Abstract
Maternal diabetes has been demonstrated to adversely affect preimplantation embryo development and pregnancy outcomes. Emerging evidence has implicated that these effects are associated with compromised oocyte competence. Several developmental defects during oocyte maturation in diabetic mice have been reported over past decades. Most recently, we further identified the structural, spatial and metabolic dysfunction of mitochondria in oocytes from diabetic mice, suggesting the impaired oocyte quality. These defects in the oocyte may be maternally transmitted to the embryo and then manifested later as developmental abnormalities in preimplantation embryo, congenital malformations, and even metabolic disease in the offspring. In this paper, we briefly review the effects of maternal diabetes on oocyte quality, with a particular emphasis on the mitochondrial dysfunction. The possible connection between dysfunctional oocyte mitochondria and reproductive failure of diabetic females, and the mechanism(s) by which maternal diabetes exerts its effects on the oocyte are also discussed.
Keywords: Diabetes, mitochondria, oocyte quality, embryo, mouse
1. Introduction
Diabetes mellitus is a metabolic condition characterized by elevated blood glucose levels secondary to absolute impairment of insulin secretion (type I diabetes). Relative impairment of insulin secretion in combination with varying degree of peripheral resistance to insulin action leads to type II diabetes (Goud et al., 2006). Women with poorly controlled type I or type II diabetes often suffer from a series of reproductive problems such as miscarriage, neonatal morbidity and mortality, and congenital malformations (Becerra et al., 1990; Greene, 1999; Sadler et al., 1988). Despite a drastic decrease in the incidence of spontaneous abortion and congenital malformations in infants of diabetic women due to improvements in glycemic control throughout pregnancy, these women still experience a 3–5 fold higher incidence of these pregnancy complications (Baccetti et al., 2002; Casson et al., 1997; El-Sayed and Lyell, 2001; Greene, 1999). These findings indicate that maternal diabetes may have permanent and irreversible effects on female reproduction. Since maternal diabetes in rodents influences embryonic and fetal development in a very similar manner to that of humans, chemically-induced or spontaneously diabetic mice and rats are commonly used as animal models to study reproduction of diabetic women (Amaral et al., 2008; Polanco Ponce et al., 2005). To date, the developmental anomalies during distinct stages induced by maternal diabetes, ranging from gamete and embryo to fetus, have been reported over past decades through animal and human studies (Amaral et al., 2008; Jungheim and Moley, 2008). In this review, we focus on the effects of maternal diabetes on oocyte quality, with a particular emphasis on the mitochondrial function.
2. Maternal diabetes and preimplantation embryo development
It has been demonstrated that mammalian embryos are vulnerable to injury during preimplantation stages of development and any damage may cause early embryo loss, embryo resorption and dysmorphogenesis (Moley, 1999). Prior to entering the uterus, pyruvate and lactate as main energy substrates of preimplantation embryo are metabolized aerobically via oxidative phosphorylation. As the developing embryo migrates from the fallopian tube into the anaerobic environment of the uterus, it adjusts to its new surroundings by increasing glucose metabolism via glycolysis (Leese, 1991). Maternal hyperglycemia has been shown to adversely affect progression from a one-cell to a blastocyst stage in rodent models (Diamond et al., 1989; Lea et al., 1996; Moley et al., 1998b; Moley et al., 1991; Moley et al., 1994). Zygotes removed from chemically-induced diabetic mice demonstrate retarded in vivo development to 2-cell stage with a lower percentage of 2-cell embryos recovered at 48 h after human chorionic gonadotropin (hCG) treatment compared with nondiabetic controls (Diamond et al., 1989). Similarly, in vitro experiments show that 2-cell embryos from control mice cultured in high glucose conditions are developmentally delayed compared with control embryos cultured in normal media (Diamond et al., 1991). It is worth noting that in vitro-cultured two-cell embryos that were recovered from diabetic mice still experience significant delay in their progression to the blastocyst stage (Diamond et al., 1989). Vesela et al. also observed that about 50% of two-cell embryos isolated from sub-diabetic rats were unable to develop to the 8-cell stage, even in a non-diabetic tract (Vesela et al., 1994). These results suggest that even brief exposure to diabetic state in periconceptual period may have lasting effects on subsequent embryonic development. Further, by conducting microanalytic assays of single embryos, Moley et al. revealed that hyperglycemia induces a downregulation of the GLUTs (facilitative glucose transporters) at the blastocyst stage in the mouse, which results in decreased glucose uptake and thus lower intraembryonic free glucose levels (Moley, 1999; Moley et al., 1998b). This decrease in glucose transport has been demonstrated to be sufficient to induce apoptosis at blastocyst stage (Chi et al., 2000; Moley et al., 1998a). In addition, an increase in fragmented embryos and a reduction in the number of cells in the inner cell mass of blastocysts recovered from diabetic rats (Lea et al., 1996) are considered to be associated with apoptosis induced by hyperglycemia through cell death effector pathways (Moley, 2001; Pampfer, 2000). Furthermore, our previous work showed the abnormal tricarboxylic acid (TCA) cycle metabolism in the glucose-induced apoptotic blastocyst, suggesting alterations in mitochondrial physiology (Chi et al., 2002). More importantly, we recently found that one-cell zygote transfer from diabetic to nondiabetic mice still results in significantly increased congenital malformations and growth retardation in the offspring (Wyman et al., 2008), indicating that exposure to maternal diabetes during oogenesis, fertilization, and the first 24h was enough to program permanently the fetus to develop morphological changes. Taking above findings together with depressed ovarian steroidogenesis (Garris et al., 1985; Vomachka and Johnson, 1982), increased granulosa cell apoptosis (Chang et al., 2005) and delayed oocyte maturation (Colton et al., 2002; Diamond et al., 1989) in diabetic mice, it is attractive to hypothesize that maternal diabetes has detrimental effects as early as the oocyte stage, and which may further predispose them to post-fertilization developmental abnormalities and even metabolic diseases in the offspring.
