %BV was found near the periphery of the defect, with generally lower %BV in the middle and central regions, consistent with prior studies using the CFMD model.8,9 In all cases, %BV was highest in the PC region of the defect and lower in the IM region (p < 0.001).
In Cohort I, higher %BV at all depths was found in the BMA sites than in the DS sites. Taken across the entire defect, the mean %BV for BMA sites was 33.7% versus 30.1% for the DS sites (SE = 4.0), but these differences were not statistically significant (p = 0.63).
In Cohort II, %BV was higher in the SR sites than in the DS-T. The mean %BV for SR sites was 26.3% versus 22.5% for the DS-T sites (SE = 3.7). However, again, these differences were not statistically significant (p = 0.36).
As a secondary analysis, the DS processing groups in Cohorts I and II were compared. DS sites presented higher %BV than the DS-T sites. However, this was not statistically significant (p = 0.20).
Table 1 provides the numerical mean and SE from each graft material by region and the p-value for each comparison.
Table 1.
Statistical Analysis of Mean %BV by Cohort and Region
| Region | Treatment | Mean %BV | SE | pa |
|---|---|---|---|---|
| Cohort 1 | ||||
| Overall defect | BMA | 33.7 | 4.0 | 0.63 |
| DS | 30.1 | 4.0 | ||
| IM | BMA | 27.4 | 4.3 | 0.56 |
| DS | 23.0 | 4.3 | ||
| PC | BMA | 39.8 | 4.3 | 0.73 |
| DS | 37.2 | 4.3 | ||
| Cohort 2 | ||||
| Overall defect | DS-T | 22.5 | 3.7 | 0.36 |
| SR | 26.3 | 3.7 | ||
| IM | DS-T | 16.0 | 3.9 | 0.50 |
| SR | 18.9 | 3.9 | ||
| PC | DS-T | 29.0 | 3.9 | 0.31 |
| SR | 33.6 | 3.9 | ||
| DS groups only | ||||
| Overall defect | DS | 30.1 | 3.8 | 0.20 |
| DS-T | 22.5 | 4.1 | ||
Statistically significant at p < 0.05 (ANOVA).
BMA, bone marrow aspirate; %BV, percent bone volume; DS, density separation; IM, intramedullary; PC, pericortical; SE, standard error; SR, selective retention.
Histology
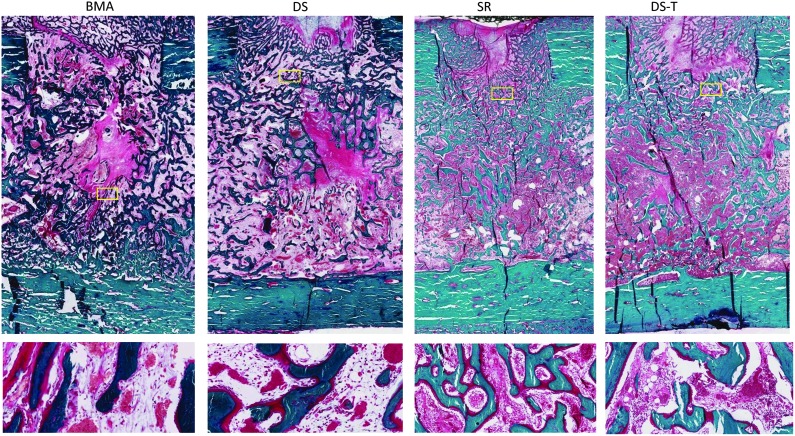
Figure 6 presents a high-power field from one sample for each treatment, illustrating the diversity of tissue subtypes that were seen in the histomorphometric analysis. Representative low-power histology images cut through the center axis of each defect are provided in Figure 6. New tissue formation occurred with little inflammation and no evidence of infection in any defect site. Figure 7 graphically summarizes the mean percent area of each tissue subtype for the two groups in each cohort in a side-by-side comparison.
FIG. 6.
Representative histology slides stained with modified Gomori's trichrome for each treatment group. Each section is cut through the center axis of one defect site. Upper images show the entire defect at low power (0.31×). Lower images show one representative high-power field (10×). Yellow boxes indicate where the higher magnification images in the lower panel are coming from. Robust bone formation was observed in all treatments with little inflammation and no evidence of infection in any defect site.
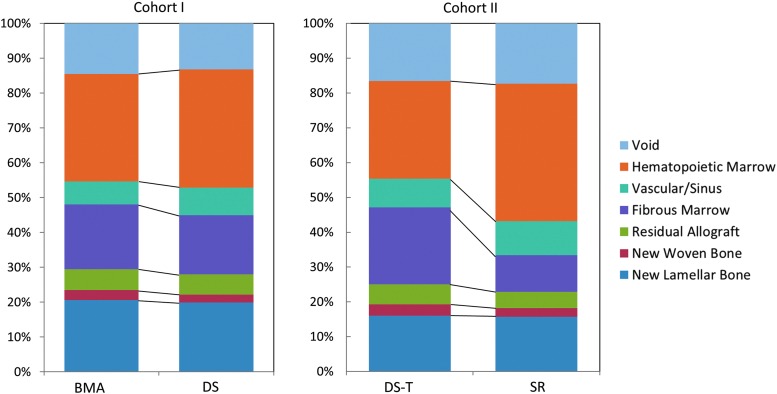
FIG. 7.
