Abstract
Behavioral and functional studies in humans suggest that attention plays a key role in activating the primary olfactory cortex through an unknown circuit mechanism. We report that a novel pathway from the anterior cingulate cortex, an area which has a key role in attention, projects directly to the primary olfactory cortex in rhesus monkeys, innervating mostly the anterior olfactory nucleus. Axons from the anterior cingulate cortex formed synapses mostly with spines of putative excitatory pyramidal neurons and with a small proportion of a neurochemical class of inhibitory neurons that are thought to have disinhibitory effect on excitatory neurons. This novel pathway from the anterior cingulate is poised to exert a powerful excitatory effect on the anterior olfactory nucleus, which is a critical hub for odorant processing via extensive bilateral connections with primary olfactory cortices and the olfactory bulb. Acting on the anterior olfactory nucleus, the anterior cingulate may activate the entire primary olfactory cortex to mediate the process of rapid attention to olfactory stimuli.
Keywords: Olfaction, attention, anterior cingulate cortex, primary olfactory cortex, anterior olfactory nucleus, posterior orbitofrontal cortex
Introduction
Traditional concepts viewed primates as anosmatic (Broca 1879) or microsmatic (Turner 1890). In the last decades, however, behavioral studies have shown that humans have good olfactory abilities. Humans are good at detecting potentially harmful odors like cooking gas (Whisman 1978), contaminated water (Widen et al. 2005) or tainted food (Siegmund and Pollinger-Zierler 2006; Carrapiso et al. 2010). Humans also have good olfactory abilities in ecologically meaningful contexts, such as mothers discriminating the specific odor of their babies (Porter et al. 1983) or subjects sorting out by smell their own T-shirt from others (Lord and Kasprzak 1989). Attention to olfactory stimuli seems to be minimal in humans under normal conditions (Sela and Sobel 2010), but when attention is directed to olfaction by an alerting signal, humans respond more rapidly in an odor discrimination task (Spence et al. 2000).
Functional neuroimaging studies in humans have shed light on the engagement of some cortical areas when directing attention to olfactory stimuli. Paradoxically, most of the earlier studies did not show activation of the primary olfactory cortex [reviewed in (Zald and Pardo 2000)], the telencephalic areas that receive a direct projection from the olfactory bulb (Price 1973; Haberly and Price 1977; de Olmos et al. 1978; Turner et al. 1978; Carmichael et al. 1994). In contrast, earlier studies consistently report activation in the multimodal posterior orbitofrontal cortex (pOFC) [reviewed in (Zald and Pardo 2000)]. More recently, studies on the time course of odorant-induced activation report that after exposure to olfactory stimuli the primary olfactory cortex shows a strong early and transient activation followed by habituation (Sobel et al. 2000; Poellinger et al. 2001; Tabert et al. 2007). Similarly, sniffing in the absence of odorants produces transient activation of the primary olfactory cortex suggesting an attentional mechanism in olfaction (Sobel et al. 1998; Sobel et al. 2000; Simonyan et al. 2007). These findings suggest that sniffs activate an attention dependent region in the primary olfactory cortex, which shows anticipatory response after instructions, and sustained activity in a working memory task with odorants (Zelano et al. 2005; Zelano et al. 2009; Zelano et al. 2011). Attempts to detect an odor also activate the primary olfactory cortex (Veldhuizen and Small 2011).
Functional studies show that the anterior cingulate cortex (ACC), which plays a key role in attention (Botvinick 2007; Pessoa 2008) and in working memory tasks (Fuster 2008), is also activated with olfactory stimuli in humans [reviewed in (Small and Prescott 2005; Seubert et al. 2012)]. Time course studies of olfactory tasks show that when human subjects are not alerted about the presentation of an odorant and are instructed not to sniff, the primary olfactory cortex is activated followed by activation in the ACC and the pOFC (Poellinger et al. 2001), or the primary olfactory cortex and ACC are co-activated (Tabert et al. 2007). Similarly, data from non-human primates show that the ACC is activated with olfactory stimuli in alert monkeys but not in sedated animals (Boyett-Anderson et al. 2003; Sasabe et al. 2003).
The behavioral and functional data thus suggest that in humans attention plays a key role in activating the primary olfactory cortex before or at the onset of olfactory perception, and in maintaining sustained activity, as needed. Activation of ACC in olfactory tasks may reflect a top-down regulation of attention to olfaction. We now report a novel monosynaptic pathway from ACC to the primary olfactory cortex, which may mediate the process of rapid attention to olfactory stimuli in primates.
Material and methods
Animal and tissue preparation
Brain imaging and surgical procedures
We conducted tract-tracing studies on six normal young adult (2–3 years) or adult (>3 years) rhesus monkeys (Macaca mulatta) of both sexes (cases DQ, AY, BG, BI, BL, and BN). We used three additional cases for cytoarchitectonic analysis of the primary olfactory cortex (cases AJ, AS, and AT). Detailed protocols describing all procedures were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and Boston University School of Medicine in accordance with NIH guidelines (DHEW Publication no. [NIH] 80-22, revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD, USA). Procedures were designed to minimize animal suffering and reduce the number of animals for research.
We obtained scans of each brain using magnetic resonance imaging (MRI) to guide the injection of neural tracers (cases AY, BG, BI, BL, and BN). Animals were anesthetized with propofol (loading dose, 2.5–5 mg/kg, intravenous; continuous rate infusion, 0.25–0.4 mg/kg −1·min −1) and positioned in a nonmetallic stereotaxic device. MRI was performed with a 3T-superconducting magnet (Phillips; or Siemens). The interaural line was rendered visible in the scan by filling the hollow ear bars of the stereotactic apparatus with Betadine salve. We then calculated the stereotaxic coordinates for each injection in three dimensions using the interaural line as reference. One to three weeks after MRI, the monkeys were sedated with ketamine hydrochloride (10–15 mg/kg, intramuscularly) and deeply anesthetized with sodium pentobarbital (~30 mg/kg, intravenous, to effect, case DQ) or isoflurane (all other cases), until a surgical level of anesthesia was accomplished. The monkeys were placed in the stereotactic apparatus and a small region of the cortex was exposed over the site for injection. Surgery to inject neural tracers was performed under aseptic conditions while heart rate, muscle tone, respiration, and pupillary dilatation were closely monitored to maintain a surgical level of anesthesia.
Injection of neural tracers
We injected neural tracers using a microsyringe (5 or 10 μl, Hamilton, Reno, NV, USA) mounted on a microdrive. The injection sites and the tracers used are listed in Table 1. Biotinylated dextran amine (BDA, 10% solution, volume of 6–10 μl, 10 kDa; Invitrogen, Carlsbad, CA, USA) and Lucifer yellow (LY, dextran Lucifer yellow, anionic, lysine fixable, 10% solution, volume of 3–4 μl, 10 kDa, Invitrogen) tracers were injected in two to four penetrations at a depth of 1.2–1.6 mm below the pial surface. The needle was left in place for 10–15 min to allow tracer penetration at the injection site and prevent upward diffusion of the tracer during retraction of the needle. We also used a case that had been injected with 3H-labeled amino acids ([3H]leucine and [3H]proline, specific activity 40–80 μCi, volume of 0.5 μl) 1.5 mm below the pial surface at each of two adjacent sites separated by 1–2 mm. BDA and LY were of 10 kDa molecular weight optimized for anterograde labeling (Veenman et al. 1992; Reiner et al. 2000); 3H-labeled amino acids are purely anterograde tracers (Cowan et al. 1972). After injection of neural tracers, the wound was closed in anatomic layers.
Table 1.
Injection sites, neural tracers and type of analysis
| Case | Sex | Hemisphere | Tracer | Injection site | LM/EM |
|---|---|---|---|---|---|
| DQ | - | Left | 3H-labeled amino acids | 32 | a |
| AY | Female | Left | BDA | 32 | a |
| BG | Female | Right | BDA | 32 | a |
| BI | Female | Right | BDA | 32 | a,b |
| BL | Male | Right | LY | 32 | a |
| BN | Male | Left | LY | 32/24 | a,b |
32, anterior cingulate area 32; 24, anterior cingulate area 24
Light microscopy (LM):
Electron microscopy (EM)
Perfusion and tissue processing
The monkeys injected with BDA and LY were anesthetized with a lethal dose of sodium pentobarbital (>50 mg/kg, intravenous, to effect) and transcardially perfused with 4% paraformaldehyde, 0.2% glutaraldehyde in 0.1M PB (pH 7.4) 19 days after tracer injection. The brains were removed from the skull, cryoprotected in a series of sucrose solutions (10–30% in 0.01 M PBS), photographed, frozen in −75 °C isopentane (Fisher Scientific, Pittsburg, PA, USA) for rapid and uniform freezing, and cut in the coronal plane on a freezing microtome at 40 or 50 μm to produce ten matched series. Tissue was stored in −20°C in anti-freeze solution until processing (30% ethylene glycol, 30% glycerol, 40% 0.05 M PB, pH 7.4 with 0.05% azide) to preserve the ultrastructure. In a case with injection of 3H-labeled amino acids, the monkey was anesthetized with a lethal dose of sodium pentobarbital (>50 mg/kg, intravenous, to effect) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) 10 days after tracer injection. The brain was removed from the skull, embedded in paraffin, cut at 10 μm and processed for autoradiography as described (Cowan et al. 1972; Ghashghaei and Barbas 2001).
Immunohistochemistry for optical microscopy
To view pathways labeled with BDA, free-floating sections were rinsed in 0.01M PBS, incubated in 0.05 M glycine, pre-blocked in 5% normal goat serum (NGS) and 5% bovine serum albumin (BSA) with 0.2% Triton-X and then incubated for 1 h in an avidin-biotin horseradish peroxidase (AB-HRP) complex (Vectastain PK-6100 ABC Elite kit, Vector Laboratories, Burlingame, CA, USA; diluted 1:100 in 0.01M PBS with 0.1% Triton X-100). Sections were then rinsed and processed for 2–3 min for the peroxidase-catalyzed polymerization of diaminobenzidine (DAB; DAB kit, Vector or Zymed Laboratories Inc., South San Francisco, CA, USA; 0.05% DAB, and 0.004% H2O2 in PBS).
To view pathways labeled with LY, sections were processed to visualize the fluorescent tracer by peroxidase-catalyzed polymerization of DAB. Sections were incubated overnight in primary antibody against LY (1:800, in PBS, 1% NGS, 1% BSA, 0.1% Triton-X, rabbit polyclonal, Molecular Probes), followed by secondary biotinylated goat anti-rabbit IgG (1:200, for 2 h; Vector), followed by AB-HRP and DAB as described for BDA. In cases with injection of LY that also had an injection of BDA in other brain areas, sections were incubated in avidin-biotin blocking reagent (AB blocking kit, Vector) before immunobinding to prevent cross-reaction with BDA, as described (Medalla et al. 2007).