3. Effects of maternal diabetes on oocyte quality
In most mammals, oocytes are arrested within ovarian follicles at the diplotene stage of the first meiotic prophase, which is also termed the germinal vesicle (GV) stage, around the time of birth. Fully-grown oocytes are stimulated to reinitiate meiosis by the pituitary luteinising hormone (LH) surge in vivo at puberty, as indicated by GV breakdown. As the microtubules become organized into a bipolar spindle and all chromosomes align at the spindle equator, the oocytes proceed to the metaphase I stage and subsequently extrude the first polar body into the perivitelline space, followed by entry into meiosis II and a second arrest at metaphase II (Miao et al., 2009; Wang and Sun, 2007). Full developmental competence of an oocyte requires synchronous nuclear maturation and cytoplasmic maturation (Krisher, 2004). Any dysfunction or dislocation of oocyte components, such as spindle, cortical granules or mitochondria could impair oocyte quality (Combelles and Racowsky, 2005; Coticchio et al., 2004; Sun et al., 2001b). Mounting evidence has suggested that oocyte quality profoundly affects fertilization, early embryonic survival, the establishment and maintenance of pregnancy, fetal development, and even adult disease (Krisher, 2004; Sirard et al., 2006). Thus, investigation of effects of maternal diabetes on oocyte quality may inform us on the origin of reproductive failure in diabetic females. Several developmental abnormalities in oocytes from diabetic animals have been reported. The following sections will give a brief summary of developmental abnormalities, and then focus on our recent findings of mitochondrial dysfunction in oocytes from diabetic mice (Wang et al., 2009).
3.1. Maternal diabetes delays meiotic progression of oocytes
Diamond et al. first reported that germinal vesicle breakdown (GVBD), a marker of oocyte meiotic maturation, is attenuated in superovulated oocytes from diabetic mice (Diamond et al., 1989), which has been further confirmed by several other different laboratories (Chang et al., 2005; Colton et al., 2002; Kim et al., 2007; Ratchford et al., 2007). Nevertheless, it is interesting to note that cumulus-enclosed oocytes (CEOs) from diabetic mice exhibit both accelerated spontaneous maturation kinetics and restricted hormone-induced maturation in vitro. In addition, ova from diabetic mice were also found to be less likely to progress to metaphase II after induction of ovulation compared with controls (Colton et al., 2002). In vitro studies have shown that both the meiosis-inducing and -suppressing effects of glucose on oocyte maturation appear to be mediated by the gap junctional communication pathway that metabolically couples the oocyte with the somatic compartment of the follicle (Downs, 1995; Downs, 2000; Fagbohun and Downs, 1991). By performing coupling assays on freshly isolated CEOs, Colton and colleagues showed that the cell-cell communication between the oocyte and the cumulus cells was reduced in diabetic mice (Colton et al., 2002). In support of this observation, we recently identified that expression of two gap junction proteins (Cx26 and Cx43) were markedly decreased in diabetic cumulus cells when compared to controls. The levels of Cx37, a gap junction protein known to be predominantly expressed in the oocyte, were also significantly lower in oocytes from control mice than those from diabetic mice (Chang et al., 2005; Ratchford et al., 2008). Moreover, incubating the CEOs with a gap junction blocker carbenoxolone (CBX) in vitro dramatically delayed the onset of GVBD in mouse oocytes (Ratchford et al., 2008), although disruption of gap junctional communication with the rat ovarian follicle induces oocyte maturation (Sela-Abramovich et al., 2006). Thus, this decrease in gap junction and connexin expression in CEOs may be responsible for the impaired oocyte maturation in diabetic mice.
In addition, defects in glucose, purine, cAMP metabolism and changes in hydroxyacyl-CoA dehydrogenase (Hadh2), glutamic pyruvate transaminase (Gpt2) and AMP-activated protein kinase (AMPK) activity were detected in CEOs and denuded oocytes from diabetic mice (Colton et al., 2003; Colton et al., 2002; Ratchford et al., 2007). Diabetic rat models demonstrated the altered prostaglandin (PGE2) production in both ovulated and immature CEOs isolated from ovaries, as well as in in vitro-matured CEOs (Jawerbaum et al., 1999; Jawerbaum et al., 1996). Each of these conditions is thought to contribute to the disrupted meiotic behavior in oocytes from diabetic animals.
3.2. Maternal diabetes causes mitochondrial dysfunction in oocytes
Mitochondria play a primary role in cellular energetic metabolism, homeostasis, and death. They are the most abundant organelles in mammalian oocyte (Van Blerkom, 2004). Mitochondria are directly involved at several levels in the reproductive process since their functional status influences the quality of oocytes and contributes to the process of fertilization and embryonic development (May-Panloup et al., 2007). Recently, we used a streptozotocin (STZ)-induced diabetic mouse model to investigate the effects of maternal diabetes on the mitochondrial status in oocytes. Herein we give a brief summary of our findings, and detailed information can be found in Wang et al. (Wang et al., 2009)
Using transmission electron microscopy, we observed marked structural aberrations in mitochondria of diabetic oocytes including a narrowed intermembrane space and rupture of the outer membrane. These ultrastructural alterations suggest swelling of mitochondria, which in somatic cells is thought to herald mitochondrial dependent apoptosis and degradation (Senoo-Matsuda et al., 2005). Interestingly, we previously discovered the decreased heat shock protein 90 (HSP90) expression in either CEOs or denuded oocytes from diabetic mice (unpublished data), which has been suggested to be able to activate the apoptotic program mediated by mitochondrial pathway (Neckers et al., 2007; Pandey et al., 2000; Sato et al., 2000).
By performing immunofluorescence microscopy, we found that the distribution pattern of mitochondria during meiotic maturation was disrupted in oocytes from diabetic mice. One pronounced tendency is that the percentage of the perinuclear distribution pattern at GV stage and polarized distribution pattern at MII stage was decreased relative to control whereas the proportion of the homogenous distribution pattern was increased accordingly. In addition, at both GV and MII stages, oocytes from diabetic mice displayed a much higher percentage of clustering mitochondrial distribution. Given that the spatial remodeling of mitochondria may allow maturing oocytes to cater to differing energy requirements of various key events, such as germinal vesicle breakdown and metaphase spindle formation (Van Blerkom, 2004), inadequate translocation of mitochondria therefore perhaps serves as an important factor contributing to the maturation delay (Chang et al., 2005; Colton et al., 2002; Diamond et al., 1989) and spindle defects (Wang et al., 2009) observed in diabetic oocytes.
