Abstract
The two splice variants of human glucose transporter 9 (hGLUT9) are targeted to different polarized membranes. hGLUT9a traffics to the basolateral membrane, whereas hGLUT9b traffics to the apical region. This study examines the sorting mechanism of these variants, which differ only in their N-terminal domain. Mutating a di-leucine motif unique to GLUT9a did not affect targeting. Chimeric proteins were made using GLUT1, a basolaterally targeted transporter, and GLUT3, an apically targeted protein whose signal lies in the C-terminus. Overexpression of the chimeric proteins in polarized cells demonstrates that the N-terminus of hGLUT9b contains a signal capable of redirecting GLUT1 to the apical membrane. The N-terminus of hGLUT9a, however, does not contain a basolateral signal sufficient enough to redirect GLUT3. Portions of the GLUT9a N-terminus were substituted with corresponding portions of the GLUT9b N-terminus to determine the motif responsible for apical targeting. The first 16 amino acids were not found to be a sufficient apical signal. The last ten amino acids of the N-termini differ only in amino acid class at one location. In the B-form, leucine, a hydrophobic residue, is substituted for lysine, a basic residue, found in the A-form. However, mutation of the leucine in hGLUT9b to a lysine resulted in retention of the apical signal. We therefore believe the apical signal exists as an interplay between the final ten amino acids of the N-terminus and another motif within the protein such as the intracellular loop or other motifs within the N-terminus.
Keywords: glucose transporter, plasma membrane, targeting
Introduction
Glucose transport across epithelial cell layers is critical for the maintenance of glucose homeostasis within mammals. While a small amount of glucose can diffuse through the phospholipid bilayer, the majority is transported down a concentration gradient via a class of proteins known as facilitative glucose transporters (GLUTs). These proteins are characterized by 12 transmembrane spanning domains, intracellular N- and C-termini, multiple glycosylation sites and a host of sugar transporter signatures consisting of conserved sequence motifs (1). Currently, there are fourteen known members of this protein family, which have been subdivided into three different classes. The first four transporters to be cloned (GLUT1–4) and GLUT14 are members of class I. Class II is comprised of GLUTs 5, 7, 9, and 11 while class III encompasses GLUTs 6, 8, 10, 12, and 13. GLUT9 is among the more recently cloned glucose transporters, and while evidence suggests it is a high affinity glucose transporter and high capacity urate transporter, relatively little is known regarding its function (2; 3).
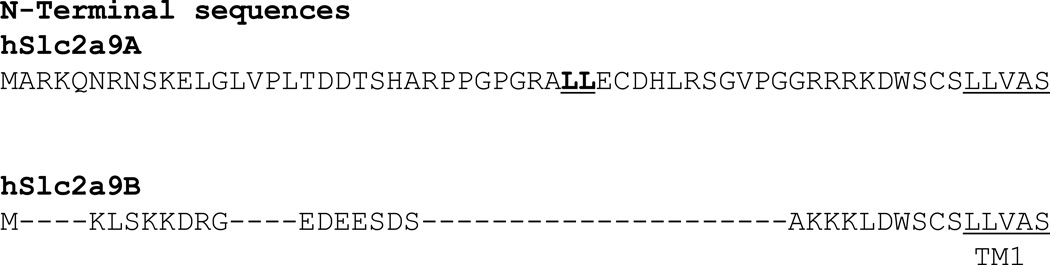
GLUT9 is a unique transporter in a number of ways. GLUT9 exists as two splice variants in both mouse and human (4; 5). The only difference between the sequences of these two forms is in the N-terminus, with the B-form N-terminus containing 29 fewer amino acids than the A-form (Figure 1). At 55 amino acids, the N-terminus of the A form is the longest of those in the GLUT family, where the average N-terminal length is approximately 18 amino acids (1). Preliminary evidence suggests this variation is dependent upon different promoter regions with the B-form region upstream of the A-form region. Two other GLUTs have demonstrated N-terminus splice variation thought to be regulated by different promoter sites – GLUT11, also a member of class II, and GLUT14, a member of class I. However, there are notable difference in the relationships of these splice variants and those of GLUT9.
Figure 1. Sequence alignment of the two hGLUT9 splice variant N-termini.
Clustal W was used to align the sequences of the N-termini, the only regions where the splice variants differ.
The expression of the two forms of hGLUT9 is unique in that the B-form is only expressed in tissues also expressing the A-form. RT-PCR has shown expression of the A-form in many human tissues. However, B-form expression is limited to human kidney and placenta (4). This expression pattern differs from the mouse homolog of GLUT9 in that both splice variants of mouse GLUT9 are found exclusively in the liver and kidney (5). In the case of GLUT11, while there is some overlap amongst tissues with expression, each splice variants has at least one tissue where it is predominant (6). Conversely, the two splice variants of GLUT14 are only expressed in one location, the testis (7). Furthermore, GLUT9 is the only glucose transporter shown to have splice variants and homologs of these variants expressed in mouse tissue. This unique aspect suggests a more generalized function of the splice variation.
Many studies have investigated the polarized membrane targeting motifs within the class I GLUTs. GLUT1 is a known basolateral sorting protein, however little is known regarding the sequence responsible for that sorting motif. GLUT 2 also goes to the basolateral membrane (8). GLUT3 is known to sort to the apical membrane, attributed to an apical signal located in the C-terminus (9). All of the members of class III have a di-leucine motif that is suggested to play a role in the recycling pathway by promoting endocytosis (10; 11; 12). To date, the two GLUT9 splice variants are the only glucose transporter splice variants shown to have different intracellular expression patterns. When overexpressed in a polarized epithelial cell line, hGLUT9a is targeted to the basolateral membrane, while hGLUT9b is targeted to the apical membrane (4). This differential targeting is also unique to the human form of GLUT9, as both splice variants of mouse GLUT9 are found at the basolateral membrane in native tissue and overexpression cell culture studies (5).
In the case of hGLUT9, the A-form harbors a di-leucine motif but is not targeted to a recycling compartment. Other membrane spanning transport proteins, such as aquaporins, rely on a di-leucine motif as a basolateral signal, thus this motif may be responsible for the basolateral sorting seen in the A-form. While, N-linked glycosylation sites are often cited as apical sorting signals, these do not exist in the N-termini of the GLUT9 variants (13). This differential sorting in mammalian cells could have profound implications on glucose homeostasis, thereby making the mechanism by which it occurs very intriguing. As a result of past studies, we hypothesized that targeting motifs might exist in either N-terminus of the hGLUT9 splice variants.
