Abstract
Small RNAs play an important role in plant immune responses. However, their regulatory function in induced systemic resistance (ISR) is nascent. Bacillus cereus AR156 is a plant growth-promoting rhizobacterium that induces ISR in Arabidopsis against bacterial infection. Here, by comparing small RNA profiles of Pseudomonas syringae pv. tomato (Pst) DC3000-infected Arabidopsis with and without AR156 pretreatment, we identified a group of Arabidopsis microRNAs (miRNAs) that are differentially regulated by AR156 pretreatment. miR825 and miR825* are two miRNA generated from a single miRNA gene. Northern blot analysis indicated that they were significantly downregulated in Pst DC3000-infected plants pretreated with AR156, in contrast to the plants without AR156 pretreatment.miR825 targets two ubiquitin-protein ligases, while miR825* targets toll-interleukin-like receptor (TIR)-nucleotide binding site (NBS) and leucine-rich repeat (LRR) type resistance (R) genes. The expression of these target genes negatively correlated with the expression of miR825 and miR825*. Moreover, transgenic plants showing reduced expression of miR825 and miR825* displayed enhanced resistance to Pst DC3000 infection, whereas transgenic plants overexpressing miR825 and miR825* were more susceptible. Taken together, our data indicates that Bacillus cereus AR156 pretreatment primes ISR to Pst infection by suppressing miR825 and miR825* and activating the defense related genes they targeted.
Keywords: Induced systemic resistance, ISR, microRNA, plant innate immunity, small RNA
INTRODUCTION
To protect from pathogen infections, plants have evolved multiple types of immune response mechanisms (Chisholm et al. 2006; Jones and Dangl 2006). The first type is initiated by the recognition of conserved pathogen-associated molecular patterns (PAMPs), such as flagellin, lipopolysaccharides, glycoproteins and chitin, by Pattern-Recognition Receptors (PRRs) located on the cell membrane (Dangl and Jones 2001). Perception of PAMP triggers PAMP-triggered immunity (PTI), including MAPK (mitogen-activated protein kinase) activation, oxidative burst, callose deposition, induction of defense-related genes, and accumulation of antimicrobial compounds (Altenbach and Robatzek 2007; Schwessinger and Zipfel 2008; Niu et al. 2015). Successful pathogens can suppress PTI by secreting effectors, such as proteins and small RNAs, into the host cells to suppress host PTI (Speth et al. 2007; Padmanabhan et al. 2009; Katiyar-Agarwal and Jin 2010; Weiberg et al. 2013). In turn, plants have also evolved resistance (R) proteins to recognize specific pathogen effectors, resulting in effector-triggered immunity (ETI). ETI is much more rapid and robust than PTI, and triggers a very similar set of defense responses as in PTI but in an accelerated and potentiated manner (Chisholm et al. 2006; Jones and Dangl 2006; Göhre and Robatzek 2008; Niu et al. 2015).
The onset of PTI or ETI from the infected loci often triggers an induced resistance in distal tissues that confers resistance against an abroad spectrum of pathogens (Pieterse et al. 2014). This systemic acquired resistance (SAR) is often associated with increased level of salicylic acid (SA) and coordinate activation of pathogenesis related (PR) genes, and involves one or more long-distance signals that propagate an enhanced defensive capacity in still undamaged plant parts (Fu and Dong 2013). Systemic resistance can also be induced by beneficial microbes that are normally associated with plant roots, which is called induced systemic resistance (ISR). In many cases, ISR is SA-independent and develops without accumulation of PR proteins. This is evidenced that in NahG, a SA deficient Arabidopsis mutant, P. fluorescens is still able to induce ISR that does not coincide with enhanced SA levels (Pieterse et al. 1996). There are a few exceptions where identified ISR occurs in a SA-dependent manner. For example, Pseudomonas aeruginosa 7NSK2 confers enhanced disease resistance in wild-type bean and tomato but not in SA-non accumulating NahG tomato (Audenaert et al. 2002; Kachroo and Robin 2013). We have shown in a previous study that Bacillus cereus strain AR156 can induce ISR by using both the SA and jasmonic acid (JA) signaling pathways. When AR156-pretreated Arabidopsis was challenged with Pst DC3000, noticeable ISR was observed that was accompanied with concurrently expressed defense-related genes such as PR1, PR2, PR5, and PDF1.2, which are markers of the SA and JA/ethylene (ET) signaling pathways, respectively. This experiment clearly demonstrated that in this AR156-induced ISR, the SA- and JA/ET-dependent signaling pathways were simultaneously activated (Niu et al. 2011). In a SA-independent ISR scenario, JA and ET are central players in the regulation of systemic immunity conferred by beneficial soil borne microbes (De Vleesschauwer et al. 2008; Hase et al. 2008). NONEXPRESSOR OF PR GENES1 (NPR1) is a common regulator of both SAR and ISR since it is required for both resistances (Fu and Dong 2013). However, the roles that NPR1 play in the two resistances seem to be different: NPR1 functions as a transcriptional co-activator in SAR that activate SA-responsive PR gene, whereas in ISR it functions in a PR-independent pathway (Ramirez et al. 2010; Pieterse et al. 2012).
Small RNAs are key regulators of plant defense responses against pathogens (Padmanabhan et al. 2009; Katiyar-Agarwal and Jin 2010; Duan et al. 2012; Pumplin and Voinnet 2013; Seo et al. 2013). The first identified miRNA involved in plant innate immunity is miR393, which is induced by a PAMP (flg22) and contributes to PTI by suppressing Auxin receptors (Navarro et al. 2006). Katiyar-Agarwal and colleagues identified the first endogenous small interfering RNA (siRNA), nat-siRNAATGB2, which is specifically induced by Pst DC3000 carrying an avrRpt2 effector, and positively regulates R gene RPS2-mediated ETI responses (Katiyar-Agarwal et al. 2006). Many miRNAs and siRNAs have been found to be involved in plant defense responses. Examples of such regulatory small RNAs include miR158 and miR168, which are involved in antiviral defenses (Zhang et al. 2006), miR160, miR398b, miR393b*, and long siRNA-1, which are involved in bacterial pathogen defense (Katiyar-Agarwal et al. 2007; Li et al. 2010; Zhang et al. 2011), and miR1536 and miR1917 in cotton root against Verticillium dahlia, a fungal plant pathogen (Yin et al. 2012).
It has been demonstrated in recent studies that miRNAs are able to regulate R genes (Zhai et al. 2011; Li et al. 2012; Shivaprasad et al. 2012). In particular, tomato miR482 and miR2118 can target the coding sequence of some R genes with NBS-LRR motifs. Being targeted by these 22-nt miRNAs, these mRNAs are degraded and subsequently generate arrays of secondary siRNAs (2nd siRNAs) in a RNA-dependent RNA polymerase 6 (RDR6)-dependent manner. Consequently, one of these 2nd siRNAs can target mRNAs of a defense-related protein. This defense mechanism is activated upon viruses or bacteria infection, by which the expression of miR482 or miR2118 is suppressed. Similarly, tobacco miR6019 targets tobacco mosaic virus (TMV) resistance gene N and triggers RDR6- and Dicer-like 4 (DCL4)-dependent 2nd siRNA formation (Li et al. 2012). In Medicago, three highly abundant 22-nt miRNAs target the conserved domains of at least 114 defense-related NB-LRR-encoding genes to induce production of 2nd siRNAs at these loci (Zhai et al. 2011). In Arabidopsis, miR472 represses both basal resistance and RPS5-mediated ETI through targeting some coiled-coil (CC)-NBS-LRR genes (Boccara et al. 2014). Collectively, these miRNAs and 2nd siRNAs function as negative regulators of plant innate immunity that maintain plant defense responses to minimum when there is no pathogen infection. Upon pathogen infection, they are downregulated and subsequently activate the defense responses by releasing the suppression of R genes (Ruiz-Ferrer and Voinnet 2009; Seo et al. 2013).
Despite the importance in plant innate immunity, the role of small RNAs in rhizobacteria-induced ISR has hardly been explored. To identify and characterize plant small RNAs that regulate systemic immune responses induced by Bacillus cereus AR156, we sequenced small RNA species by using Illumina Hi-Seq deep sequencing methodology and found that miR825 and miR825* were clearly differentially regulated by AR156 treatment. Upon Pst DC3000 infection, the expression of miR825 and miR825* was more strongly reduced in plants pretreated with AR156 than that in plants without pretreatment. We found that transgenic plants overexpressing miR825 and miR825* were more susceptible when inoculated with Pst DC3000. In contrast, the miR825 and miR825* knockdown lines displayed enhanced resistance to Pst DC3000. We further demonstrated that miR825* modulates ISR by initiating 2nd siRNAs at the At5G38850 locus, which is an R gene targeted by miR825*. Taken together, our data demonstrate that miR825 and miR825* play an important role in modulating ISR by negatively regulating a group of resistance-related genes.