Mitochondrial DNA (mtDNA) is an essential component of mitochondrial function. Low mtDNA content has been reported to be associated with oocyte incompetence, fertilization failure and even ovarian insufficiency (May-Panloup et al., 2007). Surprisingly, by performing quantitative real time-PCR on single fully-grown oocytes, we found that the average mtDNA copy number in oocytes from diabetic mice was significantly increased as compared with controls. Similarly, mtDNA copy number in oocytes from older women was significantly greater than those from young women (Steuerwald et al., 2000). Such an increase in mitochondrial biogenesis was attributed to a compensatory phenomenon to guarantee sufficient ATP production in the event of either an increased demand or due to a respiratory chain dysfunction. It is also possible to explain this as a decrease in mitochondrial degradation or autophagy (Mammucari et al., 2008; Tsukamoto et al., 2008a; Tsukamoto et al., 2008b). On the other hand, nitric oxide (NO) has been shown to have an effect upon mitochondrial biogenesis. HeLa cells expressing endothelial nitric oxide synthase (eNOS) displayed an increase in mtDNA content (Nisoli et al., 2003; Reznick and Shulman, 2006). It is interesting to point out that NOS activity is also elevated in the ovaries of mild and severe diabetic rats (Jawerbaum et al., 1999; Jawerbaum et al., 1996). Nevertheless, similar experiments have not been performed on diabetic mice yet.
Finally, by conducting microanalytical enzymatic cycling assays, we identified that the levels of ATP and TCA cycle metabolites are dramatically decreased in ovulated oocytes from diabetic mice. Our previous studies also showed the ATP content was significantly lower in preovulatory oocytes and CEOs from diabetic mice (Colton et al., 2003; Ratchford et al., 2007). These results suggest that maternal diabetes results in the reduction of mitochondrial function, which is likely related to their structural abnormalities. Variations in the ATP content have been suggested to significantly affect oocyte quality, embryonic development and implantation process (Quinn and Wales, 1973; Van Blerkom et al., 1995).
Collectively, by performing molecular, cellular and biochemical analysis, we reveal the structural, spatial, genetic and metabolic dysfunction of mitochondria in oocytes from diabetic mice.
4. Is mitochondrial dysfunction in oocytes related to reproductive failure of diabetic females?
It is obvious that maternal diabetes has adverse effects on multiple meiotic events during oocyte development. In the following parts, we will just list some correlative evidence to discuss the possible connection between dysfunctional oocyte mitochondria and reproductive failure of diabetic females. However, whether these reproductive problems are mediated through developmental defects in oocytes remains to be determined.
4.1. Mitochondrial dysfunction and meiotic defects in diabetic oocytes
The processes of spindle formation and chromatin organization are believed to be particularly sensitive to physical, chemical and endocrine environments (Hunt and Hassold, 2008). Abnormalities in the meiotic spindle and chromosome alignment are known to result in improper chromosome segregation and nondisjunction of the chromatids at the first or second meiotic division, contributing to an increased incidence of aneuploidy (Hassold and Hunt, 2001). By confocal scanning, we revealed that maternal diabetes induces an increased frequency of spindle disorganization and chromosome misalignment in oocytes, as shown in Fig 1. Several lines of evidence have suggested that these meiotic defects may be associated with mitochondrial dysfunction. First, we consistently observed chromosomal congression failure in the ovulated oocytes from diabetic mice that display clustered mitochondria (Fig. 2; lower panel, red arrows), which strongly suggests that deficient chromosome alignment may be directly linked to abnormal mitochondrial distribution. In vitro experiments also showed that exposure of maturing mouse oocytes to diazepam to disrupt mitochondrial distribution results in predivision of homologue and aneuploidy (Sun et al., 2001a; Yin et al., 1998). Most recently, association of mitochondria with spindle poles was found to facilitate spindle alignment in S. pombe (Kruger and Tolic-Norrelykke, 2008). Second, reduction of mitochondrial function evidenced by decreased ATP content may contribute to the meiotic defects in diabetic oocytes (Wang et al., 2009). Chromatin condensation during meiosis is an ATP-dependent process (Hirano, 2005). Microtubule assembly and chromosome movement also requires ATP (Inoue and Salmon, 1995). It has been suggested that mammalian mature oocytes display a high ATP turnover and that the ATP consumed is supplied by mitochondrial respiration (Dumollard et al., 2004; Igarashi et al., 2005). A recent study revealed that injury of mitochondria in MII oocytes reduces ATP content and disrupts the meiotic spindle (Zeng et al., 2007; Zhang et al., 2006). Pdha1-deficient (pyruvate dehydrogenase a1) mouse oocytes experience inadequate ATP levels along with chromatin and microtubular abnormalities (Johnson et al., 2007). Senescence accelerated mice also demonstrated that spindle defects and disturbances in chromosome alignment are associated with mitochondrial dysfunction in oocytes (Liu et al., 2002). Finally, by karyotypic analysis, we confirmed the increased aneuploidy rate in ovulated oocytes from diabetic mice. This is in line with previous reports showing a high frequency of chromosomal numerical anomalies in embryos from diabetic mice (Tatewaki et al., 1995; Yamamoto et al., 1971). Furthermore, mitochondrial dysfunction has been proposed as a factor in the increased incidence of aneuploidy in oocytes from older women (Brenner et al., 1998; Keefe et al., 1995). Collectively, above data support the idea that meiotic defects and aneuploidy in diabetic oocytes may be associated with mitochondrial dysfunction, which may be involved in reproductive failure and congenital birth defects of diabetic females.
Figure 1. Spindle defects and chromosome misalignment in oocytes from diabetic mice.
