Abstract
Secondary ion mass spectrometry (SIMS) is a technique capable of imaging tissues, single cells, and microbes revealing chemical species with sub-micrometer spatial resolution. The recently developed Fourier transform ion cyclotron resonance (FTICR) SIMS instrument provides high mass resolving power and mass accuracy, ToF-SIMS can generate chemical maps with an order of magnitude better lateral resolution than the FTICR-SIMS, and the NanoSIMS instrument offers sub-100 nm spatial resolution in chemical imaging. Many commercial ToF-SIMS instruments are also capable of depth profiling that allows three-dimensional reconstructions of cell and tissue structure.
Introduction
Mass spectrometry imaging (MSI) techniques are increasingly being utilized within many biological fields, including medicine, pathology, and microbial ecology. There are a variety of desorption/ionization methods used in these efforts to generate mass spectra and create ion images of samples of interest [1–4]. Each of these MSI methods has their own virtues. Here, we focus on the surface sensitive technique of secondary ion mass spectrometry (SIMS). Of the MSI methods available, SIMS offers the highest lateral resolution of any technique. Moreover, SIMS versatility in the number of different operating modes and types of mass spectrometers available has made it an increasingly popular method for bio-related measurements.
Traditionally, SIMS has been most heavily used for inorganic semiconductor research, but with recent technologies it has become increasing popular for analysis of biologically relevant samples. In the SIMS technique, a focused ion beam is used to bombard a sample, ejecting and ionizing molecules from the sample surface that are subsequently analyzed in a mass spectrometer (Figure 1) [5]. Currently, SIMS provides micrometer to nanometer scale lateral spatial resolution, which is the highest lateral resolution reported of any MSI method [6]. To represent the range of analytical capabilities of SIMS, we focus in this article on three specific types of SIMS instrumentation: Fourier transform ion cyclotron resonance SIMS (FTICR-SIMS), time-of-flight SIMS (ToF-SIMS), and a specialized dynamic SIMS instrument (NanoSIMS), which offers the highest lateral image resolution of any MSI method (Figure 2). These instruments provide information at different dimensional scales that can be matched to the problem of interest.
Figure 1.
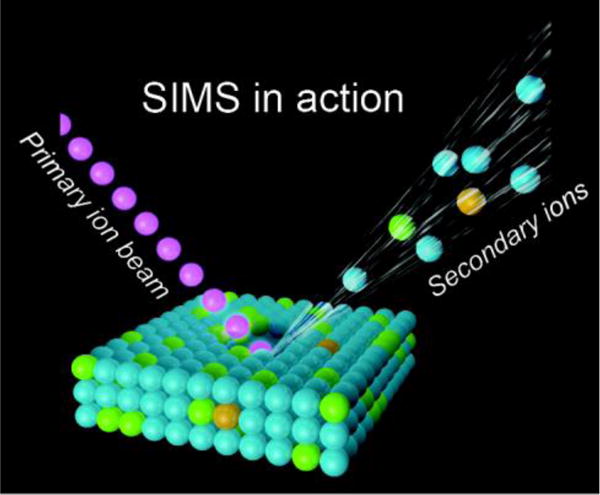
Schematic of SIMS mode of operation, where a primary ion beam is used to eject atoms, molecules, and secondary ions from the surface. The secondary ions are then analyzed based upon their mass-to-charge ratio in a mass spectrometer.
Figure 2.
