Abstract
The relationship between dopamine (DA) tone in the prefrontal cortex (PFC) and PFC-dependent cognitive functions (e.g., working memory, selective attention, executive function) may be described by an inverted-U-shaped function, in which both excessively high and low DA is associated with impairment. In the PFC, the COMT val158met single nucleotide polymorphism (rs4680) confers differences in catechol-O-methyltransferase (COMT) efficacy and DA tone, and individuals homozygous for the val allele display significantly reduced cortical DA. Many studies have investigated whether val158met genotype moderates the effects of dopaminergic drugs on PFC-dependent cognitive functions. A review of 25 such studies suggests evidence for this pharmacogenetic effect is mixed for stimulants and COMT inhibitors, which have greater effects on D1 receptors, and strong for antipsychotics, which have greater effects on D2 receptors. Overall, COMT val158met genotype represents an enticing target for identifying individuals who are more likely to respond positively to dopaminergic drugs.
Keywords: dopamine, COMT, amphetamine, antipsychotic, pharmacogenomics, catecholamines, attention-deficit/hyperactivity disorder, psychosis, schizophrenia
Introduction
Dopamine (DA) signaling underlies many neural functions. Dopaminergic afferents from the midbrain DA nuclei innervate the striatum, amygdala, hippocampus, and prefrontal cortex (PFC), where D1- and D2-like receptors differentially regulate neuronal function. D1 receptors are exclusively post-synaptically expressed, and D1 binding activates intracellular signaling cascades that tend to increase the likelihood of neuronal firing (1). In contrast, D2 receptors are expressed both pre-synaptically, where they act as autoreceptors that regulate DA release, and post-synaptically, where their binding inhibits the same intracellular cascades enhanced by D1 binding (2, 3). In the PFC, where D1 expression predominates (4), the “dual state” theory holds that D1 and D2 receptors oppose each other in their effects on cognitive function: when PFC signaling is dominated by D2 binding, cortical networks favor flexible processing, whereas when signaling is D1-dominated, networks favor stabilizing information and protecting it from interference (5). Striatal DA signaling, which is more D2-dependent, has greater effects on the former kind of cognition (6), although corticostriatal connections allow interactions between striatal and cortical DA neurons (7). In contrast, phasic DA release in the PFC, which increases D1 and reduces D2 activation (8), modulates cognitive functions that depend upon the PFC, including working memory, selective attention, and executive function (9). Many theories posit that the relationship between cortical DA and PFC-dependent cognitive function is inverted-U-shaped, with both high and low cortical DA tone associated with impaired function (9–12).
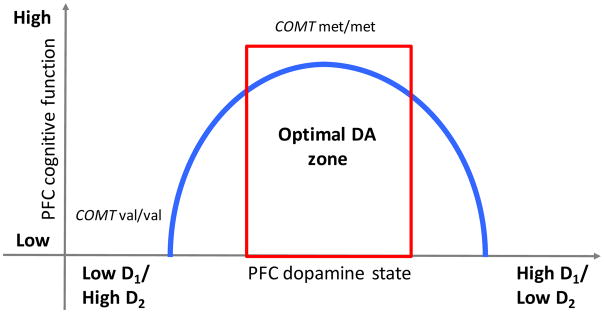
In most brain areas, including the striatum, synaptic DA is rapidly inactivated primarily through active reuptake at the presynaptic dopamine transporter (DAT). In the PFC, however, the DAT is not highly expressed (13), and the principal method of DA inactivation is enzymatic degradation by catechol-O-methyltransferase (COMT) (14). COMT inactivates DA more slowly than the DAT, causing DA effects to persist much longer in the PFC and allowing the DA signal to stabilize and protect information. A common single nucleotide polymorphism (SNP) at codon 158 in COMT, the gene that encodes this enzyme, has been associated with differential COMT function, and, accordingly, differential cortical synaptic DA accumulation. Specifically, the met (A) allele of the val158met SNP (rs4680), which causes a valine to methionine amino acid substitution, is associated with a three- to four-fold reduction in COMT efficacy, and thus greater cortical DA tone, relative to the val (G) allele (15, 16). The higher DA met allele may also be associated with a more optimal D1/D2 balance, while the lower DA val allele may be associated with a low D1/high D2 state (5, 17) (see Figure 1).
Figure 1.
Schematic of the hypothetical inverted-U-shaped relationship between cortical DA state and PFC-dependent cognitive function.
The val158met SNP is among the most thoroughly studied genetic variants in psychiatry (18, 19). Disruption of corticostriatal DA signaling is a core feature of neuropsychiatric disorders characterized by cognitive symptoms, including attention-deficit/hyperactivity disorder (ADHD) and schizophrenia (20, 21), and early studies tested the association between val158met variation and these disorders. However, meta-analyses ultimately revealed no associations with these diagnoses (22, 23). Attention then turned to associations between val158met and cognitive function, an intermediate phenotype theoretically more proximal to the neuronal level than diagnostic phenotypes. Although initial studies suggested an association between val158met variation and cognition in both clinical samples and healthy controls (24–26), subsequent meta-analyses also found no effect on this phenotype (27). Thus, despite the SNP’s clear proximal effects on enzymatic function, its distal effects on behavior have remained ambiguous (28).