Sections were counterstained with thionin for cytoarchitectonic identification of primary olfactory cortex areas and layers. For detailed cytoarchitectonic study we used three more cases (AJ, AS, and AT) with a complete series of sections stained with thionin. Sections were mounted on gelatin-coated slides, dried, dehydrated in graded alcohols, cleared in xylenes and coverslipped.
Triple pre-embedding immunohistochemistry for electron microscopy
We used pre-embedding immunohistochemistry to view synapses of ACC labeled boutons in the electron microscope (EM), as described previously (Medalla et al. 2007; Medalla and Barbas 2009, 2010, 2012). Neurons were labeled for the expression of calcium-binding proteins parvalbumin (PV), calretinin (CR), and calbindin (CB), which label non-overlapping neurochemical classes of inhibitory neurons in the primate cortex (DeFelipe 1997). In the primary olfactory cortex of rats and mice they label neurochemical classes of inhibitory neurons that overlap to some degree (Kubota and Jones 1993; Meyer et al. 2006; Gavrilovici et al. 2010; Suzuki and Bekkers 2010; Kay and Brunjes 2011) as in the rest of the cortex of rats (Kubota et al. 1994). In primates, only a minority of excitatory cortical pyramidal neurons expresses CR or CB (DeFelipe et al. 1989; del Rio and DeFelipe 1997). Triple immunohistochemical methods were used to label the tracer and two calcium-binding proteins: BDA and LY were labeled with DAB while PV, CR, or CB neurons were labeled either with silver-enhanced gold conjugated, secondary antibodies or tetramethylbenzidine (TMB), as described previously (Medalla et al. 2007; Medalla and Barbas 2009, 2010, 2012). The three methods show distinct labeling at the EM: DAB appears as a dark uniform precipitate, silver-enhanced gold particles appear as circular clumps of variable size, and TMB as rod-shaped crystals (Gonchar and Burkhalter 2003; Pinto et al. 2003; Moore et al. 2004; Medalla et al. 2007; Zikopoulos and Barbas 2007; Medalla and Barbas 2009, 2010, 2012; Zikopoulos and Barbas 2012). After processing for BDA and LY using DAB (as described above but with reduced amounts (0.025%) of Triton-X to preserve the ultrastructure), sections were incubated in AB blocking reagent to prevent cross-reaction with TMB. For BDA-labeled tissue, sections were co-incubated overnight in two primary antibodies for PV or CR (1:2000, rabbit polyclonal, Swiss Antibodies, Bellinzona, Swizerland) and CB or CR (1:2000, mouse monoclonal, Swiss Antibodies). For LY-labeled tissue, sections were incubated in two mouse monoclonal primary antibodies for PV, CR, and/or CB (1:2000, mouse monoclonal, Swiss Antibodies), processed successively, using the Mouse-on-Mouse blocking kit (M.O.M. basic kit, Vector) in between to prevent cross-reaction.
After incubation with the primary antibody for each calcium binding protein, sections were incubated overnight in the appropriate secondary gold-conjugated IgG (1:50, 1 nm gold particle diameter; GE Healthcare) or in secondary biotinylated anti-mouse IgG (1:200, Vector), followed by AB-HRP. The tissue was postfixed in 6% glutaraldehyde with 2% paraformaldehyde using a variable wattage microwave (3–6 min at 150 W in the Biowave; Ted Pella, Redding, CA, USA) until the fixative reached 30°C, and then intensified with silver (6–12 min; IntenSE M kit; GE Healthcare). Sections were then processed for TMB and stabilized with DAB-cobalt chloride solution, as described (Medalla et al. 2007; Medalla and Barbas 2009, 2010, 2012). In control experiments, we omitted the primary antibodies to test the specificity of secondary antibodies, and used the AB blocking reagent prior to AB binding, or the M.O.M. kit prior to secondary antibody binding. In all control experiments there was no immunohistochemical labeling.
Tissue sections were mounted on slides and quickly viewed under the light microscope, and images were captured with a CCD camera. Small blocks of sections with anterograde and PV-CR-CB labeling were cut under a dissecting microscope, postfixed in 1% osmium tetroxide with 1.5% potassium ferrocyanide in PB, washed in PB and water, and dehydrated in an ascending series of alcohols (50–100%). While in 70% alcohol, blocs were stained for 30 min with 1% uranyl acetate (EM Sciences, Hatfield, PA, USA). Subsequently, they were infiltrated with propylene oxide and flat embedded in araldite at 60°C. Pieces of the araldite-embedded tissue were cut and re-embedded in resin blocks. Serial ultrathin sections (50 nm) were cut with a diamond knife (Diatome USA, Hatfield, PA, USA) using an ultramicrotome (Ultracut, Leica, Wein, Austria) and collected on single slot pioloform-coated grids.
Data analysis
Mapping of primary olfactory areas and anterograde labeling at the light microscope
We first studied the cytoarchitecture of the primary olfactory cortex in series of coronal sections through the olfactory areas stained for Nissl (cases AJ, AS, and AT). We then determined the areal and laminar boundaries of olfactory areas with label from ACC axons in tissue sections that were processed for BDA and LY immunohistochemistry or autoradiography and counterstained for Nissl (thionin stain). We studied the distribution of anterograde label under brightfield illumination (Olympus optical microscope, BX 60). We mapped ACC labeled boutons in the primary olfactory cortex in precise register with respect to anatomic landmarks using a work-station with an encoded microscope stage interfaced to a computer using commercial software (Neurolucida, MicroBrightField, Williston, VT, USA). Plots of labeled boutons were made at 1,000X in a series of sections spanning the whole extent of the primary olfactory cortex. In the case with 3H-labeled amino acids sections were examined under darkfield illumination.
Stereological procedures
We analyzed ACC anterograde labeling in the primary olfactory cortex at high magnification (1,000X) in the BDA and LY cases using unbiased stereological methods [for a review, see (Howard and Reed 1998)]. We acquired image stacks of several focal planes in each area of interest. Stacks of images were then combined to create a composite image using ImageJ (NIH, USA), and scaled as described (Medalla and Barbas 2006). This method yields images with high depth of field focused throughout the z-axis extent. All labeled boutons were traced manually using the open source program Reconstruct (www.bu.edu/neural; (Fiala 2005), and data were exported to a database in Excel (n=9,950 boutons measured for major diameter). The mean bouton diameter for each case was obtained using the method of progressive means analysis which is based on calculating the mean in successive smaller samples from the total. The systematic, random sampling fraction was 1/100 of the total volume of the region studied through the primary olfactory cortex. We measured the major diameter of >1000 labeled bouton profiles per layer in each case. We used k-means cluster analysis on the major diameter of ACC labeled boutons (SPSS 16.0 for Windows) to generate a cutoff point and separate the population of labeled boutons into size groups (large and small).
We estimated the number and density of ACC labeled boutons in the primary olfactory cortex areas and layers using the unbiased stereological method of the optical fractionator (Gundersen 1986; Howard and Reed 1998) with the aid of a commercial system (StereoInvestigator; MicroBrightField). We systematically distinguished large and small boutons based on the results of the bouton population analysis described above. For each case, we selected a minimum of five evenly spaced brain sections using systematic random sampling to count boutons in different laminar and areal compartments of the primary olfactory cortex. The stereological data included volume calculation for each olfactory area and layer, which takes into consideration the sampled area and thickness of each section. The top and bottom of each section (minimum 2 μm for 15 μm sections after shrinkage) were used as guard zones. In BDA cases, boutons were counted using an optical disector restricted to the central fraction of the tissue thickness (11 μm). In LY cases the penetration of the antibodies did not reach the complete thickness of the section leaving a central band of tissue unstained, thus, the optical disector was restricted to the upper stained portion of the section (5 μm) with a guard zone of 2 μm. The actual mounted section thickness was measured by the program at each counting site. The counting frame/disector size (area 25×25 μm; height=11 μm for BDA sections and 5 μm for LY sections) and grid spacing (ranging from 150×150 μm) were set to employ a fraction to yield a coefficient of error of ≤ 10%, as recommended (Gundersen 1986; Howard and Reed 1998). The stereological analysis yielded estimates of the total number of boutons in each area and layer of the primary olfactory cortex. These estimates and the volume calculation for each layer were used to calculate bouton density per layer.
We employed one-way ANOVA (SPSS 16.0 for Windows) for statistical comparisons of labeled bouton major diameter, and for the overall number and proportion of large and small boutons among cases in the primary olfactory areas and layers.
Ultrastructural analysis
To process brain tissue to view in the electron microscope (EM) we first identified five coronal sections with the densest anterograde label through AON based on brightfield maps of the pattern of label, as described previously (Medalla et al. 2007; Medalla and Barbas 2009, 2010, 2012). We then processed sections from matched levels in adjacent series for immunohistochemistry (as described above), which included AON layers I and II. We then cut and processed for EM 100-μm-wide tissue blocks (BI: 12 blocks from 5 sections; BN: 10 blocks from 4 sections), which included either layer I or II of AON. We flat embedded the sections in resin (as described above). Using an ultramicrotome (Ultracut; Leica), the blocks were trimmed with a diamond trim tool and then cut into serial ultrathin sections (50 nm) with a diamond knife (Diatome). We viewed fields of the primary olfactory cortex with ACC labeled boutons in the EM (100CX, JEOL, Peabody, MA, USA) in two cases (BI and BN).
We used classical criteria to determine synapse type and the postsynaptic profile of ACC boutons (Peters et al. 1991) as described previously (Germuska et al. 2006; Zikopoulos and Barbas 2006; Medalla et al. 2007; Zikopoulos and Barbas 2007; Medalla and Barbas 2009, 2010; Bunce and Barbas 2011; Medalla and Barbas 2012). In the cortex synapses are made with spines, which are enriched on excitatory pyramidal neurons, or with aspiny (smooth) or sparsely spiny dendritic shafts, which are characteristic of inhibitory neurons in the cerebral cortex (Peters et al. 1991; Fiala and Harris 1999).
For 2D analysis we sampled exhaustively ACC labeled boutons from series of ten to sixty consecutive sections obtained from each of several blocks. Each bouton was followed and photographed through a minimum of ten adjacent serial sections for each synapse. Major diameter of ACC labeled boutons was measured at the level of the synapse using Reconstruct (www.bu.edu/neural; (Fiala 2005). We also followed labeled boutons that formed synapses with presumed inhibitory postsynaptic targets (40 or more sections) and reconstructed them in 3D (n=6). Section thickness was calibrated through measurements of the diameter of mitochondria which yields the same estimate of section thickness as the method of minimal folds (Fiala and Harris 2001b). Object contours of boutons and postsynaptic elements were manually traced section-by-section. Postsynaptic targets were considered as belonging to presumed inhibitory neurons if they were labeled for any of the calcium-binding proteins PV, CR or CB, and/or were part of aspiny or sparsely spiny dendrites. The latter were characterized by computing a density index for spines (number of spines/μm) and synapses (number of synapses/μm) of reconstructed dendrites as described previously (Fiala and Harris 2001a; Medalla and Barbas 2009, 2010, 2012).