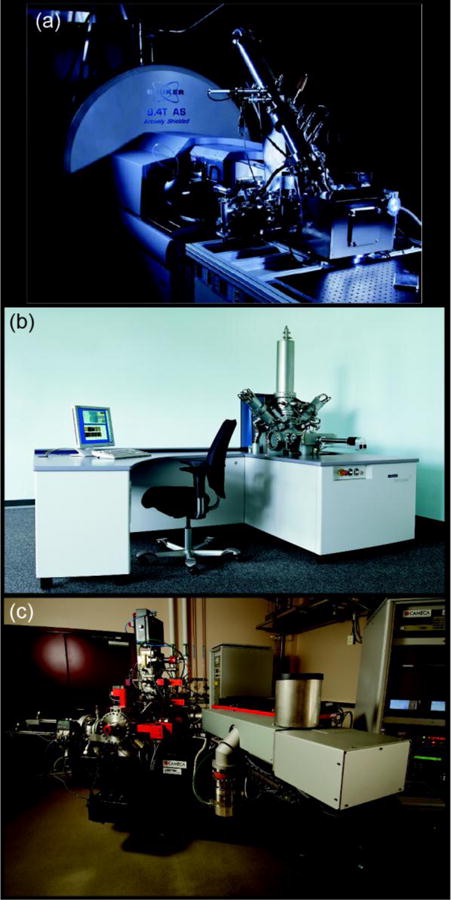
Three SIMS instruments. (a) FTICR-SIMS at PNNL, (b) IONTOF TOF.SIMS5 at PNNL and the University of Washington (Image courteous of IONTOF), and (c) a Cameca NanoSIMS 50L located at PNNL.
Materials and Methods
Primary and secondary ions
The SIMS technique requires samples to be analyzed in a high vacuum environment. The basic SIMS technique uses an ion source, where primary ions are accelerated towards a sample surface. As a result of the impact of the primary ions with the surface, molecules and atoms are ejected into the vacuum environment (Figure 1). While most of the ejected molecules are neutral, a percentage of them are ionized and carry either a positive or negative charge (secondary ions).
Dynamic and static SIMS
Dynamic SIMS instruments operate in a regime where the primary ion beam is constantly bombarding the sample. This typically causes excessive fragmentation of the surface molecules providing unmatched elemental analysis of sample-limited material [5]. Using dynamic mode, one can ‘erode’ the sample during analysis to produce a chemical depth profile of the sample. In contrast, instrumentation employing a pulsed primary ion beam is often used in the ‘static’ mode, where the percentage of the surface that is damaged by the incoming (primary) ion beam is kept to a minimum. To stay within the so-called static mode, the primary ion dose must remain below ~1012 ions/cm2. Static mode provides highly surface sensitive molecular information (typically ~ top 2 nm) [7].
Analysis and imaging
Secondary ions are collected and separated by their mass-to-charge ratio (m/z) in a mass analyzer. For imaging, the focused incoming primary ion beam is moved across the sample, either by stage movement or beam rastering, and a mass spectral measurement is produced from each area of impact. Lateral resolution of 100 nm or better can be attainable by utilizing a liquid metal ion gun (LMIG) source. The adoption of polyatomic ion sources has been shown to increase the secondary ion yield as well as increase the molecular weight of the secondary ions [8]. In addition, they are proving to be very useful in obtaining 3D information from biological systems. These capabilities and ongoing improvements are discussed in more detail below and are the principal reasons why SIMS is now a viable tool for solving problems related to biological cells and tissues.
SIMS Instruments and Applications
FTICR-SIMS of brain tissue (the large scale)
Fourier transform-based mass spectrometers (FTMS) measure the mass-to-charge of a molecule by its frequency of oscillation within a magnetic or electric field. These mass spectrometers offer the highest available mass resolution and mass accuracy. The C60 FTICR-SIMS platform described here and elsewhere [9–11] is a reworking of a commercially available MALDI configuration on a Bruker SolariX FTICR MS system (Figure 2a). The C60 primary ion source is a thermal effusive source, where C60 powder is heated under vacuum, and evaporated C60 molecules are ionized and focused through a series of ion optics as they move towards the sample. As a result of using a polyatomic primary ion source like C60, a softer bombardment of the sample surface occurs increasing the ionization efficiency of intact secondary ion species in comparison to LMIG sources.
For analysis with FTICR-MS, collected secondary ions must be collisionally cooled in order to trap them within the magnetic field. As a result, the FTICR-SIMS does not suffer from topographical effects (signal increasing or decreasing with increased or decreased sample height) like many ToF instruments. However, this trapping process and measuring the oscillation of trapped molecules (over 100s to 1000s of milliseconds) prolongs the duty cycle of FTMS instruments, which is compounded over the course of an image acquisition. This reason and the high cost of these instruments are limiting factors for their application.
