Abstract
Acoustic Pharyngometry (APh) is a method for quantifying oropharyngeal tract configuration using sound wave reflection and is commonly used in diagnostics and research of sleep apnea. The standard preset output of APh (minimal cross-sectional area) has been established as reliable. However, by conducting post-processing measures on specific breathing tasks, APh data can also reveal oral length, oral volume, pharyngeal length and pharyngeal volume. Given that these measures may have utility in dysphagia research, the reliability of these measures is unknown and is the focus of the current study. 10 young healthy female volunteers completed two sessions of APh data collection to obtain measures of oral length, oral volume, pharyngeal length and pharyngeal volume one week apart. Two-way mixed intra-class correlation coefficients were calculated to establish intra-rater reliability, inter-rater reliability and test-retest reliability. Results revealed excellent levels of agreement within and across raters for all oropharyngeal tract parameters. Levels of test-retest agreement for oral length and oral volume indicated these parameters are appropriate for monitoring change within an individual. All parameters were deemed to have acceptable test-retest values as outcome measures in group-level analysis.
Keywords: deglutition, deglutition disorders, oral cavity, pharynx, acoustic pharyngometry, reliability
INTRODUCTION
Acoustic Pharyngometry (APh) is an FDA-approved, non-invasive diagnostic method used in sleep apnea clinics and research. APh works much like sonar, where reflected sound waves are used to define a space. In APh, sound waves (produced by the device’s wavetube) are introduced into the oral and pharyngeal cavities via a snorkel-like mouthpiece and reflected back to a microphone housed in the wavetube providing a method to quantify the size/shape of the upper airway (see Figure 1). APh has been validated against MRI [1] and CT [2,3]. The standard APh preset measurements obtained using the ECCOVISION® Acoustic Pharyngometer™ (Sleep Group Solutions) according to the operator manual provides physicians/clinicians with a minimum cross-sectional area of the pharynx, which is an important variable in the study of obstructive sleep apnea (OSA). Minimal cross-sectional area obtained via APh has been established to have acceptable intra-session and test-retest reliability [4,5].
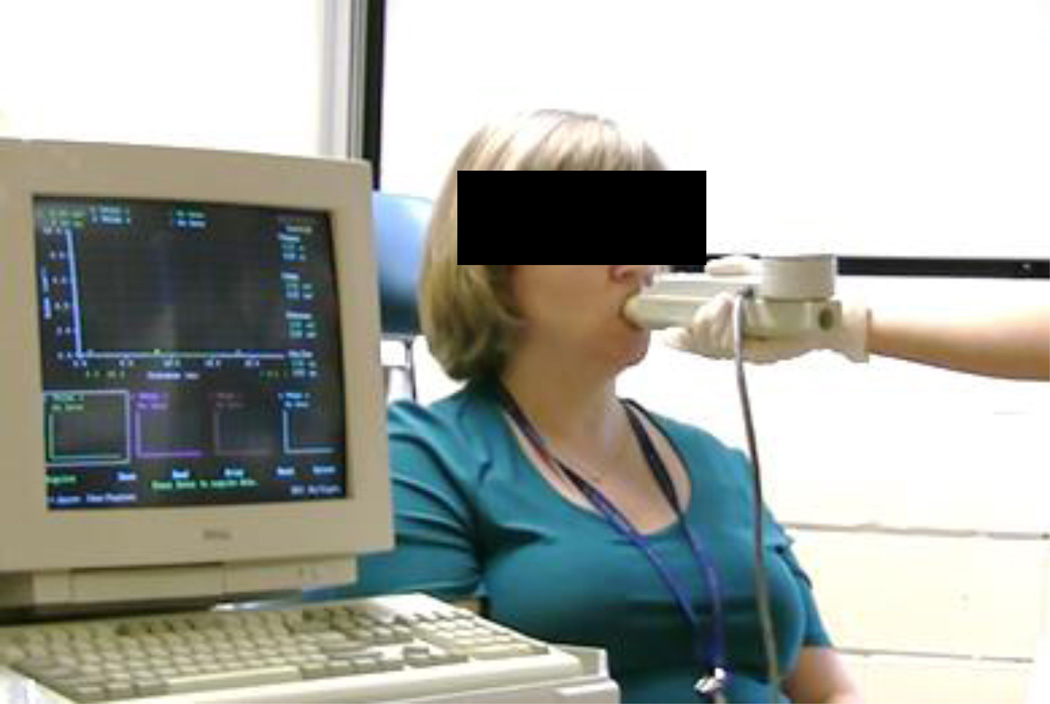
Figure 1.
Photograph of a subject participating in Acoustic Pharyngometry measurement.