RESULTS
The expression of a group of miRNAs is differentially regulated in AR156-induced ISR in Arabidopsis
To identify small RNAs involved in AR156-induced ISR, we set up a system using Arabidopsis and a classic bacterial pathogen, Pst DC3000. By using this system, we were able to show that root pretreatment with AR156 could effectively protect systemic leaves against Pst DC3000 infection. Three days after infection, bacteria propagated significantly more in plants without pretreatment than with AR156-pretreatment (Figure 1A). In order to test whether miRNAs contributed to AR156-induced ISR, we profiled and analyzed small RNA expressions by using Pst DC3000-infected Arabidopsis plants with and withoutAR156-pretreatment. Four types of small RNA libraries were constructed: Arabidopsis leaves from plants pretreated with or without AR156 for 7 days but without Pst DC3000 inoculation (named as AR156/none or control/none, respectively henceforth); and leaves from plants with AR156 or control pretreatment followed by Pst DC3000 infection for 14 h (AR156/Pst or control/Pst henceforth). Our hypothesis is that if a miRNA is involved in ISR, then its expression may show a regulated pattern during the priming process or upon Pst DC3000 infection. According to this speculation, we compared the miRNA accumulation patterns between plants with control- or AR156-treatment only (control/none vs AR156/none), and between plants with either control- orAR156-pretreatment and the following Pst DC3000 infection (control/Pst vs AR156/Pst) (Table S1). Extra attention was paid on the miRNAs with significant change (at least over 3-fold induction or reduction on normalized read number) between the compared libraries, especially on the miRNAs that may target genes with defense-related functions (Table 1).
Figure 1. AR156 pretreatment induces ISR to Pst DC3000 in Col-0 plants.
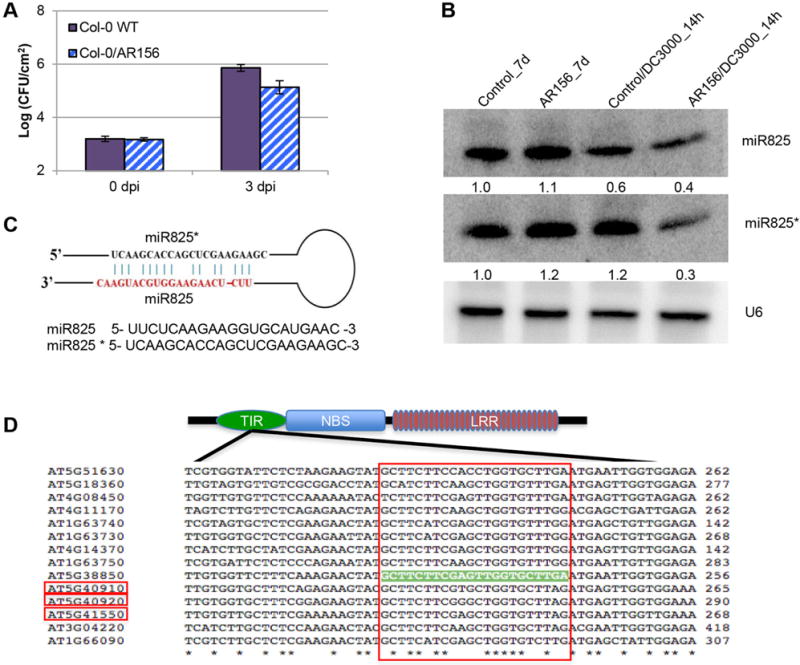
(A) Induction of systemic resistance to Pst DC3000 in Arabidopsis Col-0 plants by Bacillus cereus AR156. Plants are treated with AR156 at 5 × 107 CFU/ml and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3 days after inoculation (dpi). (B) Expression of miR825 and miR825* in different treatments is examined by Northern blotting. Plants pre-treated with AR156 at 5 × 107 CFU/ml and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/ml concentration. Total RNA is extracted from leaves with line numbers and time points indicated. RNA blots are hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 is used as a loading control. (C) Predicted secondary stem loop structure for miR825 and miR825* with mature miRNA sequences indicated. (D) Nucleotide sequences targeted by miR825* are aligned to corresponding genes, with the TIR-NBS-LRR gene structure depicted at the top. TIR: the toll-interleukin-like receptor domain; NBS: the nucleotide binding site domain; LRR: the leucine-rich repeat domain. Red boxes on gene IDs represent these genes that are validated by using real-time PCR in this study. The big red box shows the predicted target region, with the target site on AT5G38850 highlighted in light green. “*” represents conserved nucleotide.
Table 1.
Identified miRNAs with significant differential expression
| ID | Sequence | Control/DC3000_14h | AR156/DC3000_14h | Fold change: AR156/DC3000_14h Vs Control/DC3000_14h | Control_7d | AR156_7d | Fold change: AR156_7d Vs Control_7d |
|---|---|---|---|---|---|---|---|
| ath-miR848 | UGACAUGGGACUGCCUAAGCUA | 374.48 | 1.03 | 0.003 | 120.48 | 165.66 | 1.38 |
| ath-miR773 | UUUGCUUCCAGCUUUUGUCUC | 67.46 | 1.03 | 0.02 | 83.47 | 0.81 | 0.01 |
| ath-miR5629 | UUAGGGUAGUUAACGGAAGUUA | 44.98 | 1.03 | 0.02 | 72.29 | 123.43 | 1.71 |
| ath-miR864-5p | UCAGGUAUGAUUGACUUCAAA | 132.97 | 3.10 | 0.02 | 96.38 | 114.50 | 1.19 |
| ath-miR863-3p | UUGAGAGCAACAAGACAUAAU | 796.86 | 79.52 | 0.10 | 440.60 | 166.47 | 0.38 |
| ath-miR825* | UCAAGCACCAGCUCGAAGAAGC | 341.23 | 76.43 | 0.22 | 137.52 | 136.83 | 1.00 |
| ath-miR847 | UCACUCCUCUUCUUCUUGAUG | 1,282.80 | 300.54 | 0.23 | 525.79 | 36.54 | 0.07 |
| ath-miR395 | CUGAAGUGUUUGGGGGAACUC | 736.24 | 179.70 | 0.24 | 132.52 | 142.11 | 1.07 |
| ath-miR825 | UUCUCAAGAAGGUGCAUGAAC | 159.53 | 42.84 | 0.27 | 681.89 | 1,120.62 | 1.64 |
| ath-miR844* | UUAUAAGCCAUCUUACUAGUU | 123.20 | 373.87 | 3.03 | 753.83 | 119.37 | 0.16 |
| ath-miR160 | UGCCUGGCUCCCUGUAUGCCA | 3,833.75 | 12,828.16 | 3.35 | 3,813.05 | 1,461.72 | 0.38 |
| ath-miR159c | UUUGGAUUGAAGGGAGCUCCU | 1,419.69 | 4,764.22 | 3.36 | 9,000.39 | 3,344.89 | 0.37 |
| ath-miR165 | GGAAUGUUGUCUGGAUCGAGG | 4,489.82 | 16,407.78 | 3.65 | 22,617.57 | 7,995.59 | 0.35 |
| ath-miR865-5p | AUGAAUUUGGAUCUAAUUGAG | 28.35 | 110.51 | 3.90 | 112.73 | 41.42 | 0.37 |
| ath-miR844 | UGGUAAGAUUGCUUAUAAGCU | 63.55 | 253.03 | 3.98 | 1,160.01 | 73.09 | 0.06 |
| ath-miR319c | UUGGACUGAAGGGAGCUCCUU | 2,072.83 | 8,883.98 | 4.29 | 6,625.30 | 664.27 | 0.10 |
| ath-miR858b | UUCGUUGUCUGUUCGACCUUG | 114.40 | 588.68 | 5.15 | 212.55 | 245.24 | 1.15 |
| ath-miR158a | UCCCAAAUGUAGACAAAGCA | 99,445.74 | 593,309.50 | 5.97 | 349,637.34 | 29,318.78 | 0.08 |
| ath-miR775 | UUCGAUGUCUAGCAGUGCCA | 275.72 | 1,721.64 | 6.24 | 1,149.68 | 52.78 | 0.05 |
| ath-miR398b | UGUGUUCUCAGGUCACCCCUG | 751.89 | 5,138.08 | 6.83 | 24,408.35 | 8,167.74 | 0.33 |
| ath-miR172b* | GCAGCACCAUUAAGAUUCAC | 120.26 | 853.08 | 7.09 | 3,419.79 | 993.15 | 0.29 |
| ath-miR158b | CCCCAAAUGUAGACAAAGCA | 367.63 | 2,996.10 | 8.15 | 1,721.94 | 64.97 | 0.04 |
| ath-miR5654 | AUAAAUCCCAACAUCUUCCA | 57.69 | 550.47 | 9.54 | 703.06 | 35.73 | 0.05 |
| ath-miR319 | UUGGACUGAAGGGAGCUCCCU | 863.35 | 10,989.81 | 12.73 | 19,203.81 | 1,313.11 | 0.07 |
| ath-miR779.2 | UGAUUGGAAAUUUCGUUGACU | 19.55 | 269.56 | 13.78 | 210.83 | 41.42 | 0.20 |
| ath-miR858 | UUUCGUUGUCUGUUCGACCUU | 46.93 | 705.39 | 15.03 | 229.76 | 488.86 | 2.13 |
| ath-miR393b | UCCAAAGGGAUCGCAUUGAUCC | 327.55 | 6,807.05 | 20.78 | 15,241.88 | 283.41 | 0.02 |
| ath-miR398a | UGUGUUCUCAGGUCACCCCUU | 32.27 | 803.50 | 24.90 | 2,147.05 | 687.01 | 0.32 |
| ath-miR394b | UUGGCAUUCUGUCCACCUCC | 19.55 | 1,422.14 | 72.73 | 427.69 | 57.66 | 0.13 |
miRNAs with more than 3-fold variation (both induction and reduction) are listed. Reads are normalized to library size and presented in read per million (RPM). miR825 and miR825* are highlighted in red. Note: miRNAs with more than 3-fold variation (both induction and reduction) are listed. Reads are normalized to library size and presented in read per million (RPM).
