Abstract
Current therapeutic approaches for treatment of exposure to radiation involve the use of antioxidants, chelating agents, recombinant growth factors and transplantation of stem cells (e.g., hematopoietic stem cell transplantation). However, exposure to high-dose radiation is associated with severe damage to the vasculature of vital organs, often leading to impaired healing, tissue necrosis, thrombosis and defective regeneration caused by aberrant fibrosis. It is very unlikely that infusion of protective chemicals will reverse severe damage to the vascular endothelial cells (ECs). The role of irradiated vasculature in mediating acute and chronic radiation syndromes has not been fully appreciated or well studied. New approaches are necessary to replace and reconstitute ECs in organs that are irreversibly damaged by radiation. We have set forth the novel concept that ECs provide paracrine signals, also known as angiocrine signals, which not only promote healing of irradiated tissue but also direct organ regeneration without provoking fibrosis. We have developed innovative technologies that enable manufacturing and banking of human GMP-grade ECs. These ECs can be transplanted intravenously to home to and engraft to injured tissues where they augment organ repair, while preventing maladaptive fibrosis. In the past, therapeutic transplantation of ECs was not possible due to a shortage of availability of suitable donor cell sources and preclinical models, a lack of understanding of the immune privilege of ECs, and inadequate methodologies for expansion and banking of engraftable ECs. Recent advances made by our group as well as other laboratories have breached the most significant of these obstacles with the development of technologies to manufacture clinical-scale quantities of GMP-grade and human ECs in culture, including genetically diverse reprogrammed human amniotic cells into vascular ECs (rAC-VECs) or human pluripotent stem cells into vascular ECs (iVECs). This approach provides a path to therapeutic EC transplantation that can be infused concomitantly or sequentially with hematopoietic stem cell transplantation more than 24 h after irradiation to support multi-organ regeneration, thereby improving immediate and long-term survival, while limiting long-term morbidity resulting from nonregenerative damage repair pathways.
INTRODUCTION
Exposure to high-dose ionizing radiation is often fatal because of multi-organ failure. Radiation-induced injury to the vasculature of vital organs is responsible for diffuse organ malfunction and impaired healing. Surprisingly, the consequence of radiation damage to endothelial cells (ECs) that line blood vessels, in mediating complications associated with acute or chronic radiation syndromes is largely ignored and poorly studied. Vascular damage and apoptosis represent the initial pathological manifestation of radiation injury resulting in tissue necrosis and malfunction. To uncover the mechanism by which ECs protect against tissue injury, our group and others have shown that ECs are not just passive conduits in the delivery of oxygen and nutrients, but also establish an instructive “vascular niche” that supports organ regeneration by production of paracrine/juxtacrine (angiocrine factors) (1–4). Therefore, loss of EC function not only leads to hypoxia and tissue necrosis but also impairs healing by depriving the regenerative cells of the appropriate signals and growth factors necessary for organ regeneration and repair. Indeed, the microvascular capillary ECs of bone marrow, spleen, intestines, skin, lungs and brain are very sensitive to ionizing radiation. Radiation damage to the ECs is irreversible and contributes to acute radiation syndrome and aberrant healing by maladaptive fibrosis. This can also lead to long-term complications, including fibrotic scarring that predisposes to tumorigenesis.
None of the current therapeutic interventions has been shown to repair injured vasculature in irradiated organs. For example, radiation-induced injury to bone marrow ECs that establish an instructive vascular niche can be so great that transplanted hematopoietic stem cells fail to engraft or rescue hematopoiesis. Radiation injury to brain and lung vasculature results in terminal organ failure as well. It is unlikely that delivery of protective chemicals including antioxidants, chelators or growth factors could repair radiation-induced ECs injury. Therefore, one approach is to replace the injured ECs by transplantation of normal ECs.
To this end, we have developed technologies and approaches to engineer ECs that can engraft into the injured blood vessels of irradiated organs and promote organ repair. We have shown that abnormal EC function causes failed regeneration, fibrosis and death, while transplantation of ECs orchestrates bone marrow, liver, lung and even gonadal regeneration after injury by producing paracrine, “angiocrine” growth factors (Fig. 1). To restore multi-organ function to injured organs, we have devised novel approaches to propagate abundant ECs that meet the Good Manufacturing Practices guidelines (GMP grade) enforced by the FDA and are functionally immunocompatible. We have shown that intravenously transplanted ECs home to and engraft to injured tissues, and by producing instructive factors, repair injured organs with minimal fibrosis (1–4).
