Abstract
Background
Today, it is proved that isoenzymes CYP2D6 and CYP3A4 are involved in metabolism of haloperidol. In our previous investigation, we found a medium correlation between the efficacy and safety of haloperidol and the activity of CYP3A4 in patients with alcohol abuse.
Objective
The aim of this study was to evaluate the correlation between the activity of CYP2D6 and the efficacy and safety of haloperidol in patients with diagnosed alcohol abuse.
Methods
The study involved 70 men (average age: 40.83±9.92 years) with alcohol addiction. A series of psychometric scales were used in the research. The activity of CYP2D6 was evaluated by high-performance liquid chromatography with mass spectrometry using the ratio of 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline to pinoline. Genotyping of CYP2D6 (1846G>A) was performed using real-time polymerase chain reaction.
Results
According to results of correlation analysis, statistically significant values of Spearman correlation coefficient (rs) between the activity of CYP2D6 and the difference of points in psychometric scale were obtained in patients receiving haloperidol in injection form (Sheehan Clinical Anxiety Rating Scale =−0.721 [P<0.001] and Udvald for Kliniske Undersogelser Side Effect Rating Scale =0.692 [P<0.001]) and in those receiving haloperidol in tablet form (Covi Anxiety Scale =−0.851 [P<0.001] and Udvald for Kliniske Undersogelser Side Effect Rating Scale =0.797 [P<0.001]).
Conclusion
This study demonstrated the correlations between the activity of CYP2D6 isozyme and the efficacy and safety of haloperidol in patients with alcohol addiction.
Keywords: haloperidol, biotransformation, CYP2D6, side effects, alcohol addiction
Background
Haloperidol is one of the most commonly used typical antipsychotics. It has a powerful antipsychotic effect owing to its ability to antagonize mesolimbic postsynaptic D-2 receptor. According to the guidelines, haloperidol is recommended for patients with alcohol-related psychosis.1,2 Haloperidol in combination with a benzodiazepine is used to treat severe psychotic symptoms. Under the guidelines, the recommended dose of haloperidol is 1–5 mg two to three times a day.3 The exacerbation of addiction to psychoactive substances with psychomotor agitation is the main indication for use of haloperidol in patients with addictive disorders in the Russian Federation.4 The use of haloperidol can cause various side effects (dyskinesia, dystonia, reduced blood pressure, orthostatic hypotension, and arrhythmias). Therefore, alcohol abusers’ attitudes toward haloperidol are ambiguous and often negative, which sometimes limits its administration in patients with addictive disorders.
Cytosolic carbonyl reductase reduces haloperidol to its reduced form that has 10%–20% of activity of the parent molecule. CYP3A4 catalyzes the metabolism of haloperidol to haloperidol 1,2,3,6-tetrahydropyridine.5 Haloperidol 1,2,3,6-tetrahydropyridine is further metabolized to haloperidol pyridinium by both CYP3A4 and CYP2D6.5 CYP3A4 and CYP2D6 are also responsible for the N-dealkylation of haloperidol.5 The N-dealkylation of reduced haloperidol is catalyzed by CYP3A4.5 CYP3A4 also catalyzes the oxidation of reduced haloperidol back to haloperidol.5 In the investigation of Van der Weide and van der Weide,6 no difference was found in serum (dose-corrected) concentrations of haloperidol between CYP3A4*22 wild-type and carrier groups.6 In our previous investigation, we confirmed the relationship between the activity of CYP3A4 and the efficacy and safety of haloperidol in patients with alcohol abuse.7 The correlation between CYP2D6 activity and the rate of biotransformation of haloperidol was demonstrated in a number of studies on patients with schizophrenia.6,8,9 At the same time, some studies demolished the presence of this correlation.10 There were no studies about the relationship between the rate of biotransformation of haloperidol and the activity of CYP2D6 in patients with alcohol addiction, and there is lack of data on the relationship between the activity of CYP2D6 and the efficacy and safety of haloperidol in patients with any addictions to psychoactive substances.
The purpose of the current investigation was to evaluate the correlation between the activity of CYP2D6 and the efficacy and safety of haloperidol in patients with alcohol addiction.