We found that 29 miRNAs that were induced or repressed for at least 3 folds, i.e., ≥3 or ≤0.33, respectively, between AR156 (AR156/Pst) and control treatments (control/Pst) when both were challenged by Pst DC3000 (Table 1). Seven of these 29 miRNAs (miR395, miR5629, miR848, miR858b, miR864-5p, as well as miR825 and miR825* from the miR825 precursor) were differentially expressed between control/Pst and AR156/Pst, but not between control/none and AR156/none (at least 3 folds) (Table 1).
We further predicted the putative targets of each miRNA by using bioinformatics approach (Table S2), out of which miR825* was the most outstanding miRNA that might play a regulatory role in plant innate immunity. miR825* was downregulated by Pst DC3000 infection, which was strictly dependent on AR156 pre-treatment: there was no obvious expression difference between the AR156/none and the control/none samples, whereas significant downregulation was observed in the AR156/Pst samples in reference to the control/Pst samples (Figure 1B; Table 1). Sequence alignment suggested that miR825* might target a region encoding the TIR domain a group of R proteins, implying a potential role in innate immunity (Figure 1D). miR825* can be readily detected by Northern blot analysis (Figure 1B), making it a great candidate for function study. Equally outstanding is miR773, which has been reported as a negative PTI regulator against bacterial pathogens (Li et al. 2010). miR773 was 50-times downregulated under Pst DC3000 infection (Table 1). Nevertheless, we didn’t pursue this gene further due to its very weak expression (Figure S1). We were also interested in investigating what roles miR825 might play in ISR, which was originated from the same precursor molecule as miR825* (Figure 1C). miR825 showed no obvious expressional variation between the AR156/none and the control/none samples, which is similar to miR825*. However, its expression was slightly downregulated in the control/Pst plants but significantly downregulated in the AR156/Pst plants (Figure 1B; Table 1). miR825 targets a RING/U-box superfamily protein and a F-box/RNI-like superfamily protein (Table 2). Some RING/U-box proteins are well-characterized E3 ubiquitin ligase that directly catalyze the ubiquitin transfer from the E2 ubiquitin conjugating enzyme to substrate proteins (Wang and Deng 2011). An F-box/RNI-like superfamily protein is normally associated with ubiquitin-mediated protein degradation. All the putative targets of miR825 and miR825* are likely to be involved in plant defense signaling pathways. Hence, our function study of the roles miRNAs playing in ISR focused mostly on miR825 and miR825*.
Table 2.
miR825 and miR825* target predication
| miRNAs | Target gene | Symbols | Score | Alignment |
|---|---|---|---|---|
| 825* | AT5G38850 | Disease resistance protein (TIR-NBS-LRR class) | 1 |
|
| AT4G14370 | Disease resistance protein (TIR-NBS-LRR class) family | 1.5 |
|
|
| AT5G40920 | pseudogene, disease resistance protein (TIR-NBS-LRR class) | 2.5 |
|
|
| AT1G63750 | Disease resistance protein (TIR-NBS-LRR class) family | 2.5 |
|
|
| AT4G11170 | Disease resistance protein (TIR-NBS-LRR class) family | 2.5 |
|
|
| AT1G63740 | Disease resistance protein (TIR-NBS-LRR class) family | 2.5 |
|
|
| AT1G63730 | Disease resistance protein (TIR-NBS-LRR class) family | 2.5 |
|
|
| AT4G08450 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| AT5G41550 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| AT5G51630 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| AT5G40910 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| AT3G04220 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| AT5G40060 | Disease resistance protein (NBS-LRR class) family | 3 |
|
|
| AT1G66090 | Disease resistance protein (TIR-NBS class) | 3 |
|
|
| AT5G18360 | Disease resistance protein (TIR-NBS-LRR class) family | 3 |
|
|
| 825 | AT5G55970 | RING/U-box superfamily protein | 4.5 |
|
| AT5G44940 | F-box/RNI-like superfamily protein | 4.5 |
|
Prediction targets of miR825 and miR825* are listed, with gene ID, predicted function, and base-paring patterns shown. Real-time PCR validated targets of miR825 and miR825* are highlighted in red. A “:” represents a perfect match; a “.” represents a wobble (G: U pairs); a “·”(a space) represents an imperfect match. Note: Prediction targets of miR825 and miR825* are listed, with gene ID, predicted function, and base-paring patterns shown. A “:” represents a perfect match; a “.” represents a wobble (G: U pairs); a “·”(a space) represents an imperfect match.
Transgenic Arabidopsis that knock down or overexpress miR825/miR825* alter the defense responses
To investigate the function of miR825/825*, we generated transgenic plants that knockdown or overexpress miR825 and 825* for ISR test. We hypothesized that if these miRNAs were modulators of ISR, we should be able to observe altered phenotype on induced defense responses, when the function of these two miRNAs was blocked or enhanced. We successfully knocked down miR825 and miR825* function by using the short tandem target mimic strategy (STTM) (Yan et al. 2012). In STTM transgenic plants, the detectable expression abundance of miR825/825* was significantly lower than that in wild type plants (Col-0) (Figure 2A). The transgenic plants had smaller stature compared to the wild type plant (Figure 2B). Consistent with the blocked miR825 and miR825* function, the target genes (At5G44940 for miR825, as well as At5G38850, At3G04220 and At5G40910 for miR825*) were upregulated in the STTM825/825* transgenic plants (Figure 2C). The other predicted targets such as AT5G55970 of miR825 and AT1G66090 and AT5G40060 of miR825* were not significantly influenced (data not shown). Because miRNAs can regulate target genes by mRNA cleavage, degradation, or translational inhibition, whether other putative target genes are genuine targets of miR825/825* but are regulated at translational inhibition level, or they are not direct targets of miR825/miR825*, needs further verification. Upon infection of Pst DC3000, the miR825/825* knockdown plants behaved similarly to the AR156 pretreated plants and displayed enhanced resistance as compared with wild type plants, indicating that reduced expression level of miR825/825* could mimic ISR phenotype primed by AR156 and display enhanced resistance overall (Figure 2D).
Figure 2. Knocking down R825/825* enhances resistance to Pst DC3000.