In this study, we show that the apical targeting of human GLUT9b is due to a signal in the N-terminus. Using chimeric proteins we show the ability of the B-form N-terminus to redirect GLUT1 to the apical membrane, while the N-terminus of the A-form is unable to redirect GLUT3. Furthermore, substitution constructs and mutations within the B-form have narrowed the targeting signal to the 10 amino acids preceding the first transmembrane domain.
Materials and Methods
Reagents and Chemicals
Human GLUT9A and GLUTB are also referred due by their HGNC (#13446) nomenclature as SLC2A9a and SLC2A9b and sequence AF210317.1. All primers were synthesized by Integrated DNA Technology (Coralville, IA). Fusion primers, substitution primers, and mutagenesis primers were all either HPLC or PAGE purified. Unless otherwise noted, all chemicals were obtained from Sigma (St. Louis, MO).
Construct Development
Constructs were made piece-wise using a series of PCR reactions with specified primers. (See Table 1 for fusion primer sequences; Figure 2. for schematic of chimeric proteins). Once fusion reactions were done, the constructs were amplified using primers pcDNAF2 (5’AAATGTCGTAACAACTCCGC3’) and BGH (5'TAGAAGGCACAGTCGAGGC3'). Digestion of the PCR products and pcDNA 3.1(+) with KpnI and XbaI yielded sticky ends and thus facilitated introduction of the construct into the plasmid via ligation using the Rapid DNA Ligation Kit (Roche Diagnostics Corp., Indianapolis, IN) and following manufacturer protocol. Constructs were sequenced to ensure correct fusion of components. For substitution chimeras, the following primers were used:
1–9F 5’AAAGGTACCGCCACCATGAAGCTCAGTAAAAAGGACCGAGGACTAGTTCCCCTCACA3’
1–16F 5’AAAGGTACCGCCACCATGAAGCTCAGTAAAAAGGACCGAGGAGAAGATGAAGAAAGTGATTCAGGGCCTCCAGGGCCA3’.
The reverse primer used for both was BGH. For construct mutagenesis, the QuikChange II: Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) was used with HPLC purified primers. For LL→AA mutagenesis of hGLUT9a, the following primers were used:
Forward-5’GCCAGGGAGGGCAGCGGCGGAGTGTGACCACCTGAGGAGTG3’
Reverse-5’TCAGGTGGTCACCTCCGCCGCTGCCCTCCCTGGCCCTGGAG3’
For the L→K mutagenesis in hGLUT9b, the following primers were used:
Forward-5’GATTCAGCGAAAAAGAAAAAGGACTGGTCCTGC3’
Reverse- 5’GCAGGACCAGTCCTTTTTCTTTTTCGCTGAATC3’.
Table 1.
Fusion Primers for Chimeric Protein Generation
| Slc2a | N-terminus | Backbone | |
|---|---|---|---|
|
9A /1 |
For | 5' CATTGACGTCAATGGGAGTTTG 3' | 5' GGAAGGAGAAGAAAGGACTGGTCCTGCTCGCTCATGCT GGCTGTGGGAGGAGCAGTGCTT3' |
| Rev | 5’AAGCACTGCTCCTCCCACAGCCAGCATGAGCGAGCAGGA CCAGTCCTTTCTTCTCCTTCC3' |
5' TAGAAGGCACAGTCGAGGC 3' | |
|
9A /3 |
For | 5' CATTGACGTCAATGGGAGTTTG 3' | 5' AGAAGAAAGGACTGGTCCTGCTCGCTGATATTTGCCATC ACAGTTGCT3' |
| Rev | 5' AGCAACTGTGATGGCAAATATCAGCGAGCAGGACCAG TCCTTTCTTCT3' |
5' TAGAAGGCACAGTCGAGGC 3' | |
|
9B /1 |
For | 5' CATTGACGTCAATGGGAGTTTG 3' | 5' AAATTGGACTGGTCCTGCTCGCTCATGCTGGCTGTGGGA GGAGCAGTG3' |
| Rev | 5' CACTGCTCCTCCCACAGCCAGCATGAGCGAGCAGGAC CAGTCCAATTT3' |
5' TAGAAGGCACAGTCGAGGC 3' | |
|
9B /3 |
For | 5' CATTGACGTCAATGGGAGTTTG 3' | 5' AAGAAATTGGACTGGTCCTGCTCGCTGATATTTGCCATC ACAGTTGCT3' |
| Rev | 5'AGCAACTGTGATGGCAAATATCAGCGAGCAGGACCAGTC CAATTTCTT3' |
5' TAGAAGGCACAGTCGAGGC 3' |
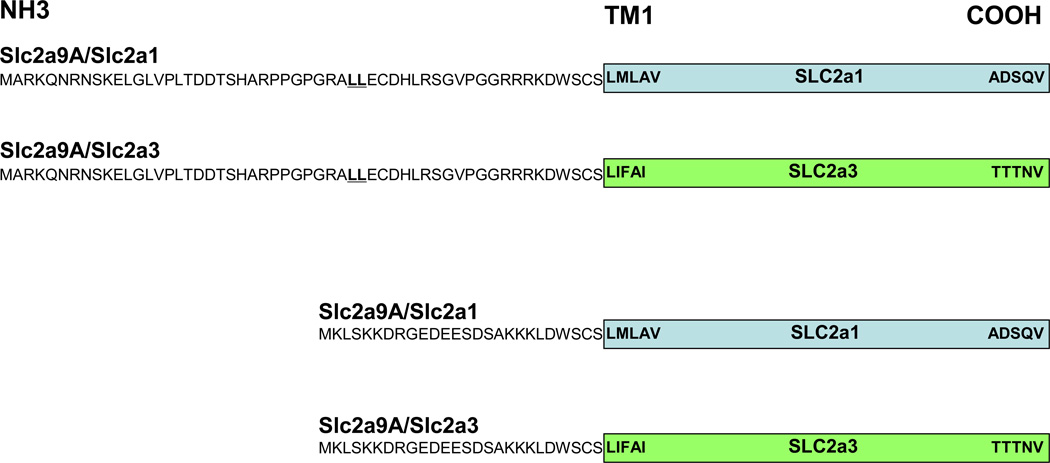
Figure 2. A graphical representation of the chimeric protein strategy.
Using fusion PCR primers, each of these four constructs were developed and cloned into mammalian expression vectors: Slc2a9A/Slc2a1, Slc2a9A/Slc2a3, Slc2a9B/Slc2a1, and Slc2a9B/Slc2a3. In addition, the wildtype constructs Slc2a9A and Slc2a9B are displayed.