Patients and methods
The study involved 70 men with alcohol addiction, who were hospitalized in Moscow Research and Practical Centre for Narcology of the Department of Public Health. The study was approved by the local ethics committee of the Peoples’ Friendship University of Russia (No 8 Minutes of February 18, 2016) and all patients provided written informed consent. During the exacerbation of the addiction, patients received haloperidol in tablet form (OOO Ozon, Ghigulevsk, Russia) at a dose of 4.34±2.38 mg/d (38 patients, once a day) and injectable forms (ZAO Bryntsalov-A, Moscow, Russia) at a dose of 6.09±2.10 mg/d (32 patients, once a day). Inclusion criteria were 5-day haloperidol therapy in tablet or injection form and no concomitant mental illness in anamnesis. Exclusion criteria were presence of any other antipsychotics in treatment, creatinine clearance <50 mL/min, concentration of creatinine in the blood plasma ≥1.5 mg/dL (133 mmol/L); weight <60 kg or >100 kg; age 75 years or older; and contraindications for haloperidol.
The activity of CYP2D6 was evaluated by determining urinary concentration of endogenous substrate of the enzyme and its metabolite – the ratio of 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline (6-HO-THBC) to pinoline11 using high-performance liquid chromatography with mass spectrometry on an Agilent 1290 Infinity. The higher the ratio, the higher the activity of CYP2D6. The results are demonstrated in arbitrary units.
Venous blood drawn in vacuum tubes Vacuette® (Greiner Bio-One, Kremsmünster, Austria) on the sixth day after the start of the therapy was used for genotyping. The real-time polymerase chain reaction on a DNA amplifier “Dtlite” of DNA Technology (Moscow, Russia) and CFX96 Touch Real Time System with CFX Manager software of Bio-Rad Laboratories Inc. (Hercules, CA, USA) and sets “SNP-Screen” of Syntol (Moscow, Russia) was used to determine polymorphism 1846G>A of CYP2D6 gene (rs3892097). In every “SNP-Screen” set, two allele-specific hybridizations were used, which allowed us to separately determine two alleles of studied polymorphism on two channels of fluorescence.
The efficacy of haloperidol was evaluated by international psychometric scales: Scale of Pathological Addiction (SoPA), Hamilton Anxiety Rating Scale (HARS), Beck Anxiety Inventory (BAI), Covi Anxiety Scale (CARS), Zung Self-Rating Anxiety Scale (ZARS), Sheehan Clinical Anxiety Rating Scale (SARS), and Hamilton Rating Scale for Depression (HDRS). The safety of haloperidol was evaluated by Udvald for Kliniske Undersogelser Side Effect Rating Scale (UKU) and Simpson–Angus Scale for Extrapyramidal Symptoms (SAS). Scaling of patients was performed the day before haloperidol therapy and after 5-day therapy. The higher the difference in scores was, the more unsafe the therapy was.
Statistical analysis of the results was done by nonparametric methods using the “Statsoft Statistica V10.0” program (Dell Statistica, Tulsa, OK, USA). The normality of distributions of samples, that was evaluated using W-Shapiro–Wilk test, was taken into account when choosing a method. The differences were considered as statistically significant at P<0.05 (statistical power in excess of 80%). Spearman rank correlation coefficient (rS) was calculated to determine the correlation between quantitative characteristics. The value of correlation coefficient rs from 0.3 to 0.7 (P<0.05) indicated positive moderate, but significant correlation between the characteristics, rs>0.7 (P<0.05) – strong and significant correlation, negative value of rs indicated inverse correlation. Regression analysis was performed in “Multiple Regression module” to determine the influence of CYP2D6 activity (as measured by the ratio of 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline to pinoline) on the effectiveness of treatment (as measured on a scale of SoPA, as the most complete method of assessing the severity of a pathological inclination) and safety of treatment (measured on a scale of UKU, as the most complete method of assessing the severity of extrapyramidal disorders).
All quantitative data are presented as arithmetic mean ± SD.
Results
As a result of CYP2D6 genotyping (polymorphic marker 1846G>A [rs3892097]) in 70 patients with alcohol addiction, the following data were obtained:
The number of patients with no mutant CYP2D6 gene (genotype GG) accounted 53 (75.71%); 23 of them (32.8%) received haloperidol in injection form, and 30 (42.85%) received haloperidol in tablet form.