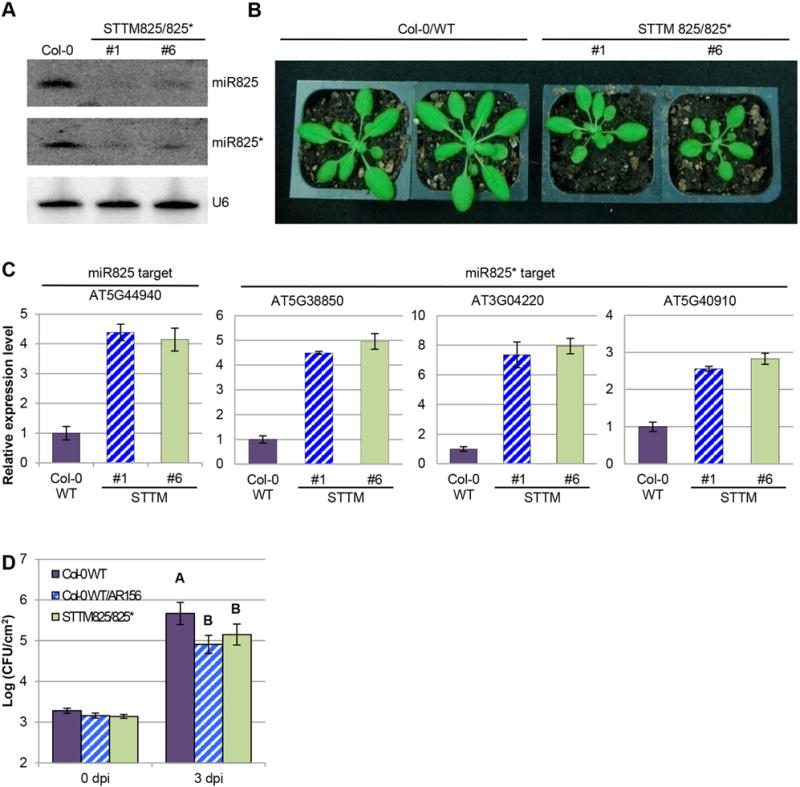
(A) Expression of miR825 and miR825* in STTM transgenic plants is examined by Northern blotting assay. RNA blots are hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 is used as a loading control. (B) Phenotype of STTM825/825* transgenic plants (line 1 and 6) are compared to Col-0 WT plants. Pictures are taken 4 weeks after germination. (C) miR825 and miR825* target genes in the indicated transgenic lines is examined by qRT-PCR assay. Target genes and line numbers (#1 and #6) are as indicated. (D) AR156-mediated ISR in Col-0 and transgenic lines. Plants pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3 days after inoculation (dpi). Error bars represent standard deviation for at least 12 leaf discs. Statistical difference among samples is represented by using letters: different letters (A and B) represent significant difference (P < 0.01) while same letters represent insignificant differences (P > 0.01).
We also generated transgenic plants overexpressing the miR825 and miR825* genes driven by the constitutive CaMV 35S promoter. The overexpression lines (OE) had no obvious phenotypes in term of development (Figure 3B). Two independent transgenic lines (#50 and #56) with high expression levels of both miR825 and miR825* were selected for further analysis (Figure 3A). Consistently, the expressions of the four target genes were reduced in reference to the control lines (Figure 3C). As expected, these transgenic lines were more susceptible to Pst DC3000 infection (Figure 3D). These results further support the negative regulatory role of miR825 and miR825* in ISR.
Figure 3. miR825/825* overexpression plants are more susceptible to Pst DC3000.
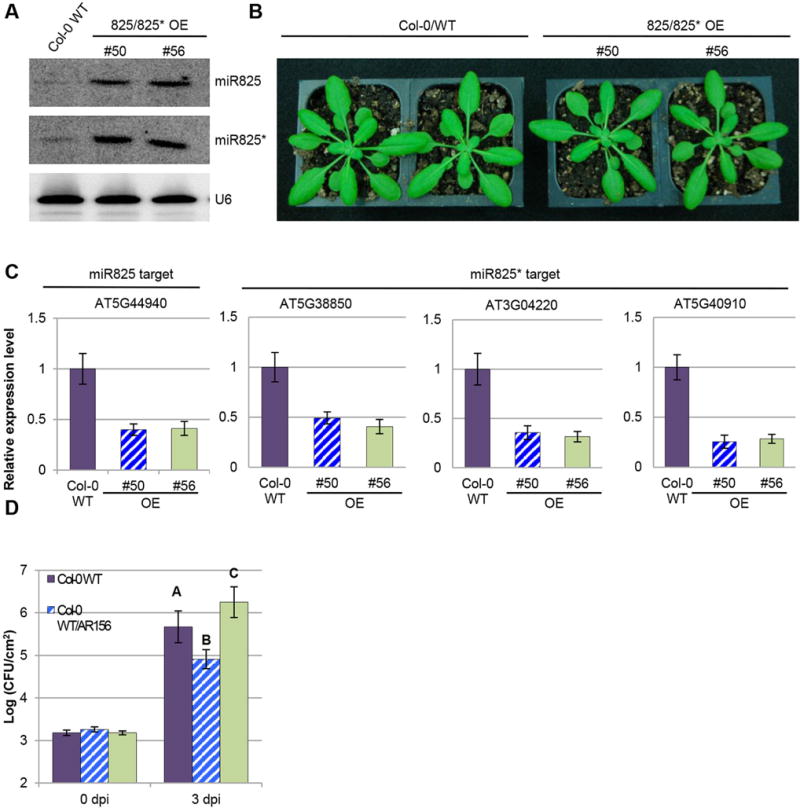
(A) Expression of miR825 and miR825* in overexpression transgenic plants are examined by Northern blotting assay. RNA blots are hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 is used as a loading control. (B) Phenotype of transgenic plants overexpressing miR825/825* (line 50 and 56) are compared to Col-0 WT plants. Pictures are taken 4 weeks after germination. (C) Expression of miR825 and miR825* target genes in the indicated transgenic lines is examined by qRT-PCR assay. Target genes and line numbers (#50 and #56) are as indicated. (D) AR156-mediated ISR in Col-0 and transgenic lines. Plants pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3 days after inoculation (dpi). Error bars represent standard deviation for at least 12 leaf discs. Statistical difference among samples is represented by using letters: different letters (A, B, and C) represent significant difference (P < 0.01) while same letters represent insignificant differences (P > 0.01).
We further asked whether miR825/825* might modulate ISR through regulating their putative targets. For this purpose, we examined the in vivo expression abundances of these miR825/825* targets in wild type plants using qRT-PCR. We anticipated seeing opposite expression patterns between these targets and their cognate miRNAs in plant with ISR. Our results showed that At5G38850, At3G04220 and At5G40910 (target genes of the miR825*) and At5G44940 (a target gene of the miR825) were indeed upregulated in plants with both AR156 pretreatment and Pst DC3000 inoculation. In contrast, in plants with AR156 pretreatment but not Pst DC3000 inoculation, or plants with Pst DC3000 inoculation but without AR156 pretreatment, the induction of these genes were negligible (Figure 4). These results, together with the results from transgenic plants, indicated that these genes were the direct targets of miR825 and miR825*, and were involved in the modulation of AR156-primed ISR against Pst DC3000.
Figure 4. miR825/825* target genes is coordinately expressed with AR156-indued ISR.
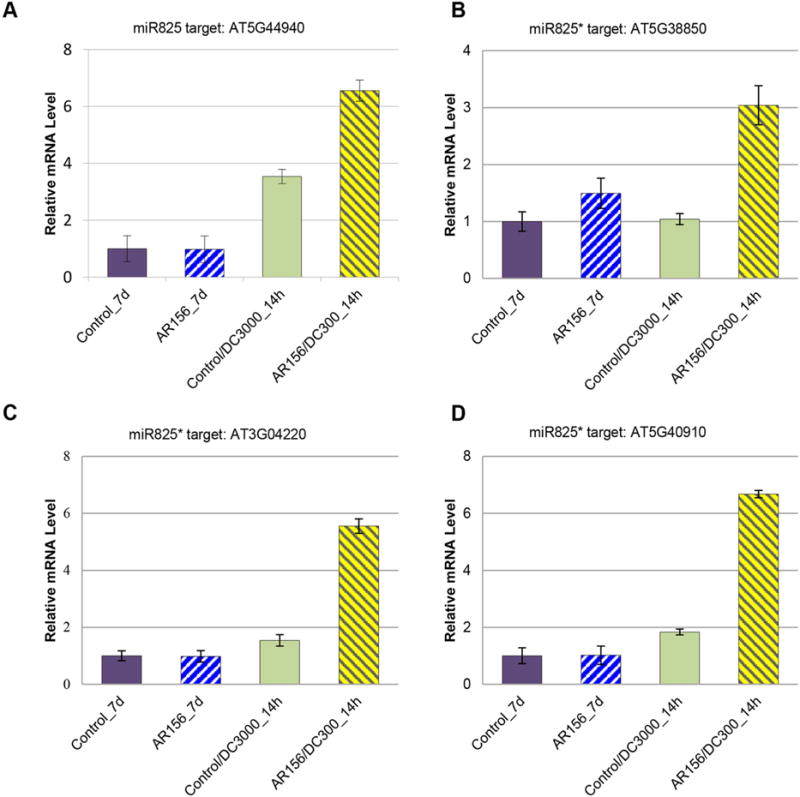
(A) Expression profiles of AT5G44940 (a miR825 target) and AT5G38850, AT3G04220 and AT5G40910 (miR825 targets). (B–D) in plants with AR156-induced ISR are measured. Plants are pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. Total RNA is extracted from leaves with line numbers and time points indicated. Expression of miR825/825* target gene is examined by qRT-PCR: Error bars indicate standard deviation from three technical replicates. The experiments are repeated two times with similar results.