One factor that may account for these mixed findings is that COMT val158met variation is only one of many influences on cognitive function, which, although “intermediate” between neuronal signaling and disease outcomes, is a highly complex phenotype (29). In contrast, drug response phenotypes, while themselves complex, are, relative to diagnostic phenotypes, potentially more strongly affected by variation in genes that directly control the neurobiological systems the drugs entrain (30). Broadly speaking, D1 agonists enhance PFC-dependent cognitive functions (31–33), while D1 antagonists impair them (34). D2 antagonist effects are more mixed (35, 36), perhaps due to these drugs’ dose-dependent effects on pre- vs. post-synaptic D2 receptors (37). Recent reviews have summarized the interaction between val158met genotype and the effects of tolcapone (38) and risperidone (39), but neither addressed drug effects on cognitive function specifically, and the most recent systematic review of val158met effects on all dopaminergic drugs was published a decade ago (40). Thus, this manuscript critically reviews studies that have tested the pharmacogenetic interaction between COMT val158met genotype and the effects of dopaminergic drugs on PFC-dependent cognitive functions.
Method
Study identification and selection
Studies were identified via PubMed searches conducted in April 2016 that included pairwise combinations of the terms “COMT”, “catechol-O-methyltransferase”, “val158met”, or “rs4680”, and “dopamine”, “medication”, “drug”, “stimulant”, or “antipsychotic”. Studies that tested an interaction between val158met genotype and therapeutic drug effects on PFC-dependent cognitive functions (working memory, selective attention, and/or executive function) were included, whether these functions were evaluated in isolation or as part of larger cognitive batteries or symptom measures (e.g., a broader IQ assessment that included a working memory subtest or an ADHD symptom measure that assessed inattention). Studies of non-cognitive therapeutic effects (e.g., mood symptoms, COMT blood levels) were not included, nor were those of adverse effects, such as antipsychotic-induced tardive dyskinesia. This process ultimately identified 25 studies, which were grouped according to whether the effects of the drugs used were more D1- (e.g., stimulants, COMT inhibitors) or D2-dependent (e.g., antipsychotics).
Study designs
Identified studies employed both double-blind, placebo-controlled designs and quasi-experimental designs, which used no placebo and compared cognitive function either between genotype groups or as a function of genotype and time. Most of the placebo-controlled studies also used within-subjects crossover designs. Nearly all studies employed longitudinal designs, but treatment length and number of assessment points varied widely. Several studies used prospective genotyping to employ an “extreme groups” design in which only individuals with homozygous genotypes (e.g., val/val or met/met) were included, but most genotyping was conducted post hoc, suggesting that experimenters were blind to genotype during outcome measure assessment. However, the use of genotype blinding was inconsistently reported across studies, precluding identification of those that used a double blind approach.
Outcome measures
Symptom measures rated by clinicians, parents, and/or teachers were common for studies of individuals with ADHD, and were analyzed both continuously and categorically, with a range of categorical cut-points used to define symptom reduction for the latter approach. Neuropsychological tests were frequently used in studies of healthy controls and individuals with schizophrenia spectrum disorders, including both full neuropsychological batteries, such as the Wechsler Adult Intelligence Scale (WAIS), and subtests intended to measure specific PFC-dependent cognitive functions. Several studies used neuroimaging methods, including functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), to measure brain response during cognitive task performance.
Results
Stimulants and COMT inhibitors
Two classes of drugs acutely increase DA concentrations, leading to increased cortical D1 binding: stimulants (e.g., amphetamine, methylphenidate), which increase DA directly in the striatum through competitive reuptake at the DAT and indirectly in the PFC through downstream D1 effects (41, 42); and COMT inhibitors, which, in the treatment of Parkinson’s disease, are commonly co-administered with L-DOPA to prevent its peripheral metabolism, but which also increase brain DA (primarily in the PFC) if they cross the blood-brain barrier (43). Table 1 lists studies that have tested moderation of the cognitive effects of these drugs by COMT val158met variation.
Table 1.