In recent years, research has surfaced that uses APh to quantify other parameters of the oropharyngeal tract (beyond traditional, preset OSA measures). By having participants conduct specific breathing tasks and by conducting post-processing measurement, researchers can extract oral length, oral volume, pharyngeal length and pharyngeal volume using this quick, non-invasive and inexpensive tool. Indeed, several recent studies have established normative data of these oropharyngeal tract parameters according to sex, race, body position, development and aging derived using APh [6–9]. These derived measures may have significant relevance in dysphagia research. Changes in oro-pharyngeal tract morphology across the lifespan, as the well as changes in response to disease and/or treatment may explain aspects of swallowing physiology. APh provides a non-invasive and efficient method for capturing oro-pharyngeal measures of length and volume to be incorporated in dysphagia research. Unfortunately, previous research that reports these derived APh parameters either fails to report reliability data or does not employ methodology that allows us to quantify the strength of agreement (i.e. reports significant p-values for Pearson Correlations between ratings). Given that these derived parameters are collected through post-processing and involve human measurement, a careful investigation of the reliability of these measures is warranted to establish their utility in future dysphagia research.
The purpose of this study is to establish the reliability of oral and pharyngeal parameters derived from APh according to the Vorperian protocol [10]. Specifically, this work will establish the intra-rater, inter-rater reliability and test-retest reliability for measures of oral length, oral volume, pharyngeal length and pharyngeal volume.
MATERIALS AND METHODS
Participants
Data was collected from a convenience sample of ten healthy young female volunteers (mean: 25.3 years old, SD: 3.2 years). Exclusion criteria included prior neurological disease, head and/or neck surgery, dental appliances and sleep apnea. For test-retest reliability, the data collection protocol was completed twice for each participant, exactly one week apart. Height, weight, neck circumference and tongue strength measures were also collected during the first visit but are not the focus of the current study. This study was approved by the local Institutional Review Board. All participants signed a consent form prior to completing the study.
Acoustic Pharyngometry
Data was collected with the ECCOVISION® Acoustic Pharyngometer™ (Sleep Group Solutions) following a published protocol for collecting derived APh measures [10]. Participants were seated upright in a chair and instructed to maintain a comfortable neutral head position while the wave tube was held parallel to the floor by the examiner. With the mouthpiece in place (lips closed, teeth resting in the guard, tongue underneath the guard), data was captured on an exhale of four breathing tasks (oral, oral, nasal and ‘modified valsalva’). Exhalation was determined by observing the participant’s shoulders, chest and abdomen. Data capture was achieved by the manual press of the acquisition button on the wave tube by the examiner. First, two waveforms of regular, oral breathing were captured. Two samples were collected to ensure a representative waveform was obtained. Representativeness was confirmed (online during data collection) by comparing the total volume between the two oral samples and insuring that less than 6% difference between existed between them (as per ECCOVISION® Acoustic Pharyngometer™ Operator Manual). This is readily available from the APh output when the two oral breathing tasks are superimposed. Typically, if a participant does not move or change their breathing pattern, both trials are within 6% on the first attempt. In order to obtain the derived measures during later post-processing, two other breathing tasks must be conducted to locate anatomical landmarks representing the velum and the glottis. To locate the velum during later post-processing, a waveform on a nasal breathing task is captured. To locate the glottis during later post-processing, a waveform on a modified valsalva task is captured. Modified valsalva breathing requires participants to situate their vocal folds in a medial position (for later identification of the glottis from the waveform). Participants are instructed to visualize a silent ‘o’ vowel (adduction without phonation) and/or to bear down and only let a small amount of air escape through their vocal folds (valsalva with air escape). Correct interpretation of instructions was confirmed prior to inserting the mouthpiece by having participants demonstrate all three breathing tasks and can be further confirmed during data collection by examining the waveform for their characteristic shapes (i.e. 3rd trough for modified valsalva, see details below). The graph for each task was saved using a unique alphanumeric code for offline randomized and blinded analysis. Total time for collecting APh measures from each participant was approximately 5 minutes.
Measurement
All measurement was conducted according a detailed published protocol [10]. First, the rater identifies the most appropriate of the two oral breathing graphs to be used for measurement based on three criteria: the oral graph with smallest error bars, the oral graph that best overlaps with the oral cavity of the nasal graph, and the oral graph that is higher than the modified valsalva graph. Next, the rater identifies the location of the velum and the glottis. The velum is located at the base of the first peak of the nasal graph where the difference between consecutive cross-sectional areas is found to be less than 0.15cm2. This is achieved by advancing the cursor from left to right at the first peak of the nasal task, and observing the change in cross-sectional area (automatically calculated at each cursor location associated with the offset). The glottis is located at the lowest point of the 3rd trough in the modified valsalva graph by observing the lowest value in cross-sectional area associated with the cursor location. These locations are overlaid on the chosen oral breathing graph to calculate oral length (distance from velum – teeth), oral volume (area-under-the-curve from velum to teeth), pharyngeal length (distance from glottis – velum) and pharyngeal volume (area-under-the-curve from glottis to velum). See Figure 2 for a schematic representation of the cross-sectional area along the length of the oropharyngeal tract. The figure highlights how the velum is located from the nasal breathing waveform and the glottis is located from the modified valsalva waveform. These landmarks are then used to calculate oral and pharyngeal length (distance) and oral and pharyngeal volume (area-under-the-curve) from the oral breathing waveform.