Photography
We captured images of ACC labeled boutons and axons in the primary olfactory cortex at the optical microscope using a CCD camera (Olympus DP70) attached to a microscope (Olympus optical microscope, BX 60) connected to a personal computer using a commercial imaging system (DPController). ACC labeled boutons and their postsynaptic profiles were captured at the EM using a digital camera (ES1000W, Gatan, Pleasanton, CA, USA) at a magnification of 10,000–50,000X. Images were imported into Canvas X software (ACD Systems, Miami, FL, USA) to assemble in figures. Minor adjustment of overall brightness and contrast were made but images were not retouched.
Results
Injection sites of tracers encompassed several sites of ACC
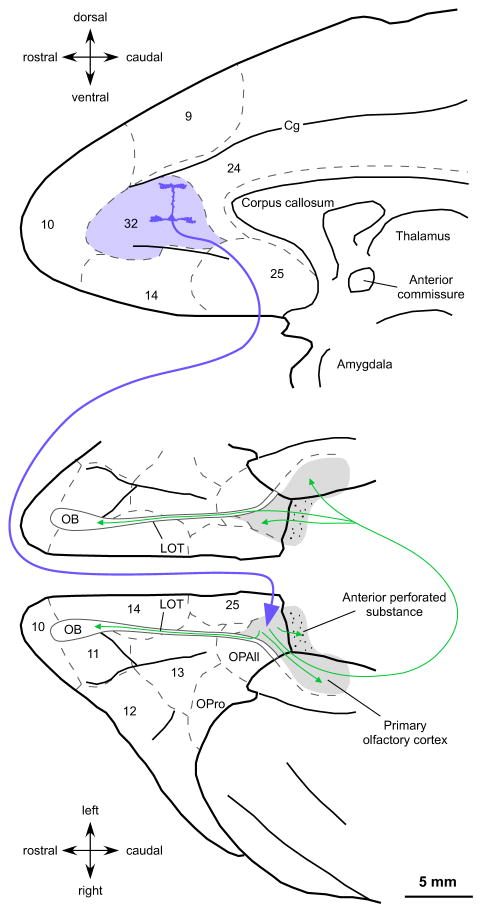
We investigated the terminations of labeled pathways from ACC to the primary olfactory cortex at the level of the system and the synapse. Figure 1a shows a composite diagram of the injection sites on a map of the medial prefrontal cortex (Barbas and Pandya 1989). Neural tracer injections in ACC involved dorsal and ventral portions of area 32 along its antero-posterior axis. In three cases the tracer was restricted to area 32 (cases AY, BI, and BL). In one case (BG) the tracer included the dorsal portion of area 32 within the lower bank of the cingulate sulcus and impinged on the medial part of area 9. In another case (BN) the injection was within the posterior part of area 32 and spread posteriorly into adjacent cingulate area 24. In another case (DQ), the tracer was mostly in area 32, but spread slightly to the adjacent area 14. In all cases the tracer occupied both superficial and deep layers of area 32.
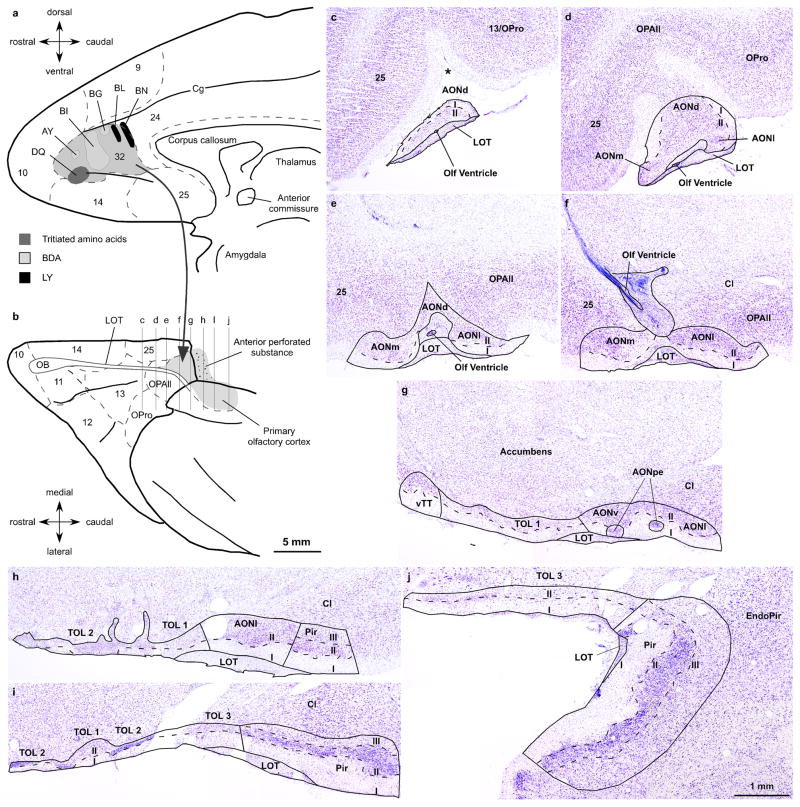
Fig. 1.
Experimental design and cytoarchitectonic parcellation of the primary olfactory cortex. a, Cortical injection sites are shown on a map of the medial surface of the rhesus monkey brain; area 32 is shaded in grey. b, Projection of the primary olfactory cortex (grey) on a view of the orbital cortex. Architectonic borders of medial and orbital prefrontal cortices (dotted lines) are according to the map of Barbas & Pandya (1989). c–j, Cytoarchitectonic parcellation of the primary olfactory cortex. The levels of the Nissl stained coronal sections are shown on a map of the orbital surface of the rhesus monkey brain (b). Asterisk in c shows the olfactory sulcus. Calibration bar in b applies to a and b; calibration bar in j applies to c–j. Abbreviations: AONd, anterior olfactory nucleus, dorsal sector; AONm, anterior olfactory nucleus, medial sector; AONl, anterior olfactory nucleus, lateral sector; AONpe, anterior olfactory nucleus, pars externa; AONv, anterior olfactory nucleus, ventral sector; Cg, cingulate sulcus; Cl, claustrum; EndoPir, endopiriform nucleus; I, layer I; II, layer II; III, layer III; LOT, lateral olfactory tract; TOL 1, olfactory tubercle, sector 1; TOL 2, olfactory tubercle, sector 2; TOL 3, olfactory tubercle, sector 3; OB, olfactory bulb; Olf ventricle, olfactory ventricle; OPAll, orbital periallocortex; OPro, orbital proisocortex; Pir, piriform cortex; vTT, ventral tenia tecta
The primary olfactory cortices have two or three layers
We briefly describe the organization of the primary olfactory cortex in the rhesus monkey to provide a structural context for projections from ACC. The primary olfactory cortex includes all telencephalic areas that receive a direct projection from the olfactory bulb through the lateral olfactory tract (LOT) (Price 1973; Haberly and Price 1977; de Olmos et al. 1978; Turner et al. 1978; Carmichael et al. 1994). In primates, the olfactory cortex is composed of several areas that have either two or three layers. The chief recipients of olfactory bulb projections are the anterior olfactory nucleus (AON), the piriform cortex (Pir) and the olfactory tubercle (TOL). In the primate brain these areas are found along the LOT, situated medial or posterior to the orbitofrontal cortex, and on the medial surface of the temporal lobe anterior to the amygdala (Fig. 1b). For areal and laminar parcellation of the monkey primary olfactory cortex we followed the cytoarchitectonic descriptions from previous studies focusing on AON, TOL and Pir (Crosby and Humphrey 1939; Meyer and Allison 1949; Turner et al. 1978; Carmichael et al. 1994). Figure 1c–j shows the areal and laminar boundaries of the primary olfactory cortex in a series of coronal sections through the right hemisphere (case AT) whose levels are shown in Figure 1b on a map of the orbital prefrontal cortex (Barbas and Pandya 1989).
The AON is the most anterior part of the primary olfactory cortex. It rests on the LOT and extends medial and posterior to the pOFC. The principal AON can be divided into several sectors by their position on the basal surface, all characterized by a superficial molecular layer I and a subjacent cellular layer II (Fig. 1c–h). The dorsal sector (AONd) is situated beneath the olfactory sulcus (asterisk in Fig. 1c), also known as the olfactory trigone based on its shape. More posteriorly, AONd loses its triangular shape as it abuts the orbital periallocortex (OPAll), situated in the posterior orbitofrontal cortex (Fig. 1d). At posterior levels there are two nests of densely packed cells between LOT and layer I that make up the AON pars externa (AONpe; Fig. 1g), which receives a spatially organized projection from the olfactory bulb and sends feedback projections to the contralateral bulb (Reyher 1988; Carmichael et al. 1994). On the medial side, the ventral tenia tecta (vTT) shows two layers similar to other AON sectors, which we included as part of AON in the stereological analysis (Fig. 1g).
The TOL is found on the ventral surface of the brain and can be identified as the anterior perforate substance medial to the LOT. The three sectors of TOL have a molecular layer I only at some levels and a deep layer II that fuses with the accumbens nucleus (Fig. 1g–j).
The Pir has a horizontal (frontal) limb posterior to pOFC and a vertical limb on the medial side of the temporal lobe anterior to the cortical nuclei of the amygdala. The Pir is distinguished by a characteristic layer II, packed with neurons below the molecular layer I, and by a deep polymorphic layer III (Fig. 1h–j).
ACC boutons in the primary olfactory cortex innervate robustly AON
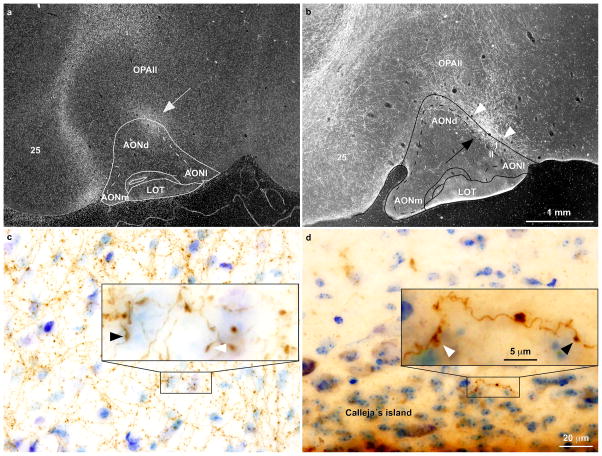
Labeled pathways from ACC terminated in distinct parts of the primary olfactory cortex. Pathways from ACC area 32 terminated mostly in AON and to a lesser extent in TOL, while only a few labeled axon terminals were seen in Pir. In most cases pathways from ACC terminated as dense patches of label in layers I and II of the dorsal sector of AON (AONd), and the label extended to the superficial and deep layers of the adjacent orbitofrontal cortex (area OPAll; Fig. 2a, b). At this level, the boundary between layer I of AONd and layer I of OPAll is marked by multiple small vessels interposed between the molecular layer of AONd and the molecular layer of OPAll (Fig. 2b). Labeled boutons from ACC axons in the primary olfactory cortex appeared as axon swellings, which were either terminaux or en passant varicosities (Fig. 2c, d). The largest BDA injection labeled numerous boutons in layer I of AONd forming dense patches that extended to layer II (case BI, Fig. 3). Layers I and II of the other AON sectors also had a moderate to light density of labeled boutons. The vTT had sparse label. Layer II of TOL included some sites with moderate label. In Pir, labeled axon terminals were sparsely distributed in the three layers in rostral levels. In another case (BN), a more posterior injection within area 32, which extended to adjacent area 24, labeled a dense plexus of boutons in layer I of all AON sectors. In this case the TOL also showed moderately dense label, while in Pir we found only a few labeled boutons from ACC axons which were restricted to its most anterior levels (Fig. 4). ACC labeled boutons and axons in the other cases followed a similar distribution. In one case (BG), in which the injection was in the lower bank of the cingulate sulcus, anterograde label was light in all primary olfactory areas. In all cases, label in AONpe was scant.