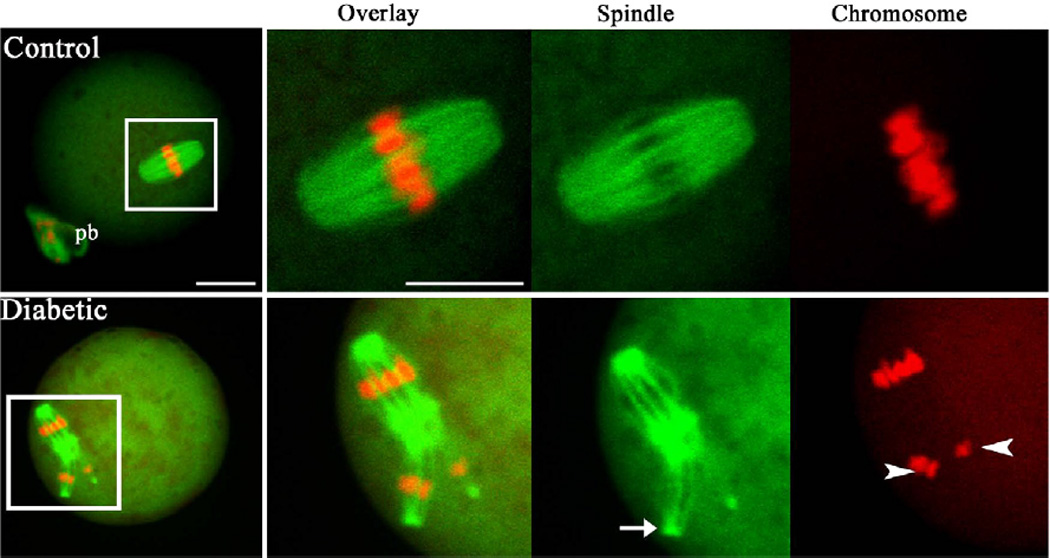
Ovulated MII oocytes from control and diabetic mice were stained with β-tubulin antibody to visualize the spindle (green) and counterstained with TO-PRO to visualize chromosomes (red). MII oocytes from control mice present a typical barrel-shape spindle and well-aligned chromosomes on the metaphase plate. In MII oocytes from diabetic mice, spindle defects (arrows) and chromosome misalignment (arrowheads) were readily observed. Representative confocal sections are shown. Scale bars: 20 µm. (From Wang Q, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 2009, 23:1603–1612, with minor modifications.)
Figure 2. Mitochondrial clustering and its relationship with chromosome congression failure in diabetic oocytes.
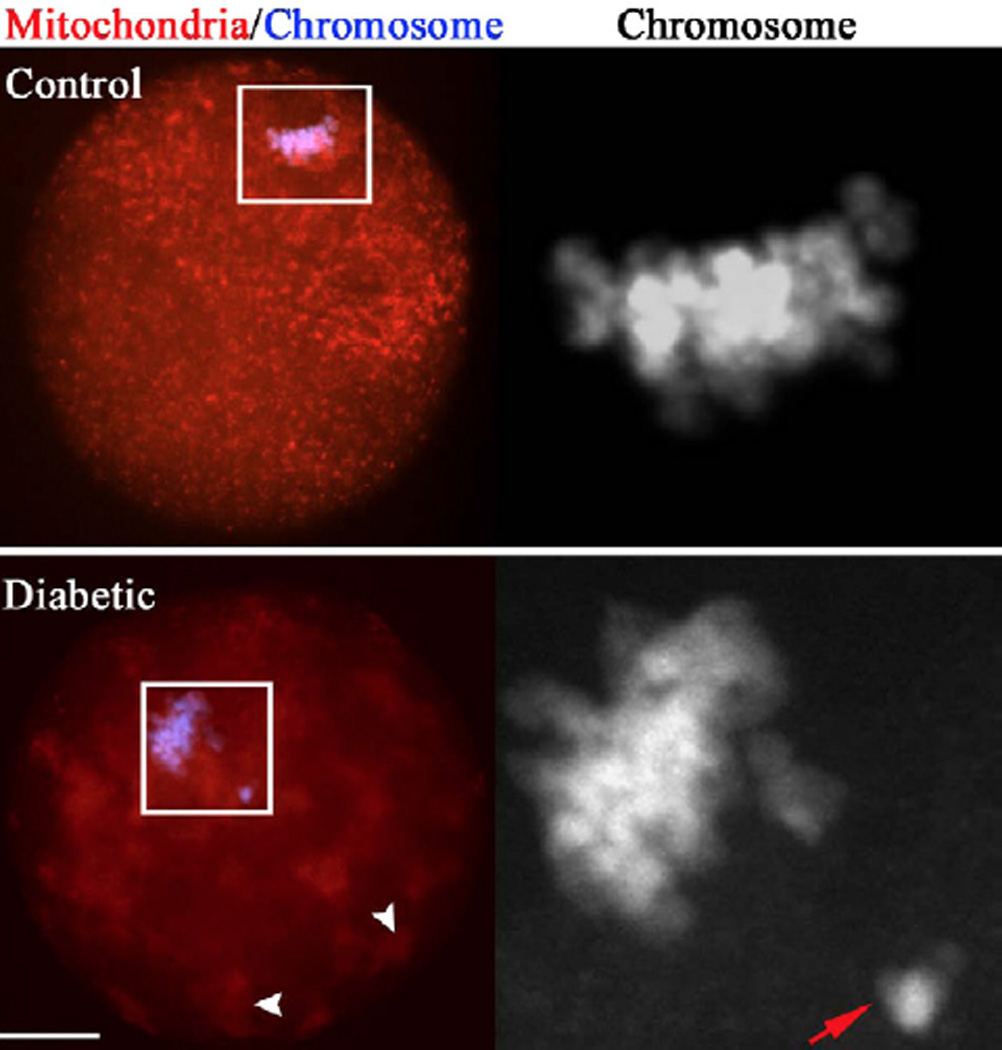
Ovulated MII oocytes from control and diabetic mice were labeled with MitoTracker Red to visualize mitochondrial localization and counterstained with DAPI for nuclear status. In most control oocytes, mitochondria display a polarized distribution pattern and chromosomes well align on the metaphase plate. However, we consistently detected chromosomal congression failure (abnormal alignment; lower panel, red arrows) in diabetic oocytes that display clustered mitochondria (white arrowheads). Scale bars: 20 µm. (From Wang Q, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 2009, 23:1603–1612, with minor modifications.)
4.2. Mitochondrial dysfunction in oocytes and developmental abnormalities of diabetic embryos
Mature mammalian oocytes are maternally endowed with thousands of mitochondria that act as the founding population of all daughter-cell mitochondria of the developing embryo (Jansen, 2000; Schatten et al., 2005). Increasing evidence indicates that oocyte mitochondrial dysfunction may be a critical determinant of embryo developmental competence (Fissore et al., 2002; Ramalho-Santos et al., 2004; Ramalho-Santos et al., 2009; Van Blerkom, 2004).