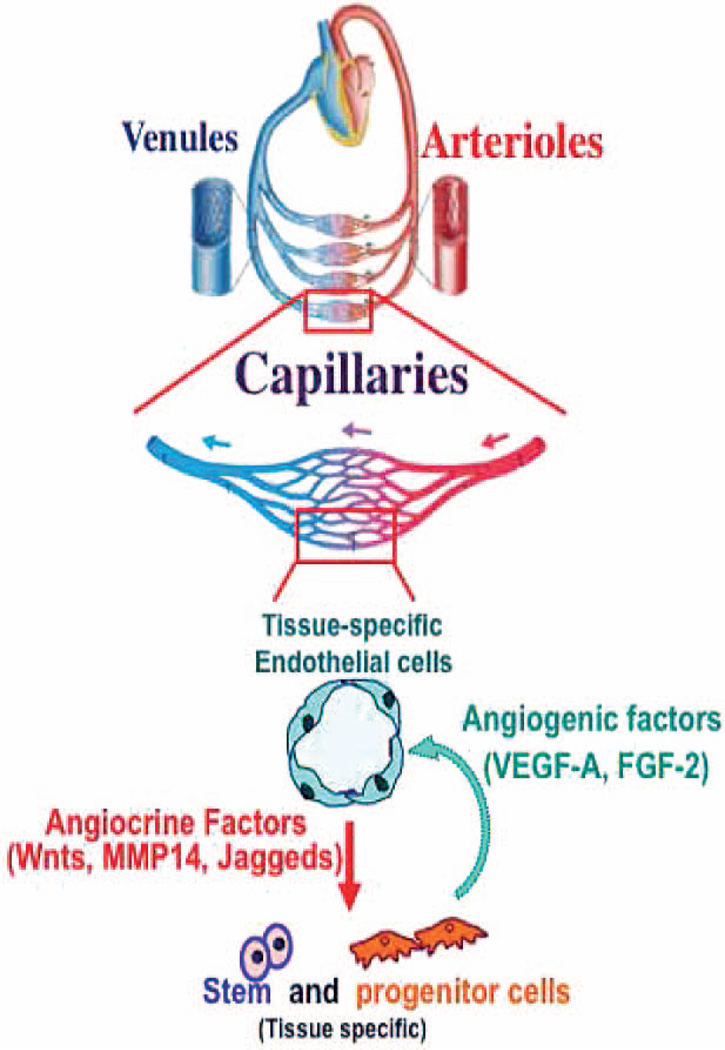
FIG. 1.
Cross-talk between microvascular ECs and repopulating stem and progenitor cells supports organ regeneration and repair. Angiogenic factors produced in the injured organ regulate the activation state and angiocrine factors elaborated by tissue-specific ECs to orchestrate organ regeneration.
Based on these studies, we have formulated the transformative notion that radiation damage to the vasculature deprives injured organs of EC-derived instructive signals leading to failed or delayed organ repair and predisposition to scarring and tumorigenesis. Transplantation and engraftment of healthy ECs during the days after radiation-induced damage can restore the inductive signals that promote organ repair, while preventing aberrant fibrosis and scarring. This approach is attractive because using transplanted ECs to rejuvenate damaged bone marrow vascular niche is expected to also facilitate hematopoietic stem cell homing and engraftment and enhance hematopoietic-driven organ repair as well.
We have been able to reprogram human amniotic cells into vascular ECs (rAC-VECs) (5). In addition, we are able to expand human organ-specific ECs with the capacity to undergo clinical-scale expansion. These approaches enable manufacturing of compatible clinical GMP-grade “vascular product” that could be infused concomitantly or sequentially with hematopoietic stem and progenitor cells (HSPCs) ≥24 h after radiation exposure to support recovery of multi-organ function. This approach will have a major impact on reversing radiation injury to the blood vessels that lead to collapse of the endothelial cells, fibrosis and bleeding as well as coagulapathies.