Figure 2.
Schematic representation of Acoustic Pharyngometry set-up, the three breathing tasks and the measurement method.
This author conducted all measurements in a blinded, randomized fashion immediately following completion of data collection (original ratings). One hundred percent of data were immediately re-rated by a trained research assistant (inter-rater reliability) and also by this author after three weeks had passed (intra-rater reliability).
Statistical Analyses
All statistical analyses were conducted in IBM SPSS version 22. First, descriptive statistics were calculated. Next, all reliability measures were computed using two-way mixed intraclass correlation coefficients (ICCs) for consistency. A priori determined cut-offs for acceptable reliability depended on the type of reliability measure. Intra- and inter-rater reliability assess agreement of the same rater with themselves (intra) or with a second rater (inter). Intra- and inter-rater reliability ICCs of 0.75 are considered to have ‘excellent’ reliability [11]. Test-retest reliability assesses whether the instrument (APh) produces a similar result on repeated administrations. According to Fitzpatrick et al [12], ICCs > 0.7 are the minimum acceptable level of agreement between two testing sessions when assessing group-level data. Higher minimum values (ICC > 0.90) are recommended for measures that will assess outcomes in a single individual [12].
RESULTS
Descriptive statistics for the four parameters of interest are displayed in Table 1.
Table 1.
Descriptive statistics including mean and standard deviation (SD) for the four variables of interest.
| ORAL LENGTH (cm) | ORAL VOLUME (ml) | |||||
| Original | Retest | Pooled | Original | Retest | Pooled | |
| MEAN | 8.48 | 8.49 | 8.49 | 42.70 | 39.82 | 41.26 |
| SD | 0.46 | 0.41 | 0.42 | 7.28 | 5.87 | 6.60 |
| PHARYNGEAL LENGTH (cm) | PHARYNGEAL VOLUME (ml) | |||||
| Original | Retest | Pooled | Original | Retest | Pooled | |
| MEAN | 12.18 | 12.62 | 12.40 | 33.93 | 31.89 | 32.91 |
| SD | 0.86 | 0.57 | 0.75 | 9.11 | 4.98 | 7.22 |
Reliability scores appear in Table 2. Encouragingly, all intra- and inter-rater reliability values for all four parameters easily achieved ‘excellent’ reliability (ICC > 0.75). While all test-retest reliability values meet the minimum acceptable level of agreement for use as an outcome measure in group analyses (ICC > 0.70)[14], only the oral measures (length and volume) appear to be appropriate for detecting individual change (ICC > 0.90) [12].
Table 2.
Reliability results presented using Intra-class Correlation Coefficients (ICC) and 95% Confidence Intervals
| INTRA-RATER RELIABILITY | INTER-RATER RELIABILITY | TEST-RETEST RELIABILITY | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||
| Oral Length | 0.944 | 0.858 | 0.978 | 0.917 | 0.790 | 0.967 | 0.936 | 0.743 | 0.984 |
| Oral Volume | 0.996 | 0.991 | 0.999 | 0.986 | 0.965 | 0.994 | 0.913 | 0.649 | 0.978 |
| Pharyngeal Length | 0.953 | 0.881 | 0.981 | 0.817 | 0.537 | 0.927 | 0.726 | −0.102 | 0.932 |
| Pharyngeal Volume | 0.966 | 0.914 | 0.987 | 0.977 | 0.941 | 0.991 | 0.780 | 0.113 | 0.945 |
DISCUSSION
Acoustic pharyngometry (APh) is a low-cost, easy to administer, non-invasive tool primarily used to capture minimal cross-sectional area of the pharynx in the assessment and management of OSA [4,5]. However, post-processing of APh waveforms can be completed to quantify additional oropharyngeal tract parameters including oral length, oral volume, pharyngeal length and pharyngeal volume. While several reports of these variables have been reported in the speech science and physiology literature [6–9], none has adequately quantified reliability of the parameters. Determining the reliability of these parameters within and across raters, as well as across sessions is of paramount importance before APh in incorporated into dysphagia research.
This work establishes that vocal tract parameters extracted from APh waveforms have acceptable levels of inter-rater, intra-rater and test-retest reliability. Non-invasive methods for capturing these measures have potential for use in future dysphagia research. The relationship between oropharyngeal tract parameters and swallowing physiology can be studied. These parameters could be incorporated into statistical analyses to control for variation attributable to size/length of the oropharynx. They might be used as a non-invasive method to monitor change in oropharyngeal muscles – for example atrophy of the tongue or pharynx. These measures may also have a role to play in the monitoring post-surgical edema of the oropharynx. Finally, they could be used to monitor morphological changes in the context of maturation or aging and/or explain changes in swallowing physiology in the context of maturation or aging.