miR825* exerts its regulatory function in ISR by triggering secondary siRNAs
miR825* is a 22-nt small RNA that can potentially initiate production of 2nd siRNAs, which in turn target transcripts by sequence complementarity (Zhai et al. 2011; Li et al. 2012; Shivaprasad et al. 2012). It has been reported that 22-nt miRNAs in tobacco, tomato and potato can trigger a secondary siRNA processing cascade specifically targeting R genes upon viral or bacterial pathogen infection (Zhai et al. 2011; Li et al. 2012; Shivaprasad et al. 2012). These reports also indicate that RDR6 and DCL4 are necessary components for the biogenesis of 2nd siRNAs. RDR6 has also been implicated in modulating both PTI and ETI through post-transcriptional gene silencing (Katiyar-Agarwal et al. 2006; Boccara et al. 2014). Thus, we examined the ability of AR156-induced ISR against Pst DC3000 in Arabidopsis rdr6 mutant.
As shown in Figure 5A, without AR156 pretreatment, the rdr6 mutant exhibited a significantly increased resistance relative to its corresponding wild-type (Col-0) plants, in agreement with previous study (Boccara et al. 2014). After Pst DC3000 infection, the rdr6 mutant with AR156 pretreatment was moderately more resistant (insignificant under condition of P-value < 0.01 but significant under condition of P-value < 0.05) than mutant without AR156 pretreatment, but to a lesser significant extent to the difference between wild type plant with and without AR156 pretreatment (comparing the difference between Col-0 and rdr6, Figures 5A, S3). These results indicate that the AR156-induced ISR is partially dependent on RDR6. In order to exclude the possibility that this reduced AR156-mediated ISR is due to altered AR156 rhizosphere colonization, we compared root AR156 colonization associated with the rdr6 mutant line along with Col-0 7 days after AR156 treatment. No significant differences were observed between rdr6 and Col-0 (Figure 5B). The number of AR156 in the rhizosphere of each tested line of Arabidopsis plants was around 5×107 colony-forming unit per gram (CFU/g) of rhizosphere soil (Figure 5B), indicating that the reduced ISR was not due to different extent of AR156 colonizing the roots. These results suggested that RDR6 might play a regulatory role in the AR156-triggered ISR against Pst DC3000 infection.
Figure 5. AR156-induced ISR to Pst DC3000 is partially dependent on RDR6.
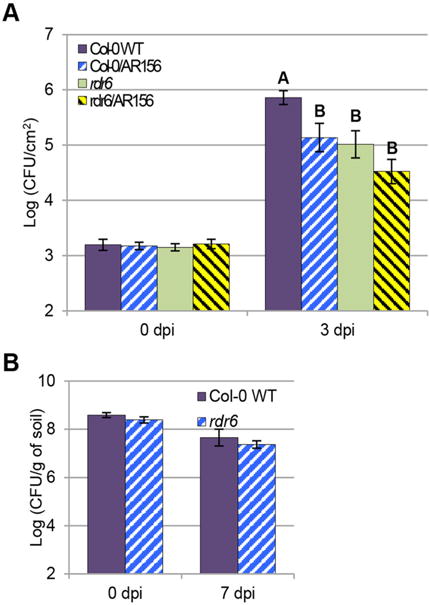
(A) AR156-induced ISR is measured in wild type and rdr6 mutant plants. Arabidopsis Col-0 and rd6 mutant plants are pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3. Error bars represent standard deviation for at least 12 leaf discs. Statistical difference among samples is represented by using letters: different letters (A and B) represent significant difference (P < 0.01) while same letters represent insignificant differences (P > 0.01). (B) Rhizosphere colonization is measured seven days after AR156 treatment. Data represent average numbers of CFU per gram of rhizosphere soil with standard deviation from three experiments.
To further confirm that the observed induced ISR is indeed a function of miR825* -mediated gene silencing through generating 2nd siRNA, we analyzed 2nd siRNA along the miR825* targets. Our rationale is that if miR825* -triggered 2nd siRNAs was functional in ISR, we should observe 2nd siRNAs originated from the miR825* cleavage site; correlated expression patterns of the 2nd siRNAs and miR825*; and that the abundance of these 2nd siRNAs is correlated to ISR. As shown in Figure 6, we were indeed able to identify 2nd siRNAs along the At5g38850 transcript, although the phasing registry was not perfect and there were also non-phased siRNAs (Table S3). In both the AR156/Pst and the control/Pst plants, 2nd siRNAs were induced from the putative cleavage site of miR825*, which were located to the 251st nt from the 5′ end. The induction of 2nd siRNAs was most intense in the first 500 bp from the cleavage site, and then remained but in a declined manner until the most 3′ end of the transcript (Figure 6A). It should be noted that 2nd siRNAs were generated from both the plus and minus strands, with about 50% more from the plus strand (Figure 6B). Similar observation has been made in other independent studies (Zhai et al. 2011; Boccara et al. 2014). It’s interesting to point out that although the general patterns were similar between the AR156/Pst and the control/Pst plants, there were more 2nd siRNAs induced in the control/Pst plants when compared with plants from the AR156/Pst treatment (Figure 6B). At5g38850 encodes a disease resistance protein (TIR-NBS-LRR class), which was also identified as a siRNA-targeted gene in a RDR6/DCL4 dependent manner in an independent study (Howell et al. 2007). Taken together, our results demonstrated that in the AR156/Pst plants, there were significantly less than 2nd siRNAs induced by miR825* at the At5g38850 locus when compared to the control/Pst treatment. The resulting boosted expression of At5g38850, together with other not yet uncharacterized R gene as wellQ2, contributes to the induced ISR.
Figure 6. Identify 2nd siRNAs at the At5g38850 locus.
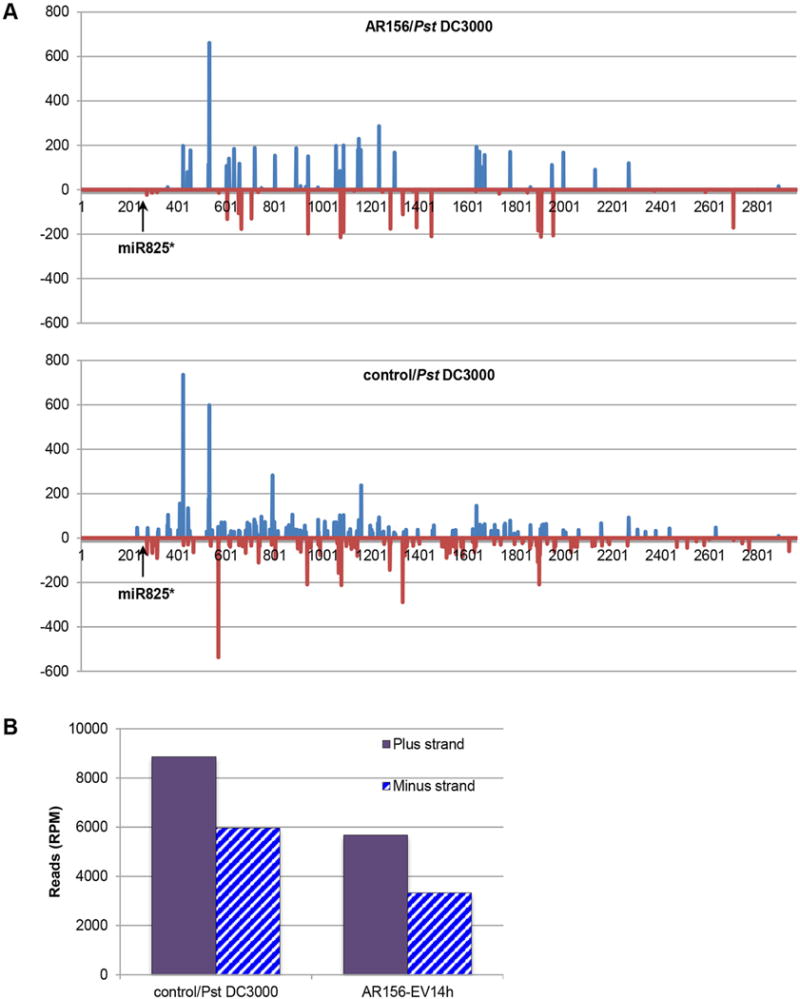
(A) siRNAs are aligned to the Arabidopsis genome (version TAIR 10) by sequence similarities. Reads (read per million; RPM) is shown as bars at the Y-axis whereas locations of the siRNAs are presented at the X-axis. Light blue represents alignment to the plus strand while red to the minus strand. miR825* cleavage site is marked by an arrow, which is the 251 nt to the 5′ end. Up: AR156/Pst; bottom: control/Pst. (B) The total reads aligned to the plus or the minus strand in each sample is calculated by adding all the reads up and plotted to the right side of the corresponding figure.