Mechanism of Vascular Endothelial Cell-Mediated Mitigation in Radiation-Induced Injury
Acute radiation syndrome is associated with vascular, neurological, hematopoietic and gastrointestinal dysfunction. HSPC transplantation has been the mainstay of treatment for patients, who have been exposed to moderate doses of irradiation. However, higher doses cause severe damage to the bone marrow vascular niche, thereby inhibiting the engraftment of the transplanted HSPCs. Furthermore, radiation targets every tissue-specific micro-vasculature as well as repopulating stem and progenitor cells, including neuronal, hepatocytes, lung alveolar, intestinal and dermal epithelial cells. To restore multi-organ function in patients exposed to radiation, it will be essential to transplant multiple cell types, including tissue-specific epithelial cells. Given the difficulty in multiple-lineage transplantation, most studies have focused on delivery of growth factors and HSPCs, with no tangible benefit so far.
Endothelial Cell Transplantation for Organ Repair after Irradiation
Therapeutic endothelial cell transplantation for organ regeneration challenges the prevailing approaches for stimulating organ repair after irradiation and sets forth an innovative alternative approach to direct organ regeneration by infusion of ECs that function as an instructive niche to promote multi-organ repair without provoking fibrosis.
Developmental biologists were the first to show that ECs provide functions beyond that of a passive vascular sheath because ECs instructively induce pancreatic and hepatic organogenesis prior to the initiation of circulation (6, 7). Until now, the instructive role of ECs in promoting organ repair has not been well studied, but several lines of evidence point to the central importance of microvascular ECs in organ regeneration (Fig. 1). Thus, transplanted ECs by reconstruction of the bone marrow vascular niche augment hematopoietic reconstitution, amplifying the rate of organ repair. Similarly, transplantation of other organ-specific ECs will repair end-stage organ damage.
Model Systems to Study the Contribution of Tissue-Specific Endothelial Cells to Organ Regeneration
Our group and others have pioneered the concept that organotypic mature ECs are unique instructive niche cells that produce tissue-specific angiocrine growth factors, to sustain organ homeostasis and to directly induce regeneration by guiding and supporting the adult stem/progenitor cells after organ injury.
We originally found support for this idea using hematopoietic regeneration after irradiation or chemotherapy-induced myeloablation as a model (2, 9–5). We have since found that ECs supply stimulatory and antifibrotic angiocrine factors to support regeneration of other organs, including lung (3), liver (4, 6, 7) and testis (8–20). Herein, we elaborate on the technologies and approaches developed in our laboratory that define mechanisms by which ECs could adapt to a tissue-specific microenvironment and by elaboration of various inductive growth factors to promote organ repair.
BONE MARROW SINUSOIDAL VASCULAR NICHE CELLS BY DEPLOYMENT OF KIT-LIGAND AND NOTCH LIGANDS RECONSTITUTE HEMATOPOIESIS AFTER MYELOSUPPRESSION
During homeostasis, marrow sinusoidal ECs produce angiocrine factors, such as Kit-ligand, Notch ligands and IGFBP2 to sustain hematopoietic stem and progenitor cell (HSPC) self-renewal and differentiation (Fig. 2). As the bone marrow vascular niche regenerates after chemotherapy or irradiation, expression of angiogenic, vascular endothelial growth factor (VEGF-A) is induced in the marrow and this activates its’ tyrosine kinase receptor VEGFR2 on marrow ECs upregulating the expression of Notch-ligand Jagged-1. Conditional deletion of either VEGFR2 (10) or Jagged-1 (21) leads to stem cell exhaustion and death due to hematopoietic failure. Similarly, pleiotrophin and EGF, a heparin binding factor that is secreted by bone marrow sinusoidal ECs and is necessary for maintenance of the HSPC pool in vivo and for hematopoietic recovery and survival of mice after lethal dose irradiation (22–26).