The number of patients with heterozygous polymorphism 1846G>A of CYP2D6 gene (genotype GA) accounted for 17 (24.29%); eight of them (11.43%) received haloperidol in injection form, and nine (12.86%) received haloperidol in tablet form.
There were no patients with homozygous polymorphism 1846G>A of CYP2D6 gene (genotype AA).
The distribution of genotypes corresponded to Hardy–Weinberg equilibrium in the European population (Fisher’s exact test χ2=1.34; P=0.25).
The results of psychometric scales and scales of severity of side effects in patients who received haloperidol in injectable form are presented in Table 1 and in tablet form are presented in Table 2.
Table 1.
The general characteristics of participants
| Characteristics | All participants | CYP2D6 1846G>A (genotype GG) | CYP2D6 1846G>A (genotype GA) | P-value |
|---|---|---|---|---|
| N (%) | 70 (100) | 53 (75.71) | 17 (24.29) | |
| Age, years | 40.83±9.92 | 41.34±10.14 | 39.24±9.3 | 0.556 |
| Weight, kg | 81.57±12.04 | 82.08±12.54 | 80±10.53 | 0.589 |
| Height, cm | 176.26±7.06 | 176.23±7.3 | 176.35±6.46 | 0.951 |
| Body mass index, kg/m2 | 26.44±4.85 | 26.65±5.15 | 25.8±3.79 | 0.646 |
Notes: aMann–Whitney U-test. Data shown as mean ± standard deviation unless otherwise indicated.
Table 2.
Scores of psychometric scales and scales of severity of side effects in patients who received haloperidol in injectable form
| The name of the scale | The score of the scale before the therapy | The score of the scale after 5-day therapy | The difference in scores | P-value |
|---|---|---|---|---|
| SoPA | 24.84±2.87 | 14.03±3.21 | 10.61±1.47 | <0.001 |
| HARS | 39.78±4.3 | 25.94±5.04 | 14.02±2 | <0.001 |
| BAI | 34.75±4.08 | 13.97±5.62 | 20.92±3.38 | <0.001 |
| CARS | 9±1.16 | 4.72±1.44 | 4.28±0.59 | <0.001 |
| ZARS | 40.31±3.61 | 23.47±4.87 | 16.94±2.31 | <0.001 |
| SARS | 73.88±3.88 | 38.88±6.99 | 34.54±4.96 | <0.001 |
| HDRS | 21.69±2.9 | 9.34±4.15 | 12.64±2.92 | <0.001 |
| UKU | 11.94±3.56 | 20.19±2.56 | −7.96±2.11 | <0.001 |
| SAS | 4.31±1.2 | 8.47±1.24 | −4.24±0.74 | <0.001 |
Note: Data shown as mean ± SD.
Abbreviations: SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms; SD, standard deviation.
The results of CYP2D6 phenotyping are presented in Table 3.
Table 3.
Scores of psychometric scales and scales of severity of side effects in patients who received haloperidol in tablet form
| The name of the scale | The score of the scale before the therapy | The score of the scale after 5-day therapy | The difference in scores | P-value |
|---|---|---|---|---|
| SoPA | 24±2.56 | 13.24±2.7 | 10.87±1.29 | <0.001 |
| HARS | 40.29±5.19 | 26.63±5.63 | 13.92±1.5 | <0.001 |
| BAI | 34.16±3.34 | 13.37±4.05 | 20.66±2.46 | <0.001 |
| CARS | 8.87±1.07 | 4.55±1.01 | 4.34±0.51 | <0.001 |
| ZARS | 38.79±3.65 | 21.87±4.12 | 17.17±1.82 | <0.001 |
| SARS | 73.92±4.98 | 39.37±7.01 | 34.36±4.27 | <0.001 |
| HDRS | 22.29±2.47 | 10.74±3.42 | 11.71±2.3 | <0.001 |
| UKU | 12.03±2.81 | 20.29±2.12 | −8.33±1.65 | <0.001 |
| SAS | 3.87±1.04 | 8.08±0.71 | −4.33±0.65 | <0.001 |
Note: Data shown as mean ± SD
Abbreviations: SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms; SD, standard deviation.
The differences in scores (the efficacy and safety of haloperidol therapy) in groups of patients with the GG and GA genotype of CYP2D6 gene (1846G>A) were compared using nonparametric Mann–Whitney test, thus working out the profile of efficacy and safety of the therapy (Tables 4 and 5).