Cell Culture and Transfection
Madin-Darby Canine Kidney (MDCK) cells were used as an epithelial cell model. The cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2% glutamine, 1% sodium pyruvate, 1% penicillin/streptomycin, and 10% fetal calf serum. Prior to transfection, cells were split and seeded for 24 h. Transfection was carried out using FuGENE 6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Stable expression was achieved by splitting the cells 24 h after transfection into medium supplemented with 0.8 mg/ml G418. Clonal colonies were obtained by serial dilution. To investigate the localization of each construct in polarized epithelial cells, expressing MDCK cells were plated at high density (1.6 × 106 cells) onto 0.45-µm Falcon cell culture inserts (catalog number 3090, BD Biosciences, San Jose, CA) and cultured for five days under daily media renewal. After achieving stable transfection, all polarization experiments were repeated between 3 and 5 times for each construct. Localization of each construct was done using immunohistochemical staining.
Immunofluorescent Staining
Cells were washed twice with PBS, fixed for 10 min in 3% paraformaldehyde, and quenched by three washes with 50 mM NH4Cl in PBS. Cells were permeabilized with 0.2% Saponin and washed. Nonspecific antiserum binding was blocked with 2% BSA in PBS/0.01% Saponin for 30 min. Cells were incubated for 1 h with antiserum for the respective construct. Constructs containing the GLUT9a N-terminus and GLUT9b N-terminus were incubated with peptide purified antibodies raised to a portion of those specific sequences at a concentration of 1.5 µm/mL in 2% BSA/0.01% Saponin/PBS. Constructs which contain mutations or substitutions in the N-terminus were probed with an antibody to the C-terminus of hGLUT9 diluted 1:500 in 2% BSA/0.01% Saponin/PBS. The cells were then washed with PBS/0.01% Saponin, and probed with goat anti-rabbit Alexa 488 antibody (1:200 in 2% BSA in PBS/0.01% Saponin). Cells were washed with PBS/0.01% Saponin, and nuclei were stained with 1 mMTopro-3 iodide (1:500 in PBS, Molecular Probes, Eugene, OR) for 10 min. After a final PBS/0.01% Saponin wash, cells were mounted using Secure Seal imaging spacers (Sigma Chemical, St. Louis, MO) and Vectashield (Vector Laboratories, Burlingame, CA). Specimens were examined by confocal microscopy using a Bio-Rad MRC-600 or Nikon C1 confocal microscope. All these immunostaining experiments were repeated at least 3 times with the stably transfected polarized cell lines.
Total Membrane Extraction and Western Blots
Total membrane extraction and western blotting were performed to confirm expression of each construct. Cells were washed with PBS and homogenization buffer (HB-20mM Tris/HCl pH 7.4, 1mM EDTA, 255mM Sucrose). The cells were then homogenized in HB containing protease inhibitors using 15 passages of a 25G needle. The slurry was then centrifuged at 4° C 600g for 10 minutes. Total membrane proteins were then separated from the cytosol by centrifugation for 30 minutes at 200,000g using a Beckman TLA 100.4 rotor (Beckman Coulter, Fullerton, CA, USA). Total membrane proteins were run on 10% polyacrylamide gels, transferred onto nitrocellulose, blocked with 5% dry milk in Tris-buffered saline/Tween 20, and probed with an antibody raised against the GLUT9a N-terminus (5 micrograms/mL in 1% dry milk), or the GLUT9b N-terminus (5 µg/mL in 1% dry milk), depending on the components of the constructs. Western blots were probed with a horseradish peroxidase-coupled goat anti-rabbit secondary antibody (1:10,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and developed using the SuperSignal Dura Western kit (Pierce Biotechnology, Inc., Rockfort, IL).
ATB-BMPA Photolabeling and Electrophoresis
Cells were washed two times with Krebs-Ringer-Hepes buffer (KRH buffer, 136 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 10 mM Hepes, pH 7.4). The cells then incubated with 40 µM Bio-ATB-BMPA in 300 µl KRH buffer for 10 min at 18°C, and irradiated for 1 minute in Rayonet photoreactor with 300 nm lamps. Following irradiation the cells were washed four times with 1% BSA/Hepes buffer, four times with 0.1% BSA/Hepes buffer, and once in KRH buffer to remove excess photolabel.
The cells were then solubilized in 1 ml of detergent buffer containing 2% C12E9, PBS, pH 7.4, and proteinase inhibitors, and maintained at 4°C for 10 min, following centrifugation of solubilization cells at 20,000 g for 20 min at 4°C. The supernatant was separated from unsolubilized pellet and then mixed with 50 µl of 50% slurry of immobilized streptavidin on 6% beaded agarose (Pierce) by slow rotation overnight at 4°C. The precipitates were washed three times in 1% C12E9 detergent buffer, three times in 0.1% (w/v) C12E9 detergent buffer, and once with PBS, pH 7.4. The biotinylated protein was eluted at 95°C for 20 min in electrophoresis sample buffer (62.5 mM Tris-HCl, pH 6.7, 2 % SDS, 50 % glycerol, 0.02% Coomassie Blue and 10% mercaptoethanol) and subjected to electrophoresis on 10% polyacrylamide gels, transferred onto nitrocellulose (Bio-rad), blocked for 1 hour in phosphate/saline buffer, 0.1% (v/v) Tween 20 and 5% (w/v) non-fat dry milk, and then immunoblot with GLUT9 overnight at 4°C. The bound antibody was detected using enhanced chemiluminescence detection reagents (Amersham Biosciences UK) after incubation for 1 hour with ECL anti-rabbit, horseradish peroxidase antibody (1:5000, Amersham Biosciences UK).
Results
The GLUT9a N-terminal di-leucine motif is not responsible for the basolateral sorting of this protein
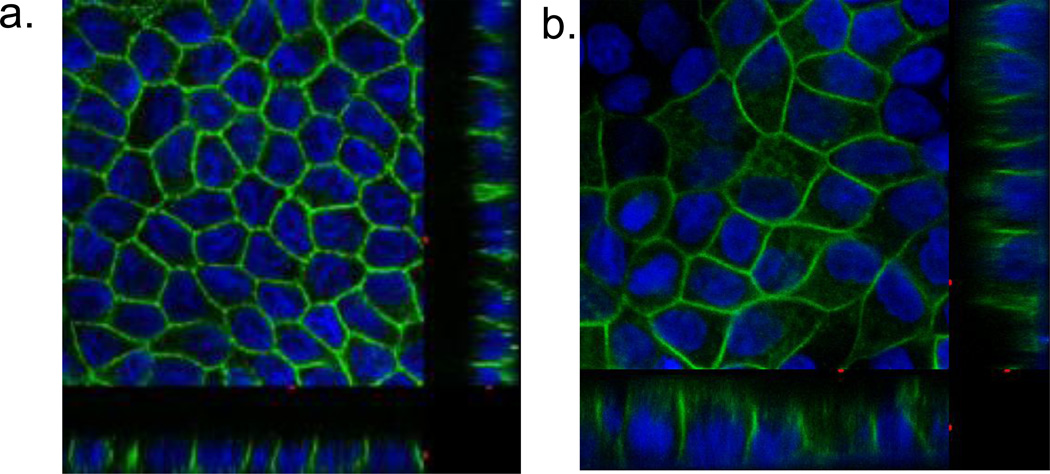
In order to investigate the role of the N-terminal di-leucine motif in hGLUT9a, mutation of leucines 33 and 34 both to alanines was performed. The resulting transporter still targets to the basolateral membrane (Figure 3A and B). The observed targeting pattern suggests that the di-leucine motif is not responsible for the basolateral targeting in hGLUT9a.