An FTICR-SIMS analysis of a rat brain tissue section is shown in Figure 3 as an example of SIMS analysis of a “larger” biological sample. Many tissue sections are large compared to the typical SIMS analysis areas of ~ 500×500 μm for ToF-SIMS and ~ 100 × 100 μm for NanoSIMS. For these instruments, stitching together multiple SIMS images to create a mosaic image is required to image an entire tissue section. However, for the FTICR-SIMS, as configured, the primary ion beam of C60 stays in one location and the sample stage is mechanically moved between beam pulses, defining the pixel size. With this setup, images can be taken across multiple centimeters, making it well suited for analysis of ‘larger’ tissue areas. Figure 3a illustrates the approximate region of analysis of the rat brain tissue section, based on a serial section that has been H & E stained to elucidate the different anatomical areas. One can see the spectral complexity generated from SIMS analysis of the tissue section, where 2783 separate peaks are detected over the entire sampling area (Figure 3b). The mass resolving power and mass accuracy of this SIMS instrument is unmatched, where in this case a mass resolution of ~97,000 is measured at m/z= 634.601 (the most abundant peak) with < 5 ppm error. However, mass resolving power >1,000,000 and mass errors <1 ppm have been reported using this instrument [9]. While there is extreme spectral richness evident in the FTICR-SIMS analysis of rat brain (Figure 3b), many of the peaks detected are not all molecular species. Some of the peaks are either fragment ions of a parent species that are created by the impact of the primary ion beam, or some peaks are isotopologues (e.g., naturally occurring 13C-speices) of abundant molecules in the sample.
Figure 3.
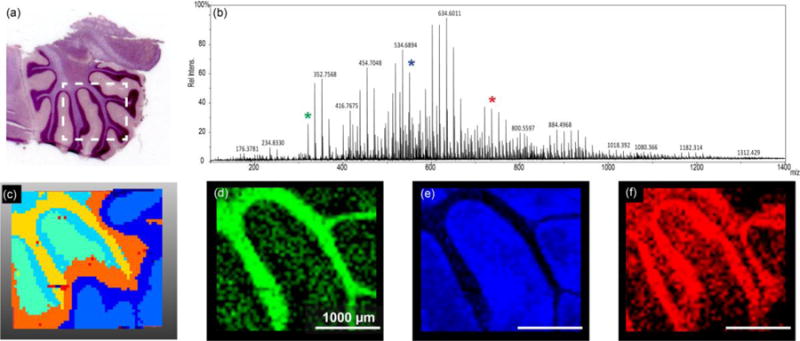
C60 FTICR-SIMS analysis of sagittal sectioned rat brain. (a) Optical image of a serial section, which has been H & E stained, taken of the brain sample analyzed. The white box indicates the approximate area of analysis within the cerebellum. (b) The average overall spectrum of the entire rat brain area imaged, with a measured mass resolving power of approximately 97,000 at m/z= 634.601. (c) A dendrogram illustrating pixels that have similar spectra, created using SCiLS Lab. From the dendrogram we can see unique peaks that co-localize with areas identified in the H & E stain image (a). (d) N-glycolylneuraminic acid at m/z= 326.109 co-localizes with the white matter of the cerebellum. (e) m/z= 550.663 a possible lysolipid co-localizes in the grey matter area. And (f) either PC (32:2) or PE (35:2)—same nominal mass— at m/z= 730.541 co-localizes within the granular cell region. A 20 μm beam size was used with 50 μm steps between spectra.
Recent MSI analysis software tools, like SCiLS Lab and the NBToolbox, have been developed to assist in handling the complex data streams that are generated from MSI data. In this example, SCiLS Lab is used in spatial segmentation analysis of the FTICR-SIMS of the rat brain. This analysis identifies pixels in the image that have similar spectra, producing a dendrogram (Figure 3c) mapping the location of pixels with correlated spectra. The areas in the dedrogram correspond well with the anatomical features seen in Figure 3a, indicating that the FTICR-SIMS analysis is detecting different biochemical areas in this sample. The most prominent mass peaks that co-localize with each of these features can be determined. Figure 3d shows the ion image of m/z= 326.109 corresponding to n-glycolylneuraminic acid, which co-localized with the yellow clusters in Figure 3c and with the white matter of the cerebellum. Figure 3e is the ion image of a possible lysolipid at m/z= 550.663 that correlates to the orange clusters in Figure 3c and the grey matter of the rat brain. Finally, Figure 3f is the ion image of an intact lipid, PC (32:2) or PE (35:2) at m/z= 730.541 that co-localizes to the light blue clusters in Figure 3c and with the granular cell region of the rat brain section. Tandem MS would be required for discrimination between PC and PE lipids with the same monoisotopic mass.