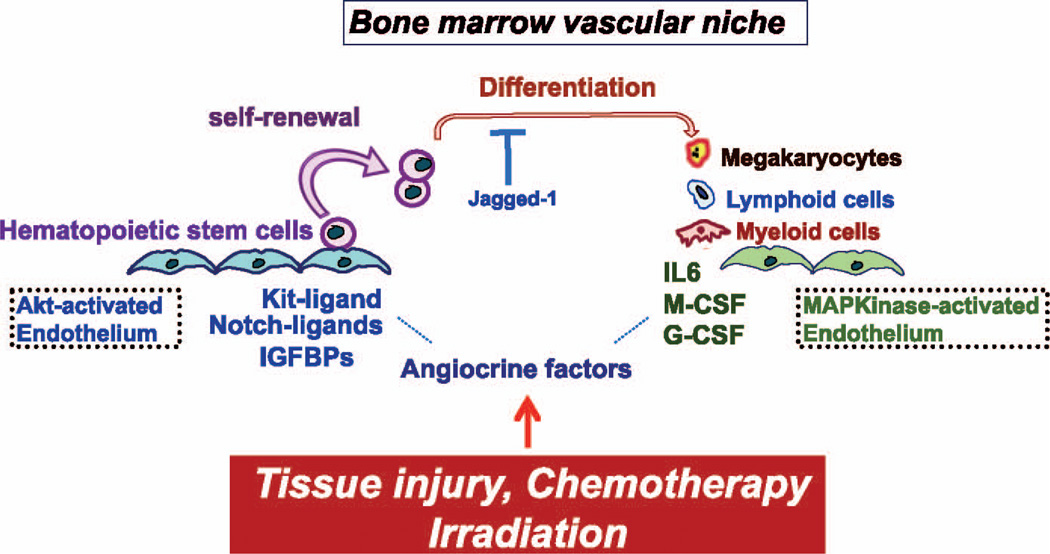
FIG. 2.
Differential activation of Akt and MAPKinase in the ECs balance the rate of self-renewal and differentiation of HSPCs. Under steady-state conditions, ECs maintain the homeostasis of the HSPCs. Irradiation or chemotherapy activates the Akt-mTOR pathway in ECs, which in turn induces angiocrine factors that support the expansion of HSPCs. Activation of the MAPkinase pathway in ECs promotes differentiation of the HSPCs. Therefore, the activation state of the ECs through manufacturing of defined stimulatory and inhibitory angiocrine factors balances proliferation and differentiation of HSPCs.
Furthermore, ECs produce a wide variety of other stimulatory and inhibitory cytokines that coordinate self-renewal and differentiation of HSPCs (9). Therefore, regeneration of the marrow vascular niche, potentially accelerated by transplantation of ECs, is critical for subsequent engraftment and reconstitution of HSPCs and hematopoiesis. A recently published study has shown that intravenous transplantation of ECs facilitates reconstitution of hematopoiesis after sublethal irradiation (27).
INTRAVENOUS TRANSPLANTATION OF PULMONARY CAPILLARY ENDOTHELIAL CELLS, RESTORES ALVEOLAR REGENERATION AFTER PNEUMONECTOMY
After left lung pneumonectomy, activation of VEGFR2 and FGFR1 signaling in pulmonary capillary ECs (PCECs) upregulates MMP14 and stimulates alveolar regeneration in the right lung. PCEC-derived MMP14 acts by cleaving HB-EGF and laminin5-γ2 chain to unmask the cryptic EGF-receptor-ligands driving proliferation of alveolar epithelial cells. In Vegfr2- and Fgfr1-deficient mice, lung regeneration is impaired due to diminished production of MMP14 by the PCECs (3). Remarkably, intravenous transplantation and engraftment of normal lung PCECs (that can produce MMP14), into the right lung restores alveolar regeneration in Vegfr2- and Fgfr1-deficient mice without inducing fibrosis. Transplanted liver ECs did not home and engraft into the lungs failing to promote alveolar repair.
STEPS NECESSARY TO ENABLE EC-MEDIATED ORGAN REPAIR
The major hurdles with transplantation of ECs for therapeutic organ repair after radiation-induced tissue injury are as follows (Fig. 3).