Histomorphological analysis of bone and soft tissue composition in defects grafted within the overall defect area for each group in Cohort I (n = 24 sections) and Cohort II (n = 24 sections). Mean percent area of each tissue for the two groups in each cohort is presented for side-by-side comparison. Bone formation was not different between BMA and DS processing, but DS sites tended to show more vascularity and less fibrosis in the regenerated marrow space. However, the retention of DS processed cells and CTP-Os in the mineralized cancellous allograft matrix was low. In Cohort II, SR sites contained more hematopoietic and vascular tissues and less fibrosis, residual allograft, particularly in the IM cavity, suggesting a more advanced stage of remodeling (p = 0.04). See Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea) for detailed numeric data.
In Cohort I, the distribution of tissues in the two groups was similar. In both groups, the mean area for tissue types was approximately as follows: new lamellar bone ∼20%, new woven bone ∼3%, residual allograft ∼6%, fibrous marrow ∼18%, vascular/sinus ∼7%, hematopoietic marrow ∼32%, and void ∼14%.
In Cohort II, the two groups were comparable in new lamellar bone area ∼16%, new woven bone ∼3%, vascular sinus area ∼9%, and void area ∼17%, accounting for 45% of the area. However, SR sites contained a larger area of hematopoietic tissue (40% vs. 28% [SE = 4]) and vascular tissues (10% vs. 8% [SE = 2]), offset by less fibrous marrow (11% vs. 22% [SE = 6]) and residual allograft (4.7 vs. 5.8% [SE = 1]). This pattern was statistically significant in the IM region (p = 0.04).
Total cells and CTP-Os delivered into the defect sites on MCA matrix
Table 2 provides a summary of the number and concentration of cells and CTP-Os, as well as prevalence of CTP-Os (PCTP-O) delivered into the defect site as a result of matrix loading.
Table 2.
Summary of Total Cells and CTP-Os Delivered into the Defect Sites on MCA Matrix Separated in Cohort I and in Cohort II
| Cohort I | Cohort II | |||||||
|---|---|---|---|---|---|---|---|---|
| BMA | DS | DS-T | SR | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Initial BMA sample | ||||||||
| Ncell (millions) | 67 | 14 | 204 | 83 | 326 | 84 | 275 | 141 |
| NCTP-O | 1130 | 1026 | 10,918 | 7029 | 34,641 | 12,415 | 36,893 | 19,104 |
| Volume (mL) | 1 | 0 | 1 | 0 | 1 | 0 | 6 | 0 |
| [Cell] (million cells per mL) | 67 | 14 | 204 | 83 | 326 | 84 | 46 | 23 |
| [CTP-O] (CTP-O per mL) | 1130 | 1026 | 10,918 | 7029 | 34,641 | 12,415 | 6149 | 3184 |
| PCTP-O (CTP-O per million cells plated) | 18 | 18 | 61 | 38 | 105 | 30 | 139 | 55 |
| Matrix loading process | ||||||||
| Fold Δ[Cell] | 0.7 | 0.0 | 0.4 | 0.1 | 0.7 | 0.1 | 1.5 | 0.5 |
| Fold Δ[CTP-O] | 0.7 | 0.2 | −0.3 | 0.9 | 0.8 | 0.01 | 4.9 | 0.1 |
| RECell (%) | 86.3 | 5.7 | 51.5 | 15.0 | 83.6 | 8.1 | 28.3 | 10.2 |
| RECTP-O (%) | 83.6 | 18.0 | −39.8 | 103.4 | 97.7 | 1.3 | 95.5 | 1.5 |
| Selection ratio | 1.0 | 0.2 | −0.5 | 1.8 | 1.2 | 0.1 | 3.8 | 1.3 |
| Implanted cells | ||||||||
| Ncell (millions) | 58 | 15 | 110 | 63 | 276 | 86 | 76 | 42 |
| NCTP-O | 887 | 681 | 62 | 6488 | 33,965 | 12,297 | 35,411 | 18,545 |
| Volume (mL) | 1.17 | 0 | 1.17 | 0 | 1.17 | 0 | 1.17 | 0 |
| [Cell] (million cells per mL) | 50 | 12 | 94 | 54 | 236 | 73 | 65 | 36 |
| [CTP-O] (CTP-O per mL) | 758 | 582 | 53 | 5545 | 29,030 | 10,510 | 30,266 | 15,850 |
| PCTP-O (CTP-O per million cells plated) | 16 | 13 | 10 | 65 | 121 | 31 | 560 | 355 |
“Load” sample is the sample that was initially loaded in each cell concentration device. For the unprocessed BMA clot, the load sample is a raw BMA. “Implanted cells” is the BMA preparation that was added to the MCA graft.