Studies of stimulants and COMT inhibitors and moderation by COMT val158met genotype
| First author, year | Drug | Placebo-controlled | Population | Total N (val/val, val/met, met/met) | Outcome measure(s) | Significant COMT x drug interaction | Group(s) w/best outcome: |
|---|---|---|---|---|---|---|---|
| Stimulants: healthy controls | |||||||
| Mattay, 2003 | Amphetamine | Yes (crossover) | Healthy adults | 25 (9, 10, 6) | Executive function (WCST), working memory (fMRI during N-back) | Better WCST and N-back performance and reduced DLPFC activation during N- back in val/val | Val/val |
| Hamidovic, 2010 | Amphetamine | Yes (crossover) | Healthy adults (all Caucasian) | 161 (36, 72, 53) | Selective attention (DSST, Deviation from the Mode task) | Better performance on all measures in val/val, val/met | Val/val, val/met |
| Hart, 2013 | Amphetamine | Yes (crossover) | Healthy adults (all Caucasian) | 176 (50, 77, 49) | Selective attention (DSST) | None | None |
| Wardle, 2013 | Amphetamine | Yes (crossover) | Healthy adults | 193 (56, 89, 48) | Selective attention (DSST), executive function (WCST), working memory (N-back) | None | None |
| Stimulants: psychiatric populations | |||||||
| Cheon, 2008 | Methylphenidate | No | Children w/ ADHD (all Korean) | 124 (68, 48, 8) | ADHD sx (≥50% decrease in teacher-rated ADHD-RS) | Greater likelihood of sx decrease in val/val | Val/val |
| Kereszturi, 2008 | Methylphenidate | No | Children w/ ADHD (all Caucasian) | 122 (39, 59, 24) | ADHD sx (≥25% decrease in clinician-rated ADHD-RS and CGI-S ≤ 2) | Greater likelihood of sx decrease in val/val | Val/val |
| Sengupta, 2008 | Methylphenidate | Yes (crossover) | Children w/ ADHD (all Caucasian) | 188 (46, 104, 38) | ADHD sx (experimenter- rated RASS) | None | None |
| McGough, 2009 | Methylphenidate | Yes (crossover) | Children w/ ADHD | 82 (24, 39, 19) | ADHD sx (composite of teacher-rated ADHD-RS, SWAN, and PERMP) | None | None |
| Froehlich, 2011 | Methylphenidate | Yes (crossover) | Children w/ ADHD | 89 (30, 40, 19) | ADHD sx (composite of Vanderbilt ADHD Parent and Teacher Rating Scales) | Trend (p = .09) for greater likelihood of hyperactive- impulsive sx decrease in in val/val | Val/val |
| Salatino- Oliveira, 2011 | Methylphenidate | No | Male children w/ ADHD | 112 (35, 77)@ | ADHD sx (parent-rated SNAP-IV) | Greater reduction in oppositional sx after 1 month of treatment in val/met, met/met; no effect at 3 months | Val/met, met/met |
| Contini, 2012 | Methylphenidate | No | Adults w/ ADHD | 164 (47, 117) | ADHD sx (≥30% decrease in SNAP-IV and CGI-S ≤ 2) | None | None |
| McCracken, 2014 | Methylphenidate | Yes (crossover) | Children w/ autism spectrum disorders | 57 (19, 38)@ | Hyperactive-impulsive sx (≥ 25% decrease in parent- /teacher-rated ABC and clinician-rated CGI-C ≤ 2) | Greater likelihood of hyperactive-impulsive sx decrease in val/val | Val/val |
| Park, 2014 | Methylphenidate | No | Children w/ ADHD (all Korean) | 120 (70, 43, 7) | ADHD sx (≥50% decrease in parent-rated ADHD-RS), selective attention (≥10% decrease in CPT errors, RT, | Greater likelihood of hyperactive-impulsive sx decrease and CPT RT variability in val/val | Val/val |
| COMT inhibitors: healthy controls | |||||||
| Apud, 2007 | Tolcapone | Yes (crossover) | Healthy adults | 47 (15, 21, 11) | and RT variability) Executive function (CANTAB ID/ED, Trails B, WCST), selective attention (CPT), working memory (N- back, LNS), verbal fluency, verbal episodic memory | Better CANTAB ID/ED performance in val/val; no effects on other tasks | Val/val |
| Giakoumaki, 2008 | Tolcapone | Yes (crossover) | Healthy adult males | 23 (12, 0, 11)* | Working memory (N-back, letter-number sequencing), cognitive efficiency (prepulse inhibition) | Better performance on all measures in val/val | Val/val |
| Farrell, 2012 | Tolcapone | Yes | Healthy adult males | 67 (33, 0 34)* | Working memory (N-back), decision-making (gambling | Better performance on both measures in val/val | Val/val |
| COMT inhibitors: psychiatric populations | |||||||
| Ashare, 2013 | Tolcapone | Yes (crossover) | Adults w/ nicotine dependence | 20 (9, 11)@ | task) Working memory (fMRI during N-back) | Better N-back performance and increased medial frontal, DLPFC activation during N- back in val/met and met/met | Val/met, met/met |
Abbreviations: ABC = Aberrant Behavior Checklist; ADHD = Attention-deficit/hyperactivity disorder; ADHD-RS = ADHD Rating Scale IV; CANTAB ID/ED = Cambridge Neuropsychological Test Automated Battery Intradimensional/Extradimensional set-shifting test; CGI-C = Clinical Global Impression Global Improvement; CGI-S = Clinical Global Impression Severity; CPT = Continuous Performance Test; DLPFC = dorsolateral prefrontal cortex; DSST = Digit Symbol Substitution Test; LNS = letter-number sequencing; PERMP = Permanent Product Measure of Performance math test; POMS = Profile of Mood States; RASS = Restricted Academic Situation Scale; RT = response time; SNAP-IV = Swanson, Nolan and Pelham Scale-Version IV; SWAN = Strengths and Weaknesses of ADHD-Symptoms and Normal Behavior scale; sx = symptoms; WCST = Wisconsin Card-Sorting Task
Excluded val/met subjects
Second figure refers to the combined total of val/met and met/met subjects; study combined these groups for analysis.