DISCUSSION
The molecular mechanisms underlying ISR have been studied extensively, especially in the model plant Arabidopsis. Many molecular processes and signaling components at the interface between hosts and ISR-eliciting mutualists have been illustrated (Van Wees et al. 2008; Pieterse et al. 2014). However, whether R genes are used in ISR remains known. How many miRNAs contribute to the modulation of ISR is not explored either. In our previous study, we found that Bacillus cereus AR156 could trigger systemic resistance in Arabidopsis thaliana. PR1 gene expression was induced to a higher level upon Pst DC3000 infection in AR156/Pst samples, when compared to control/Pst (Niu et al. 2011). Increasing evidence suggests that some miRNAs act as negative regulators of plant innate immunity: miRNAs suppress defense responses when plants are not challenged; upon infection, a healthy plant is capable of deploying a line of immune response by removing this small RNA-mediated suppression (Ruiz-Ferrer and Olivier Voinnet 2009; Seo et al. 2013; Niu et al. 2015). Our data showed that when plants with and without AR156 pretreatment were compared, there were many small RNAs significantly differentially expressed after Pst DC3000 infection, indicating a potential role they play in modulating ISR (Table S1).
We explored which small RNAs might be involved in modulating ISR by identifying small RNAs that were significantly differentially expressed (≥ 3 or ≤1/3) between the AR156- and control-pretreated plants, with and without Pst DC3000 infection. We also paid extra attention to small RNAs that may target genes directly involved in plant defense, such as R genes, transcription factors, and protein ubiquitination pathway components. We finally focused on miR825* that satisfy the two abovementioned criteria. What made it more interesting was that the cognate miRNA generated from the same duplex with miR825*, miR825, was also significantly differentially expressed (3.7 fold reduction in AR156/Pst vs control/Pst). It would be interesting to see whether the miR825/825* pair represents a common innate immunity regulatory mechanism, as we previously found in the miR393/393b case (Zhang et al. 2011).
miR825* can target a disease resistance gene family belonging to the TIR-NBS-LRR class (Table 2). The target region of miR825* is embedded in a region encoding the TIR domain, which is relatively conserved in the TIR-NBS-LRR family proteins (Figure 1D). It is possible that multiple R genes are targeted by this specific miRNA. We observed that when miR825* was knocked down, these R genes were activated (Figure 2C), whereas when miR825* was overexpressed these R genes were suppressed (Figure 3B), supporting the idea that these genes are genuine targets of miR825*. miR825 may target At5G55970, a putative RING/U-box superfamily protein, and At5G44940, a F-box/RNI-like superfamily protein. Despite the fact that the functions of these two genes are unknown currently, an amino acid sequence analysis suggested that they might function in defense-related signaling. Some RING/U-box superfamily protein can function as E3 ubiquitin transferases that are involved in the protein ubiquitination pathway (Wang and Deng 2011). Meanwhile, F-box/RNI-like superfamily proteins are also known for their involvement in protein ubiquitination. An example of this protein group is TIR1 that mediates Auxin signaling by triggering Aux/IAA proteasomal degradation and activating ARFs from the repressive effects (Yang et al. 2013). TIR1 is a target of miR393, which is induced by elicitor flg22 and contributes to PTI (Navarro et al. 2006). Based on the functional domains these proteins possess, we predicted that miR825 might contribute to ISR by specifically activating certain signaling pathway via protein ubiquitination.
We further validated our findings by manipulating the expression levels of miR825 and miR825* in transgenic plants and examining the performance of ISR. miR825/825* knockdown mutants were more resistant (Figure 2D) whereas overexpressing plants were more susceptible than wild type plants (Figure 3C), indicating a negative role they may play on ISR. Neither transgenic plants overexpressing nor knocking-down miR825/825* responded to AR156 priming significantly (Figure S2). We believe this observation is in line with our conclusion that miR825/825* are negatively involved in priming plants from pathogen infection in a potentiated manner. When miR825/825* function is blocked, the priming activity is turned on such that further AR156 treatment won’t additionally induce ISR in a significant manner; on the other hand, overexpressing miR825/825* shuts down the priming ability such that transgenic plants are unable to respond to AR156 treatment anymore. In plants that ISR is effectively primed, the expression of At5G38850, At3G04220 and At5G40910 (target genes of the miR825*) and At5G44940 (a target gene of the miR825) was dramatically increased (Figure 4), suggesting a negative regulatory role miR825/825* playing in ISR.
Boccara and colleagues reported that a 22-nt miRNA, miR472, could initiate a cascade of 2nd siRNAs and target R genes through a RDR6/DCL4 dependent manner. Their results showed that miRNA472- and RDR6 could mediate both PTI and ETI responses when infected with Pst DC3000 (Boccara et al. 2014). We studied ISR further in a rdr6 mutant because miR825* is also a 22-nt miRNA, and it is likely to function through a RDR6/DCL4 dependent manner by initiating 2nd siRNAs as well (Li et al. 2012; Shivaprasad et al. 2012; Zhai et al. 2012;Q3 Boccara et al. 2014). Under a stringent statistical condition (i.e., P < 0.01), the AR156-primed ISR was not significantly affected in the rdr6 mutant, when compared to Col-0 wild type (Figure 5A). This observation is in agreement with our hypotheses that miR825* may participate in ISR regulation through an RDR6-mediated pathway. However, when a lesser stringent statistical criteria is applied (e.g. P < 0.05), significantly different ISR was observed on rdr6 plants without and with AR156 pretreatment (Figure S3). Therefore, it’s likely that there are alternative regulatory machineries besides RDR6-mediated 2nd siRNAs. We then analyzed generation of 2nd siRNAs on potential miR825* cleavage loci. We were able to find arrays of phased siRNAs (≥98% are 21 nt) aligned at the cleavage site on target gene At5g38850, peaked in the first 500 nt to the 5′ end, and remained but declined until the most 3′ end (Figure 6A). In agreement with our hypothesis that AR156 pretreatment induced their generation, there were more siRNAs in the control/Pst then in the AR156/Pst plants. Almost no siRNA was identified upstream the miR825* target site, supporting our prediction that these siRNAs were generated due to the cleavage of At5g38850 by miR825*. At5g38850 encodes a disease resistance protein (TIR-NBS-LRR class), which has been previously observed as a siRNA-targeted gene in a RDR6/DCL4 dependent manner (Howell et al. 2007). Taken together, our results demonstrated a correlation between AR156/Pst treatments and 2nd siRNAs on the At5g38850 locus. We speculate that the reduced siRNAs is going to lead to a boosted expression of this R gene, which may contribute to the induced ISR observed.
Previous studies investigating the molecular mechanism of ISR have been focused on the roles of protein regulators, such as NPR1 and MYB72, and crucial signaling pathways such as JA and ET, where the involvement of R genes has been ignored. Our data demonstrated that plants were able to modulate ISR through miRNAs by specifically activating certain subgroups of R genes, whose activities were suppressed under normal conditions. This suppression is reinforced by using both miRNA (i.e., miR825*) and arrays of 2nd siRNAs. Upon infection in a primed tissue, this suppression can be released in a very rapid manner since once the expression of the initiating miRNA is quenched, all the 2nd siRNAs triggered by it will be diminished as well. This quick switch between an idle and engaged mode is in such a potentiated manner that best fit the defense responses employed by ISR.