Fig. 2.
Labeled terminals and boutons from ACC axons terminate in the olfactory AON. a, Darkfield photomicrograph shows dense patch of anterograde label (white grain, white arrow) found at the level of AONd extending to the adjacent orbitofrontal area OPAll (case DQ, tracer 3H-labeled amino acids). b, Darkfield photomicrograph shows dense patches of labeled axons (white patch, black arrow) in the same location as in A, and in the adjacent orbital cortex in another case (case BI, tracer BDA); white arrowheads point to two layers of small vessels that mark the limit between layer I of AONd and layer I of orbital area OPAll. c, Brightfield photomicrographs at higher magnification show labeled axons and boutons (brown dots) in layer I of AON (case AY, tracer BDA); and d, in the islands of Calleja of the olfactory TOL 2 (case BG, tracer BDA). The insets in C and D show en passant (black arrow heads) and terminaux (white arrow heads) boutons on labeled axons. Photomicrographs are from coronal sections through the primary olfactory cortex. Calibration bar in b applies to a and b. Calibrations bars in d apply to c and d.
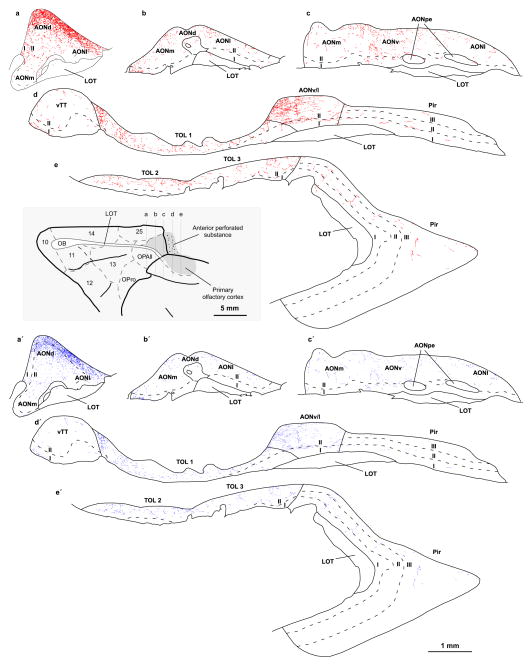
Fig. 3.
Distribution of ACC axon terminations in the primary olfactory cortex. Maps from consecutive coronal sections through the primary olfactory cortex show the distribution of labeled boutons from ACC axons in the primary olfactory cortex (case BI, tracer BDA). Each red dot (top) represents a small bouton and each blue dot (bottom) represents a large bouton. The level of the images is shown on a map of the orbital surface of the rhesus monkey brain (grey box). For abbreviations see caption to Figure 1. Small and large boutons form ACC axons innervate chiefly AONd where they are densely distributed in layer I at this level (a, a′). There is moderate label from ACC axons in other AON sectors and vTT (b–d, b′–d′) and TOL (d–e, d′–e′). Fewer boutons are found in the piriform cortex (d–e, d′–e′)
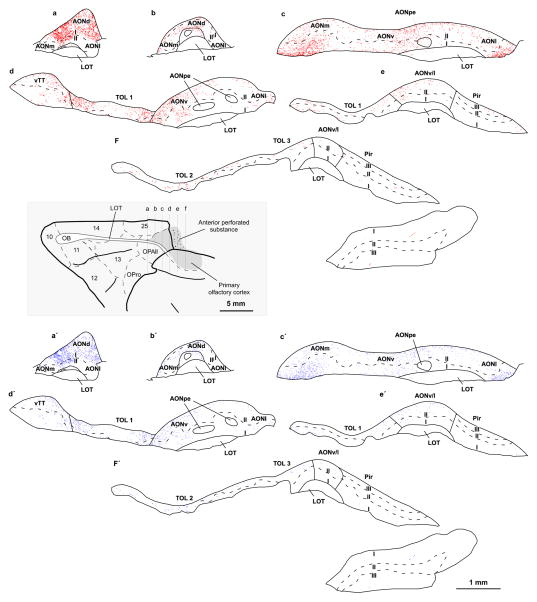
Fig. 4.
Distribution of ACC axon terminations in the primary olfactory cortex. Maps from consecutive coronal sections through the primary olfactory cortex show labeled boutons from ACC axons (case BN, tracer LY). Each red dot (top) represents a small bouton and each blue dot (bottom) represents a large bouton. The level of the images is shown on a map of the orbital surface of the rhesus monkey brain (grey box). For abbreviations see caption to Figure 1. Most axon boutons from ACC pathway were found in AONd, where they were densely distributed in layer I (a, a′) but also in layer I and layer II of other AON sectors (b–f, b′–f′). vTT and TOL also showed moderate label (d–f, d′–f′). Only a few labeled boutons from ACC axons innervated the piriform (Pir) cortex (e–f, e′–f′)
Projections from ACC to the primary olfactory cortex thus targeted mostly AON, to a minor extent TOL, and sparsely Pir. There was no evidence of ACC labeled boutons in other areas that also receive projections from the olfactory bulb in the macaque monkey brain, including the periamygdaloid cortex or the cortical nuclei of the amygdala. We found labeled axon terminals in several layers of the anterior part of the entorhinal cortex, which receives a small projection from the olfactory bulb in layer I. Further description here is restricted to those olfactory areas within the frontal lobe that are the major recipients of olfactory bulb afferents in the macaque monkey brain (Carmichael et al. 1994).
A significant proportion of ACC boutons in the primary olfactory cortex are large
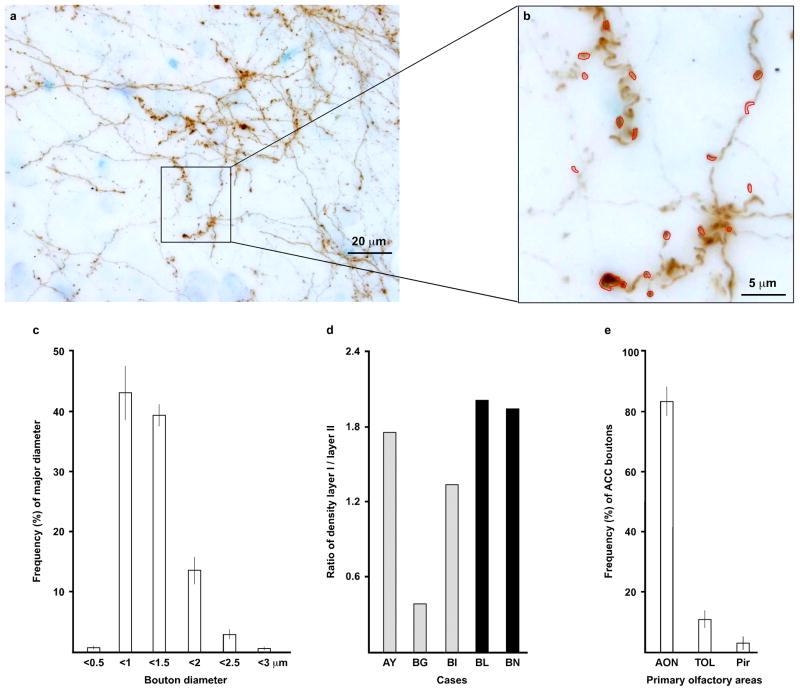
The size of axon boutons is positively correlated with the number of synaptic vesicles (Germuska et al. 2006; Zikopoulos and Barbas 2006, 2007) and with the probability of neurotransmitter release with each action potential (Murthy et al. 1997; Stevens 2003). We thus traced and measured the major diameter of ACC labeled boutons in all areas and layers of the primary olfactory cortex at the light microscope (Fig. 5a, b). Figure 5c shows the distribution of labeled ACC boutons by size (cases AY, BG, BI, BL, and BN; ± standard deviation). Because analysis (ANOVA) showed no significant difference in size of major diameter among cases for different olfactory areas or layers, we pooled data into a single group. The mean major diameter across cases was 1.12 μm (sd ± 0.39).
Fig. 5.
Presynaptic characteristics of the ACC pathway to the primary olfactory cortex. a, Compressed stack of images taken at several focal planes in an area of dense ACC anterograde label in layer I of the AON (case BI, tracer BDA). b, Labeled boutons were traced manually using the open source program Reconstruct (Fiala, 2005; five cases: AY, BG, BI, BL, and BN). c, Distribution of labeled ACC boutons in olfactory areas by size (bars show the mean frequency of the five cases, as above; vertical line on bars shows the standard deviation). The figures are based on the major diameter of boutons measured at the light microscope as described in b. d, Ratio of bouton density in layer I to layers II–III, based on values estimated using stereology (five cases, as above). e, Proportion of estimated ACC boutons in distinct primary olfactory areas using stereology (bars show the mean frequency of the five cases, as above; vertical line on bars shows the standard deviation); most ACC boutons were found in AON. Abbreviations: AON, anterior olfactory nucleus; Pir, piriform cortex; TOL, olfactory tubercle
In each case, k-means cluster analysis segregated ACC labeled boutons into small and large clusters separated by a cutoff value (SPSS 16.0 for Windows). Because ANOVA yielded no significant effects of case, area, or layer on the cutoff value and cluster centers, we pooled data into a single group. The cutoff value was 1.26 μm; the small cluster center was 0.92 μm and the large cluster center was 1.59 μm. We next investigated the distribution of large (≥1.26 μm) and small (<1.26 μm) ACC boutons in the primary olfactory cortex areas and layers using unbiased stereological methods. The overall mean proportion of small boutons was 72 ± 0.02% across cases and of large boutons 28 ± 0.02%. Analysis showed no significant effects by case, area or layer on percentage of large and small boutons (ANOVA).