Figure 3. Localization of hGLUT9A in overexpressing MDCK cells (A) compared to cells overexpressing hGLUT9A with the N-terminus LL→AA Mutation (B).
hGLUT9A cDNA with the WT dileucine motif was cloned into a mammalian expression vector and stably overexpressed in MDCK cells. Immunofluorescent staining with a polyclonal antibody to the C-terminus reveals a basolateral membrane sorting pattern (A). The same pattern is seen in the construct containing a LL→AA mutation in the N-terminus (B).
GLUT9a N-terminus does not contain a basolateral signal capable of redirection
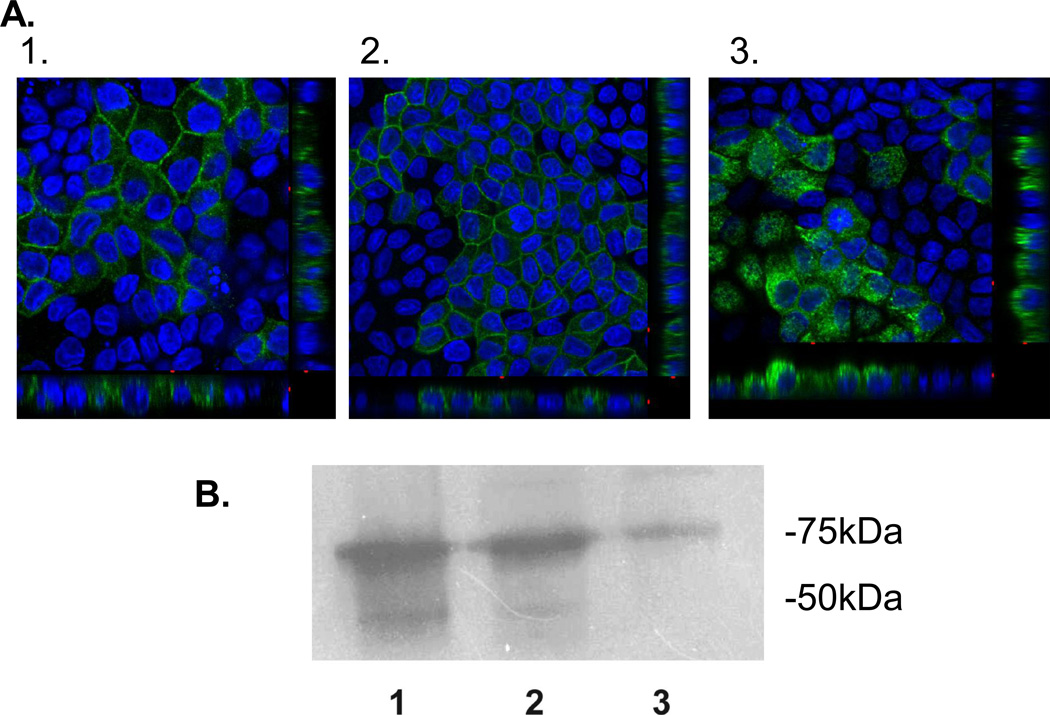
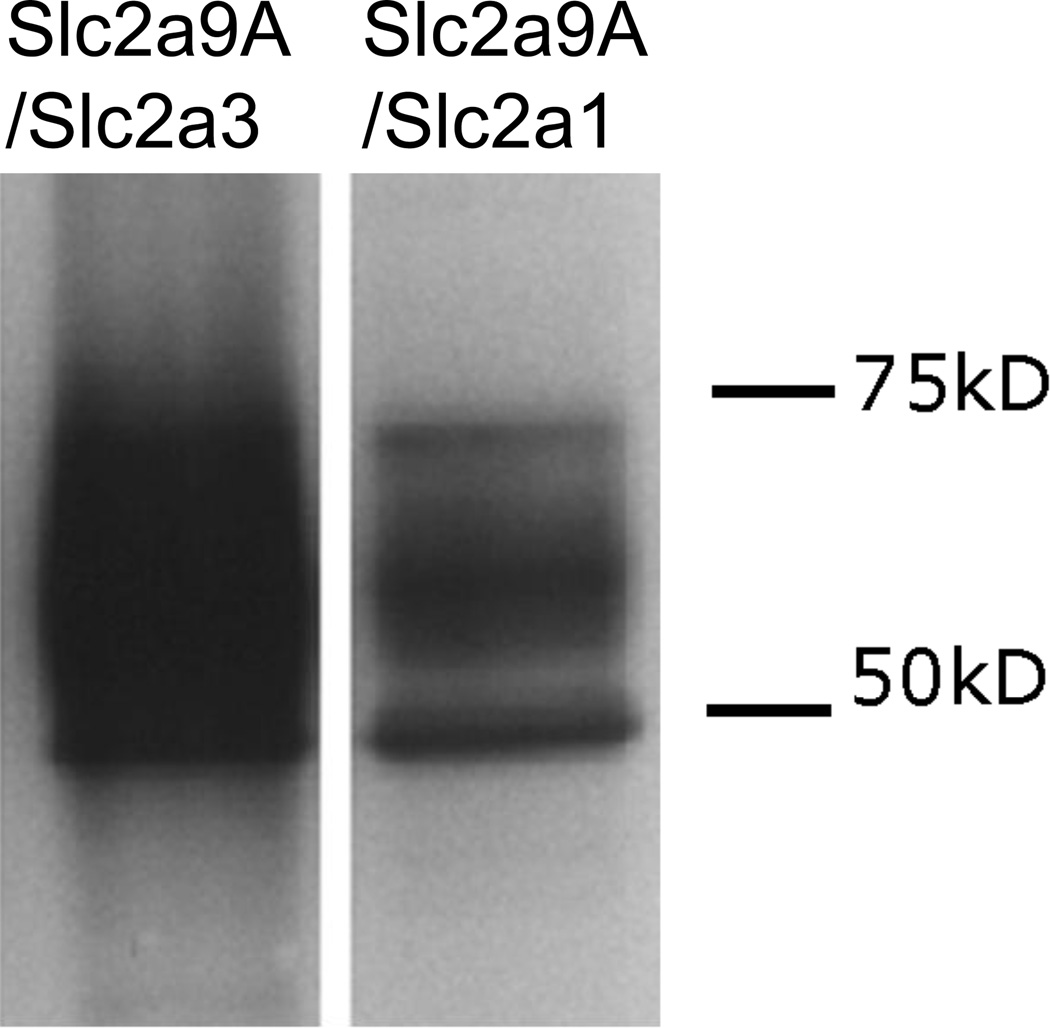
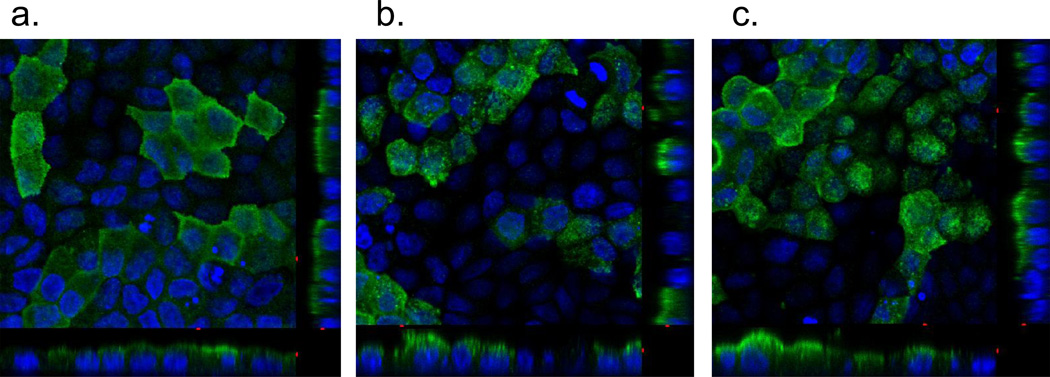
The first set of chimeras was made using the N-terminus of hGLUT9a cloned on to the GLUT1 and GLUT3 backbones. These constructs are referred to as G9A/G1 and G9A/G3, respectively. Orthogonal projections of the confocal microscopy z-series demonstrate that the G9A/G1 chimera localizes to the basolateral membrane (Figure 4B) as would be expected for either G9A or G1 in entirety (Figure 4A). However, G9A/G3 localizes to the apical membrane as would be expected with the C-terminus apical signal harbored in GLUT3 (Figure 4C). The failure of this chimera to localize to the basolateral membrane suggests the lack of a dominant basolateral signal within the N-terminus of G9A. ATB-BMPA photolabeling reagent was used to tag the basolateral surfaces and the labeled protein was only seen on these surfaces in the GLUT9 wildtype and G9A/G1chimera as shown by Western immunoblot (highly glycosylated band appears as smear between 50–75kDa (Figure 4D). Western immunoblots were also performed on the MDCK cell using antibody to the N-terminus of GLUT9a to confirm the overexpression of these chimeric transporters (Figure 5).
Figure 4. hGLUT9A chimeric proteins show an inability of the GLUT9A N-terminus to redirect an apical sorting signal.
The chimeric proteins were stably overexpressed in MDCK cells and visualized by immunofluorescence using a polyclonal antibody to the N-terminus of hGLUT9A. (A) hGLUT9A seen basolaterally (top) (B) hGLUT9A N-terminus spliced onto GLUT1, seen basolaterally (middle) (C) hGLUT9A N-terminus spliced onto GLUT3, seen apically (bottom). D. ATB-BMPA photolabeling of the basolateral surfaces was consistent with the confocal finding. GLUT9a protein was seen on the basolateral surface in Slc2a9A (1) and Slc2a9A/Slc2a1 (2), but not Slc2a9A/Slc2a3 (3). GLUT9 protein is detected as a highly glycosylated set of bands appearing as a smear between 50–75 kDa.