MATERIALS AND METHODS
Small-RNA library construction and deep sequencing
Bacterium treatment and pathogen infection was carried out on 4-week-old Arabidopsis Col-0 as described previously (Niu et al. 2011) with some modifications. Briefly, 10 mL of Bacillus cereus AR156 cell suspension at 5 × 107 CFU/mL was applied to the soil around the roots of Arabidopsis plants in each pot. For two control treatments, an equal volume of sterile 0.85% NaCl was applied to the soil around the roots of Arabidopsis in each pot. Seven days after induction treatment, plants of both treatments were inoculated by spraying the leaves with cell suspension of the virulent pathogen Pst DC3000 at 1 × 108 CFU/mL until all the leaves were covered with fine droplets. Leaves were collected at 7 days post-treatment by AR156 if there was no following Pst DC3000 inoculation, or after 14 h post-inoculation by Pst DC3000.
Small RNA extraction and library construction was carried out as described previously (Katiyar-Agarwal and Jin 2007; Chellappan and Jin 2009). Briefly, total RNA was isolated from infiltrated leaves and fractionated on a 15% denaturing polyacrylamide gel. RNA molecules ranging from 18 to 26 nt were excised and ligated to 5′- and 3′-RNA adaptors using T4 RNA ligase, followed by RT-PCR and gel purification as instructed by Illumina. The small RNA libraries were sequenced by Illumina Inc. and the UCR core facility.
Generation of transgenic plants
To generate the overexpression construct of miR825 and miR825*, the miR825 precursors were cloned using a miR319 backbone (based on Web MicroRNA Designer) into a Gateway destination vector, pEG100 (Earley et al. 2006; Schwab et al. 2006). The STTM825 and 825*constructs used to inactivate miR825 and miR825* were generated according to Yan and colleagues (2012). Briefly, the sub-cloning vectors were amplified by using the following two primers: (i) 825–825* -STTMSwa48ntlink-PF: GccATTTAAATatggtctaaagaagaagaatGTTCA-TGCACctaCTTCTTGAGAAgaattcggtacgctgaaatcaccag; (ii) 825-825-STTMSwa48ntlink-PR: GccATTTAAAT-tagaccataacaacaacaacTTCTCAAGAAtagGGTGCATGAACaagcttgggctgtcc-tctccaaatg. The PCR product that includes the pOT2 backbone (−3.6 kb) was purified and cleaved by SwaI, followed by purification and self-ligation. The recombinant plasmids (−3.6 kb) were further amplified by a pair of primers that contain PacI sites: (i) origin-del-PacI-PF: TCCCTTAATTAAGTTTGCAAGCAGCAGATTACGCG; and (ii) Origin-del-PacI-PR: TCCC-TTAATTAAGAAAGGCGGACAGGTATCCGGTAAG. The PCR products that contain STTM and a chloramphenicol selection marker were introduced into a modified pFGC5941 binary vector through the unique PacI site. All constructs described were electroporated into Agrobacterium tumefaciens GV3101, and used to transform Arabidopsis by the floral dipping method.
Pathogen infection
Bacterial growth assay and ISR assay were performed as previously described (Katiyar-Agarwal et al. 2006; Katiyar-Agarwal et al. 2007; Niu et al. 2011). For bacterial growth assay, 1 × 108 CFU/mL of Pst DC3000 was used for spray inoculation. Leaf samples were collected by acock borer, and the bacterial titer were determined by plating and counting the number of colonies at 0 and 3 dpi, respectively. At least 12-leaf discs were collected for each growth assay. Student’s t-test was used to determine the significant differences between mutants and control plants.
Quantification of AR156 within Arabidopsis rhizosphere
The colonization of AR156 within Arabidopsis rhizosphere were performed as previously described (Niu et al. 2011). Briefly, 1 g of mixed rhizosphere sample (fw) was resuspended in 9 mL of sterile 0.85% NaCl and was vortexed for 5 min. Serial dilutions of a cell suspension of the rhizosphere was plated on LB medium. The number of CFU per gram of rhizosphere soil was determined after incubation at 28 °C for 24 h.
Identification of target genes of miRNA
To identify miR825 and miR825* target genes, the guideline for target prediction was followed as previously described with several modifications (Allen et al. 2005). Only one gap located in the small RNA was allowed, but with double penalty. Nucleotides at positions 10 and 11 of the small RNA must be a perfect match with its target. A maximum of three continuous mismatches was allowed if the mismatch region contained at least two G:U pairs and the penalty score of the region was multiplied by 1.5. We used 4.5 as the cutoff score for selecting the miRNA targets and 3.0 for miR825* targets selection.
qRT-PCR
To analyze the expression of target genes, total RNA was extracted from Arabidopsis leaves using TRIzol reagent. Reverse transcription to cDNA was conducted by using the SuperScript first-strand synthesis system (Invitrogen). SYBR Green mix was used in real-time PCR to determine the expression level of miRNAs target genes. ACTIN2 was used as a quantitative control in the qRT-PCR analysis.
Supplementary Material
Figure S1. Expression of miR773 after AR156 treatment and Pst DC3000 inoculation.
Plants are pre-treated with AR156 at 5 × 107 CFU/ml and 0.85% NaCl for seven days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/ml concentration. Total RNA is extracted from leaves with line numbers and time points indicated. RNA blots are hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 is used as a loading control.
Figure S2. miR825/825* knockdown and overexpression diminish response to AR156 priming.
(A) AR156-mediated ISR in Col-0 and STTM825/825* transgenic plants. (B) AR156-mediated ISR in Col-0 and transgenic plants over-expressing miR825/825*. Plants pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3 days after inoculation (dpi). Error bars represent standard deviation for at least 12 leaf discs.
Figure S3. AR156-induced ISR to Pst DC3000 is partially dependent on RDR6.
(A) AR156-induced ISR is measured in wild type and rdr6 mutant plants. Arabidopsis Col-0 and rd6 mutant plants are pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3. Error bars represent standard deviation for at least 12 leaf discs. Statistical difference among samples is represented by using letters: different letters (a, b, and c) represent significant difference (P < 0.05) while same letters represent insignificant differences (P > 0.05). (B) The statistical value is listed in a table. Value in the 3rd column corresponds to Figure 5A.
Table S1. Summary of miRNAs identified in this research miRNAs identified in this research are listed.
Reads are normalized to library size and presented in read per million (RPM).
Table S2. Summary of predicted target genes from miRNAs with significant differential expression.
Prediction targets of miRNAs with significant expression alteration (Table 2) are listed, with gene ID, predicted function, and base-paring patterns shown. A “:” represents a perfect match; a “·” represents a wobble (G: U pairs); a “·”(a space) represents an imperfect match.
Table S3. siRNA aligned to the At5G38850 locus.
siRNAs aligned to the At5G38850 locus are listed. Reads (#) indicate the copy number of the specific small RNA in column D5. “+” strand indicates the same orientation of small RNA as that of At5G38850 while “−” indicates the reversed strand. Register is the position (column C) module 21.
Acknowledgments
We are grateful to Dr Kendall for his proofreading of the manuscript. This work was supported by a Joint Research Fund for Overseas, Hong Kong and Macao Scholars (31228018) to HJ and JG, an NIH grant (R01GM093008) to HJ, a grant from Natural Science Foundation of Jiangsu Province of China (BK20141360) and a PhD Programs Foundation of Ministry of Education of China (B0201300664) to HZ, an NIH grant-(R01GM100364) and an National Science Foundation grant (DBI-0743797) to WZ, and a Talent Development Program of Wuhan, the municipal government of Wuhan, Hubei, China (2014070504020241), and an internal research grant of Jianghan University, Wuhan, China to WZ.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
AUTHOR CONTRIBUTIONS
H.J. and J.G. conceived this project; H.J., W.Z. and H.Z. designed experiments and analyzed data. D.N., J.X., C.J., B.Q., X.L., and S.L. performed the experiments. H.Z. and H.J. wrote the manuscript. All authors discussed the results and made comments on the manuscript.