SIMS of insects (the medium scale)
Although different SIMS instrumentations have varying capabilities in spatial and mass resolution, their capabilities can overlap. In this section insects are examined using both C60 FTICR-SIMS and TOF-SIMS providing a comparison of instrumentation capabilities.
In the first example, the C60 FTICR-SIMS instrument was used to collect images from a drosophila imaginal wing disc. Figure 4a shows a schematic of this feature and Figure 4b shows an FTICR-SIMS image of the same area. These discs undergo tremendous changes during metamorphosis to give rise to the adult structure [12]. With the FTICR-SIMS we can visualize chemical patterns within the wing disc, which begin to form during this period. Because of the size of these discs, a smaller step size (35 μm) was required than in Figure 3, in order to laterally resolve regions within the wing disc. However, because of stage scanning used produce the image, the lateral resolution of the FTICR-SIMS is significantly coarser than that of a ToF-SIMS instrument.
Figure 4.
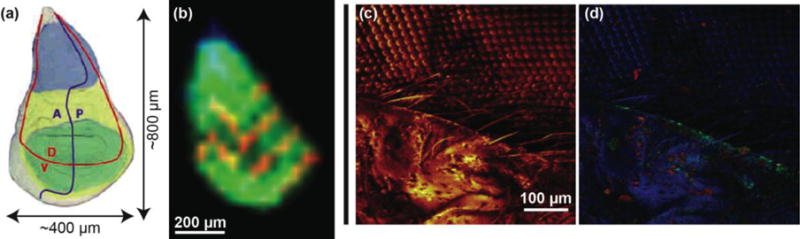
SIMS analysis of insects. (a) Anatomy of the drosophila wing imaginal disc during the 3rd instar larva developmental period, where A is the anterior, P is posterior, D is dorsal, and V is ventral. (b) C60 FTICR-SIMS image of drosophila wing disk (35 μm step size) following m/z= 554.7240 (blue), 570.7001 (green), and 980.4017 (red). Sample courteous of Florian Marty. (c) ToF-SIMS image of an adult house fly eye taken with the IONTOF5 instrument at NESACBIO (image from Dan Graham) showing signal from all emitted positive ions. (d) ToF-SIMS image showing an overlay of image for 3 positive ion masses: Na+ peak in red, Si2C5H15O+ indicative of PDMS in green, and C4H5N+ peak in blue.
An example of the lateral resolution capabilities of ToF-SIMS is shown in Figure 4c, where an IONTOF TOF.SIMS5 instrument (Figure 2b) was used to image part of a common house fly eye. The LMIG in SIMS systems (such as the bismuth LMIG of the IONTOF data shown in Figure 4c) use electrostatic beam deflection to raster the tightly focused liquid metal ion beam across the surface, achieving submicron lateral resolution. In an image showing the intensity of the total positive ions emitted from the surface (Figure 4c) the fly’s bristles as well as details of the compound eye can easily be identified. As with the FTICR-SIMS, the ToF-SIMS image data results in a large number of mass spectral secondary ion peaks. In Figure 4d a three-color overlay is used to represent position and intensity of three of the secondary ions detected in the ToF-SIMS spectra: sodium, polydimethyl siloxane (PDMS), and a C4H5N+ ion fragment. The salt (shown in red) is scattered across the fly eye. However, while the PDMS (green) is usually a contaminant that spreads across surfaces in vacuum, this effect is not apparent on the compound eye part of the fly. Note that the organic peak attributed to C4H5N+ (blue in Figure 4d) appears to best represent the compound eye and other main features of the fly.