Figure 5. Western Blot analysis confirms expression of the chimeric proteins containing the hGLUT9A N-terminus.
Total Membrane extracts from the overexpressing cell lines were prepared and run on a 10% polyacrylamide gel. Visualization was done using a peptide purified antibody to the N-terminus of hGLUT9A and Dura SuperSignal from Pierce.
GLUT9b N-terminus contains an apical signal capable of redirection
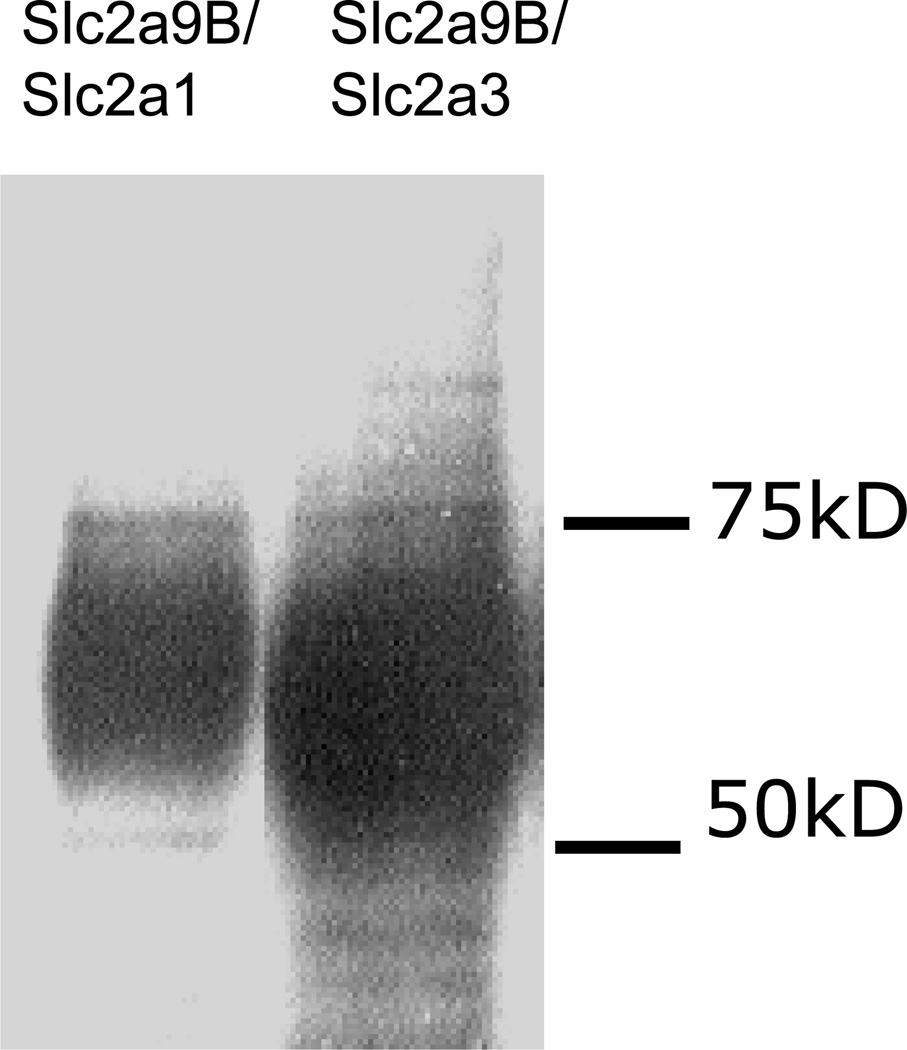
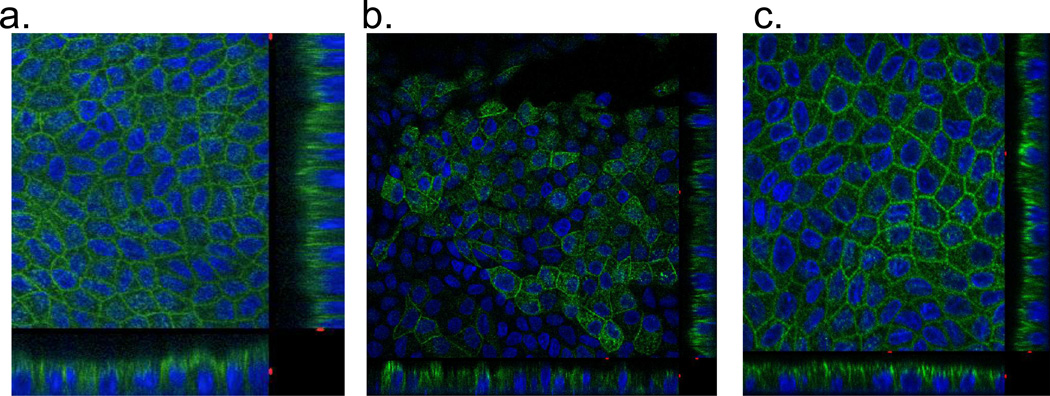
The next set of chimeras was made with the hGLUT9b N-terminus fused on to the GLUT1 and GLUT3 backbones. These constructs are referred to as G9B/G1 and G9B/G3, respectively. Orthogonal projections of the confocal microscopy z-series demonstrate that the G9B/G3 chimera localizes to the apical membrane (Figure 6C) as would be expected for either G9B or G3 (Figure 6A) in entirety. Yet, G9B/G1 also localizes apically (Figure 6B). Since GLUT1 is natively a basolateral sorting protein with the signal expected to be in the C-terminal region, the observed location of the chimeric transporter suggests the N-terminus of G9B is capable of redirecting the chimeric protein to the apical membrane, and thus may harbor an apical sorting motif. Western immunoblots of total membrane preparation from each of these overexpressing cell lines show that the transporters are expressed and glycosylated at varying degrees resulting in bands ranging from 50kDa to 75kDa (Figure 7).
Figure 6. hGLUT9B chimeric proteins show the N-terminus to be sufficient to redirect a basolateral sorting protein to the apical membrane.
The chimeric proteins were stably overexpressed in MDCK cells and visualized using a polyclonal antibody to the N-terminus of hGLUT9B. (A) hGLUT9B, seen on the apical membrane (top) (B) hGLUT9B N-terminus spliced onto GLUT1, seen on the apical membrane (middle) (C) hGLUT9A N-terminus spliced onto GLUT3, seen on the apical membrane (bottom). The entire N-terminus of hGLUT9B redirected GLUT1.
Figure 7. Western Blot analysis confirms expression of the chimeric proteins containing the hGLUT9B N-terminus.
Total Membrane extracts from the overexpressing cell lines were prepared and run on a 10% polyacrylamide gel. Visualization was done using a peptide purified antibody to the N-terminus of hGLUT9B and Dura SuperSignal from Pierce.