The data analyzed for this study was restricted to young, healthy women. While we do not anticipate altered reliability based on age or sex, it may be worth ruling out in future studies. Of course, the active participation of the subject to conduct the breathing tasks will limit the youngest of age groups from being studied. Future work should compare these oropharyngeal parameters to a gold-standard imaging method in patients with known impairments to velar and/or glottal function, given that this approach may not be valid in these patient populations.
CONCLUSIONS
Acoustic Pharyngometry can be used to calculate measures of oral length, oral volume, pharyngeal length and pharyngeal volume and is relatively inexpensive, easy-to-administer and non-invasive. This research confirms that the oral and pharyngeal measures derived from Acoustic Pharyngometry output have satisfactory inter-rater, intra-rater and test-retest reliability, thus establishing these measures as reproducible and acceptable for monitoring outcomes in group-level data.
Acknowledgments
I would like to thank Danielle Brates for assistance with data collection and analysis. The research reported in this publication was supported by National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number 1R21DC015067-01 and by the American Speech Language Hearing Association’s Advancing Academic Research Careers Award (ASHA AARC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or ASHA.
Footnotes
CONFLICTS OF INTEREST
I have no conflicts of interest to disclose.
REFERENCES
- 1.Marshall I, Maran NJ, Martin S, Jan MA, Rimmington JE, Best JJK, et al. Acoustic reflectometry for airway measurements in man: Implementation and validation. Physiol Meas. 1993;14(2):157–169. doi: 10.1088/0967-3334/14/2/007. [DOI] [PubMed] [Google Scholar]
- 2.D'Urzo AD, Rubinstein I, Lawson VG, Vassal KP, Rebuck AS, Slutsky AS, et al. Comparison of glottic areas measured by acoustic reflections vs. computerized tomography. J Appl Physiol. 1988;64(1):367–370. doi: 10.1152/jappl.1988.64.1.367. [DOI] [PubMed] [Google Scholar]
- 3.D'Urzo AD, Lawson VG, Vassal KP, Rebuck AS, Slutsky AS, Hoffstein V. Airway area by acoustic response measurements and computerized tomography. Am Rev Respir Dis. 1987;135(2):392–395. doi: 10.1164/arrd.1987.135.2.392. [DOI] [PubMed] [Google Scholar]
- 4.Brooks LJ, Castile RG, Glass GM, Griscom NT, Wohl ME, Fredberg JJ. Reproducibility and accuracy of airway area by acoustic reflection. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1984;57(3):777–787. doi: 10.1152/jappl.1984.57.3.777. [DOI] [PubMed] [Google Scholar]
- 5.Kamal I. Test-retest validity of acoustic pharyngometry measurements. Otolaryngology - Head and Neck Surgery. 2004;130(2):223–228. doi: 10.1016/j.otohns.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Xue SA, Cheng RWC, Ng LM. Vocal tract dimensional development of adolescents: An acoustic reflection study. Int J Pediatr Otorhinolaryngol. 2010;74(8):907–912. doi: 10.1016/j.ijporl.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Xue SA, Hao GJP, Mayo R. Volumetric measurements of vocal tracts for male speakers from different races. Clinical Linguistics and Phonetics. 2006;20(9):691–702. doi: 10.1080/02699200500297716. [DOI] [PubMed] [Google Scholar]
- 8.Xue SA, Hao GJ. Changes in the human vocal tract due to aging and the acoustic correlates of speech production: A pilot study. Journal of Speech, Language, and Hearing Research. 2003;46(3):689–701. doi: 10.1044/1092-4388(2003/054). [DOI] [PubMed] [Google Scholar]
- 9.Vorperian HK, Kurtzweil SL, Fourakis M, Kent RD, Tillman KK, Austin D. Effect of body position on vocal tract acoustics: Acoustic pharyngometry and vowel formants. J Acoust Soc Am. 2015;138(2):833–845. doi: 10.1121/1.4926563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vorperian HK. VTLab Acoustic Pharyngometry (APh) Protocol: Data Collection (Part I) & Data Analysis (Part II) [Accessed 09/09, 2014];2013 Available at: http://www.waisman.wisc.edu/vocal/APh-Protocol-Part%20I%20&%20II-Running%20Participants-Copyright-v11-FINAL2.pdf. [Google Scholar]
- 11.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- 12.Fitzpatrick R, Davey C, Buxton M, Jones D. Evaluating patient based outcome measures for use in clinical trial. Health Technol Assess. 1998;2:1–74. [PubMed] [Google Scholar]