SIMS of bacteria (small scale)
The Cameca NanoSIMS 50/50L (Figure 2c) is a recently developed dynamic SIMS instrument capable of achieving a lateral resolution of 50 nm; this is the highest lateral resolution currently available in current MSI instruments. In practice, lateral resolutions of 70 nm to 100 nm for measuring biological features are more common [13]. This high lateral resolution is made possible by a number of factors including the type of primary ion source, special primary and secondary ion beam optics, and a specific mass analyzer. The NanoSIMS uses the most electropositive and electronegative sources available (Cs+ and O−) that achieve the highest ionization efficiencies in negative and positive ion modes, respectively. The last focusing optic of the primary ion beam has a very short working distance to the sample (100s of μm), and the primary beam enters the sample orthogonal to the plane of the sample [6]. The secondary ions are then analyzed using a magnetic sector mass spectrometer, where up to 5 or 7 (depending on NanoSIMS model) preselected ions can be detected simultaneously using electron multipliers. By finely tuning the magnetic field and aperture slits along the secondary ion path, mass resolutions capable of discriminating 12CHH− v. 12CD− and 12C15N− v 13C14N− are possible.
Figure 5 shows the usefulness of this instrument in an image of unicyanobacterial consortia from Hot Lake, Washington, grown on a silicon wafer and feed 15NH4+ for 4 hrs before NanoSIMS analysis. Here, consumption of NH4+ is localized primarily within the cyanobacteria filaments. With the NanoSIMS, individual heterotrophs were resolvable (typically less than 1 μm in size), and enrichment in individual heterotrophs was observed and quantifiable. These observations indicate either direct consumption of 15NH4+ or consumption of a secondary metabolite made by the cyanobacteria utilizing the 15NH4+. These NanoSIMS measurements, and those described elsewhere [14], were invaluable in determining actual metabolic processes within microbial communities.
Figure 5.
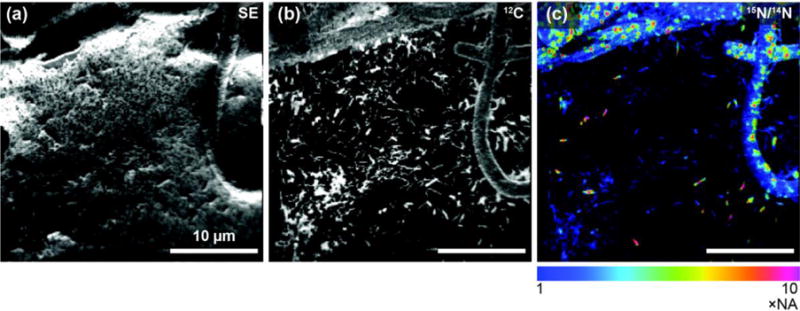
NanoSIMS image of the cyanobacteria community illustrates the high lateral resolution of SIMS. The secondary electron image (a) was collected simultaneously with the ion image (b and b). The ion image of 12C− (b) illustrates the location of biomaterial created by the community on the silicon wafer. (c) Ratio image showing the locations of nitrogen enrichment within the sample, where enrichment is determine by 12C15N−/12C14N− over the natural abundance (0.00367). This is a 256 pixel × 256 pixel image using a beam size of approximately 110 nm. The lateral resolution of this image is modestly low for a NanoSIMS image, which here is defined by the pixel size (156 nm).
Three-dimensional SIMS
The surface sensitivity of SIMS limits analysis to two-dimensional images; however, it is possible to use the dynamic sputtering capabilities of the incoming ion beam to etch away part of the sample and reveal a lower layer (or slice) of the sample. Figure 6 shows a schematic of this where a sputter ion source (usually a different primary ion beam than that used for analysis) is used to erode a layer of the sample followed by two-dimensional image analysis using the analysis ion beam. In this manner a series of separate layers can be etched and then imaged. By reconstructing these serial 2D images a 3D representation of the sample can be formed, even the 3D reconstruction of a single cell [15]. Figures 7a and 7b show an example of this with a reconstruction of C2C12 confluent cells grown on a silicon wafer (image provided by the Dr. Daniel Graham of the NESACBIO group). A bismuth LMIG analysis source in a ToF-SIMS was used to analyze the cells (acquire 2D images) and a C60 cluster ion source was used to sputter away the layers. Figure 7a shows a 3D reconstruction image using the m/z= 58 (choline) + m/z= 184 (phosphocholine) peaks which are headgroup components of phosphatidylcholine (a component of the cell wall) showing the outer membrane of the cells. Figure 7b shows the same reconstruction, but only showing the lower half of the cell membranes. The position of the interior of these cells are seen as empty ‘holes’ since there are now cell wall phospholipids in the interior of the cells. This type of data reconstruction demonstrates the possibility for ToF-SIMS to be used to create a three dimensional molecular maps of cells and other biologically relevant samples with sub-micron resolution.