The first 16 amino acids of hGLUT9b are not sufficient for apical targeting
Based on sequence alignment, substitutions of the GLUT9a N-terminus were made using portions of the GLUT9b N-terminus. The first substitution involved the first nine amino acids of GLUT9b for the first seventeen amino acids of GLUT9a. Similar to wildtype GLUT9a (Figure 8A), this protein localizes to the basolateral membrane (Figure 8B), suggesting the first nine amino acids of GLUT9b are not sufficient for apical membrane localization. Substitution of the first sixteen amino acids of GLUT9b for amino acids 1–45 of GLUT9a still confers a basolateral sorting pattern (Figure 8C), suggesting the apical sorting motif of GLUT9b must rely on the last ten amino acids in the N-terminus. Five of these ten amino acids are homologous to those of GLUT9a, leaving five candidate amino acids for an apical signal. Of these five amino acids, only one site shows a difference in amino-acid type.
Figure 8. Substitution of portions of the N-terminus of hGLUT9b onto aligned portions of the N-terminus of hGLUT9a reveals no specific apical targeting domain in the first 16 amino acids.
Using primers engineered to add the B-form sequence onto the corresponding portions of the A-form, PCR /was done to create these substitution constructs. (A) hGLUT9A (top), (B) hGLUT9B1–9/hGLUT9A (middle), (C) hGLUT9B1–16/hGLUT9A (bottom). The constructs were cloned into a mammalian overexpression vector and used to stably transform MDCK cells. The proteins were visualized using immunofluorescence with a polyclonal antibody to the C-terminus of hGLUT9 and confocal microscopy. None of the substitutions were sufficient to redirect a basolateral sorting protein to the apical membrane.
Leucine21 is not responsible for apical targeting of hGLUT9b
Based on the sequence alignment, lysine50, a basic residue in GLUT9a, is substituted for leucine21, a hydrophobic residue, in GLUT9b. However, when the leucine in GLUT9b is mutated to a lysine, the resulting transporter still targets to the apical membrane (Figure 9A and B) similar to the GLUT9a wildtype. Using ATB-BMPA photolabeling reagent to tag the apical surface, this conclusion is confirmed in Figure 9C.
Figure 9. Leucine to Lysine mutation in hGLUT9b does not cause a loss of apical signal.