Table 4.
Results of determination of the urinary concentration of pinoline and 6-HO-THBC using HPLC-MS/MS
| Group | N (%) | Concentration of pinoline (pg/mL) | Concentration of 6-HO-THBC (pg/mL) | Ratio 6-HO-THBC/pinoline |
|---|---|---|---|---|
| Haloperidol in injection form | 32 (45.71) | |||
| Median | 1,565.150 | 1,756.070 | 1.037 | |
| Quartile 1 | 1,057.780 | 1,036.080 | 0.745 | |
| Quartile 3 | 2,502.815 | 2,634.660 | 1.803 | |
| Haloperidol in tablet form | 38 (54.29) | |||
| Median | 1,887.885 | 1,943.025 | 0.913 | |
| Quartile 1 | 1,234.440 | 1,346.520 | 0.566 | |
| Quartile 3 | 2,710.360 | 2,527.180 | 1.868 | |
| Total group | 70 (100) | |||
| Median | 1,612.180 | 1,917.490 | 0.942 | |
| Quartile 1 | 1,136.770 | 1,127.000 | 0.708 | |
| Quartile 3 | 2,585.700 | 2,558.580 | 1.811 |
Abbreviations: 6-HO-THBC, 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline; HPLC-MS/MS, high-performance liquid chromatography with mass spectrometry.
Table 5.
Difference in scores on scales in patients who received haloperidol in injection form with the GG and GA genotype of CYP2D6 gene by polymorphic marker 1846G> A
| Scale | Genotype GG (N=23) | Genotype GA (N=9) | P-value |
|---|---|---|---|
| SoPA | 10.28±1.36 | 12.08±0.80 | <0.001 |
| HARS | 13.31±1.86 | 15.56±1.39 | <0.001 |
| BAI | 19.53±3.05 | 23.89±1.84 | <0.001 |
| CARS | 4.05±0.57 | 4.63±0.42 | <0.001 |
| ZARS | 16.04±2.09 | 18.84±1.55 | <0.001 |
| SARS | 33.51±4.56 | 39.04±3.68 | <0.001 |
| HDRS | 11.46±2.85 | 14.76±1.38 | <0.001 |
| UKU | −7.44±1.84 | −10.25±1.22 | <0.001 |
| SAS | −3.94±0.72 | −4.72±0.48 | <0.001 |
Note: Data shown as mean ± SD.
Abbreviations: SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms; SD, standard deviation.
The Spearman correlation analysis demonstrated a statistically significant negative moderate correlation between the concentrations of pinoline, 6-HO-THBC, their ratio and the difference in scores on the SoPA, HARS, BAI, CARS, ZARS, SARS, HDRS scales (the efficacy of therapy) and a positive moderate correlation between the concentrations of pinoline, 6-HO-THBC, their ratio and the difference in scores on the UKU and SAS scales (the safety of therapy) in patients who received haloperidol in injection (Table 6) and tablet (Table 7) forms.
Table 6.
Difference in scores on scales in patients who received haloperidol in tablet form with the GG and GA genotype of CYP2D6 gene by polymorphic marker 1846G>A
| Scale | Genotype GG (N=30) | Genotype GA (N=8) | P-value |
|---|---|---|---|
| SoPA | 10.55±1.31 | 11.72±0.64 | <0.001 |
| HARS | 13.38±1.34 | 15.42±0.86 | <0.001 |
| BAI | 20.14±2.31 | 23.31±0.88 | <0.001 |
| CARS | 4.16±0.54 | 4.51±0.20 | <0.001 |
| ZARS | 16.52±1.76 | 18.66±0.72 | <0.001 |
| SARS | 33.04±3.43 | 40.18±1.25 | <0.001 |
| HDRS | 11.47±2.19 | 12.43±2.71 | <0.01 |
| UKU | −7.79±1.55 | −9.9±0.62 | <0.001 |
| SAS | −4.08±0.68 | −4.6±0.26 | <0.001 |
Note: Data shown as mean ± SD.
Abbreviations: SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms; SD, standard deviation.
Table 7.