Figure 6.
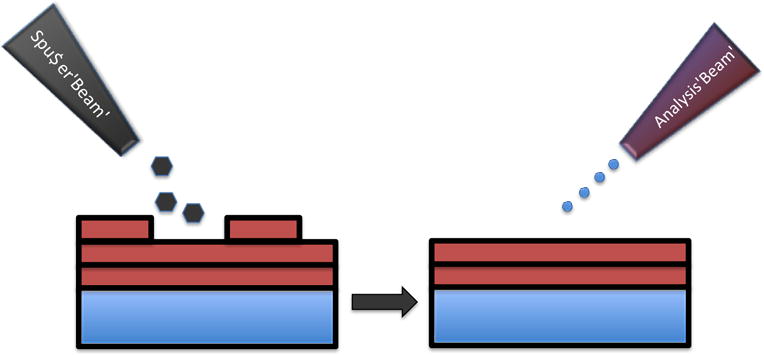
Schematic of the SIMS sputter-then-image method to create separate two-dimensional images. A series of these 2D images can be reconstructed to create a 3D representation of the sample.
Figure 7.
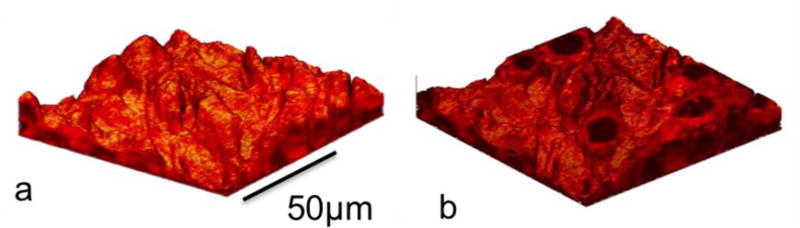
(a) 3D reconstruction of a ToF-SIMS depth profile of C2C12 cells taken with an IONTOF TOF.SIMS5 instrument at NESACBIO. A summation of the m/z= 58 (choline) + m/z= 184 (phosphocholine) peaks is used to show the position of the outer cellular membrane. (b) The same 3D reconstruction data as in (a) showing only the lower half of the cells.
Discussion
The use of SIMS imaging techniques to analyze biologically relevant samples has increased in recent years as evidenced by the increasing number of published manuscripts and research groups using this MSI technique to characterize biologically related samples. There are different strengths and weaknesses in the different types of SIMS instrumentation and analysis modes. However, new instruments are being developed that combine capabilities. For example, coupling ToF-SIMS with tandem MS capabilities would allow for rapid imaging of a sample followed by verification of the structural confirmation of ions of interest. The introduction of cluster ion sources over the past decade has improved the mass analysis range of SIMS by increasing ion yields of higher mass fragments and intact molecular species. Cluster sources have also improved the three dimensional capabilities of the SIMS imaging by allowing thinner ‘slices’ to be sputtered off the sample for higher z-dimension lateral resolution. Data processing techniques (e.g., multivariate analysis) are now more commonly applied to MSI data analysis, allowing for more information to be mined from the large amount of data produced with SIMS techniques [16].
Conclusion
SIMS has been a staple method of inorganic surface analysis for now approaching half a century. However, SIMS has recently experienced a renaissance in pursuit of providing novel chemical information about tissues, single cells, and microbial systems. During this period new SIMS instrumentation, methods, and data analysis approaches have been and are continuing to be developed to address biochemical information previously unattainable. Here, we demonstrated the high mass resolving power and mass accuracy measurement FTICR-SIMS can provide. We show the value in ToF-SIMS to generate high information content chemical maps with an order of magnitude better lateral resolution than the FTICR-SIMS. We demonstrate that NanoSIMS is capable of producing very high spatial resolution information of submicron environments. Finally, we establish that reconstruction of depth profiles with SIMS allows three-dimensional images of cell and tissue structure. Each of these methods has their limitation, but multi-technique approaches can overcome the knowledge gap produced by using only a single SIMS method. Lastly, the columniation of improved primary ion beams, advances in mass spectrometers, and increased sophistication of data processing methods suggest a very bright future for SIMS imaging in applications of biochemical analysis.