A. N-terminal sequences of hSlc2a9A and hSlc2a9B highlighting Leucine to Lysine substitution made. B. Confocal immunofluorescent microscopy with antibody to hGLUT9b revealed that the mutation did not result in an alteration of the apical signal, indicating the leucine is not responsible for the apical targeting of hGLUT9b. C. ATB-BMPA photolabeling was used to tag the apical surfaces of the polarized cells and confirmed the cell surface protein express of both hGLUT9b and LhGLUT9bK.
Discussion
This study attempts to elucidate the amino-acid signal responsible for the differential targeting of the two human GLUT9 splice variants. The only sequential difference between the two proteins lies in the N-terminus, therefore our targeting investigation started with looking for targeting motifs within the two N-termini. Due to variation in model systems within published studies, it was unclear whether an apical or basolateral signal would be responsible for the sorting as the discussion on what is a default pathway continues to provide evidence that both basolateral and apical sorting may act as default.
Polarized membrane targeting signals for the basolateral membrane are most often found in a cytoplasmic domain of membrane spanning proteins (14). All members of the glucose transporter family have cytoplasmic N-termini, thereby further facilitating the possibility of a basolateral signal in the N-terminus of GLUT9a. Sequence analysis of hGLUT9a revealed a di-leucine motif within the cytoplasmic N-terminus. Both di-leucine and tyrosine motifs have been shown to cause basolateral sorting. This targeting is not exclusive to membrane transporters as both E-cadherin, a protein that plays a role in maintaining cell polarity (15) and NPPase I, an enzyme which is thought to be functionally related to alkaline phosphatase (16) contain di-leucine motifs which, when mutated, cause misrouting to the apical membrane. These data, along with other studies on cell polarity, support the idea that apical sorting is a default pathway and only in the presence of a basolateral signal will proteins be directed to the basolateral membrane. Based on this theory, mutating the di-leucine motif to di-alanine in hGLUT9a would result in an apically sorted protein. This, however, was not the case as the mutated protein continued to localize to the basolateral membrane. From these results, it can be concluded that the di-leucine motif within hGLUT9a is not responsible for the basolateral membrane targeting of this protein. Interestingly, GLUT4 and all the members of class III glucose transporters contain di-leucine motifs in the C-terminus which is believed to be the cause of the intracellular compartmentalization seen in these proteins (11; 12; 17). While di-leucine motifs are common basolateral sorting signals, it may be the case that in glucose transporters they serve a role in the recycling pathway.
While not as fully characterized as basolateral signals, apical signals have been reported. Yet in sequence examination and structure prediction, the best known apical signals are not relevant to GLUT9. N- or O-linked glycosylation is one of the most extensively studied apical targeting motifs. However, there are no additional or unique N-terminal glycosylation sites on hGLUT9b as would be expected if glycans were responsible for apical targeting (14). GPI-anchored proteins are another mechanism by which proteins are targeted to the membrane, however these posttranslational modifications occur on exofacial regions of transmembrane proteins (14). The exofacial region of GLUT9a is exactly identical to that of GLUT9b. Therefore, this apical sorting mechanism should not preferentially treat the B-form over the A-form.
The number of characterized amino-acid apical sorting motifs is in fact quite low, however there are a few examples within the area of membrane transporters. Aquaporin-2, a member of the aquaporin family of water transport proteins contains an apical signal in the C-terminus (18) as does the sodium dependent Vitamin C transporter (19). SGLT1, a sodium dependent glucose transporter also relies on an apical signal but this signal is found in the N-terminus (19). While the sorting of this type of glucose transporter seems to be dependent upon a tyrosine in the N-terminus, it is also noted that a mutation in the aspartic acid residue near the first transmembrane domain will lead to retention of the transporter in intracellular compartments.
Chimeric proteins are often used for the investigation of sorting signals. Because the first four members of the GLUT protein family have been more extensively studied in regards to structure and functional elements, they are a useful source for building chimeric proteins. GLUT1 is a known basolaterally sorting protein. While the location of the signaling motif within the protein is still not fully known, data suggests it lies in the C-terminal region. In fact, C-terminus signaling domains seem to be common throughout the GLUT family. GLUT3 contains an apical signal in the C-terminus which consists of 12 amino acids (DRSGKDGVMEMN) (20), while GLUT4’s endocytotic targeting signal also seems to lie in the C-terminus (10; 17).
The results of our study, however, suggest that a signal in the N-terminus of hGLUT9b directs this protein to the apical membrane. Attempts to narrow down the sequence responsible for the apical targeting resulted in the isolation of the ten amino acids preceding the first transmembrane domain. The only major amino-acid difference between these two regions in the A and B forms of the protein is in the sixth amino acid from the first transmembrane domain. In hGLUT9a, the amino acid is lysine, a basic residue, whereas in hGLUT9b, it is leucine, a hydrophobic residue. Interestingly, both forms of GLUT9 in the mouse have a lysine in the same position and both are targeted to the basolateral membrane. We therefore hypothesized that the single leucine may be responsible for apical sorting. While a single leucine has been implicated as a membrane targeting signal, it has only been shown to be a basolateral signal, not an apical signal as would be expected in this context (21). Nevertheless, mutation of the construct was completed, but failed to change the apical location of hGLUT9b.
Based on the substitution constructs, it appeared as though the final ten N-terminal amino acids were responsible for the apical targeting signal in hGLUT9b. Interestingly, the amino acid sequence in that region of the two forms is more homologous than any other region in the N-terminus. Mutation of the amino acid most different within that region yielded no difference in sorting. We therefore believe that the apical sorting signal relies on interplay between regions of the N-terminus. The need for interplay between the last region and a region preceding it would explain why the addition of just the first nine or first 16 amino acids was not able to redirect hGLUT9a.
Furthermore, while these regions are similar, they are not exactly homologous. It could be that the interplay depends on a series of lysines, found in hGLUT9b and not arginines found in the same position in hGLUT9a. While both these amino acids are basic, the difference in their chemical structures may be a crucial element in their recognition as a sorting signal.
Another explanation for these findings is that the final 10 amino acids of the N-terminus of hGLUT9b may interact with the large intracellular loop within GLUT9 and common to both GLUT9a and GLUT9b in such a way to direct sorting to the apical surface, thus individual substitution of the single leucine did not alter this interaction, whereas substitution of the entire 10 amino acids does alter the targeting. Similar conclusions were drawn from a targeting study in polarized intestinal epithelium overexpression chimeric proteins of GLUT1 and GLUT5 (22). To test this hypothesis, chimeras of GLUT9a or GLUT9b with the cytoplasmic loop of GLUT1 could be created and tested to see if altering the interaction between the N-terminus of GLUT9b and the large intracellular loop of GLUT9 affects apical trafficking.