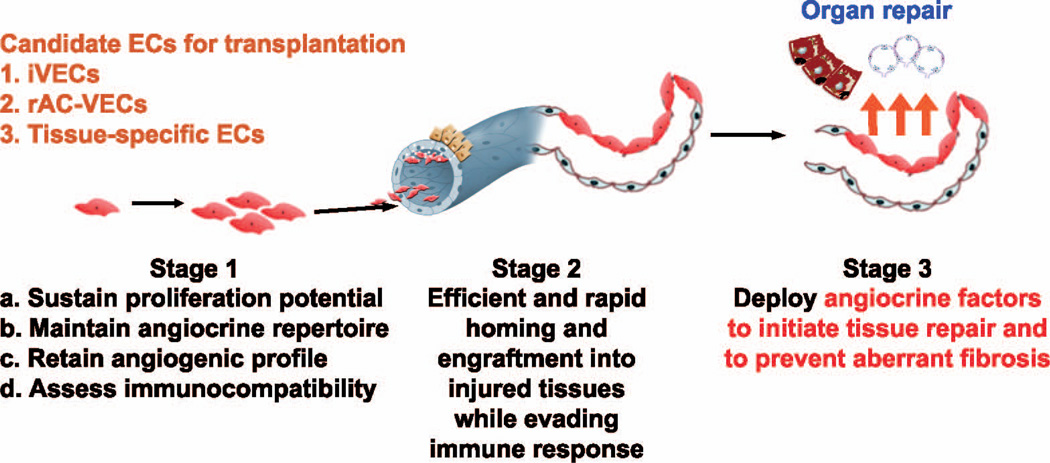
FIG. 3.
Sources of ECs for therapeutic EC transplantation. Mouse and human ECs, including iVECs, rAC-VECs 4 will be used to enable transplantation of ECs for organ repair. Experiments are proposed to optimize survival (stage 1) homing and engraftment (stage 2) and functional incorporation of ECs (stage 2) into injured organs without provoking fibrosis and immune response.
Survival, Expansion and Engraftment of Transplanted ECs
Expansion and maintenance of generic immunocompatible ECs requires constitutive stimulation with numerous angiogenic growth factors, including serum, VEGF-A, FGF-2, IGF, EGF and other chemical additives. As such, transplantation of the ECs into circulation, which is devoid of any of these prosurvival signals, results in rapid apoptosis and exhaustion of transplanted ECs.
Transplanted ECs have the difficult task of navigating through a vast pool of the circulatory volume and home specifically to the injured vascular bed. Upon landing in vessels of the injured organ, transplanted ECs need to properly engraft and deploy angiocrine growth factors to enhance the repair process without inducing fibrosis.
Requirements for Immunological Compatibility of the Allogeneic ECs for Proper Engraftment
As ECs express various human leukocyte antigens (HLAs), one expects that a high level of HLA mismatch might result in immune rejection of the transplanted ECs. Surprisingly, very few studies have rigorously interrogated the potential of HLA-mismatched ECs to mount immune response. Notably, several vascular beds, including heart and kidney, are spared from the wrath of immune rejection during graft-vs.-host disease, suggesting that ECs from different organs may behave differently in eliciting immune response. For certain EC types that might trigger uncontrolled immune reaction, it’s conceivable to employ cutting-edge technology such as genome editing to selectively modulate their immunogenic molecules, such as MHCII. To this end, guided editing of immunogenic determinant in transplanted ECs might potentially circumvent long-term rejection after EC transplantation.
Sources of Mouse and Human ECs for Transplantation
While purification of organ-specific ECs from mouse organs is feasible, it is technically difficult to cultivate abundant and stable autologous adult human tissue-specific ECs for transplantation. To circumvent this problem, we have developed new strategies to generate human ECs:
Generic ECs derived from pluripotent stem cells (PSCs)
We have developed a strategy to differentiate human and mouse PSCs into induced vascular endothelial cells (iVECs) (28). Incubation of PSCs with angiogenic growth factors concomitant with TGFβ inhibition allows for differentiation of PSCs into ECs that are mature, functional and stable. TGFβ inhibition is essential to prevent endothelial-to-mesenchymal transition. This strategy allows for the generation of autologous mouse and human generic ECs for organ repair.