We have observed mitochondrial swelling and even rupture of the outer membrane in ovulated oocytes from diabetic mice (Wang et al., 2009). Similar characteristics were also found in mitochondria of rat embryos exposed to maternal diabetes (Yang et al., 1995; Yang et al., 1998). These ultrastructural alterations have been correlated with an increase of mitochondrial membrane permeability, which is directly regulated by the Bcl-2 family of proteins (Adams and Cory, 2001). Interestingly, we previously identified that expression of Bax, a pro-apoptotic member of the Bcl-2 family, is increased in blastocyst embryos recovered from diabetic mice and that these changes correlate morphologically with increased DNA fragmentation (Moley et al., 1998a). These findings indicate that the structural abnormalities of oocyte mitochondria may be transmitted to the embryo and therefore be involved in the apoptosis in preimplantation embryos in diabetic mice (Chi et al., 2002; Eng et al., 2007; Keim et al., 2001). More importantly, because all mitochondria are maternally inherited, the proportion of genetically compromised mitochondria capable of replication largely determines the extent to which post-implantation development may be compromised, and the probability that certain cytopathologies will develop later in life (Christodoulou, 2000; Jacobs et al., 2006; Van Blerkom and Davis, 2007).
The localization of mitochondria in the egg during maturation and their segregation to blastomeres in the cleaving embryos are strictly regulated. Remarkably, we found that mitochondrial clustering was dramatically increased in oocytes from diabetic mice (Wang et al., 2009). Ovulated eggs by non-diabetic aged mice also displayed a higher percentage of mitochondrial aggregates (Tarin et al., 2001). Such an abnormal distribution pattern may lead to disproportionate mitochondrial segregation during cleavage in embryo, which has been reported to associate with arrested cytokinesis and lysis in the blastomeres that inherited a significantly reduced organelle complement (El Shourbagy et al., 2006; Nagai et al., 2006; Van Blerkom et al., 2000).
In vitro studies demonstrated that mitochondrial dysfunction in mouse oocytes induced by photosensitization results in developmental arrest and apoptotic degeneration of preimplantation embryo (Thouas et al., 2004). Treatment of one-cell zygotes with protonophore carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP) to disrupt mitochondrial function also markedly delayed the cleavage of early embryos (Liu et al., 2002). In addition, sublethal mitochondrial injury in mouse oocyte resulted in the aberrant cytoplasmic patterning of mitochondria (Thouas et al., 2006) and upon IVF these resultant embryos demonstrated increased miscarriages, growth retardation and neural tube defects similar to that seen with diabetic embryopathy. In combination with other results showing the structural, metabolic and biogenetic alterations in mitochondria of diabetic embryos (Akazawa, 2005; Alcolea et al., 2007; Chi et al., 2002; Yang et al., 1995; Yang et al., 1998), it is possible that dysfunctional mitochondria in diabetic oocyte may be maternally transmitted to the embryo, contributing to the developmental retardation in preimplantation embryos (Diamond et al., 1989; Moley et al., 1991), congenital malformations, and even metabolic disease in the offspring.
5. How does maternal diabetes exert its effects on oocyte quality?
Although the adverse effects of maternal diabetes on embryo development have been associated with compromised oocyte competence, however, to date, the pathway(s) by which maternal diabetes exerts its effects on the oocyte remains obscure.
Mammalian ovarian follicles are highly specialized structures that support the growth and development of oocytes. Bidirectional communication between oocytes and their associated follicular somatic cells, granulose cells, constitutes a regulatory loop essential for the development of both cell types (Eppig, 2001). The oocyte and granulosa cells are metabolically coupled throughout follicular development by membrane specializations known as gap junctions (Albertini and Anderson, 1974; Sugiura and Eppig, 2005). Glucose is a necessary energy substrate for oocyte maturation in the presence of cumulus cells (Downs and Utecht, 1999; Preis et al., 2005; Zheng et al., 2007; Zuelke and Brackett, 1992). Nevertheless, oocytes carry out glycolysis poorly and require cumulus cells to metabolize glucose into products that can be used by oocytes as energy production substrates to support maturation (Biggers et al., 1967; Sugiura and Eppig, 2005). Hence, the somatic compartment is a critical mediator in the interaction of glucose in oocyte development. Notably, significant reductions in metabolic coupling and gap junction communication have been demonstrated in cumulus-oocyte complexes from diabetic mice (Colton et al., 2003; Ratchford et al., 2008). These alterations may be responsible for the delay in oocyte growth and maturation and the altered energy resources seen in diabetic mice (Diamond et al., 1989; Ratchford et al., 2007). In addition, we detected an increased apoptosis in granulose cells from diabetic mice (Chang et al., 2005), which has been correlated with compromised oocyte quality and poor pregnancy outcome (Lee et al., 2001; Nakahara et al., 1997). Recently, we revealed the structural and metabolic dysfunction of mitochondria in cumulus cells of diabetic mice, which was further demonstrated to be involved in the increased apoptosis of cumulus cells exposed to maternal diabetes (Wang Q, et al., unpublished data). Together these data suggest that maternal diabetes may indirectly impair oocyte competence by disturbing the metabolism in granulosa cells and their communications with the oocyte.
We previously found that glycogen levels were significantly higher in oocytes from diabetic mice than controls (Ratchford et al., 2007), which indicates that the hyperglycemic environment may lead to accumulation of glucose stored as glycogen. In support of this idea, our most recent data showed around 2-fold increase in glucose levels in oocytes from diabetic mice compared with those from control mice (Ratchford A, et al., unpublished data). The deleterious effects of high glucose on mitochondria have been widely documented in various cell types (Rolo and Palmeira, 2006; Russell et al., 2002; Yu et al., 2008). It is interesting to point out that elevated glycogen levels have been reported to inhibit AMPK activation (Kawanaka et al., 2000; Polekhina et al., 2003; Wojtaszewski et al., 2002), which is in line with our result showing the drop of AMPK activity in diabetic mouse oocytes (Ratchford et al., 2007). Moreover, activation of AMPK was demonstrated to participate in the regulation of oocyet maturation (Chen and Downs, 2008; Chen et al., 2006; Downs et al., 2002). On the other hand, Wellen et al. recently identified that glucose availability can influence histone acetylation in an adenosine triphosphate-citrate lyase (ACL) dependent manner (Wellen et al., 2009). Histone acetylation is required for meiotic resumption, spindle assembly and chromosome segregation during oocyte development (Akiyama et al., 2006; Kageyama et al., 2007; Nagashima et al., 2007; Wang et al., 2006). Importantly, epigenetic modifications linked to early nutrition can be transmitted to subsequent generations and may contribute to “intergenerational programming” of diabetes risk (Drake and Walker, 2004; Woo and Patti, 2008). Taking above data together with our results showing mitochondrial dysfunction and meiotic defects in diabetic oocytes, it is plausible to speculate that maternal hyperglycemia could directly disrupt meiotic events by inducing glucose accumulation in the oocyte.