CTP-O, osteogenic connective tissue progenitors; MCA, mineralized cancellous allograft; SD, standard deviation.
Cohort I—BMA versus DS processing: In Cohort I, the overall number of cells retained in the DS implant was less than expected. Only 51.5% of the 204 ± 83 million cells loaded on the matrix were retained on the MCA matrix. A mean of only 62 ± 6488 CTP-Os were retained out of the total mean of 10,918 ± 7029 that were loaded in the DS process. In comparison, the BMA implants contained a mean of 58 ± 15 million nucleated cells and 887 ± 681 CTP-Os. When the clotted unprocessed BMA sample was mixed with the MCA, 86% of the 67 ± 14 million cells and 83% of the 1130 ± 1026 CTP-Os that were added to the MCA were retained.
Cohort II—DS-T versus SR processing: In Cohort II, in the DS-T group, where a calcium and thrombin clotting step was added after loading DS processed cells on the matrix, the retention of cells and CTP-Os improved dramatically. The DS-T implants contained a mean of 276 ± 86 million nucleated cells and 33,965 ± 12,297 CTP-Os. This represented a mean retention efficiency for cells (RECell) of 83.6% and a retention efficiency for CTP-Os (RECTP-O) of 97.7%. This mean selection ratio of 1.2 ± 0.1 for DS-T suggested that CTP-Os were slightly more likely to be retained than nonprogenitor nucleated cells. This CTP-O retention was associated with a nonsignificant increase in the mean PCTP-O in the implant from 105 to 121 CTP-Os per million nucleated cells.
The SR- and DS-T-processed implants contained essentially the same number of CTP-Os, 35,441 ± 18,545 and 33,965 ± 12,297, respectively, retaining a mean of 95.5% of the CTP-Os that were loaded into the MCA matrix. SR implants retained a mean of only 76 ± 42 million nucleated cells, however, the mean prevalence (PCTP-O) in the SR processed implants was increased to 560 ± 355 CTP-Os per million nucleated cells. The RECell was 28.3% compared to an RECTP-O of 95.5%. This represented a mean selection ratio of 3.8 ± 1.3, that is, CTP-Os were almost four times more likely to be retained than nonprogenitor nucleated cells.
A 10 mL marrow aspirate sample processed by SR delivered comparable numbers of CTP-Os (about 35,000 per defect site) as a 30 mL of marrow aspirate processed by DS. However, the SR implants contained almost four times fewer nucleated nonprogenitor cells and a significantly higher PCTP-O than DS-T implants (560 ± 355 vs. 121 ± 31 CTP-Os per million nucleated cells, respectively).
DS processing performance and yield estimates
Table 3 provides a summary of the measured effect of DS processing from the time of initial sample harvest to the preparation of the concentrate sample that was loaded on the MCA matrix in both cohorts. DS processing, both in Cohort I and II, resulted in a significant increase in the measured concentration of both cells and CTP-Os.
Table 3.
DS Processing Performances and Yield Estimates Showing Direct Comparison Between DS from Cohort I Versus DS-T from Cohort II
| Cohort I | Cohort II | |||
|---|---|---|---|---|
| DS | DS-T | |||
| Mean | SD | Mean | SD | |
| Initial BMA sample | ||||
| Ncell (millions) | 1874 | 421 | 1445 | 361 |
| NCTP-O | 31,358 | 29,856 | 59,988 | 24,017 |
| Volume (mL) | 30 | 0 | 30 | 0 |
| [Cell] (million cells per mL) | 62 | 14 | 48 | 12 |
| [CTP-O] (CTP-Os per mL) | 1045 | 995 | 2000 | 801 |
| PCTP-O (CTP-Os per million cells plated) | 18 | 18 | 47 | 25 |
| DS process description | ||||
| Fold Δ[Cell] | 3.3 | 1.3 | 7.2 | 2.5 |
| Fold Δ[CTP-O] | 19.7 | 19.3 | 17.9 | 5.9 |
| Fold Δ[PCTP-O] | 5.3 | 4.0 | 2.7 | 1.1 |
| RECell (%) | 38.7 | 12.4 | 80.3 | 36.4 |
| RECTP-O (%) | 215.1 | 200.1 | 190.2 | 42.4 |
| Selection Ratio | 5.3 | 4.0 | 1.2 | 0.1 |
| Concentrate sample after DS processing | ||||
| Ncell (millions) | 710 | 253 | 1058 | 258 |
| NCTP-O | 37,399 | 22,268 | 113,781 | 42,908 |
| Concentrate volume output after DS processing (mL) | 3.6 | 0.5 | 3.3 | 0.6 |
| [Cell] (millions per mL) | 204 | 83 | 326 | 84 |
| [CTP-O] (CTP-Os per mL) | 10,918 | 7029 | 34,641 | 12,415 |
| PCTP-O (CTP-Os per million cells plated) | 61 | 38 | 105 | 30 |
Cohort I: In Cohort I, a 30 mL volume of ACD anticoagulated marrow was reduced to a mean of 3.6 mL in volume. This was associated with a mean 3.3-fold increase in cell concentration ([Cell]) from 204 ± 83 million to 710 ± 253 million nucleated cells per mL. This represented a mean yield of nucleated cell harvest of only 39%, however. The measured concentration of CTP-Os ([CTP-O]) also increased by a remarkable 19.7 fold, from 1045 to 10,914 per mL. This represented a 215% yield compared to CTP-Os measured in the starting sample.