Several large, placebo-controlled studies of amphetamine among healthy controls suggest weak evidence for pharmacogenetic moderation of stimulant effects. Although one small early study of amphetamine demonstrated greater improvement in executive function and working memory, relative to placebo, among val/val subjects administered amphetamine (44), subsequent larger studies have mostly failed to find this interaction. Notably, although one larger study reported greater amphetamine-induced improvements in selective attention among val-allele carriers (45), an attempted replication of this finding by the same group was negative (46, 47). The authors attributed this discrepancy to a failure to fully correct for multiple testing and to their own bias for publishing a positive pharmacogenetic effect but not negative ones; these issues represent challenges for many pharmacogenetic studies.
Evidence for pharmacogenetic moderation of stimulant effects is somewhat stronger among psychiatric populations. Most studies have focused on methylphenidate effects on ADHD symptoms among children with this disorder. Of seven such studies, four found greater reduction in ADHD symptoms among val/val subjects administered methylphenidate (48–51). All four studies used ADHD symptom rating scales, which combine inattentive and hyperactive symptoms, as endpoints; two (49, 51) analyzed these symptom clusters separately, and identified pharmacogenetic effects specifically for hyperactive symptoms, while the other two analyzed only total scale scores. Park et al. (51) also reported greater methylphenidate effects on a measure of inattention (response time variability on a continuous performance task) among val/val children. Of the remaining three studies of children with ADHD, two reported no pharmacogenetic interaction for combined symptoms (52, 53), and one found greater reduction in oppositional symptoms among male children with the val/met and met/met genotypes administered methylphenidate, although this effect did not persist beyond one month of treatment (54). A large study of adults with ADHD reported no pharmacogenetic effect on combined symptoms (55), but a smaller study of methylphenidate effects on hyperactivity among children with autism spectrum disorders found greater drug effects among val/val subjects (56). Study size and outcome measures did not predict whether studies reported a pharmacogenetic effect, but study design and population did. Only two of the four studies that used a stronger placebo-controlled crossover design reported a pharmacogenetic effect. Further, although the two studies that included only Korean subjects both reported pharmacogenetic effects (48, 51), the significantly lower frequency of the val158met met allele among individuals of East Asian descent raises the possibility that the small number of met-allele homozygotes in these studies may have driven these effects.
In contrast to the findings for stimulants, there is stronger evidence of pharmacogenetic moderation of COMT inhibitors, although studies have been limited by small samples, and have included mostly healthy controls. Three placebo-controlled studies of tolcapone among healthy controls found better performance on executive function, working memory, and decision-making tasks, as well as greater prepulse inhibition of the startle response, among val/val subjects administered tolcapone (57–59). However, one of these studies also examined a large number of cognitive measures, including other executive function and working memory measures, for which no pharmacogenetic effects were found. The two others included only homozygous subjects; thus, val/val subjects’ tolcapone-induced cognitive improvement was in comparison to met/met subjects’ decline. However, the only COMT inhibitor study that included a psychiatric population (treatment-seeking cigarette smokers) found better working memory performance and greater medial and dorsolateral PFC (DLPFC) activation during a working memory task among tolcapone-treated met-allele carriers, relative to val/val subjects (60).
Overall, extant data indicate weak evidence for val158met moderation of stimulant and COMT inhibitor effects on cognitive function. Strong evidence is limited to two studies of methylphenidate among Korean children with ADHD and three studies of tolcapone among healthy adults. Notably, the strongest and most consistent evidence of a pharmacogenetic effect is for tolcapone, which more specifically increases cortical DA, in contrast to stimulants, which increase DA throughout the brain.
Antipsychotics
Antipsychotic effects on cortical DA concentrations are complex. First-generation, “typical” antipsychotics (e.g., haloperidol, sulpiride) act most strongly as D2 antagonists, and second- and third-generation, “atypical” antipsychotics (e.g., clozapine, olanzapine), in addition to D2 antagonism, also act as serotonergic antagonists or partial agonists. At therapeutic doses, these drugs have greater effects on post- than pre-synaptic D2 receptors (37), suggesting that, since post-synaptic D2 receptors are more prevalent in PFC, antipsychotics may bias PFC networks towards D1-dominated states (61). However, this effect may depend upon an individual’s baseline DA tone, such that antipsychotics may also reduce tonically increased DA (62) (although this mechanism may be unique to drugs with DA partial agonist properties). Table 2 lists studies that have tested moderation of the cognitive effects of antipsychotics by COMT val158met variation.