ACC boutons are denser in layer I of the primary olfactory cortex
We estimated the laminar distribution and density of labeled boutons from the ACC to the primary olfactory cortex using unbiased stereological methods to compare ACC projections with other afferents to primary olfactory areas. Previous studies in rats have shown that axons from the olfactory bulb terminate in the superficial part of layer I of the primary olfactory cortex, while associational intracortical axons are concentrated mostly in the deep part of layer I and the superficial part of layer II of all primary olfactory areas and in layer III of Pir (Valverde 1965; Price 1973; Haberly and Price 1978a, b; Luskin and Price 1983a, b).
For the laminar analysis, we included layer III of Pir with layer II of AON, TOL and Pir because it had only a few labeled boutons. The density of labeled boutons was higher in layer I than in layers II–III in all cases, except for the case with an injection restricted to the lower bank of the cingulate sulcus (BG), in which labeled boutons were denser in layers II–III. Figure 5d shows the ratio of the density of boutons in layer I and layers II–III in the five cases. Figure 5e shows that ACC boutons (cases AY, BG, BI, BL, and BN) were found mostly in the AON (84 ± 10%), with the rest found in TOL (11 ± 6%) and the Pir.
ACC boutons in AON form synapses mostly with spines of putative excitatory neurons
We then studied the pathway from ACC to primary olfactory areas at the synaptic level in layers I and II of AON, the largest recipient of ACC terminals among primary olfactory cortices (cases BI and BN). This analysis made it possible to achieve two objectives. First, to compare results with the findings obtained at the level of the system; second, to identify the postsynaptic sites of ACC boutons and their potential excitatory or inhibitory nature (Fig. 6a). Serial sections helped identify and classify postsynaptic elements with a high degree of confidence.
Fig. 6.
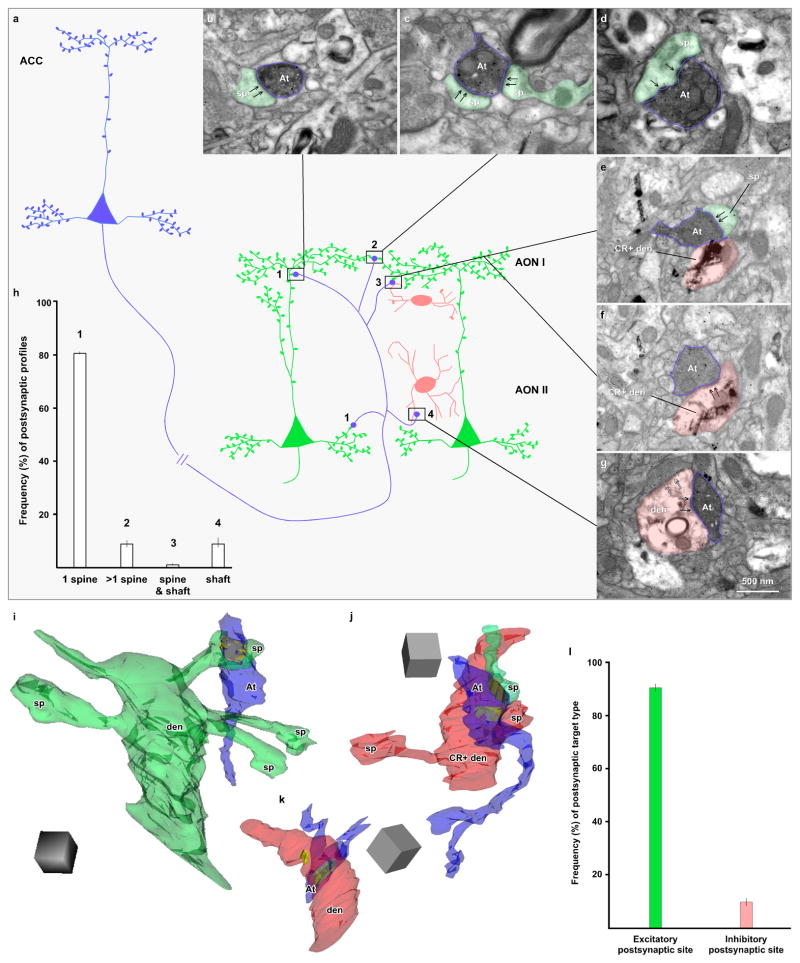
The fine structure of ACC boutons that form synapses in AON. a, Schematic representation of synaptic relationships between ACC boutons and AON excitatory and inhibitory neurons: blue depicts ACC projection neurons and axon terminals, green depicts excitatory postsynaptic pyramidal neurons in the olfactory AON, pink shows inhibitory postsynaptic interneurons in AON. ACC projection neurons and AON pyramidal neurons have spiny dendrites with sparsely spiny dendritic shafts proximal to the cell body, while AON inhibitory neurons have smooth dendrites or only a few spines. 1 depicts an ACC axon terminal that forms a synapse on one spine; 2 shows an ACC synapse on two spines; 3 shows ACC synapse on one spine and one dendritic shaft; and 4 depicts ACC synapse on a dendritic shaft. b–g, Photomicrographs through synapses made by ACC axon boutons in olfactory areas. ACC boutons were labeled with DAB, which appears as a dark uniform precipitate. b, ACC bouton (blue outline, At) forms a synapse (black arrows) with a single spine (green shade, sp) in AON layer I. c, ACC bouton (blue outline, At) forms two synapses (black arrows) with two spines (green shade, sp) in AON layer I. d, ACC bouton (blue outline, At) forms a perforated synapse (black arrows) with a single spine (green shade, sp) in AON layer I. e–f, ACC bouton (blue outline, At) forms one synapse (black arrows) with a spine (green shade, sp) (e) and, at another level (f), it forms another synapse (black arrows) with a dendritic shaft in layer I of AON (pink shade); the shaft (pink) is labeled with TMB (rod-shaped crystals) for calretinin (CR+). g, ACC bouton (blue outline, At) forms a synapse (black arrows) with a dendritic shaft (pink shade) in layer II of AON; the shaft is not labeled for calcium binding proteins and receives a synapse from another unlabeled bouton (silhouette arrows). Calibration bar in g applies to b–g. h, Proportion of all labeled boutons with distinct postsynaptic targets (two cases: BI and BN; vertical line on bars shows standard deviation). ACC boutons formed synapses mostly with one spine in the primary olfactory cortex. ACC boutons innervating dendritic shafts made up ~10% of the total. i, 3D reconstruction of a synapse between an ACC bouton (blue) and a spiny dendrite (green); the postsynaptic density is shown in yellow. j, 3D reconstruction of an ACC bouton (blue) forms a synapse with a spine (green, sp) and a sparsely spiny dendrite (pink) labeled for calretinin (CR+); postsynaptic densities are shown in yellow. k, 3D reconstruction of a synapse between an ACC bouton (blue, At) and a smooth dendrite (pink) in layer II of AON; postsynaptic densities are shown in yellow. Scale cube in i–k is 0.5 μm. l, Proportion of presumed excitatory and inhibitory postsynaptic targets of ACC boutons in AON (two cases, as above; vertical line on bars shows standard deviation). ACC boutons formed synapses mostly with spines of excitatory pyramidal neurons of AON. At, axon terminal; CR, calretinin; den, dendritic shaft; sp, spine
Two dimensional analysis showed that the average major diameter of ACC labeled boutons in AON was comparable in the two cases at the level of the synapse (Case BI, 0.96 μm, sd ± 0.31μm, n=61; case BN, 0.92 μm, sd ± 0.26 μm, n=16). These figures are within the range of the average mean diameter of ACC labeled boutons measured at the optical microscope in AON, as described above.
All labeled boutons formed asymmetric synapses (presumed to be excitatory) as in other ACC corticocortical pathways (Medalla et al. 2007; Medalla and Barbas 2009, 2010; Bunce and Barbas 2011; Medalla and Barbas 2012). Labeled boutons from ACC mainly innervated single spines in AON (case BI: 82%, n=50; case BN: ~81%, n=13; Fig. 6b), and a few formed synapses with two spines (case BI: 11.5%, n=7; case BN: ~6%, n=1; Fig. 6c). These patterns resemble other ACC pathways directed to prefrontal and temporal cortices (Medalla et al. 2007; Medalla and Barbas 2009, 2010; Bunce and Barbas 2011; Medalla and Barbas 2012), or between other cortices (Anderson et al. 1998; Melchitzky et al. 1998). Some ACC boutons formed perforated synapses with spines, characterized by segmented postsynaptic densities (case BI: 24.6%, n=15; case BN: 17.6%, n=3; Fig. 6d). Perforated synapses are thought to be more efficacious than non-perforated synapses (Greenough et al. 1978; Sirevaag and Greenough 1985; Geinisman et al. 1987; Ganeshina et al. 2004). Innervated spines were not labeled for calcium-binding proteins and were presumed to be on dendrites of excitatory neurons.
A minority of ACC boutons in AON form synapses with inhibitory neurons
Inhibitory neurons are either devoid of spines or have a low spine density in the cerebral cortex. Inhibitory neurons thus receive synapses on their shafts, a feature that can be used to identify synapses on inhibitory neurons by morphology. Inhibitory neurons in the cerebral cortex can also be identified neurochemically by label with the calcium binding proteins PV, CR, or CB. We used one or both criteria to identify synapses of ACC axons with inhibitory neurons in the primary olfactory cortex.
A small number of boutons from ACC axons formed synapses on spines and dendritic shafts (case BI: 1.6%, n=1; Fig. 6e, f), or only on dendritic shafts (case BI: ~5%, n=3; case BN: 12.5%, n=2; Fig. 6g); one of the latter formed a perforated synapse (case BI, Fig. 6g). In layer I of AON all boutons from ACC that formed synapses on dendritic shafts were labeled for CR (case BI: n=2; case BN: n=2), but in layer II they were not labeled for any of the three non-overlapping neurochemical classes of presumed inhibitory neurons that are positive for the calcium-binding proteins (PV, CR, and CB; case BI: n=2). Figure 6h shows the percentages of each postsynaptic target.
We performed 3D analysis of the six postsynaptic dendrites innervated by ACC boutons to investigate if their morphologic features are consistent with presumed inhibitory or excitatory neurons (Peters et al. 1991; Fiala and Harris 1999). We reconstructed each dendrite and computed a density index for spines/μm and synapses/μm, as described previously (Fiala and Harris 2001a; Medalla and Barbas 2009, 2010). Figure 6i shows the 3D reconstruction of a typical spiny dendrite with an average of 2 spines/μm and one ACC labeled terminal forming a synapse on one spine; this segment of a spiny dendrite was devoid of synapses on its shaft. Two CR labeled dendrites in layer I (case BI) were sparsely spiny (with 0.42 and 1.25 spines/μm, respectively), and had ~0.4 asymmetric synapses/μm; one example is shown in a reconstructed synapse in Figure 6j. Two other CR labeled dendrites in layer I (case BN) were smooth and had 1 asymmetric synapse/μm. Non-labeled dendrites in layer II (case BI: n=2) were smooth and had ~1 asymmetric synapses/μm, similar to the one reconstructed in Figure 6k.