Cohort II: In Cohort II, a 30 mL volume of ACD anticoagulated marrow was reduced to a mean of 3.3 mL in volume. This was associated with a mean 7.2-fold increase in [Cell] from 48 ± 12 million to 326 ± 88 million nucleated cells per mL. This represented a mean yield of nucleated cell harvest of a respectable 80.3%. The measured [CTP-O] again increased by a remarkable 17.9 fold, from 2000 to 34,641 CTP-Os per mL. This represented a 190% yield compared to CTP-Os measured in the starting sample.
Overall, DS processing of ACD anticoagulated marrow in Cohort I and II increased the concentration of nucleated cells in a manner that is consistent with a roughly sixfold increase in cell concentration that is commonly reported using DS processing. However, roughly twice as many CTP-Os were measured in the final concentrate than in the initial aspirate. This raised the possibility that the prevalence of CTP-Os in the starting samples of DS processed marrow may have been systematically underestimated due to a reduction of colony-forming efficiency (CFE), either resulting from the abundance of RBCs (previously noted to compromise CFE)19 or a potential effect of the ACD anticoagulant.
Effect of ACD and RBCs on CFE and measurement of CTP-Os in initial aspirate samples
As described in the ASTM methods used for colony assay,29 the observed prevalence of CTP-Os (oPCTP-O) and the true prevalence of CTP-Os (tPCTP-O) are related by the variable of CFE, where oPCTP-O = tPCTP-O CFE. When CFE = 1.0, essentially all cells that are biologically capable of forming a colony do form a colony and are detected. However, if a colony formation assay is performed under conditions in which only half of the cells capable of forming a colony will form colonies, then the CFE falls to 0.5 and oPCTP-O drops in half, resulting in an underestimate of the true prevalence (tPCTP-O).
We have previously published that high concentrations of RBCs can reduce CFE by as much as 50%. Therefore, RBCs could account for the entire suspected effect on CFE.19 However, in this case, there was also the possibility that the ACD used as an anticoagulant may have an adverse effect on CFE. To assess the possibility of an adverse effect of ACD on CFE, an additional follow-up experiment was performed. In vitro colony formation was assessed under conditions that exposed cells to ACD or heparin at the initial concentration used for anticoagulation as well as the concentration that would persist in the initial culture environment after being diluted by isolation of the buffy coat and plating. For ACD, this diluted concentration was 3.78 mg/mL. For heparin, this was 1000 IU/mL. Under an IRB-approved protocol, samples of discarded human cancellous bone were collected from the proximal femur (n = 6) during elective hip arthroplasty surgery. Marrow-derived cells were suspended from these samples, providing a source of marrow-derived cells and CTP-Os containing minimal contaminating RBCs. To eliminate the confounding effects of variation in tPCTP-O from sample to sample, the oPCTP-O in each sample using ACD was normalized to oPCTP-O in the same sample using heparin. These data revealed no evidence of an adverse effect of ACD on CFE. The ratio of colony-forming units for ACD versus Heparin under the diluted conditions of primary culture (oPACD/oPHep) was 0.97 ± 0.25.
Discussion
Treatment of large bone defects remains an unsolved clinical challenge in both military and civilian settings, despite a wide array of existing graft materials and strategies.31 The biological challenge of bone regeneration is made more complex as the size of the defect increases and if regional tissues are compromised (e.g., soft tissue loss, reduced local vascularity, and regional scarring). All bone formation requires a source of osteogenic progenitors. Local deficiency in osteogenic connective tissue progenitors (CTP-Os) due to bone or soft tissue compromise is one of the central biological barriers to bone regeneration. Transplantation of cells from autogenous bone and marrow are the primary clinical means by which this CTP-O deficiency is addressed.5,14
Many variables may contribute to clinical success of marrow cell transplantation, including the site of harvest, the technique of harvest, processing methods, delivery environment (scaffold, local surface chemistry, and local delivery of bioactive factors), local wound environment, and systemic environment. Isolating, understanding, and optimizing these variables in the setting of cell therapy require a rigorously controlled and biologically relevant large animal system as well as quantitative methods for assessment of bone regeneration and resulting tissue quality, like those provided by the CFMD model.8,9,26,27
In this study, the following variables were held constant: harvest site location (canine proximal humerus), harvest technique (2 cc aspirates using a Lee-Lok needle), delivery environment (mineralized cancellous chips—MCA), local wound environment (10 mm diameter × 15 mm long cylindrical unicortical/IM femoral defects of the CFMD model), and systemic environment (adult purposely bred male coonhounds). This allowed isolation of the variables of anticoagulant selection and processing methods.