Table 2.
Studies of antipsychotics and moderation by COMT val158met genotype
| First author, year | Drug | Placebo-controlled | Population | Total N (val/val, val/met, met/met) | Outcome measure(s) | Significant COMT x drug interaction | Group(s) w/best outcome: |
|---|---|---|---|---|---|---|---|
| Bertolino, 2004 | Olanzapine | No | Adults w/ SZ or schizophreniform disorder | 30 (8, 17, 5) | Working memory (fMRI during N-back) | Greater improvement on N-back, less DLPFC activation during N- back in met/met | Met/met |
| Weickert, 2004 | Antipsychotics* | Yes (crossover) | Adults w/ SZ or schizoaffective disorder | 20 (5, 11, 4) | Working memory (N-back), executive function (WCST), verbal fluency, overall cognitive function (WAIS-R FSIQ, WMS-R) | Better N-back performance in met/met; no effects on other measures | Met/met |
| Mata, 2006 | Antipsychotics* | No | Adults w/ non- affective psychotic disorders | 87 (23, 47, 17) | Overall cognitive function (WAIS-III Vocabulary, Information, Digit-Symbol) | Less cognitive deterioration (score on Digit-Symbol relative to Vocab and Info) in met/met | Met/met |
| Woodward, 2007 | Clozapine | No | Adults w/ SZ | 86 (29, 35, 21) | Attention and verbal fluency (CIGT, COWAT, DSST), memory (ACTT, BSRT, WISC-R Mazes), executive function (WCST) | Greater improvement in attention and verbal fluency in met/met and val/met; no effects on memory, executive function | Met/met, val/met |
| Rebollo- Mesa, 2011 | Antipsychotics* | No | Adults w/ SZ (some concordant identical twins) | 68 (17, 36, 15) | Overall cognitive function (WAIS-III VIQ, PIQ) | Greater antipsychotic dose associated with greater VIQ in met/met and val/met; no effect on PIQ | Met/met, val/met |
| Arts, 2013 | Antipsychotics* | No | Adults w/ bipolar spectrum disorders | 51 (7, 32, 12) | Verbal learning and memory (VLT), selective attention (Flanker CPT), working memory (WAIS-III Digit Span Backward) | Less deterioration on composite of all three measures in met/met | Met/met |
| Bosia, 2014 | Antipsychotics* + cognitive remediation therapy | No | Adults w/ SZ (all Caucasian) | 98 (24, 50, 24) | Verbal memory, working memory, motor coordination, processing speed, verbal fluency, executive function (BAC-S) | Greater improvement on processing speed in met/met, val/met treated w/ antipsychotics other than clozapine; no effects on other subtests | Met/met, val/met |
| Healthy controls | |||||||
| Mueller, 2011 | Sulpiride | Yes | Healthy adult males | 169 (33, 86, 50) | Selective attention (EEG during Flanker CPT) | Smaller error-related negativity, delta/theta power, and post-error slowing in val/val, val/met | Val/val, val/met |
Abbreviations: ACTT = Auditory Consonant Trigram Test; BAC-S = Brief Assessment of Cognition in Schizophrenia; BSRT = Buschke Selective Reminding Task; CIGT = Category Instance Generation Test; CPT = Continuous Performance Test; COWAT = Controlled Oral Word Association Test; DLPFC = dorsolateral prefrontal cortex; DSST = Digit Symbol Substitution Test; EEG = electroencephalogram; FSIQ = Full Scale Intelligence Quotient; GAF = Global Assessment of Functioning; PIQ = Performance Intelligence Quotient; SZ = schizophrenia; VIQ = Verbal Intelligence Quotient; VLT = Verbal Learning Test; WAIS = Wechsler Adult Intelligence Scale (R = Revised, III = Third Edition); WCST = Wisconsin Card-Sorting Task; WISC-R = Wechsler Intelligence Scale for Children—Revised; WMS-R = Wechsler Memory Scale—Revised
Subjects prescribed various first- and second-generation antipsychotics were included.
There is strong evidence for val158met pharmacogenetic moderation of antipsychotic effects on cognitive function. Seven studies have examined this phenomenon among psychiatric populations (primarily adults with psychotic disorders), and one has tested it among healthy controls. Since these disorders are relatively rare and subjects are often recruited from clinics, most studies have been small (range = 20–98 subjects) and have examined pharmacogenetic effects among patients already taking antipsychotics. All seven psychiatric studies have reported better drug effects among met/met subjects; three have also reported better effects among val/met, relative to val/val, subjects.