Such specific regulation of a transporter’s membrane targeting signal would not be surprising. The body relies on a number of differentially targeted transport proteins to maintain homeostasis of elements, including water, sodium, urate and glucose. Without tight regulation of such transport protein locations, homeostasis would not be obtained. This regulation is evident as seen in glucose-galactose malabsorption syndrome, a disease in which evidence suggests an amino acid mutation may prevent SGLT1 from interacting with sorting rafts and thus keeping the protein from being expressed on the apical membrane (23). This situation has also been linked to some familial forms of hypercholesterolemia, where a mutation in the basolateral sorting signal of the LDL receptors results in misrouting of the protein and therefore a decrease in the cell’s ability to clear cholesterol from the blood (24). In a recent paper, Witkowska et al. demonstrates, using heterologous expression in Xenopus oocytes, that the two isoforms display differential urate handling profiles in response to hexoses available in the culture conditions, thus shedding new light on the possible mechanisms responsible for kidney urate handling (25). Moreover, the authors speculate and present some data to suggest that the two isoforms may exhibit asymmetric solute interactions within the same cell type depending on exofacial and endofacial motif binding and affinity to the N-terminal amino acids. They also note the existence of PKA serine phosphorylation site in the N-terminus of GLUT9a and speculate that kinase activity at this site may change the hexose binding profile of the transporter or its interaction with other intracellular binding proteins yet to be discovered (25, 26).
In conclusion, this study attempted to elucidate the transport function and membrane targeting signals within the two splice variants of hGLUT9. The two splice variants differ only in the N-terminal region of the protein, making it a candidate region for differential targeting motifs. We have shown that the N-terminus contains a signal capable of redirecting GLUT1 from the basolateral to apical membrane and that a specific signal lies within a stretch of ten amino acids. Further studies will attempt to investigate the exact sequence or interplay of sequences, which are responsible for the apical targeting of hGLUT9b.
Acknowledgments
The authors would like to thank Dr. Irving Boime for his thoughtful advice and critical reading of this manuscript. This work was supported by a Research Grant from the American Diabetes Association awarded to KHM.
REFERENCES
- 1.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008 Oct 7;5(10):e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab. 2009 Oct;297(4):E831–E835. doi: 10.1152/ajpendo.00296.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279:16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 5.Keembiyehetty C, Augustin R, Carayannopoulos MO, Steer S, Manolescu A, Cheeseman CI, Moley KH. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol. 2006;20:686–697. doi: 10.1210/me.2005-0010. [DOI] [PubMed] [Google Scholar]
- 6.Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–371. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553–557. doi: 10.1006/geno.2002.7010. [DOI] [PubMed] [Google Scholar]
- 8.Pascoe WS, Inukai K, Oka Y, Slot JW, James DE. Differential targeting of facilitative glucose transporters in polarized epithelial cells. Am J Physiol. 1996;271:C547–C554. doi: 10.1152/ajpcell.1996.271.2.C547. [DOI] [PubMed] [Google Scholar]
- 9.Inukai K, Shewan AM, Pascoe WS, Katayama S, James DE, Oka Y. Carboxy terminus of glucose transporter 3 contains an apical membrane targeting domain. Mol Endocrinol. 2004;18:339–349. doi: 10.1210/me.2003-0089. [DOI] [PubMed] [Google Scholar]
- 10.Garippa RJ, Johnson A, Park J, Petrush RL, McGraw TE. The carboxyl terminus of GLUT4 contains a serine-leucine-leucine sequence that functions as a potent internalization motif in Chinese hamster ovary cells. J Biol Chem. 1996;271:20660–20668. doi: 10.1074/jbc.271.34.20660. [DOI] [PubMed] [Google Scholar]
- 11.Aerni-Flessner LB, Otu MC, Moley KH. The amino acids upstream of NH(2)-terminal dileucine motif play a role in regulating the intracellular sorting of the Class III transporters GLUT8 and GLUT12. Mol Membr Biol. 2011;28:30–41. doi: 10.3109/09687688.2010.508196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flessner LB, Moley KH. Similar [DE]XXXL[LI] motifs differentially target GLUT8 and GLUT12 in Chinese hamster ovary cells. Traffic. 2009;10:324–333. doi: 10.1111/j.1600-0854.2008.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zegers MM, Hoekstra D. Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem J. 1998;336(Pt 2):257–269. doi: 10.1042/bj3360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 15.Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276:22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- 16.Bello V, Goding JW, Greengrass V, Sali A, Dubljevic V, Lenoir C, Trugnan G, Maurice M. Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis. Mol Biol Cell. 2001;12:3004–3015. doi: 10.1091/mbc.12.10.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhey KJ, Birnbaum MJ. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J Biol Chem. 1994;269:2353–2356. [PubMed] [Google Scholar]
- 18.Kuwahara M, Asai T, Terada Y, Sasaki S. The C-terminal tail of aquaporin-2 determines apical trafficking. Kidney Int. 2005;68:1999–2009. doi: 10.1111/j.1523-1755.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J Biol Chem. 2004;279:27719–27728. doi: 10.1074/jbc.M400876200. [DOI] [PubMed] [Google Scholar]
- 20.Inukai K, Shewan AM, Pascoe WS, Katayama S, James DE, Oka Y. Carboxy Terminus of Glucose Transporter GLUT3 Contains an Apical Membrane Targeting Domain. Mol Endocrinol. 2003 doi: 10.1210/me.2003-0089. [DOI] [PubMed] [Google Scholar]
- 21.Wehrle-Haller B, Imhof BA. Stem cell factor presentation to c-Kit. Identification of a basolateral targeting domain. J Biol Chem. 2001;276:12667–12674. doi: 10.1074/jbc.M008357200. [DOI] [PubMed] [Google Scholar]
- 22.Inukai K, Takata K, Asano T, Katagiri H, Ishihara H, Nakazaki M, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y. Targeting of GLUT1-GLUT5 chimeric proteins in the polarized cell line Caco-2. Mol Endocrinol. 1997;11:442–449. doi: 10.1210/mend.11.4.9873. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Fujikura K, Koyama H, Matsuzaki T, Takahashi Y, Takata K. The apical localization of SGLT1 glucose transporter is determined by the short amino acid sequence in its N-terminal domain. Eur J Cell Biol. 2001;80:765–774. doi: 10.1078/0171-9335-00204. [DOI] [PubMed] [Google Scholar]
- 24.Koivisto UM, Hubbard AL, Mellman I. A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell. 2001;105:575–585. doi: 10.1016/s0092-8674(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 25.Witkowska K, Smith KM, Yao SY, Ng AM, O'Neill D, Karpinski E, Young JD, Cheeseman CI. Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00134.2012. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blom N, Gammeltoft S, Brunak S. Sequence and structure-bases prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]