Spearman’s correlation coefficients (rs), demonstrating correlation between concentration of pinoline, 6-HO-THBC, and difference between values of scales before and after haloperidol therapy in patients receiving haloperidol in injection form
| Scale |
rs
|
|||||
|---|---|---|---|---|---|---|
| Concentration of pinoline | P-value | Concentration of 6-HO-THBC | P-value | Ratio 6-HO-THBC/pinoline | P-value | |
| SoPA | 0.310 | >0.05 | −0.359 | <0.05 | −0.629 | <0.01 |
| HARS | 0.250 | >0.05 | −0.475 | <0.05 | −0.635 | <0.01 |
| BAI | 0.482 | <0.05 | −0.305 | >0.05 | −0.675 | <0.01 |
| CARS | 0.321 | >0.05 | −0.431 | <0.05 | −0.681 | <0.001 |
| ZARS | 0.343 | >0.05 | −0.419 | <0.05 | −0.662 | <0.01 |
| SARS | 0.385 | <0.05 | −0.423 | <0.05 | −0.721 | <0.001 |
| HDRS | 0.267 | >0.05 | −0.137 | >0.05 | −0.444 | <0.05 |
| UKU | −0.322 | >0.05 | 0.425 | <0.05 | 0.692 | <0.001 |
| SAS | −0.344 | >0.05 | 0.453 | <0.01 | 0.726 | <0.001 |
Abbreviations: 6-HO-THBC, 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline; SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson-Angus Scale for Extrapyramidal Symptoms.
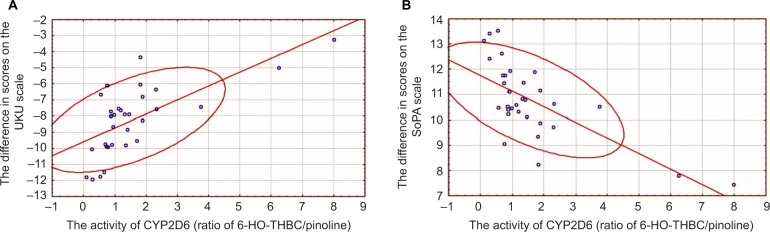
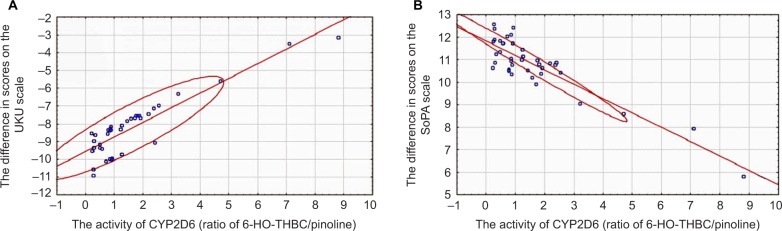
Linear regression analysis allowed us to construct a model of correlation between activity of CYP2D6 and difference in scores on SoPA (efficacy of addiction exacerbation treatment) and the UKU scale (safety of treatment) in patients receiving haloperidol in injection form (Figure 1) and tablet form (Figure 2, Table 8).
Figure 1.
Relationship between activity of CYP2D6 and difference in scores on UKU (A) and SoPA (B) scales in patients receiving haloperidol in injection form.
Abbreviations: UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SoPA, Scale of Pathological Addiction; 6-HO-THBC, 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline.
Figure 2.
Relationship between activity of CYP2D6 and difference in scores on UKU (A) and SoPA (B) scales in patients receiving haloperidol in tablet form.
Abbreviations: UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SoPA, Scale of Pathological Addiction; 6-HO-THBC, 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline.
Table 8.
Spearman’s correlation coefficients (rs), demonstrating correlation between concentration of pinoline, 6-HO-THBC, and difference between values of scales before and after haloperidol therapy in patients receiving haloperidol in tablet form
| Scale |
rs
|
|||||
|---|---|---|---|---|---|---|
| Concentration of pinoline | P-value | Concentration of 6-HO-THBC | P-value | Ratio 6-HO-THBC/pinoline | P-value | |
| SoPA | 0.554 | <0.01 | −0.386 | <0.05 | −0.667 | <0.01 |
| HARS | 0.544 | <0.01 | −0.371 | <0.05 | −0.689 | <0.01 |
| BAI | 0.339 | <0.05 | −0.404 | <0.05 | −0.558 | <0.01 |
| CARS | 0.734 | <0.001 | −0.522 | <0.05 | −0.851 | <0.001 |
| ZARS | 0.455 | <0.05 | −0.420 | <0.05 | −0.605 | <0.001 |
| SARS | 0.443 | <0.05 | −0.359 | <0.05 | −0.586 | <0.01 |
| HDRS | 0.388 | <0.05 | −0.140 | >0.05 | −0.445 | <0.05 |
| UKU | −0.615 | <0.001 | 0.539 | <0.01 | 0.797 | <0.001 |
| SAS | −0.744 | <0.001 | 0.577 | <0.01 | 0.895 | <0.001 |
Abbreviations: 6-HO-THBC, 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline; SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms.