Three of the studies of psychiatric populations reported pharmacogenetic effects on specific cognitive functions. A small placebo-controlled crossover study of antipsychotics found pharmacogenetic moderation of drug effects on the N-back working memory task, but not other cognitive domains, such that antipsychotics, relative to placebo, improved performance only among met/met subjects (63). A study of olanzapine effects on changes in working memory over four weeks of treatment also found greater N-back improvement among met/met subjects relative to val-allele carriers (64). Because reduced DLPFC activation during the N-back was accompanied by performance improvements, greater reduction in DLPFC activation among met/met subjects was interpreted as evidence of increased cortical efficiency (e.g., less activation was required to produce better performance). Additionally, a study of patients who received both an antipsychotic and cognitive remediation therapy for 12 weeks found greater improvement among met-allele carriers relative to val/val subjects in processing speed, but not other cognitive functions (65). However, the pharmacogenetic interaction was only present for patients taking antipsychotics other than clozapine, and was driven by significantly worse performance among val/val subjects. In contrast, clozapine, which has greater D1 affinity than other antipsychotics, improved cognition irrespective of COMT genotype, suggesting that val158met pharmacogenetic effects might be specific to drugs with greater D2 effects.
The other four studies of psychiatric populations found pharmacogenetic effects on broader indices of cognitive function. In contrast to the Bosia et al. (2014) finding, another clozapine study reported greater improvement on a neurocognitive factor comprised of attention and verbal fluency measures among met-allele carriers relative to val/val subjects after six months of treatment (66). A study of antipsychotic effects on cognition found less “cognitive deterioration” (i.e., scores on “hold” tests that are stable in adulthood and insensitive to acquired brain damage, such as WAIS Vocabulary and Information, relative to tests that are sensitive to brain damage, such as WAIS Digit Symbol) among met/met subjects (67). Similarly, met-allele carriers treated with greater antipsychotic doses demonstrated higher WAIS verbal IQ, but not performance IQ, scores relative to val/val subjects administered the same doses (68). Finally, a small study of patients with bipolar spectrum disorders assessed change in cognition as a function of genotype and antipsychotic use during a two-year period. For subjects who used antipsychotics, there was less deterioration over time in a composite measure comprised of verbal learning and memory, selective attention, and working memory tasks among met/met subjects relative to val-allele carriers (69). Thus, taken together, it appears that val-allele homozygotes with psychotic disorders are most susceptible to interference in cognitive function from antipsychotic medications, perhaps because these individuals’ D1/D2 balance is too low for antipsychotics to rescue.
A placebo-controlled study of the D2 antagonist sulpiride among healthy controls (70) reported contrasting findings to the antipsychotic studies among psychiatric subjects. Neurophysiological measures of error reactivity (e.g., EEG error-related negativity, error-related increases in delta/theta power, and post-error slowing) were obtained during a selective attention task. Under placebo, these measures were reduced in met/met subjects relative to val-allele carriers, suggesting more optimal cognitive function in the met/met group. Sulpiride reduced each measure in val-allele carriers but increased each in met/met subjects, suggesting that healthy controls, relative to individuals with schizophrenia spectrum disorders, might display a right-shifted inverted-U-shaped function under which D2 antagonism worsens met/met subjects’ more optimal D1/D2 balance.
Overall, extant data indicate strong evidence for val158met moderation of antipsychotic effects on cognitive function. Studies have included a broad range of individuals with psychotic disorders, as well as a broad range of drugs. However, only one study has used a placebo-controlled crossover design, likely due to the difficulty of changing or discontinuing medications among individuals with severe and persistent mental illness. Additionally, although extant studies have reported pharmacogenetic effects on a variety of cognitive outcomes, few specific findings have been replicated; there is inconsistent evidence for a pharmacogenetic effect on any specific neurocognitive domain. Nonetheless, the COMT val158met SNP holds promise for predicting the effects of antipsychotics on cognitive function.
Discussion
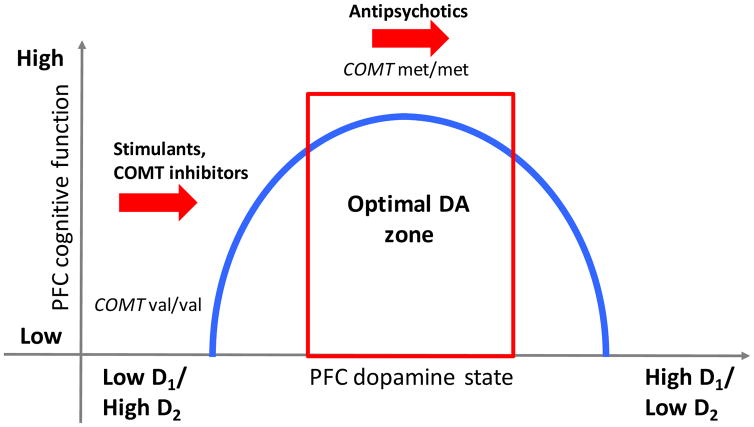
This paper reviewed 25 studies of the moderating influence of the COMT val158met SNP on dopaminergic drug effects on PFC-dependent cognitive functions. These studies examined medications that modulate cortical D1 and D2 binding among both psychiatric populations and healthy controls. There was mixed evidence of pharmacogenetic effects for stimulants and COMT inhibitors, but stronger evidence for antipsychotics. COMT inhibitors improved cognitive function the most among val-allele homozygotes, while antipsychotics improved it the most among met-allele homozygotes (see Figure 2). The implications of these findings in the context of the dual state theory of prefrontal DA and the inverted-U-shaped hypothesis are discussed below, as are directions for future work in this area.