This project was designed to assess and compare DS and SR, two promising and readily implemented methods for rapid intraoperative processing of bone marrow-derived cells, as a means of enhancing bone regeneration. The DS method used in this experiment is the Harvest® SmartPrep 2BMAC® system, which is the only point-of-care, easy-to-use, closed system specifically FDA approved and uniquely designed for concentrating cells in bone marrow, as opposed to whole blood or bone marrow mixed with whole blood in other systems in the market. The SR method is another option for a surgeon to create in the sterile field.
As described above, this study was performed as the second phase of a systematic effort to identify and develop optimal methods for regeneration of bone in large complex bone defects that combine scaffolds, sources of osteogenic cells, and bioactive scaffold modifications. In Phase I, the CFMD model was used to compare a promising range of biological scaffold materials designed for use as bone void fillers.8 This first study demonstrated that MCA was superior to a range of synthetic biodegradable scaffolds. This project represents an initial segment of Phase II, in which the added value of rapid intraoperative processing methods for bone marrow-derived cells.
The primary goal of intraoperative processing is to increase the number and concentration of marrow-derived osteoblastic connective tissue progenitors within a graft site. Both DS and SR processing accomplished that goal, delivering a mean concentration of CTP-Os of ∼30,000 CTP-Os per mL into each graft site. In the case of SR processing, this concentration was achieved by allowing CTP-Os to attach to the matrix based on their native mechanisms for attachment. In the case of DS processed cells, the retention of CTP-Os in the matrix was dependent upon the induction of a clot using calcium and thrombin.
Intraoperative processing can also provide a means of removing cells that may compete with CTP-Os within the graft site or inhibit the function of CTP-Os (e.g., nonprogenitor nucleated cells and erythrocytes). Removal of non-CTP-Os can be measured as an increase in CTP-O prevalence (PCTP-O) in the graft site (i.e., the number of CTP-Os per million nucleated cells). The unprocessed BMA clot and the SR treatment groups contain few RBCs. The primary effect of DS and DS-T bone marrow concentrates is to remove the vast majority of RBCs as well as serum volume. This BMA concentrate (commonly referred to as “BMAC” by clinicians) was mixed with the allograft matrix. In the DS group, cells and CTP-Os that would naturally attach to the MCA matrix or each other were retained. In the DS-T group, thrombin was used to induce clot formation to enhance the retention of cells and CTP-Os.
In the case of SR processing, CTP-Os attached more readily than non-CTPs to the MCA matrix (retention efficiency of 95.5% vs. 28.3%). As a result, the prevalence of CTP-Os was also increased from 139 CTP-Os per million cells in the initial heparinized sample to 560 per million cells, a fourfold increase in PCTP-O.
In the case of DS processing, when calcium and thrombin were added, cells and CTP-Os were retained with essentially equal retention efficiency for CTP-Os (97.7%), but the DS processing method had a higher retention efficiency for cells (86.3%). As a result, PCTP-O was not significantly changed and remained at a mean of 121 CTP-Os per million cells.
The harvest of marrow in this study was performed using clinically relevant methods that enable comparison of the canine proximal humerus to reports of clinical harvest of bone marrow from the human pelvis.5,13,28 The mean concentration of cells in aspirates from the canine humerus in Cohort 1 and 2 was 100 million cells/mL with a mean of ∼1500 CTP-Os/mL. This is comparable to previous reports using the canine model.7–9,26,27 In contrast, data from the human anterior iliac crest aspirates collected at the time of elective arthroplasty procedures using an essentially identical technique (needle and aspirate volume of 2 mL) by Muschler et al.28 provided a mean of 33 million cells/mL and slightly more than 1200 CTP-Os/mL.28 This suggests that the canine humerus is more cellular than the pelvis of elderly humans, but comparable in the CTP-O yield and concentration.
Hernigou5 reported a mean of 18 million nucleated cells/mL and ∼600 CTP-Os/mL. Marx and Harrell13 report a mean of 15.5 million cells/mL. The lower concentration reported in these later studies is likely the result of the larger volume of aspiration (4 and 15 cc, respectively) that was used during harvest, resulting in dilution of marrow-derived cells and CTP-Os with nonprogenitor containing peripheral blood, as previously documented.28 This highlights the importance of harvest technique in optimizing the starting concentration and prevalence of marrow-derived cells and CTP-Os.
The CFMD model with four unicortical 10 mm diameter × 15 mm cylindrical defects has been designed and used effectively as a local wound environment and systemic environment to assess the relative efficacy of scaffold materials,8 cell sourcing/processing options,9,26 and delivery of growth factors (e.g., bone morphogenetic proteins [BMPs]).26,32 The availability of data from four sites in each subject enables comparison of two materials, while controlling for variation between implant sites and between subjects. The defects are sufficient in size to create a biological environment in which the interior of the defect is profoundly hypoxic.27
The CFMD model has limits, however. It is a biologically relevant animal model, but is not designed to include the full range of biological challenges and conditions that are presented in the most severe bone injuries or segmental defects. As in virtually all of the available animal models, the CFMD model can reach a “ceiling effect.” Once grafting strategies reach a sufficient level of effectiveness, defects will heal rapidly and reliably. Beyond this point, detection of potentially clinically important differences or incremental improvement can be impossible without unacceptable increases in cohort size.