However, there are major hurdles associated with the clinical use of iVECs: 1. iVECs are unstable and their proliferative potential is limited; and 2. iVECs may give rise to teratomas. While these issues need to be resolved, we have developed another pragmatic approach to generate an expandable and safe source of genetically diverse human ECs, which are reprogrammed from amniotic cells.
Transcriptional reprogramming of amniotic cells into vascular endothelial cells (rAC-VECs)
We have recently reported that human (as well as mouse) mid-gestation, lineage-committed, amniotic-derived epithelial and mesenchymal cells (ACs) can be efficiently reprogrammed into vascular endothelial cells (5). Transient expression of the ETS transcription factor ETV2 for two weeks and TGFβ-inhibition for 3 weeks along with constitutive co-expression with Fli1/ERG1 stably endowed rAC-VECs with a vascular transcriptome and morphology matching those of mature ECs (Fig. 4).
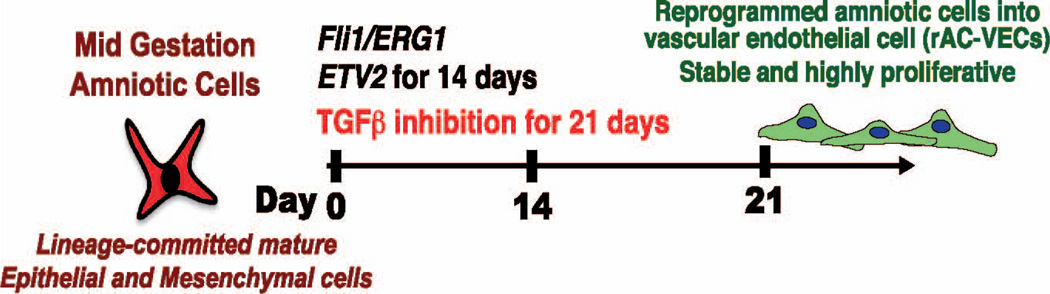
FIG. 4.
Reprogramming of amniotic cells into vascular endothelial cells (rAC-VECs). Transient expression of ETV2 and TGFβ concomitant with Fli1 and ERG1 generated abundant and stable rAC-VECs within 21 days, which will be available for intravenous transplantation.
Functionally, rAC-VECs form patent vasculature in Matrigel® plugs implanted in mice. Furthermore, transplanted rAC-VECs home and engraft into the sinusoidal vessels of the regenerating liver of mice that have undergone 70% partial hepatectomy, acquiring liver-specific vascular attributes.
Therefore, rAC-VECs generated from mice or human mid-gestation ACs represent an ideal “generic” ECs that are endowed with epigenetic plasticity undergoing tissue-specific education by the microenvironmental cues conveyed by the injured irradiated organs. Given that each year thousands of amniocenteses are performed world-wide, we expect sufficient numbers of HLA-typed rAC-VECs could be frozen and banked to be able to transplant a broad range of haploidentical individuals with organ injury.
TRANSPLANTING ENGINEERED ENDOTHELIAL CELLS TO STIMULATE REGENERATION AND BYPASS FIBROSIS
Endothelial cells are capable of regulating organ regeneration and fibrosis in divergent ways (7, 29). Coordinated stimulation of proregenerative angiocrine signaling in endothelial cells promotes organ regeneration and bypasses excessive scar formation/fibrosis. Upon hepatic and pulmonary injury, activation of chemokine receptor CXCR7 signaling in endothelial cells is essential for synchronized production of stem cell-active factors such as metalloprotease 14 (MMP14), hepatocyte growth factor (HGF) and Wnt ligands (7). Moreover, pharmacological activation of CXCR7 in endothelial cells after tissue damage prevents aberrant expression of profibrotic factors, ameliorating fibrosis. Thus, selectively stimulating proregenerative angiocrine signaling such as CXCR7 in endothelial cells can generate transplantable cells with preferential proregenerative and antifibrotic capacity. To this end, transplanting these specialized endothelial cells in the injured organs might create a proregenerative milieu, inducing regeneration and bypassing fibrosis.