It is known that the type I diabetic mouse is characterized by hyperglycemia and hypoinsulinemia. Therefore, hypoinsulinemia could also be a contributory factor affecting oocyte quality, as the insulin signaling pathway has been reported to function in chromatin remodeling during oocyte growth (Acevedo et al., 2007).
6. Concluding remarks
Maternal diabetes adversely affects oocyte developmental competence. In particular, mitochondria have been demonstrated to be dysfunctional in oocytes from diabetic mice. Those abnormal oocyte mitochondria may be maternal transmitted to the embryo and then be propagated during embryogenesis and fetal development, contributing to the reproductive problems experienced by diabetic females. More work is necessary to explore the pathways by which maternal diabetes impairs oocyte quality and to identify the potential mediators during this process. This information is essential in order to more effectively improve reproductive outcomes for diabetic women.
Acknowledgments
Part of the author’s work reported herein was supported by NIH-UO1 HD044691-03 (KHM) and NIH-R01 GM085150 (TS).
References
- Acevedo N, Ding J, Smith GD. Insulin signaling in mouse oocytes. Biol Reprod. 2007;77:872–879. doi: 10.1095/biolreprod.107.060152. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- Akazawa S. Diabetic embryopathy: studies using a rat embryo culture system and an animal model. Congenit Anom (Kyoto) 2005;45:73–79. doi: 10.1111/j.1741-4520.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci U S A. 2006;103:7339–7344. doi: 10.1073/pnas.0510946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini DF, Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974;63:234–250. doi: 10.1083/jcb.63.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea MP, Llado I, Garcia-Palmer FJ, Gianotti M. Responses of mitochondrial biogenesis and function to maternal diabetes in rat embryo during the placentation period. Am J Physiol Endocrinol Metab. 2007;293:E636–E644. doi: 10.1152/ajpendo.00120.2007. [DOI] [PubMed] [Google Scholar]
- Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4:46–54. doi: 10.2174/157339908783502398. [DOI] [PubMed] [Google Scholar]
- Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, Petraglia F, De Leo V. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002;17:2673–2677. doi: 10.1093/humrep/17.10.2673. [DOI] [PubMed] [Google Scholar]
- Becerra JE, Khoury MJ, Cordero JF, Erickson JD. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics. 1990;85:1–9. [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Wolny YM, Barritt JA, Matt DW, Munne S, Cohen J. Mitochondrial DNA deletion in human oocytes and embryos. Mol Hum Reprod. 1998;4:887–892. doi: 10.1093/molehr/4.9.887. [DOI] [PubMed] [Google Scholar]
- Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, Platt MJ, Stanisstreet M, van Velszen D, Walkinshaw S. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. Bmj. 1997;315:275–278. doi: 10.1136/bmj.315.7103.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology. 2005;146:2445–2453. doi: 10.1210/en.2004-1472. [DOI] [PubMed] [Google Scholar]
- Chen J, Downs SM. AMP-activated protein kinase is involved in hormone-induced mouse oocyte meiotic maturation in vitro. Dev Biol. 2008;313:47–57. doi: 10.1016/j.ydbio.2007.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, Downs SM. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;291:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab. 2002;283:E226–E232. doi: 10.1152/ajpendo.00046.2002. [DOI] [PubMed] [Google Scholar]
- Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252–40257. doi: 10.1074/jbc.M005508200. [DOI] [PubMed] [Google Scholar]
- Christodoulou J. Genetic defects causing mitochondrial respiratory chain disorders and disease. Hum Reprod. 2000;15(Suppl 2):28–43. doi: 10.1093/humrep/15.suppl_2.28. [DOI] [PubMed] [Google Scholar]
- Colton SA, Humpherson PG, Leese HJ, Downs SM. Physiological changes in oocyte-cumulus cell complexes from diabetic mice that potentially influence meiotic regulation. Biol Reprod. 2003;69:761–770. doi: 10.1095/biolreprod.102.013649. [DOI] [PubMed] [Google Scholar]
- Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biol Reprod. 2002;67:220–231. doi: 10.1095/biolreprod67.1.220. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Racowsky C. Assessment and optimization of oocyte quality during assisted reproductive technology treatment. Semin Reprod Med. 2005;23:277–284. doi: 10.1055/s-2005-872456. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann N Y Acad Sci. 2004;1034:132–144. doi: 10.1196/annals.1335.016. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH. Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil. 1989;86:1–10. doi: 10.1530/jrf.0.0860001. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Pettway ZY, Logan J, Moley K, Vaughn W, DeCherney AH. Dose-response effects of glucose, insulin, and glucagon on mouse pre-embryo development. Metabolism. 1991;40:566–570. doi: 10.1016/0026-0495(91)90045-x. [DOI] [PubMed] [Google Scholar]
- Downs SM. The influence of glucose, cumulus cells, and metabolic coupling on ATP levels and meiotic control in the isolated mouse oocyte. Dev Biol. 1995;167:502–512. doi: 10.1006/dbio.1995.1044. [DOI] [PubMed] [Google Scholar]
- Downs SM. Adenosine blocks hormone-induced meiotic maturation by suppressing purine de novo synthesis. Mol Reprod Dev. 2000;56:172–179. doi: 10.1002/(SICI)1098-2795(200006)56:2<172::AID-MRD8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Downs SM, Hudson ER, Hardie DG. A potential role for AMP-activated protein kinase in meiotic induction in mouse oocytes. Dev Biol. 2002;245:200–212. doi: 10.1006/dbio.2002.0613. [DOI] [PubMed] [Google Scholar]
- Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60:1446–1452. doi: 10.1095/biolreprod60.6.1446. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- El-Sayed YY, Lyell DJ. New therapies for the pregnant patient with diabetes. Diabetes Technol Ther. 2001;3:635–640. doi: 10.1089/15209150152811270. [DOI] [PubMed] [Google Scholar]
- El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- Eng GS, Sheridan RA, Wyman A, Chi MM, Bibee KP, Jungheim ES, Moley KH. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56:2228–2234. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Fagbohun CF, Downs SM. Metabolic coupling and ligand-stimulated meiotic maturation in the mouse oocyte-cumulus cell complex. Biol Reprod. 1991;45:851–859. doi: 10.1095/biolreprod45.6.851. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124:745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- Garris DR, Williams SK, West L. Morphometric evaluation of diabetes-associated ovarian atrophy in the C57BL/KsJ mouse: relationship to age and ovarian function. Anat Rec. 1985;211:434–443. doi: 10.1002/ar.1092110410. [DOI] [PubMed] [Google Scholar]
- Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Activation of the cGMP signaling pathway is essential in delaying oocyte aging in diabetes mellitus. Biochemistry. 2006;45:11366–11378. doi: 10.1021/bi060910e. [DOI] [PubMed] [Google Scholar]
- Greene MF. Spontaneous abortions and major malformations in women with diabetes mellitus. Semin Reprod Endocrinol. 1999;17:127–136. doi: 10.1055/s-2007-1016220. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hirano T. SMC proteins and chromosome mechanics: from bacteria to humans. Philos Trans R Soc Lond B Biol Sci. 2005;360:507–514. doi: 10.1098/rstb.2004.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72:1256–1261. doi: 10.1095/biolreprod.104.034926. [DOI] [PubMed] [Google Scholar]
- Inoue S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LJ, de Wert G, Geraedts JP, de Coo IF, Smeets HJ. The transmission of OXPHOS disease and methods to prevent this. Hum Reprod Update. 2006;12:119–136. doi: 10.1093/humupd/dmi042. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod. 2000;15(Suppl 2):112–128. doi: 10.1093/humrep/15.suppl_2.112. [DOI] [PubMed] [Google Scholar]
- Jawerbaum A, Gonzalez ET, Carolina P, Debora S, Christian P, Gimeno MA. Diminished levels of prostaglandin E in type I diabetic oocyte-cumulus complexes. Influence of nitric oxide and superoxide dismutase. Reprod Fertil Dev. 1999;11:105–110. doi: 10.1071/rd99033. [DOI] [PubMed] [Google Scholar]
- Jawerbaum A, Gonzalez ET, Faletti A, Novaro V, Vitullo A, Gimeno MA. Altered prostanoid production by cumulus-oocyte complexes in a rat model of non-insulin-dependent diabetes mellitus. Prostaglandins. 1996;52:209–219. doi: 10.1016/s0090-6980(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Moley KH. The impact of type 1 and type 2 diabetes mellitus on the oocyte and the preimplantation embryo. Semin Reprod Med. 2008;26:186–195. doi: 10.1055/s-2008-1042957. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85–94. doi: 10.1530/REP-06-0025. [DOI] [PubMed] [Google Scholar]
- Kawanaka K, Nolte LA, Han DH, Hansen PA, Holloszy JO. Mechanisms underlying impaired GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Am J Physiol Endocrinol Metab. 2000;279:E1311–E1318. doi: 10.1152/ajpendo.2000.279.6.E1311. [DOI] [PubMed] [Google Scholar]
- Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577–583. [PubMed] [Google Scholar]
- Keim AL, Chi MM, Moley KH. Hyperglycemia-induced apoptotic cell death in the mouse blastocyst is dependent on expression of p53. Mol Reprod Dev. 2001;60:214–224. doi: 10.1002/mrd.1080. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim CH, Moley KH, Cheon YP. Disordered meiotic regulation of oocytes by duration of diabetes mellitus in BBdp rat. Reprod Sci. 2007;14:467–474. doi: 10.1177/1933719107306228. [DOI] [PubMed] [Google Scholar]
- Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82(E-Suppl):E14–E23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- Kruger N, Tolic-Norrelykke IM. Association of mitochondria with spindle poles facilitates spindle alignment. Curr Biol. 2008;18:R646–R647. doi: 10.1016/j.cub.2008.06.069. [DOI] [PubMed] [Google Scholar]
- Lea RG, McCracken JE, McIntyre SS, Smith W, Baird JD. Disturbed development of the preimplantation embryo in the insulin-dependent diabetic BB/E rat. Diabetes. 1996;45:1463–1470. doi: 10.2337/diab.45.11.1463. [DOI] [PubMed] [Google Scholar]
- Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet. 2001;18:490–498. doi: 10.1023/A:1016649026353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the preimplantation mammalian embryo. Oxf Rev Reprod Biol. 1991;13:35–72. [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. 2002;1:40–46. doi: 10.1046/j.1474-9728.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Chretien MF, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol. 2007;77:51–83. doi: 10.1016/S0070-2153(06)77003-X. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- Moley KH. Diabetes and preimplantation events of embryogenesis. Semin Reprod Endocrinol. 1999;17:137–151. doi: 10.1055/s-2007-1016221. [DOI] [PubMed] [Google Scholar]
- Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab. 2001;12:78–82. doi: 10.1016/s1043-2760(00)00341-6. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med. 1998a;4:1421–1424. doi: 10.1038/4013. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998b;275:E38–E47. doi: 10.1152/ajpendo.1998.275.1.E38. [DOI] [PubMed] [Google Scholar]
- Moley KH, Vaughn WK, DeCherney AH, Diamond MP. Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil. 1991;93:325–332. doi: 10.1530/jrf.0.0930325. [DOI] [PubMed] [Google Scholar]
- Moley KH, Vaughn WK, Diamond MP. Manifestations of diabetes mellitus on mouse preimplantation development: effect of elevated concentration of metabolic intermediates. Hum Reprod. 1994;9:113–121. doi: 10.1093/oxfordjournals.humrep.a138298. [DOI] [PubMed] [Google Scholar]
- Nagai S, Mabuchi T, Hirata S, Shoda T, Kasai T, Yokota S, Shitara H, Yonekawa H, Hoshi K. Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J Exp Med. 2006;210:137–144. doi: 10.1620/tjem.210.137. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Maruyama T, Furuya M, Kajitani T, Uchida H, Masuda H, Ono M, Arase T, Ozato K, Yoshimura Y. Histone acetylation and subcellular localization of chromosomal protein BRD4 during mouse oocyte meiosis and mitosis. Mol Hum Reprod. 2007;13:141–148. doi: 10.1093/molehr/gal115. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Saito H, Saito T, Ito M, Ohta N, Takahashi T, Hiroi M. The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil Steril. 1997;68:312–317. doi: 10.1016/s0015-0282(97)81521-x. [DOI] [PubMed] [Google Scholar]
- Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Pampfer S. Peri-implantation embryopathy induced by maternal diabetes. J Reprod Fertil Suppl. 2000;55:129–139. [PubMed] [Google Scholar]
- Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. Embo J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco Ponce AC, Revilla Monsalve MC, Palomino Garibay MA, Islas Andrade S. Effect of maternal diabetes on human and rat fetal development. Ginecol Obstet Mex. 2005;73:544–552. [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Preis KA, Seidel G, Jr, Gardner DK. Metabolic markers of developmental competence for in vitro-matured mouse oocytes. Reproduction. 2005;130:475–483. doi: 10.1530/rep.1.00831. [DOI] [PubMed] [Google Scholar]