With respect to bone formation as an outcome, it is evident that all of the cell/scaffold constructs that were evaluated in these two cohorts pushed the limit of this ceiling effect. MCA, already identified as a highly effective matrix,8 performed well regardless of whether cells were added in the form of clotted unprocessed BMA, DS concentrated cells and CTP-Os, or cells and CTP-Os that were selectively retained through SR processing. In Cohort I, higher bone formation in the BMA group may have been the result of the very poor attachment of DS processed CTP-Os, which severely limited the number of CTP-Os transplanted. However, both groups healed very well, and this difference was not statistically different. Greater bone formation in the SR group in Cohort II may be related to the increased prevalence of CTP-Os in the SR implants and the reduced number of competing nonprogenitors in the graft site. However, this too was not a statistically significant difference.
In the case of histological assessment, sampling bias can sometimes obscure important findings. This can result either from observers selecting only fields of views that support their conclusions for presentation or analysis, systematic variation within regions of a defect site, or a failure to look for potential adverse effects. In this case, neither of the problems is present: (1) we eliminated observer bias in the histomorphometric data by sampling each defect in the reproducible manner that is described. We believe that these methods are clearly described in the current methods and in Figure 1and (2) we did not limit observation to just the regions that we subjected to quantitative histomorphometric analysis. The entire area of each slide was observed. In no slide was acute or chronic inflammation identified. Furthermore, in no case were the standardized regions of systematized histomorphometry considered to be substantially different or be nonrepresentative of the physiological state observed within the defect as a whole.
With respect to histological quality of the tissue formed, these treatments also pushed the ceiling effect in this animal model. In Cohort I, there was very similar tissue composition in both BMA and DS graft sites, despite transplantation of many fewer RBCs in the DS processed cells than in the raw BMA and a theoretically lower burden of RBC debris and the inflammatory process and macrophage response that would be associated with their removal. It should be remembered that DS implants almost doubled the mean burden of implanted nucleated cells when compared to BMA implants (110 million vs. 58 million). In Cohort II, a histological difference was seen, with more hematopoietic marrow area and less fibrosis in the SR-processed implant sites. In this case, the difference did rise to statistical significance, although p = 0.04. This finding may be the result of the significant reduction in both RBCs and nonprogenitor nucleated cells in the SR versus DS-T implant sites (76 million vs. 276 million nucleated cells, respectively), resulting in a lower burden of debris from dead cells and the associated inflammatory response.
None of these data demonstrated clear superiority of one method over the other. While the results from SR and DS processing were found comparable in the CFMD model, the SR results were achieved with the following: (1) no need to process using the centrifuge and (2) a much lower volume of initial bone marrow sample. Each of the groups tested resulted in an effective filling of the defect with healthy remodeling bone and marrow tissue. In fact, each of these methods exceeded the outcome that we have previously reported for grafts containing OP-1 (BMP-7), with and without unprocessed BMA-derived cells.26 However, these data highlighted several important differences between the BMA, DS, and SR processing options that could have important implications when the bar is raised and attention is turned to more challenging defects.
This study stimulates further thought related to the process of understanding and optimizing methods for cell processing and transplantation. In Cohort I, we found that when DS processed cells were added to MCA, the REcells increased, but RECTP-O decreased dramatically when compared to raw marrow anticoagulated using heparin. REcells and RECTP-O were restored to high levels with the addition of a clotting step in Cohort II. The generalizability of this finding needs to be further assessed and may justify modification of current practices when DS processed cells are mixed with matrix materials for implantation to preserve RECTP-O.
This study also highlights the important effect of CFE when making estimates of true CTP prevalence (tPCTP-O) based on measurement of observed CTP-O prevalence (oPCTP-O) since oPCTP-O = tPCTP-O CFE. Consistent with our previous report,19 we found evidence that the high concentration of RBCs in raw marrow aspirates appears to compromise CFE, resulting in an underestimation of tPCTP-O unless RBC concentration is reduced by centrifugation. This finding highlights a limitation in our ability to precisely characterize the effect of DS processing. However, it can also be viewed as providing a compelling justification for use of processing methods like DS and SR that will dramatically reduce the burden of RBCs that are placed in a graft site, and potentially improve the in situ CFE of transplanted CTP-Os, where the performance is most needed. We were pleased to find no evidence that heparin or ACD, both common clinical methods for anticoagulation, had any adverse effect on CFE.
The next steps that follow the current study are clear. When a given graft material or strategy reaches this ceiling effect, where further improvement becomes difficult or impossible to measure, then it is time to “raise the bar” and move to a more stringent model. Recognizing the need for generalizable standards, we recently published a review on the design rational for animal models for bone regeneration. In this review, we described both the CFMD model as well as the development of the chronic caprine tibial defect (CCTD) model.27 The CCTD model has been specifically designed to incorporate the combination of bone (5 cm), bone marrow (reaming), periosteum (9 cm gap), muscle (10 g defect), and secondary scarring around an antibiotic eluting PMMA spacer. These features characterize the severely compromised biological setting of the most challenging clinical bone defect sites. It is our intention to advance the systematic assessment and refinement of DS, SR, and other advanced cell processing methods into this much more stringent and clinically relevant CCTD model.