Excitatory neurons in the cerebral cortex have spiny dendrites and rarely receive synapses on their shafts, while inhibitory neurons have mostly smooth dendrites that receive synapses. Sparsely spiny dendrites may belong to the proximal dendritic segments of excitatory neurons, or to inhibitory neurons. The spine density of sparsely spiny dendrites overlaps with the density of spiny dendrites. However, inhibitory neurons have a significantly higher density of synapses on shafts, which are virtually absent on spiny dendrites of excitatory neurons [(Medalla and Barbas 2009, 2010); for a review see (Peters et al. 1991; Fiala and Harris 1999)]. In AON, there are pyramidal excitatory neurons with distal dendrites that are spiny and proximal dendrites that are moderately spiny. AON also included several types of non-pyramidal neurons which are presumed to be inhibitory and have smooth or sparsely spiny dendrites. The only segments of the dendritic tree of pyramidal excitatory neurons that reach layer I of AON are the distal (spiny) segments of the apical dendrites (Valverde 1965; Valverde et al. 1989; Brunjes and Kenerson 2010). Based on this evidence, we concluded that the smooth and sparsely spiny dendrites that ACC innervated in AON were on putative inhibitory neurons.
In summary, a large majority of ACC boutons in AON layers I and II (case BI: 94%; case BN: 88%) formed synapses with putative excitatory neurons, while only a small number (case BI: 6%; case BN: 12%) formed synapses on smooth or moderately spiny dendritic shafts of presumed inhibitory neurons (Fig. 6l). In the latter cases, the ACC innervated dendritic shafts that were labeled for CR in AON (layer I).
Discussion
We have found a novel monosynaptic pathway from ACC to the primary olfactory cortex in the rhesus monkey brain. The ACC pathway chiefly innervated the anterior olfactory nucleus where it formed excitatory synapses mostly on profiles of other excitatory postsynaptic neurons. Interestingly, behavioral and functional data suggest that activation of the primary olfactory cortex depends on attention (Zelano et al. 2005; Sela and Sobel 2010). Moreover, the ACC, which is activated when cognitive demand is high (Botvinick 2007; Pessoa 2008), and during working memory tasks (Fuster 2008), is also activated by olfactory stimuli (Poellinger et al. 2001; Small and Prescott 2005; Tabert et al. 2007). The novel direct pathway from ACC to primary olfactory areas has functional implications for attention to olfactory stimuli.
The ACC projects densely and efficiently to AON in the primary olfactory cortex
Axon terminals from ACC had an uneven distribution in the primary olfactory cortex, with more than two thirds concentrated in the principal AON. In AONd, ACC boutons formed dense patches in layer I that extended to layer II as well as to neighboring orbitofrontal cortex. At this level, layer I of AONd and layer I of orbitofrontal cortex (area OPAll) are apposed and separated by two layers of blood vessels (Fig. 2b). This pattern is reminiscent of the apposition formed by layer I of the dentate gyrus and layer I of the cornu ammonis in the hippocampal formation. At such junctions, the abutting structures do not interact at the dendritic level (Cajal 1893; Lorente de Nó 1934; Rihn and Claiborne 1990). Therefore, ACC boutons in AONd layer I innervate dendritic elements of AON, and not dendrites from the adjacent orbitofrontal cortex. The other sectors of AON also showed substantial ACC labeling with the exception of AONpe. Outside AON, some ACC boutons innervated the olfactory tubercle, and sparsely the piriform cortex.
The pathways from ACC to the primary olfactory cortex are probably reciprocal, like the large majority of corticocortical connections (Schmahmann and Pandya 2006). In macaque monkeys, medial prefrontal areas 25 and 14 receive projections from the primary olfactory cortex (Carmichael et al. 1994). Further studies are needed to investigate if the pathway from ACC area 32 to the primary olfactory cortex described here is also reciprocal.
Terminals from ACC axons were more densely distributed in layer I than in layer II (or III for Pir) of the primary olfactory cortex. The significance of this observation is based on the laminar specificity of innervation of primary olfactory areas by other afferents. In rats, as olfactory bulb axons leave the LOT they terminate in the superficial part of layer I (also known as layer Ia), while most associational intracortical axons are concentrated in the deep part of layer I (also known as layer Ib), and the superficial portion of layer II of all primary olfactory areas, and in layer III of Pir (Valverde 1965; Price 1973; Luskin and Price 1983a, b; Illig and Eudy 2009). This laminar distribution, which has been described most fully in rats, is also found in macaque monkeys, but the pattern does not appear to be as sharp (Carmichael et al. 1994). Nevertheless, the ACC pathway is in a key position to influence all of layer I of the primary olfactory cortex, which receives olfactory bulb and many associational intracortical axons.
A prominent feature of the ACC pathway to the primary olfactory cortex was the large size of terminals, which accounted for about one third of the ACC boutons that innervated primary olfactory areas. The significance of this finding is based on evidence that bouton size is positively correlated with the number of synaptic vesicles (Germuska et al. 2006; Zikopoulos and Barbas 2006) and with the probability of neurotransmitter release with each action potential (Murthy et al. 1997; Stevens 2003).
Our findings at the synaptic level further suggest that ACC projections to the primary olfactory cortex predominantly innervate other excitatory neurons. The ACC pathway to AON formed a higher proportion (~90%) of synapses on spines of excitatory neurons than in other cortical pathways of ACC to parahippocampal cortices (77%), or to dorsolateral prefrontal cortices (~63–88%) (Medalla and Barbas 2009, 2010; Bunce and Barbas 2011). Conversely, we found that only a small proportion (<12%) of ACC boutons to the olfactory AON formed synapses on shafts of smooth or sparsely spiny dendrites, features of inhibitory interneurons in the cerebral cortex (Peters et al. 1991; Fiala and Harris 1999). This figure is lower than the proportion of inhibitory neurons that ACC innervates in the parahippocampal (23%) or dorsolateral prefrontal cortices (~16–35%) (Medalla and Barbas 2009, 2010; Bunce and Barbas 2011). The few synapses made by ACC with presumed inhibitory neurons in layer I of AON involved exclusively the neurochemical class of CR neurons. In other cortical areas, CR neurons innervate other inhibitory neurons, at least in the upper cortical layers (Meskenaite 1997; Melchitzky et al. 2005). If this is the case for the primary olfactory cortex as well, innervation of CR neurons in olfactory cortex by ACC could lead to disinhibition of nearby excitatory neurons.
The pattern of ACC innervation of a subset of inhibitory neurons in parahippocampal or dorsolateral prefrontal cortices is consistent with the function of reducing noise and enhancing signal in cognitive operations (Medalla and Barbas 2009, 2010; Bunce and Barbas 2011). In contrast, the pattern of innervation and ultrastructural characteristics of ACC pathways to AON suggest a different function. Overall, our findings suggest that ACC axons are poised to exert a powerful postsynaptic excitatory effect on the primary olfactory cortex through dense terminations on spines of excitatory neurons, and a small number of synapses with CR inhibitory neurons.
ACC is in a key position to influence activity in the primary olfactory cortex through AON
The organization and connections of the primary olfactory cortex differ from other sensory areas. Projections from the olfactory epithelium to the olfactory bulb are spatially ordered producing a “map” of odor quality (Ressler et al. 1994; Vassar et al. 1994; Mombaerts et al. 1996). This chemotopy is preserved only in the olfactory bulb projections to AONpe (Reyher 1988; Carmichael et al. 1994; Yan et al. 2008). In contrast, olfactory bulb projections to the rest of the primary olfactory cortex lack topographic organization [(Haberly and Price 1977; de Olmos et al. 1978; Carmichael et al. 1994; Ghosh et al. 2011; Miyamichi et al. 2011; Sosulski et al. 2011); reviewed in (Shepherd 2007)]. In accord with the connectional pattern, functional studies show that odorant stimuli activate ensembles of neurons throughout the primary olfactory cortex in a non-specific spatial pattern, as individual neurons receive input from multiple glomeruli and respond to multiple odors (Illig and Haberly 2003; Lei et al. 2006; Rennaker et al. 2007; Howard et al. 2009; Poo and Isaacson 2009; Stettler and Axel 2009; Kay et al. 2011).
In addition to receiving a direct projection from the olfactory bulb, pyramidal neurons in the primary olfactory cortex receive extensive intrinsic associational and commissural projections originating in other excitatory pyramidal neurons of the primary olfactory cortex (Valverde 1965; Haberly and Price 1978a, b; Luskin and Price 1983a, b). Each pyramidal neuron is thought to target more than 1,000 other neurons in the primary olfactory cortex (Johnson et al. 2000; Franks et al. 2011). Most associational projections in layer I reach the proximal segments of the apical dendrites of pyramidal neurons close to the cell body. Consequently, activation of pyramidal neurons by olfactory bulb input is followed by a second wave of excitation mediated by intrinsic association axons, and followed by strong local inhibitory feedback (Johnson et al. 2000; Stettler and Axel 2009). In fact, odor-evoked inhibition in the piriform cortex is non-selective and widespread and may depress odor-induced excitation across the primary olfactory cortex so that only neurons that receive strong excitation are driven to spike (Poo and Isaacson 2009).
The distributed pattern of olfactory bulb projections and the extensive system of intrinsic axons suggest that the primary olfactory cortex is akin to an associative cortex. In this sense, the primary olfactory cortex may capture the significance of an odorant through non-patially organized and widely distributed neurons and recognize complex ensembles of odorant features rather than specific features (Johnson et al. 2000; Stettler and Axel 2009). In this context, we found that the predominant projection from ACC to the primary olfactory cortex was to AON, which gives rise to extensive and bilateral intrinsic associational connections. The AON projects on both sides to the primary olfactory cortices with axons that terminate directly in the deep portion of layer I and the superficial portion of layer II, where they contact the proximal segments of the apical dendrites of pyramidal neurons (Haberly and Price 1978a, b; Luskin and Price 1983a, b; Illig and Eudy 2009). The AON, like the piriform cortex, also projects back to the olfactory bulb and is the main source of contralateral bulbar projections (de Olmos et al. 1978; Haberly and Price 1978a, b; Carmichael et al. 1994). The AON is thus in a key position to influence activity in the entire primary olfactory cortex through its extensive bilateral feedforward connections with the primary olfactory cortices and bilateral feedback projections to the olfactory bulb.
Activation of as few as 1% of piriform neurons can recruit an ensemble of odor responsive neurons (Franks et al. 2011) and AON neurons show even more enhanced excitability (McGinley and Westbrook 2011). Consequently, excitation of a small number of AON excitatory neurons by the efficient ACC pathway may have widespread and significant effects throughout the primary olfactory cortex, as summarized in Figure 7. Top-down regulation of the primary olfactory cortex by ACC may underlie anticipatory events prior to presentation of stimuli, as described for the piriform cortex in rats and humans (Kay and Freeman 1998; Zelano et al. 2005; Veldhuizen and Small 2011; Zelano et al. 2011).