Acknowledgments
The authors would like to thank Dr. Daniel Graham (NESACBIO, University of Washington) for ToF-SIMS images and advice and Ljiljana Pasa-Tolic (PNNL) for advice and useful discussions. They also thank Florian Marty (University of Zurich) and Ron M. A. Heeren (Maastricht University) for the wing disc sample and Jesse Cole (PNNL) for the cyanobacteria sample. LJG acknowledges the NESACBIO funding NIH P41 EB002027. Portions of the research were performed using the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at PNNL. PNNL is a multiprogram national laboratory operated by Battelle for DOE under Contract DE-AC05- 76RL01830.
References
- 1.Chughtai K, Heeren RMA. Mass Spectrometric Imaging for biomedical tissue analysis. Chemical reviews. 2010;110(5):3237–3277. doi: 10.1021/cr100012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spengler B. Mass Spectrometry Imaging of Biomolecular Information. Analytical Chemistry. 2015;87(1):64–82. doi: 10.1021/ac504543v. [DOI] [PubMed] [Google Scholar]
- 3.Weaver EM, Hummon AB. Imaging mass spectrometry: From tissue sections to cell cultures. Advanced Drug Delivery Reviews. 2013;65(8):1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Wu CP, et al. Mass spectrometry imaging under ambient conditions. Mass Spectrometry Reviews. 2013;32(3):218–243. doi: 10.1002/mas.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams P. Secondary ion mass spectrometry. Annual Review of Materials Science. 1985;15(1):517–548. [Google Scholar]
- 6.Hillion F, et al. A new high performance instrument: the CAMECA NanoSIMS 50 Secondary ion mass spectrometry: SIMS IX Wiley, Chichester. 1993:254–257. [Google Scholar]
- 7.Benninghoven A. Surface analysis by secondary ion mass spectrometry (SIMS) Surface Science. 1994;299:246–260. [Google Scholar]
- 8.Winograd N. Imaging Mass Spectrometry on the Nanoscale with Cluster Ion Beams. Analytical Chemistry. 2015;87(1):328–333. doi: 10.1021/ac503650p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DF, et al. High mass accuracy and high mass resolving power FT-ICR secondary ion mass spectrometry for biological tissue imaging. Analytical and Bioanalytical Chemistry. 2013;405(18):6069–6076. doi: 10.1007/s00216-013-7048-1. [DOI] [PubMed] [Google Scholar]
- 10.Smith DF, et al. C60 secondary ion Fourier transform ion cyclotron resonance mass spectrometry. Analytical chemistry. 2011;83(24):9552–9556. doi: 10.1021/ac2023348. [DOI] [PubMed] [Google Scholar]
- 11.DeBord JD, et al. Secondary Ion Mass Spectrometry Imaging of Dictyostelium discoideum Aggregation Streams. Plos One. 2014;9(6) doi: 10.1371/journal.pone.0099319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiung F, et al. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437(7058):560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 13.Frisz JF, et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proceedings of the National Academy of Sciences. 2013;110(8):E613–E622. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens S, et al. Linking microbial phylogeny to metabolic activity at the single–cell level by using enhanced element labeling-catalyzed reporter deposition fluorescence in situ hybridization (EL-FISH) and NanoSIMS. Applied and environmental microbiology. 2008;74(10):3143–3150. doi: 10.1128/AEM.00191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson M, Graham D, Castner D. ToF-SIMS Depth Profiling of Cells: Z-correction, 3D Imaging, and Sputter Rate of Individual NIH/3T3 Fibroblasts. Analytical Chemistry. 2013;84(11):4880–4885. doi: 10.1021/ac300480g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham D, Castner D. Multivariate Analysis of ToF-SIMS Data from Multicomponent Systems: The Why, When, and How. Biointerphases. 2012:7. doi: 10.1007/s13758-012-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