DS and SR processing methods are not the only cell processing strategies that have proven worthy of advancement into the stringency of the CCTD model. We have recently reported on the performance of another method for rapid intraoperative processing of bone and marrow cells using MS processing.9,33 In this study, marrow-derived CTP-Os were effectively labeled and concentrated based on the presence of hyaluronan on their extracellular surface. CTP-Os were enriched in both concentration and prevalence. The bone formation response in the CFMD model was excellent and the histological outcome was significantly improved when MS-processed cells were implanted.9 BMP-2 delivered with marrow-derived cells on a ceramic tricalcium phosphate (TCP) and collagen carrier has been shown to be very effective in the CFMD model.32
The CFMD model should continue to be viewed as a valuable resource for comparative assessment of scaffold materials, drug delivery systems, and for novel cell processing strategies. The promising methods DS and SR now require assessment in more challenging settings such as the chronic caprine tibial defect (CCTD) model.27
Supplementary Material
Acknowledgments
This work was supported by the Harvest Technologies, enabling work on Cohort I to be completed and supporting DS preparation of samples for Cohort II. We are particularly grateful to Lou Blassetti, who initiated and facilitated this work. We mourn his loss. We also thank Lille Tidwell, PhD and Sherwin Kevy, PhD for assistance in critical review of the cell data. Cohort II was primarily funded by the Armed Forces Institute of Regenerative Medicine (AFIRM), which is managed and funded through the US Army Medical Research and Materiel Command (MRMC). The AFIRM has additional funding from the US Navy, the Office of Naval Research, the US Air Force Office of the Surgeon General, the National Institutes of Health, the Veterans Administration, the Cleveland Clinic, and local public and private matching funds. The AFIRM support was part of a subcontract from Rutgers University, Department of Chemistry and Chemical Biology/NJ Center for Biomaterials, under Cooperative Agreement No. WSIXWH-08-2-0034 from the US Department of Defense In US Army Medical Research Acquisition. The authors wish also to acknowledge the contributions of Kristen Shogren of Mayo Clinic for analysis of histomorphometry; Rick Rozic and Jason Bryan of ImageIQ, who support for microCT imaging and analysis, Christopher Heylman, Edward Kwee, and Vivek Raut for assistance with animal surgery and review of CTP-O colony assays; and Esteban Walker, PhD for assistance with statistical analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ito K., Aoyama T., Fukiage K., Otsuka S., Furu M., Jin Y., Nasu A., Ueda M., Kasai Y., Ashihara E., Kimura S., Maekawa T., Kobayashi A., Yoshida S., Niwa H., Otsuka T., Nakamura T., and Toguchida J. A novel method to isolate mesenchymal stem cells from bone marrow in a closed system using a device made by nonwoven fabric. Tissue Eng Part C Methods 16, 81, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Dawson J.I., Smith J.O., Aarvold A., Ridgway J.N., Curran S.J., Dunlop D.G., and Oreffo R.O.C. Enhancing the osteogenic efficacy of human bone marrow aspirate: concentrating osteoprogenitors using wave-assisted filtration. Cytotherapy 15, 242, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Naung N.Y., Suttapreyasri S., Kamolmatyakul S., and Nuntanaranont T. Comparative study of different centrifugation protocols for a density gradient separation media in isolation of osteoprogenitors from bone marrow aspirate. J Oral Biol Craniofac Res 4, 160, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodke D., Pedrozo H.A., Kapur T.A., Attawia M., Kraus K.H., Holy C.E., Kadiyala S., and Bruder S.P. Bone grafts prepared with selective cell retention technology heal canine segmental defects as effectively as autograft. J Orthop Res 24, 857, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Hernigou P., Poignard A., Beaujean F., and Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87, 1430, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Muschler G.F., Matsukura Y., Nitto H., Boehm C.A., Valdevit A.D., Kambic H.E., Davros W.J., Easley K.A., and Powell K.A. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop 242, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muschler G.F., Nitto H., Matsukura Y., Boehm C., Valdevit A., Kambic H., Davros W., Powell K., and Easley K. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res 407, 102, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luangphakdy V., Walker E., Shinohara K., Pan H., Hefferan T., Bauer T.W., Stockdale L., Saini S., Dadsetan M., Runge M.B., Vasanji A., Griffith L., Yaszemski M., and Muschler G.F. Evaluation of osteoconductive scaffolds in the canine femoral multi-defect model. Tissue Eng Part A 19, 634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caralla T., Joshi P., Fleury S., Luangphakdy V., Shinohara K., Pan H., Boehm C., Vasanji A., Hefferan T.E., Walker E., Yaszemski M., Hascall V., Zborowski M., and Muschler G.F. In vivo transplantation of autogenous marrow-derived cells following rapid intraoperative magnetic separation based on hyaluronan to augment bone regeneration. Tissue Eng Part A 19, 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann P.C., Huber S.L., Herrler T., von Hesler C., Andrassy J., Kevy S.V., Jacobson M.S., and Heeschen C. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant 16, 1059, 2008 [PubMed] [Google Scholar]