Coefficients of linear regression y=a+b×x (x – index of activity CYP2D6, y – difference in points on a scale, a and b – coefficients of the equation), that demonstrate correlation between activity of CYP2D6 and difference in scores on SoPA, HARS, BAI, CARS, ZARS, SARS, HDRS, UKU, and SAS scales, are presented in Table 9.
Table 9.
Coefficients of linear regression y=a+b×x, demonstrating correlation between activity of CYP2D6 and difference in scores on SoPA, HARS, BAI, CARS, ZARS, SARS, HDRS, UKU, and SAS scales
| Scale | Haloperidol in injection form
|
Haloperidol in tablet form
|
||||||
|---|---|---|---|---|---|---|---|---|
| a | P-value | b | P-value | a | P-value | b | P-value | |
| SoPA | 11.75 | <0.01 | −0.62 | <0.01 | 11.94 | <0.01 | −0.59 | <0.01 |
| HARS | 15.25 | <0.01 | −0.84 | <0.01 | 14.94 | <0.01 | −0.71 | <0.01 |
| BAI | 22.93 | <0.05 | −1.39 | <0.05 | 22.51 | <0.05 | −1.06 | <0.05 |
| CARS | 4.59 | <0.05 | −0.24 | <0.05 | 4.69 | <0.05 | −0.29 | <0.05 |
| ZARS | 18.21 | <0.05 | −0.88 | <0.05 | 17.92 | <0.05 | −0.78 | <0.05 |
| SARS | 37.65 | <0.01 | −1.65 | <0.01 | 36.87 | <0.01 | −1.57 | <0.01 |
| HDRS | 14.31 | <0.05 | −1.22 | <0.05 | 12.67 | <0.05 | −0.62 | <0.05 |
| UKU | −9.58 | <0.01 | 0.86 | <0.01 | −9.32 | <0.01 | 0.79 | <0.01 |
| SAS | −4.67 | <0.05 | 0.32 | <0.05 | −4.83 | <0.05 | 0.29 | <0.05 |
Abbreviations: SoPA, Scale of Pathological Addiction; HARS, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; CARS, Covi Anxiety Scale; ZARS, Zung Self-Rating Anxiety Scale; SARS, Sheehan Clinical Anxiety Rating Scale; HDRS, Hamilton Rating Scale for Depression; UKU, Udvald for Kliniske Undersogelser Side Effect Rating Scale; SAS, Simpson–Angus Scale for Extrapyramidal Symptoms.
Discussion
Today, there is information that CYP2D6 takes part in metabolism of haloperidol.5 There were some investigations about relationship of CYP2D6 polymorphism and safety and efficacy of haloperidol in patients with schizophrenia,6,8–10 but there was no investigation about relationship of CYP2D6 activity (as measured by the ratio of 6-hydroxy-1,2,3,4-tetrahydro-beta-carboline to pinoline) and CYP2D6 polymorphism and efficacy and safety of haloperidol in patients with alcohol abuse.
In this investigation, we statistically demonstrated that the efficacy and safety of haloperidol in patients with alcohol addiction depend on the activity of CYP2D6 isozyme. The higher the CYP2D6 activity is, the lower the efficacy of haloperidol is, which is probably due to acceleration of biotransformation and elimination of haloperidol. Safety indices increase with increasing activity of CYP2D6 that is also due to acceleration of elimination of haloperidol. This is confirmed by the results that were obtained in earlier investigations in patients with schizophrenia.6,8,9
Average therapeutic dose should be carefully administered in patients with high activity of CYP2D6, as high activity of CYP2D6 inhibits the achievement of therapeutic concentrations, which results in no therapeutic effect. In patients with low activity of CYP2D6 and who received an average therapeutic dose of haloperidol, its concentration in plasma may be higher, which will lead to increased frequency and severity of side effects. Haloperidol is contraindicated in patients with very high or very low activity of CYP2D6; therefore, other antipsychotics, in which biotransformation of CYP2D6 is not involved, are recommended.