References
- Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Altenbach D, Robatzek S. Pattern recognition receptors: From the cell surface to intracellular dynamics. MPMI. 2007;20:1031–1039. doi: 10.1094/MPMI-20-9-1031. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Pattery T, Cornelis P, Hofte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. MPMI. 2002;15:1147–1156. doi: 10.1094/MPMI.2002.15.11.1147. [DOI] [PubMed] [Google Scholar]
- Boccara M, Sarazin A, Thiebeauld O, Jay F, Voinnet O, Navarro L, Colot V. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 2014;10:e1003883. doi: 10.1371/journal.ppat.1003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan P, Jin H. Discovery of plant microRNAs and shortinterfering RNAs by deep parallel sequencing. Methods Mol Biol. 2009;495:121–132. doi: 10.1007/978-1-59745-477-3_11. [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer D, Djavaheri M, Bakker PAHM, Hoefte M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008;148:1996–2012. doi: 10.1104/pp.108.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan CG, Wang CH, Guo HS. Application of RNA silencing to plant disease resistance. Silence. 2012;3:5. doi: 10.1186/1758-907X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song KM, Pikaard CS. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Dong XN. Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- Göhre V, Robatzek S. Breaking the barriers: Microbial effector molecules subvert plant immunity. Annu Rev Phytopathol. 2008;46:189–215. doi: 10.1146/annurev.phyto.46.120407.110050. [DOI] [PubMed] [Google Scholar]
- Hase S, Takahashi S, Takenaka S, Nakaho K, Arie T, Seo S, Ohashi Y, Takahashi H. Involvement of jasmonic acid signalling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathol. 2008;57:870–876. [Google Scholar]
- Howell MD, Fahlgren N, Chapman EJ, Cumbie JS, Sullivan CM, Givan SA, Kasschau KD, Carrington JC. Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on miRNA- and tasiRNA-directed targeting. Plant Cell. 2007;19:926–942. doi: 10.1105/tpc.107.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Robin GP. Systemic signaling during plant defense. Curr Opin Plant Biol. 2013;16:527–533. doi: 10.1016/j.pbi.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Gao S, Vivian-Smith A, Jin H. A novel class of bacteria-induced small RNAs in Arabidopsis. Gene Dev. 2007;21:3123–3134. doi: 10.1101/gad.1595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Jin H. Discovery of pathogen-regulated small RNAs in plants. Methods Enzymol. 2007;427:215–227. doi: 10.1016/S0076-6879(07)27012-0. [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Jin H. Role of small RNAs in host-microbe interactions. Annu Rev Phytopathol. 2010;48:225–246. doi: 10.1146/annurev-phyto-073009-114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Morgan R, Dahlbeck D, Borsani O, Villegas A, Jr, Zhu JK, Staskawicz BJ, Jin H. A pathogen-inducible endogenous siRNA in plant immunity. Proc Natl Acad Sci USA. 2006;103:18002–18007. doi: 10.1073/pnas.0608258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B. MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci USA. 2012;109:1790–1795. doi: 10.1073/pnas.1118282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang QQ, Zhang JG, Wu L, Qi YJ, Zhou JM. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010;152:2222–2231. doi: 10.1104/pp.109.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- Niu D, Wang Z, Wang S, Qiao L, Zhao H. Profiling of small rnas involved in plant-pathogen interactions. In Mysore KS, Senthil-Kumar M, eds. Plant Gene Silencing Springer New York. 2015:61–79. doi: 10.1007/978-1-4939-2453-0_4. [DOI] [PubMed] [Google Scholar]
- Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY, Jin HL, Guo JH. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. MPMI. 2011;24:533–542. doi: 10.1094/MPMI-09-10-0213. [DOI] [PubMed] [Google Scholar]
- Padmanabhan C, Zhang X, Jin H. Host small RNAs are big contributors to plant innate immunity. Curr Opin Plant Biol. 2009;12:465–472. doi: 10.1016/j.pbi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, vanWees SCM, Hoffland E, vanPelt JA, vanLoon LC. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Pumplin N, Voinnet O. RNA silencing suppression by plant pathogens: Defence, counter-defence and counter-counter-defence. Nat Rev Microbiol. 2013;11:745–760. doi: 10.1038/nrmicro3120. [DOI] [PubMed] [Google Scholar]
- Ramirez V, Van der Ent S, Garcia-Andrade J, Coego A, Pieterse CMJ, Vera P. OCP3 is an important modulator of NPR1-mediated jasmonic acid-dependent induced defenses in Arabidopsis. BMC Plant Biol. 2010;10:199. doi: 10.1186/1471-2229-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ferrer V, Voinnet O. Roles of plant small rnas in biotic stress responses. Annu Rev Plantbiol. 2009;60:485–510. doi: 10.1146/annurev.arplant.043008.092111. [DOI] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Zipfel C. News from the frontline: Recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol. 2008;11:389–395. doi: 10.1016/j.pbi.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Seo J-K, Wu J, Lii Y, Li Y, Jin H. Contribution of small rna pathway components in plant immunity. MPMI. 2013;26:617–625. doi: 10.1094/MPMI-10-12-0255-IA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell. 2012;24:859–874. doi: 10.1105/tpc.111.095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth EB, Lee YN, He SY. Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr Opin Plant Biol. 2007;10:580–586. doi: 10.1016/j.pbi.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wees SC, Van der Ent S, Pieterse CM. Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Wang F, Deng XW. Plant ubiquitin-proteasome pathway and its role in gibberellin signaling. Cell Res. 2011;21:1286–1294. doi: 10.1038/cr.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Gu Y, Jia X, Kang W, Pan S, Tang X, Chen X, Tang G. Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell. 2012;24:415–427. doi: 10.1105/tpc.111.094144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang L, Yuan D, Lindsey K, Zhang X. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J Exp Bot. 2013;64:1521–1536. doi: 10.1093/jxb/ert013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Li Y, Han X, Shen F. Genome-wide profiling of miRNAs and other small non-coding RNAs in the Verticillium dahliae-inoculated cotton roots. PLoS ONE. 2012;7:e35765. doi: 10.1371/journal.pone.0035765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai JX, Jeong DH, De Paoli E, Park S, Rosen BD, Li YP, Gonzalez AJ, Yan Z, Kitto SL, Grusak MA, Jackson SA, Stacey G, Cook DR, Green PJ, Sherrier DJ, Meyers BC. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Gene Dev. 2011;25:2540–2553. doi: 10.1101/gad.177527.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Gene Dev. 2006;20:3255–3268. doi: 10.1101/gad.1495506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao H, Gao S, Wang WC, Katiyar-Agarwal S, Huang HD, Raikhel N, Jin H. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol Cell. 2011;42:356–366. doi: 10.1016/j.molcel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of miR773 after AR156 treatment and Pst DC3000 inoculation.
Plants are pre-treated with AR156 at 5 × 107 CFU/ml and 0.85% NaCl for seven days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/ml concentration. Total RNA is extracted from leaves with line numbers and time points indicated. RNA blots are hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 is used as a loading control.
Figure S2. miR825/825* knockdown and overexpression diminish response to AR156 priming.
(A) AR156-mediated ISR in Col-0 and STTM825/825* transgenic plants. (B) AR156-mediated ISR in Col-0 and transgenic plants over-expressing miR825/825*. Plants pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3 days after inoculation (dpi). Error bars represent standard deviation for at least 12 leaf discs.
Figure S3. AR156-induced ISR to Pst DC3000 is partially dependent on RDR6.
(A) AR156-induced ISR is measured in wild type and rdr6 mutant plants. Arabidopsis Col-0 and rd6 mutant plants are pre-treated with AR156 at 5 × 107 CFU/mL and 0.85% NaCl for 7 days, respectively. Leaves are spray inoculated with Pst DC3000 cell suspension at 1 × 108 CFU/mL concentration. ISR is measured by counting colonies on plates 3. Error bars represent standard deviation for at least 12 leaf discs. Statistical difference among samples is represented by using letters: different letters (a, b, and c) represent significant difference (P < 0.05) while same letters represent insignificant differences (P > 0.05). (B) The statistical value is listed in a table. Value in the 3rd column corresponds to Figure 5A.
Table S1. Summary of miRNAs identified in this research miRNAs identified in this research are listed.
Reads are normalized to library size and presented in read per million (RPM).
Table S2. Summary of predicted target genes from miRNAs with significant differential expression.
Prediction targets of miRNAs with significant expression alteration (Table 2) are listed, with gene ID, predicted function, and base-paring patterns shown. A “:” represents a perfect match; a “·” represents a wobble (G: U pairs); a “·”(a space) represents an imperfect match.
Table S3. siRNA aligned to the At5G38850 locus.
siRNAs aligned to the At5G38850 locus are listed. Reads (#) indicate the copy number of the specific small RNA in column D5. “+” strand indicates the same orientation of small RNA as that of At5G38850 while “−” indicates the reversed strand. Register is the position (column C) module 21.