Figure 2.
Summary of the interaction between COMT val158met genotype and dopaminergic drug effects.
Several factors may account for the weak evidence of pharmacogenetic effects for stimulants. First, stimulants’ mechanism of action is not PFC-specific; stimulants non-selectively increase DA throughout the brain, most notably in the striatum (71). Thus, the beneficial effect of increasing cortical DA among individuals with low D1/high D2 occupancy (e.g., val-allele homozygotes) may be counteracted by increases in striatal DA, which, although it increases behavioral flexibility (6), is also associated with impulsivity and risky decision-making (72). Data that suggest that midbrain and striatal DA concentrations are positively associated with cortical blood flow in val-allele carriers but negatively associated in met/met subjects support this notion (73). Second, and relatedly, the primary outcome in most of the stimulant studies, ADHD symptoms, includes both PFC-mediated “cognitive” symptoms (e.g., inattention, distractibility) and motor and hyperactivity symptoms that are likely striatally mediated. Finally, in contrast to the antipsychotic studies, most of the stimulant studies reviewed had large sample sizes and employed placebo-controlled crossover designs, suggesting that weaker designs could lead to false positive findings. However, these design considerations were possible in part because of the greater prevalence of ADHD relative to schizophrenia spectrum disorders and the lower clinical risk in administering placebo medications to ADHD patients.
In contrast to the findings for stimulants, there was stronger evidence of pharmacogenetic moderation of the COMT inhibitor tolcapone. Studies were small and mostly limited to healthy controls, but consistently demonstrated greater drug effects among val/val subjects. As noted previously, tolcapone acutely increases DA most prominently in the PFC; thus, it is logical that val158met genotype would moderate its effects more powerfully than stimulants. Tolcapone has been used sparingly in clinical practice due to hepatoxicity concerns, but the findings reviewed here, as well as data suggesting that tolcapone may improve cognitive function independent of COMT genotype (74), have increased interest in its potential clinical utility (38, 75).
Evidence of a pharmacogenetic effect for antipsychotics was also strong. All seven studies of antipsychotics among psychiatric populations suggested that these drugs improved cognition (or prevented its deterioration) the most among met-allele homozygotes. Since antipsychotics increase cortical D1 binding and the COMT met allele may be associated with a more optimal D1/D2 balance, this pattern of results might seem counterintuitive. One possibility is that val158met variation may differentially impact D1/D2 balance among individuals with schizophrenia spectrum disorders as a function of the reduced cortical D1 function that may characterize these disorders (76, 77). Individuals with these disorders may be shifted leftward on the inverted-U-shaped function, leaving met-allele homozygotes’ D1/D2 balance on the near left edge of the function and amenable to antipsychotic effects, but val-allele homozygotes’ balance so dysregulated that antipsychotics cannot remediate it. Interestingly, the one antipsychotic study that included healthy controls, among whom cortical DA function is presumably normal, reported deleterious drug effects in met/met subjects, suggesting the pharmacogenetic interaction may indeed be population-specific. Alternatively, the relationship between cortical DA and some of the cognitive functions measured in these studies may not be inverted-U-shaped (78).
In the antipsychotic studies, there was considerable variability regarding the specific cognitive domains affected by the pharmacogenetic interaction. Data were most consistent for working memory; three of the four studies that examined this construct found pharmacogenetic effects on it. However, despite this strong evidence, there is limited evidence (not reviewed here) that val158met genotype moderates overall antipsychotic treatment response (e.g., decrease in schizophrenia symptoms or improvement in global functioning). Impairments in cognitive function represent only one aspect of schizophrenia spectrum disorders, and improving cognition may be of limited clinical benefit for individuals with these disorders.
Several factors limit interpretation of the data reviewed here. First, publication bias remains problematic for the pharmacogenetic literature, and effect sizes are often lower in replication studies (79). The val158met SNP is a sound candidate gene for moderation of dopaminergic drug effects—it has downstream functional effects and is related to the mechanism of action for these drugs—but studies of its potential pharmacogenetic effects must adhere to strong experimental design to reduce the likelihood of false-positive findings. Second, it is unclear whether race or ethnicity may influence val158met effects. The val allele is significantly more common among individuals of African and Asian descent, and the frequency of other polymorphisms that affect DA signaling also varies by race and ethnicity. These factors could result in differential epistatic interactions between val158met and these other polymorphisms. Thus, differences in pharmacogenetic effects by race and ethnicity should be examined when possible. Finally, none of the studies reviewed here addressed pharmacogenetic moderation of adverse drug effects. The val/val genotype may confer liability to the development of antipsychotic-induced tardive dyskinesia (80). Similarly, several of the studies reviewed here suggested that val-allele homozygotes with schizophrenia spectrum disorders administered antipsychotics experienced decreased cognitive function from these drugs, but this phenomenon has not been systematically reviewed.