Conclusion
The correlation between the activity of CYP2D6 isozyme and the efficacy and safety of haloperidol was demonstrated in this research on a group of 70 patients with alcohol addiction.
Acknowledgments
The authors are grateful to KB Mirzaev, KA Rizhikova, JA Avdeeva and NE Snalina at the research center of Russian Medical Academy of Postgraduate Education, Ministry of Health of the Russian Federation for assistance in the genotyping of patients. This work was supported by the Russian Science Foundation. The project 16-15-00227 “Fundamental research and exploratory research in priority areas of research”. The abstract of this paper was presented at the First International Conference on “Personalized psychiatry: modern possibilities of genetics in psychiatry” (Russia, Moscow, 2015) as a poster presentation with interim findings. The poster’s abstract was published in The Bulletin of Russian State Medical University.12
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.National Institute for Health and Care Excellence [webpage on the Internet] Acute Alcohol Withdrawal. National Institute for Health and Care Excellence; 2015. [Accessed July 27, 2016]. Available from: http://pathways.nice.org.uk/pathways/alcohol-use-disorders. [Google Scholar]
- 2.Stewart S, Swain S, NICE. Royal College of Physicians London Assessment and management of alcohol dependence and withdrawal in the acute hospital. Clin Med (Lond) 2012;12(3):266–271. doi: 10.7861/clinmedicine.12-3-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHS [webpage on the Internet] Guidelines for the Management of Alcohol Issues in the Acute General Hospital Setting. 2006. [Accessed July 27, 2016]. Available from: http://www.alcohollearningcentre.org.uk/_library/17__Doncaster_Guidelines_For_The_Management_Of_Patients_with_Alcohol_Misuse_In_The_Acute_General_Hospital_Setting.pdf.
- 4.Ivanec NN. Cancer therapy. Med News Agency. 2008:531–580. [Google Scholar]
- 5.Fang J, Baker GB, Silverstone PH, Coutts RT. Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol. 1997;17(2):227–233. doi: 10.1023/A:1026317929335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Weide K, van der Weide J. The influence of the CYP3A4*22 polymorphism and CYP2D6 polymorphisms on serum concentrations of aripiprazole, haloperidol, pimozide, and risperidone in psychiatric patients. J Clin Psychopharmacol. 2015;35(3):228–236. doi: 10.1097/JCP.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 7.Zastrozhin MS, Smirnov VV, Sychev DA, Savchenko LM, Bryun EA, Matis OA. CYP3A4 activity and haloperidol effects in alcohol addicts. Int J Risk Saf Med. 2015;27(suppl 1):S23–S24. doi: 10.3233/JRS-150676. [DOI] [PubMed] [Google Scholar]
- 8.Butwicka A, Krystyna S, Retka W, Wolańczyk T. Neuroleptic malignant syndrome in an adolescent with CYP2D6 deficiency. Eur J Pediatr. 2014;173(12):1639–1642. doi: 10.1007/s00431-013-2208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassó P, Papagianni K, Mas S, et al. Relationship between CYP2D6 genotype and haloperidol pharmacokinetics and extrapyramidal symptoms in healthy volunteers. Pharmacogenomics. 2013;14(13):1551–1563. doi: 10.2217/pgs.13.150. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura A, Mihara K, Nemoto K, et al. Lack of correlation between the steady-state plasma concentrations of aripiprazole and haloperidol in Japanese patients with schizophrenia. Ther Drug Monit. 2014;36(6):815–818. doi: 10.1097/FTD.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 11.Jiang XL, Shen HW, Yu AM. Pinoline may be used as a probe for CYP2D6 activity. Drug Metab Dispos. 2009;37(3):443–446. doi: 10.1124/dmd.108.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sychev DA, Zastrozhin MS, Smirnov VV, et al. Association between the activity of CYP2D6 enzyme and profile of efficacy and safety of haloperidol in patients with alcohol addiction. The Bulletin of Russian State Medical University. 2015;4:36–39. [Google Scholar]