- Quinn P, Wales RG. The relationships between the ATP content of preimplantation mouse embryos and their development in vitro during culture. J Reprod Fertil. 1973;35:301–309. doi: 10.1530/jrf.0.0350301. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Amaral A, Brito R, Freitas M, Almeida Santos T. Simultaneous analysis of cytoskeletal patterns and chromosome positioning in human fertilization failures. Fertil Steril. 2004;82:1654–1659. doi: 10.1016/j.fertnstert.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15:553–572. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- Ratchford AM, Chang AS, Chi MM, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293:E1198–E1206. doi: 10.1152/ajpendo.00097.2007. [DOI] [PubMed] [Google Scholar]
- Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol Endocrinol. 2008;22:2643–2654. doi: 10.1210/me.2007-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. Faseb J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Hunter ES, 3rd, Balkan W, Horton WE., Jr Effects of maternal diabetes on embryogenesis. Am J Perinatol. 1988;5:319–326. doi: 10.1055/s-2007-999717. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Prather RS, Sun QY. The significance of mitochondria for embryo development in cloned farm animals. Mitochondrion. 2005;5:303–321. doi: 10.1016/j.mito.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Igaki T, Miura M. Bax-like protein Drob-1 protects neurons from expanded polyglutamine-induced toxicity in Drosophila. Embo J. 2005;24:2700–2713. doi: 10.1038/sj.emboj.7600721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–136. doi: 10.1016/j.theriogenology.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, Brenner CA. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote. 2000;8:209–215. doi: 10.1017/s0967199400001003. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Eppig JJ. Society for Reproductive Biology Founders' Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev. 2005;17:667–674. doi: 10.1071/rd05071. [DOI] [PubMed] [Google Scholar]
- Sun F, Yin H, Eichenlaub-Ritter U. Differential chromosome behaviour in mammalian oocytes exposed to the tranquilizer diazepam in vitro. Mutagenesis. 2001a;16:407–417. doi: 10.1093/mutage/16.5.407. [DOI] [PubMed] [Google Scholar]
- Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001b;122:155–163. [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–150. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- Tatewaki R, Hashimoto R, Tanigawa K, Furuse K, Tanaka O. Relationship between associations of NOR and chromosomal anomalies in the abnormal embryos of nonobese diabetic and STZ-diabetic mouse. Biol Neonate. 1995;67:132–139. doi: 10.1159/000244154. [DOI] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Jones GM. Developmental effects of sublethal mitochondrial injury in mouse oocytes. Biol Reprod. 2006;74:969–977. doi: 10.1095/biolreprod.105.048611. [DOI] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod. 2004;71:1936–1942. doi: 10.1095/biolreprod.104.033589. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Kuma A, Mizushima N. The role of autophagy during the oocyte-to-embryo transition. Autophagy. 2008a;4:1076–1078. doi: 10.4161/auto.7065. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008b;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod. 2007;13:759–770. doi: 10.1093/molehr/gam068. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–2633. doi: 10.1093/humrep/15.12.2621. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- Vesela J, Rehak P, Baran V, Koppel J. Effects of healthy pseudopregnant milieu on development of two-cell subdiabetic mouse embryos. J Reprod Fertil. 1994;100:561–565. doi: 10.1530/jrf.0.1000561. [DOI] [PubMed] [Google Scholar]
- Vomachka MS, Johnson DC. Ovulation, ovarian 17 alpha-hydroxylase activity, and serum concentrations of luteinizing hormone, estradiol, and progesterone in immature rats with diabetes mellitus induced by streptozotocin (41500) Proc Soc Exp Biol Med. 1982;171:207–213. doi: 10.3181/00379727-171-41500. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19:1–12. doi: 10.1071/rd06103. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yin S, Ai JS, Liang CG, Hou Y, Chen DY, Schatten H, Sun QY. Histone deacetylation is required for orderly meiosis. Cell Cycle. 2006;5:766–774. doi: 10.4161/cc.5.7.2627. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- Woo M, Patti ME. Diabetes risk begins in utero. Cell Metab. 2008;8:5–7. doi: 10.1016/j.cmet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Endo A, Watanabe G, Ingalls TH. Chromosomal aneuploidies and polyploidies in embryos of diabetic mice. Arch Environ Health. 1971;22:468–475. doi: 10.1080/00039896.1971.10665880. [DOI] [PubMed] [Google Scholar]
- Yang X, Borg LA, Eriksson UJ. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anat Rec. 1995;241:255–267. doi: 10.1002/ar.1092410212. [DOI] [PubMed] [Google Scholar]
- Yang X, Borg LA, Siman CM, Eriksson UJ. Maternal antioxidant treatments prevent diabetes-induced alterations of mitochondrial morphology in rat embryos. Anat Rec. 1998;251:303–315. doi: 10.1002/(SICI)1097-0185(199807)251:3<303::AID-AR5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Yin H, Baart E, Betzendahl I, Eichenlaub-Ritter U. Diazepam induces meiotic delay, aneuploidy and predivision of homologues and chromatids in mammalian oocytes. Mutagenesis. 1998;13:567–580. doi: 10.1093/mutage/13.6.567. [DOI] [PubMed] [Google Scholar]
- Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL, Liang XY. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod. 2007;22:1681–1686. doi: 10.1093/humrep/dem070. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16:841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- Zheng P, Vassena R, Latham KE. Effects of in vitro oocyte maturation and embryo culture on the expression of glucose transporters, glucose metabolism and insulin signaling genes in rhesus monkey oocytes and preimplantation embryos. Mol Hum Reprod. 2007;13:361–371. doi: 10.1093/molehr/gam014. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Brackett BG. Effects of luteinizing hormone on glucose metabolism in cumulus-enclosed bovine oocytes matured in vitro. Endocrinology. 1992;131:2690–2696. doi: 10.1210/endo.131.6.1446610. [DOI] [PubMed] [Google Scholar]