- 11.Fortier L.A., Potter H.G., Rickey E.J., Schnabel L.V., Foo L.F., Chong L.R., Stokol T., Cheetham J., and Nixon A.J. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am 92, 1927, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Murawski C.D., and Kennedy J.G. Percutaneous internal fixation of proximal fifth metatarsal jones fractures (zones II and III) with Charlotte Carolina screw and bone marrow aspirate concentrate: an outcome study in athletes. Am J Sports Med 39, 1295, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Marx R.E., and Harrell D.B. Translational research: The CD34+ cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Oral Craniofac Tissue Eng 2, 263, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Muschler G.F., Nakamoto C., and Griffith L.G. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86-A, 1541, 2004 [DOI] [PubMed] [Google Scholar]
- 15.McLain R.F., Fleming J.E., Jr, Boehm C.A., and Muschler G.F. Aspiration of osteoprogenitor cells from the vertebral body during pedicle screw fixation: comparison of progenitor cell concentration to iliac crest. J Bone Joint Surg Am 87, 2655, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager M., Hernigou P., Zilkens C., Herten M., Fischer J., and Krauspe R. [Cell therapy in bone-healing disorders]. Der Orthopade 39, 449, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Jager M., Jelinek E.M., Wess K.M., Scharfstadt A., Jacobson M., Kevy S.V., and Krauspe R. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther 4, 34, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lee K., and Goodman S.B. Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplasty 24, 43, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Heylman C.M., Caralla T.N., Boehm C.A., Patterson T.E., and Muschler G.F. Slowing the onset of hypoxia increases colony forming efficiency of connective tissue progenitor cells in vitro. J Regen Med Tissue Eng 2013. [Epub ahead of print]; DOI: 10.7243/2050-1218-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung C., Nakamoto C., and Muschler G.F. Factors affecting connective tissue progenitors and orthopaedic implications. Scand J Surg 95, 81, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lu C., Rollins M., Hou H., Swartz H.M., Hopf H., Miclau T., and Marcucio R.S. Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J 28, 14, 2008 [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen F.B. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand 182, 215, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jaremko K.M., Chen-Roetling J., Chen L., and Regan R.F. Accelerated hemolysis and neurotoxicity in neuron-glia-blood clot co-cultures. J Neurochem 114, 1063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann T.J., Otsuru S., Marino R., Rasini V., Veronesi E., Murgia A., Lahti J., Boyd K., Dominici M., and Horwitz E.M. Transplanted murine long-term repopulating hematopoietic cells can differentiate to osteoblasts in the marrow stem cell niche. Mol Ther 21, 1224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Kohn D.F., Lipman N.S., Locke P., Melcher J.M., Quimby F.W., Turner P.V., Wood G.A., and Wurbel H. Guide for the Care and Use of Laboratory Animals. Eighth ed. Washington, DC: Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division of Earth and Life Studies, National Research Council of the National Academies, 2011. [Google Scholar]
- 26.Takigami H., Kumagai K., Latson L., Togawa D., Bauer T., Powell K., Butler R.S., and Muschler G.F. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res 25, 1333, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Muschler G., Raut V.P., Patterson T., Wenke J.C., and Hollinger J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev 16, 123, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Muschler G.F., Boehm C., and Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am 79, 1699, 1997 [DOI] [PubMed] [Google Scholar]
- 29.ASTM Standard F2944-12, 2012. Standard test method for automated colony forming unit (CFU) assays—image acquisition and analysis method for enumerating and characterizing cells and colonies in culture. ASTM International, West Conshohocken, PA, 2012, DOI 10.1520/F2944-12 Available at www.astm.org Accessed October15, 2015 [DOI] [Google Scholar]
- 30.Raut V.P., Rivera J., Luangphakdy V., Alvarez L.A., Wells A., Griffith L., and Muschler G. Evaluation of beta-tricalcium phosphate scaffolds with tethered epidermal growth factor in the canine femoral multi-defect model. eCM 2014. (In press) [Google Scholar]
- 31.Owens B.D., Kragh J.F., Jr, Macaitis J., Svoboda S.J., and Wenke J.C. Characterization of extremity wounds in operation Iraqi freedom and operation enduring freedom. J Orthop Trauma 21, 254, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Luangphakdy V., Samaranska A., Walker E.A., Shinohara K., Pan H., Boehm C., Hefferan T.E., and Muschler G.F. Evaluation of rhBMP-2/ACS/TCP-HA bone graft with addition of bone marrow cells in the canine femoral multi defect model. Eur Cell Mater 29, 57, discussion 68, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Caralla T., Boehm C., Hascall V., and Muschler G. Hyaluronan as a novel marker for rapid selection of connective tissue progenitors. Ann Biomed Eng 40, 2559, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.