Fig. 7.
Summary of the pathway impact from ACC on the primary olfactory cortex. The circuits at the level of the system and synapse suggest that ACC pathways (blue arrow) exert a powerful postsynaptic excitatory effect on ipsilateral pyramidal neurons of AON, a critical hub in the primary olfactory cortex based on its extensive and bilateral associational connections with other primary olfactory areas and the olfactory bulb (green arrows). ACC acting on AON may influence the entire primary olfactory cortex bilaterally for rapid attention to olfactory stimuli. For abbreviations see caption to Figure 1
The ACC also has robust connections with high order association cortices involved in olfactory processing, like the pOFC and the anterior insula (Mesulam and Mufson 1982; Mufson and Mesulam 1982; Barbas 1993; Carmichael et al. 1994). The pOFC is connected with the primary olfactory cortex and high order sensory association cortices (Barbas 1993; Carmichael et al. 1994; Cavada et al. 2000). Functional studies suggest that the pOFC is poised to process ongoing olfactory information from the primary olfactory cortex for perceptual decision making (Bowman et al. 2012), as well as integrate olfactory information with other sensory modalities [(Gottfried and Dolan 2003; Small et al. 2004); reviewed in (Gottfried and Zelano 2011)]. The anterior insula receives olfactory and taste information (Mesulam and Mufson 1982; Mufson and Mesulam 1982), is activated when subjects search for an odor or taste (Veldhuizen and Small 2011), and seems to act as a chemosensory convergence zone involved in flavor perception (Small and Prescott 2005; Small et al. 2008; Lundstrom et al. 2011). Time course studies of olfactory tasks show activation of ACC, pOFC and the anterior insula along with the primary olfactory cortex (Poellinger et al. 2001; Tabert et al. 2007; Bowman et al. 2012). This evidence suggests that the ACC can modulate attention to olfactory stimuli at the primary olfactory cortex and its integration with other sensory modalities in pOFC and the anterior insula.
Conclusions: A new pathway for attention to olfaction
Pathways from ACC to the primary olfactory cortex innervate chiefly the AON and are denser in layer I where they interact synaptically with the apical dendrites of excitatory neurons. A high proportion of ACC axons terminate as large and efficient boutons that innervate mostly spines of excitatory neurons that likely receive direct projections from the olfactory bulb. In layer I of AON, ACC boutons target only a small number of putative inhibitory neurons that are labeled for CR and may have disinhibitory effects on nearby neurons, as in the upper layers of other cortical areas. These features at the level of the system and synapse suggest that the ACC pathways can exert a powerful excitatory effect on AON, a critical hub of the primary olfactory cortex through extensive and bilateral associational connections. Acting on the olfactory AON, the novel and direct ACC pathway may influence the entire primary olfactory cortex for rapid attention to olfactory stimuli (Fig. 7).
The novel pathway from ACC to olfactory areas provides the circuit basis for functional evidence showing that the anterior part of the primary olfactory cortex is activated by sniffs and instructions (Sobel et al. 1998; Sobel et al. 2000; Zelano et al. 2005; Zelano et al. 2009; Zelano et al. 2011) in the absence of odorants. Future studies on the time course of activation of ACC and the primary olfactory cortex during olfactory tasks will help clarify the functional relationship between these two cortices and the role of ACC in attention to olfaction.
Acknowledgments
We thank Isabel Park and Justin Tepes for technical assistance; Marcia Feinberg and Clare Timbie for outstanding electron microscopy assistance; Dr. Ron Killiany for assistance with imaging; Dr. Angela Carville and Dr. Leah Makaron for veterinary care and surgical assistance; Dr. Basilis Zikopoulos, Dr. Jamie Bunce, Dr. Yohan John and Dr. Maria Medalla for helpful discussion of the manuscript. This work was supported by National Institutes of Health grants from the National Institute of Neurological Disorders and Stroke (R01NS024760) and the National Institute of Mental Health (RO1MH057414); and by Center of Excellence for Learning in Education, Science and Technology (CELEST), a National Science Foundation Science of Learning Center (NSF SBE-0354378). M. Á. García-Cabezas was the recipient in 2010 of a Short Stay Grant from Fundación Alicia Koplowitz (Spain).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Miguel Á. García-Cabezas, Email: gcabezas@bu.edu.
Helen Barbas, Email: barbas@bu.edu.
References
- Anderson JC, Binzegger T, Martin KA, Rockland KS. The connection from cortical area V1 to V5: a light and electron microscopic study. J Neurosci. 1998;18(24):10525–10540. doi: 10.1523/JNEUROSCI.18-24-10525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7(4):356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Bowman NE, Kording KP, Gottfried JA. Temporal integration of olfactory perceptual evidence in human orbitofrontal cortex. Neuron. 2012;75(5):916–927. doi: 10.1016/j.neuron.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett-Anderson JM, Lyons DM, Reiss AL, Schatzberg AF, Menon V. Functional brain imaging of olfactory processing in monkeys. Neuroimage. 2003;20(1):257–264. doi: 10.1016/s1053-8119(03)00288-x. S105381190300288X [pii] [DOI] [PubMed] [Google Scholar]
- Broca P. Localisations Cérébrales: Recherches sur les centres olfactifs. Rev D’anthropol. 1879;2(2):385–455. [Google Scholar]
- Brunjes PC, Kenerson MC. The anterior olfactory nucleus: quantitative study of dendritic morphology. J Comp Neurol. 2010;518(9):1603–1616. doi: 10.1002/cne.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JG, Barbas H. Prefrontal pathways target excitatory and inhibitory systems in memory-related medial temporal cortices. Neuroimage. 2011;55(4):1461–1474. doi: 10.1016/j.neuroimage.2011.01.064. S1053-8119(11)00100-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Estructura del asta de Ammon y fascia dentata. An Soc Esp Hist Nat. 1893:22. [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346(3):403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Carrapiso AI, Martin L, Jurado A, Garcia C. Characterisation of the most odour-active compounds of bone tainted dry-cured Iberian ham. Meat Sci. 2010;85(1):54–58. doi: 10.1016/j.meatsci.2009.12.003. S0309-1740(09)00383-0 [pii] [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. 0006-8993(72)90344-7 [pii] [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telecephalon. I. The nuclear configuration of the olfactory and accessory olfactory formations and of the nucleus olfactorius anterior of certain reptiles, birds, and mammals. J Comp Neurol. 1939;71(1):121–213. [Google Scholar]
- de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978;181(2):213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989;503(1):49–54. doi: 10.1016/0006-8993(89)91702-2. 0006-8993(89)91702-2 [pii] [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14(1):1–19. doi: 10.1016/s0891-0618(97)10013-8. S0891061897100138 [pii] [DOI] [PubMed] [Google Scholar]
- del Rio MR, DeFelipe J. Synaptic connections of calretinin-immunoreactive neurons in the human neocortex. J Neurosci. 1997;17(13):5143–5154. doi: 10.1523/JNEUROSCI.17-13-05143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Harris KM. Dendrite Structure. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. Oxford University Press; Oxford: 1999. pp. 1–34. [Google Scholar]
- Fiala JC, Harris KM. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. J Am Med Inform Assoc. 2001a;8(1):1–16. doi: 10.1136/jamia.2001.0080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Harris KM. Cylindrical diameters method for calibrating section thickness in serial electron microscopy. J Microsc. 2001b;202(Pt 3):468–472. doi: 10.1046/j.1365-2818.2001.00926.x. jmi926 [pii] [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218(Pt 1):52–61. doi: 10.1111/j.1365-2818.2005.01466.x. JMI1466 [pii] [DOI] [PubMed] [Google Scholar]
- Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72(1):49–56. doi: 10.1016/j.neuron.2011.08.020. S0896-6273(11)00741-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. Neuroimaging. In: Fuster J, editor. The prefrontal cortex. Academic Press; London: 2008. pp. 285–331. [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004;125(3):615–623. doi: 10.1016/j.neuroscience.2004.02.025. S0306452204001575 [pii] [DOI] [PubMed] [Google Scholar]
- Gavrilovici C, D’Alfonso S, Poulter MO. Diverse interneuron populations have highly specific interconnectivity in the rat piriform cortex. J Comp Neurol. 2010;518(9):1570–1588. doi: 10.1002/cne.22291. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Morrell F, de Toledo-Morrell L. Axospinous synapses with segmented postsynaptic densities: a morphologically distinct synaptic subtype contributing to the number of profiles of ‘perforated’ synapses visualized in random sections. Brain Res. 1987;423(1–2):179–188. doi: 10.1016/0006-8993(87)90838-9. 0006-8993(87)90838-9 [pii] [DOI] [PubMed] [Google Scholar]
- Germuska M, Saha S, Fiala J, Barbas H. Synaptic distinction of laminar-specific prefrontal-temporal pathways in primates. Cereb Cortex. 2006;16(6):865–875. doi: 10.1093/cercor/bhj030. bhj030 [pii] [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103(3):593–614. doi: 10.1016/s0306-4522(00)00585-6. S0306452200005856 [pii] [DOI] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472(7342):217–220. doi: 10.1038/nature09945. nature09945 [pii] [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Distinct GABAergic targets of feedforward and feedback connections between lower and higher areas of rat visual cortex. J Neurosci. 2003;23(34):10904–10912. doi: 10.1523/JNEUROSCI.23-34-10904.2003. 23/34/10904 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39(2):375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zelano C. The value of identity: olfactory notes on orbitofrontal cortex function. Annals of the New York Academy of Sciences. 2011;1239:138–148. doi: 10.1111/j.1749-6632.2011.06268.x. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Haberly LB, Price JL. The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res. 1977;129(1):152–157. doi: 10.1016/0006-8993(77)90978-7. 0006-8993(77)90978-7 [pii] [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. Systems originating in the piriform cortex and adjacent areas. J Comp Neurol. 1978a;178(4):711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978b;181(4):781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology, Three-dimensional Measurement in Microscopy. Vol. 1. BIOS Scientific Publishers Limited; Oxford: 1998. [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12(7):932–938. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457(4):361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Illig KR, Eudy JD. Contralateral projections of the rat anterior olfactory nucleus. J Comp Neurol. 2009;512(1):115–123. doi: 10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20(18):6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. 20/18/6974 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112(3):541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kay RB, Brunjes PC. Society for Neuroscience Abstracts 2011. 2011. Interneurons in the anterior olfactory nucleus/cortex. [Google Scholar]
- Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation. J Comp Neurol. 2011;519(2):277–289. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Jones EG. Co-localization of two calcium binding proteins in GABA cells of rat piriform cortex. Brain Res. 1993;600(2):339–344. doi: 10.1016/0006-8993(93)91394-8. 0006-8993(93)91394-8 [pii] [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649(1–2):159–173. doi: 10.1016/0006-8993(94)91060-x. 0006-8993(94)91060-X [pii] [DOI] [PubMed] [Google Scholar]
- Lei H, Mooney R, Katz LC. Synaptic integration of olfactory information in mouse anterior olfactory nucleus. J Neurosci. 2006;26(46):12023–12032. doi: 10.1523/JNEUROSCI.2598-06.2006. 26/46/12023 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord T, Kasprzak M. Identification of self through olfaction. Percept Mot Skills. 1989;69(1):219–224. doi: 10.2466/pms.1989.69.1.219. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation study of the ammonic system. J Psychol Neurol. 1934;46(2–3):113–177. [Google Scholar]
- Lundstrom JN, Boesveldt S, Albrecht J. Central Processing of the Chemical Senses: an Overview. ACS chemical neuroscience. 2011;2(1):5–16. doi: 10.1021/cn1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983a;216(3):264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The laminar distribution of intracortical fibers originating in the olfactory cortex of the rat. J Comp Neurol. 1983b;216(3):292–302. doi: 10.1002/cne.902160306. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Westbrook GL. Membrane and synaptic properties of pyramidal neurons in the anterior olfactory nucleus. J Neurophysiol. 2011;105(4):1444–1453. doi: 10.1152/jn.00715.2010. jn.00715.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23(1):161–179. doi: 10.1111/j.1460-9568.2005.04522.x. EJN4522 [pii] [DOI] [PubMed] [Google Scholar]
- Medalla M, Lera P, Feinberg M, Barbas H. Specificity in inhibitory systems associated with prefrontal pathways to temporal cortex in primates. Cereb Cortex. 2007;17(Suppl 1):i136–150. doi: 10.1093/cercor/bhm068. 17/suppl_1/i136 [pii] [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron. 2009;61(4):609–620. doi: 10.1016/j.neuron.2009.01.006. S0896-6273(09)00045-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. J Neurosci. 2010;30(48):16068–16081. doi: 10.1523/JNEUROSCI.1773-10.2010. 30/48/16068 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. The anterior cingulate cortex may enhance inhibition of lateral prefrontal cortex via m2 cholinergic receptors at dual synaptic sites. J Neurosci. 2012;32(44):15611–15625. doi: 10.1523/JNEUROSCI.2339-12.2012. 32/44/15611 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390(2):211–224. doi: 10.1002/(SICI)1096-9861(19980112)390:2<211::AID-CNE4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neuroscience. 2005;130(1):185–195. doi: 10.1016/j.neuroscience.2004.08.046. S0306-4522(04)00782-1 [pii] [DOI] [PubMed] [Google Scholar]
- Meskenaite V. Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol. 1997;379(1):113–132. doi: 10.1002/(SICI)1096-9861(19970303)379:1<113::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the Old World Monkey. III: Efferent Cortical Output and Comments on Function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Meyer EA, Illig KR, Brunjes PC. Differences in chemo- and cytoarchitectural features within pars principalis of the rat anterior olfactory nucleus suggest functional specialization. J Comp Neurol. 2006;498(6):786–795. doi: 10.1002/cne.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Allison AC. An experimental investigation of the connexions of the olfactory tracts in the monkey. J Neurol Neurosurg Psychiatry. 1949;12(4):274–286. doi: 10.1136/jnnp.12.4.274. illust. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, Callaway EM, Horowitz MA, Luo L. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472(7342):191–196. doi: 10.1038/nature09714. nature09714 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–686. doi: 10.1016/s0092-8674(00)81387-2. S0092-8674(00)81387-2 [pii] [DOI] [PubMed] [Google Scholar]
- Moore CT, Wilson CG, Mayer CA, Acquah SS, Massari VJ, Haxhiu MA. A GABAergic inhibitory microcircuit controlling cholinergic outflow to the airways. J Appl Physiol. 2004;96(1):260–270. doi: 10.1152/japplphysiol.00523.2003. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18(4):599–612. doi: 10.1016/s0896-6273(00)80301-3. S0896-6273(00)80301-3 [pii] [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–158. doi: 10.1038/nrn2317. nrn2317 [pii] [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. Neurons and their supporting cells. Oxford University Press; New York: 1991. The fine structure of the nervous system. [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459(2):142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. Activation and habituation in olfaction--an fMRI study. Neuroimage. 2001;13(4):547–560. doi: 10.1006/nimg.2000.0713. S1053-8119(00)90713-4 [pii] [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62(6):850–861. doi: 10.1016/j.neuron.2009.05.022. S0896-6273(09)00397-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Cernoch JM, McLaughlin FJ. Maternal recognition of neonates through olfactory cues. Physiol Behav. 1983;30(1):151–154. doi: 10.1016/0031-9384(83)90051-3. 0031-9384(83)90051-3 [pii] [DOI] [PubMed] [Google Scholar]
- Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150(1):87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. S0165-0270(00)00293-4 [pii] [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27(7):1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. 27/7/1534 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79(7):1245–1255. doi: 10.1016/0092-8674(94)90015-9. 0092-8674(94)90015-9 [pii] [DOI] [PubMed] [Google Scholar]
- Reyher CK. Persistence of the pars externa system of the anterior olfactory nucleus in a microsmatic primate, Callithrix jacchus. Brain Res. 1988;457(1):169–175. doi: 10.1016/0006-8993(88)90071-6. 0006-8993(88)90071-6 [pii] [DOI] [PubMed] [Google Scholar]
- Rihn LL, Claiborne BJ. Dendritic growth and regression in rat dentate granule cells during late postnatal development. Brain Res Dev Brain Res. 1990;54(1):115–124. doi: 10.1016/0165-3806(90)90071-6. [DOI] [PubMed] [Google Scholar]
- Sasabe T, Kobayashi M, Kondo Y, Onoe H, Matsubara S, Yamamoto S, Tsukada H, Onoe K, Watabe H, Iida H, Kogo M, Sano K, Hatanaka A, Sawada T, Watanabe Y. Activation of the anterior cingulate gyrus by ‘Green Odor’: a positron emission tomography study in the monkey. Chem Senses. 2003;28(7):565–572. doi: 10.1093/chemse/bjg048. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Sela L, Sobel N. Human olfaction: a constant state of change-blindness. Exp Brain Res. 2010;205(1):13–29. doi: 10.1007/s00221-010-2348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Djordjevic J, Lundstrom JN. Statistical localization of human olfactory cortex. Neuroimage. 2012;66C:333–342. doi: 10.1016/j.neuroimage.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Annals of the New York Academy of Sciences. 2007;1121:87–101. doi: 10.1196/annals.1401.032. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Pollinger-Zierler B. Odor thresholds of microbially induced off-flavor compounds in apple juice. J Agric Food Chem. 2006;54(16):5984–5989. doi: 10.1021/jf060602n. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage. 2007;37(2):401–409. doi: 10.1016/j.neuroimage.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. II. Synaptic morphometry. Brain Res. 1985;351(2):215–226. doi: 10.1016/0165-3806(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92(3):1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166(3–4):345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57(5):786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392(6673):282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83(1):537–551. doi: 10.1152/jn.2000.83.1.537. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472(7342):213–216. doi: 10.1038/nature09868. nature09868 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Kettenmann B, Kobal G, McGlone FP. Selective attention to the chemosensory modality. Percept Psychophys. 2000;62(6):1265–1271. doi: 10.3758/bf03212128. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63(6):854–864. doi: 10.1016/j.neuron.2009.09.005. S0896-6273(09)00684-9 [pii] [DOI] [PubMed] [Google Scholar]
- Stevens CF. Neurotransmitter release at central synapses. Neuron. 2003;40(2):381–388. doi: 10.1016/s0896-6273(03)00643-3. S0896627303006433 [pii] [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518(10):1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Steffener J, Albers MW, Kern DW, Michael M, Tang H, Brown TR, Devanand DP. Validation and optimization of statistical approaches for modeling odorant-induced fMRI signal changes in olfactory-related brain areas. Neuroimage. 2007;34(4):1375–1390. doi: 10.1016/j.neuroimage.2006.11.020. S1053-8119(06)01131-1 [pii] [DOI] [PubMed] [Google Scholar]
- Turner BH, Gupta KC, Mishkin M. The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. J Comp Neurol. 1978;177(3):381–396. doi: 10.1002/cne.901770303. [DOI] [PubMed] [Google Scholar]
- Turner W. The Convolutions of the Brain: A Study in Comparative Anatomy. Journal of anatomy and physiology. 1890;25(Pt 1):105–153. [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Studies on the Piriform Lobe. Harvard University Press; Cambridge: 1965. [Google Scholar]
- Valverde F, Lopez-Mascaraque L, De Carlos JA. Structure of the nucleus olfactorius anterior of the hedgehog (Erinaceus europaeus) J Comp Neurol. 1989;279(4):581–600. doi: 10.1002/cne.902790407. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79(6):981–991. doi: 10.1016/0092-8674(94)90029-9. 0092-8674(94)90029-9 [pii] [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41(3):239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Small DM. Modality-specific neural effects of selective attention to taste and odor. Chem Senses. 2011;36(8):747–760. doi: 10.1093/chemse/bjr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman ML. Odorant evaluation: A study of ethanediol and tetrahydrothiophene as warning agents in propane. Environ Sci Technol. 1978;12(12):1285–1288. [Google Scholar]
- Widen H, Leufven A, Nielsen T. Identification of chemicals, possibly originating from misuse of refillable PET bottles, responsible for consumer complaints about off-odours in water and soft drinks. Food Addit Contam. 2005;22(7):681–692. doi: 10.1080/02652030500159987. G608Q64182X41T81 [pii] [DOI] [PubMed] [Google Scholar]
- Yan Z, Tan J, Qin C, Lu Y, Ding C, Luo M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58(4):613–624. doi: 10.1016/j.neuron.2008.03.012. S0896-6273(08)00258-4 [pii] [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36(2):165–181. doi: 10.1016/s0167-8760(99)00110-5. S0167-8760(99)00110-5 [pii] [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8(1):114–120. doi: 10.1038/nn1368. nn1368 [pii] [DOI] [PubMed] [Google Scholar]
- Zelano C, Montag J, Khan R, Sobel N. A specialized odor memory buffer in primary olfactory cortex. PLoS One. 2009;4(3):e4965. doi: 10.1371/journal.pone.0004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Mohanty A, Gottfried JA. Olfactory predictive codes and stimulus templates in piriform cortex. Neuron. 2011;72(1):178–187. doi: 10.1016/j.neuron.2011.08.010. S0896-6273(11)00731-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26(28):7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. 26/28/7348 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2(9):e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci. 2012;32(15):5338–5350. doi: 10.1523/JNEUROSCI.4793-11.2012. 32/15/5338 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]