CONCLUSIONS AND FUTURE DIRECTIONS
Exposure to high doses of radiation have catastrophic short- and long-term health consequences and a major economic impact. Current approaches to mitigate the effects of radiation are primarily focused on delivery of small-molecule compounds that perform as chelating agents, antioxidants and growth factors. Cellular therapy by transplantation of autologous or allogeneic hematopoietic cells is the mainstay of therapy to mitigate injury inflicted by high-dose irradiation.
Indeed, cellular therapy approaches to mitigate radiation injury are primarily driven by optimizing the efficacy of infusion of allogeneic HSPCs as “off-the-shelf products” to reconstitute immune response, platelets and red blood cells. However, these approaches most likely will result in only transient improvement in the recovery of the blood cells. In addition, depending on the severity of the radiation-induced damage to the bone marrow microenvironment, it is unlikely that transplanted hematopoietic cells will engraft and support long-term blood production.
Nevertheless, even if these approaches are successful in reconstituting hematopoietic system, radiation exposure to other organs, including gastrointestinal, skin, lung and liver, will lead to significant morbidity and mortality. Indeed, diffuse damage to vital organs need to be reversed and repair pathways need to be activated to foster organ repair and regeneration. However, high-dose radiation causes such severe injury to the infrastructure of the vasculature that drives organ repair, that it is unlikely that infusion of growth factors or transplantation of short-lived hematopoietic progenitors could repair injured organs.
To circumvent these hurdles, we have designed and plan to improve on highly innovative and transformative approaches to reconstitute the vasculature within every organ, which will initiate organ repair by three key mechanisms:
Transplantation and engraftment of ECs into various irradiated organs will initiate immediate repair by providing organ-specific angiocrine growth factors. Importantly, incorporation of ECs into irradiated hematopoietic organs, including bone marrow and spleen, will reconstruct the damaged microenvironment of the hematopoietic organs thereby facilitating engraftment of HSPCs.
Engraftment of the ECs will also enable formation of new blood vessels that will deliver oxygen and nutrients as well as balance the metabolism of the hypoxic injured organs.
Repair of organs by EC transplantation will also prevent radiation-induced fibrosis and scarring, thereby preventing tumorigenesis.
Collectively, if our approach of EC transplantation is successful, we expect a significant decrease in the morbidity and mortality associated with exposure to high-dose radiation. In this regard, our strategy will benefit the health and well being of civilian and military populations potentially exposed to lethal doses of radiation.
While manufacturing of clinical-grade ECs may be costlier than the delivery of pharmaceutical agents to protect against radiation injury, the short- and long-term clinical benefits afforded by the EC transplantation to accelerate organ repair will significantly decrease the morbidity and mortality associated with radiation exposure.
In fact, we anticipate that in the long run development of the EC transplantation technology will save more lives, increase quality of life and improve rehabilitation as well as prevent tumorigenesis. Therefore, when compared to other approaches, the EC transplantation platform becomes more economically appealing in mitigating radiation-induced injury, benefiting a large portion of civilian and military populations. Nevertheless, additional experimentation is necessary to enable such approaches.
REFERENCES
- 1.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 8.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S, et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 13.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafii S, Cao Z, Lis R, Siempos II, Chavez D, Shido K, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seandel M, Falciatori I, Shmelkov SV, Kim J, James D, Rafii S. Niche players: spermatogonial progenitors marked by GPR125. Cell Cycle. 2008;7:135–140. doi: 10.4161/cc.7.2.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Seandel M, Falciatori I, Wen D, Rafii S, et al. CD34+ testicular stromal cells support long-term expansion of embryonic and adult stem and progenitor cells. Stem Cells. 2008;26:2516–2522. doi: 10.1634/stemcells.2008-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himburg HA, Harris JR, Ito T, Daher P, Russell JL, Quarmyne M, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Himburg HA, Yan X, Doan PL, Quarmyne M, Micewicz E, McBride W, et al. Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J Clin Invest. 2014;124:4753–4758. doi: 10.1172/JCI76838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doan PL, Russell JL, Himburg HA, Helms K, Harris JR, Lucas J, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31:327–337. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulos MG, Crowley MJ, Gutkin MC, Ramalingam P, Schachterle W, Thomas JL, et al. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem cell reports. 2015;5(10):881–894. doi: 10.1016/j.stemcr.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]