Despite these limitations, there are many promising future directions for research in this area. First, val158met genotype will likely be most useful for predicting the cognitive effects of drugs that specifically target cortical DA. Most of the drugs used in the studies reviewed here affect DA, and other neurotransmitter systems, in areas beyond the PFC. Two notable exceptions that merit further pharmacogenetic research are COMT inhibitors, which have demonstrated some of the most promising pharmacogenetic results of any class of drugs, and second-generation antipsychotics with more prominent D1 effects (e.g., clozapine) or novel dopaminergic mechanisms of action (e.g., the D2 partial agonist aripiprazole). Second, future studies should expand the use of neuroimaging outcome measures, which are ideal intermediate phenotypes, and are arguably more likely to demonstrate genetic effects than behavioral measures (28). Only four studies reviewed here used fMRI, and all had among the smallest N’s of the identified studies. Large-scale imaging genetics studies that include drug challenges, though more difficult and expensive to conduct, would greatly improve confidence in extant pharmacogenetic findings. Related to this issue, the use of a common neurocognitive outcome measure (e.g., the NIH Toolbox multidimensional battery) in future research would facilitate comparison between studies. Third, in placebo-blinded studies, prospective genotyping should be used to ensure equal distribution of individuals with each COMT genotype to active and placebo medications; for studies of racial or ethnic populations in which one allele is significantly less frequent, this provision would be particularly useful for preventing small cell sizes, and thus imprecise estimates of drug effects. Finally, investigation of other COMT polymorphisms may be fruitful. A synonymous COMT SNP, rs4818, forms a haplotype with val158met that may predict COMT expression beyond the effects of either variant alone (81), and may moderate tolcapone effects on cognitive function (82). Other COMT SNPs have been reported to moderate risperidone response among patients with schizophrenia (83, 84), suggesting that val158met is only one of several functionally relevant COMT polymorphisms.
In conclusion, extant data suggest that variation at the COMT val158met SNP is a promising target for predicting the effects of dopaminergic drugs on PFC-dependent cognitive functions. Continued development of medications that specifically modulate cortical DA may ultimately allow this variant to guide a personalized medicine approach to cognition in a variety of neuropsychiatric disorders.
References
- 1.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 2.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 3.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9(1):53–8. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40(3):657–71. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 5.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64(9):739–49. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59(10):898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282C:248–57. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30(42):14273–83. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–25. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 11.Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 12.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17(21):8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18(7):2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116(1):127–37. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 15.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13(8):821–7. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- 18.Craddock N, Owen MJ, O'Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry. 2006;11(5):446–58. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- 19.Lachman HM. Does COMT val158met affect behavioral phenotypes: yes, no, maybe? Neuropsychopharmacology. 2008;33(13):3027–9. doi: 10.1038/npp.2008.189. [DOI] [PubMed] [Google Scholar]
- 20.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 21.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Cheuk DK, Wong V. Meta-analysis of association between a catechol-O-methyltransferase gene polymorphism and attention deficit hyperactivity disorder. Behav Genet. 2006;36(5):651–9. doi: 10.1007/s10519-006-9076-5. [DOI] [PubMed] [Google Scholar]
- 23.Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry. 2005;10(8):765–70. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- 24.Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, et al. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry. 2004;161(6):1110–2. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–4. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–96. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 27.Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64(2):137–44. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2012;88(5):418–28. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelernter J. Genetics of complex traits in psychiatry. Biol Psychiatry. 2015;77(1):36–42. doi: 10.1016/j.biopsych.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18(7):2720–8. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci U S A. 2012;109(50):20726–31. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29(9):1628–36. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- 34.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 35.Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E, et al. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology (Berl) 2009;207(1):35–45. doi: 10.1007/s00213-009-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176(3–4):331–42. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- 37.la Fougere C, Meisenzahl E, Schmitt G, Stauss J, Frodl T, Tatsch K, et al. D2 receptor occupancy during high- and low-dose therapy with the atypical antipsychotic amisulpride: a 123I-iodobenzamide SPECT study. J Nucl Med. 2005;46(6):1028–33. [PubMed] [Google Scholar]
- 38.Bitsios P, Roussos P. Tolcapone, COMT polymorphisms and pharmacogenomic treatment of schizophrenia. Pharmacogenomics. 2011;12(4):559–66. doi: 10.2217/pgs.10.206. [DOI] [PubMed] [Google Scholar]
- 39.Llerena A, Berecz R, Penas-Lledo E, Suveges A, Farinas H. Pharmacogenetics of clinical response to risperidone. Pharmacogenomics. 2013;14(2):177–94. doi: 10.2217/pgs.12.201. [DOI] [PubMed] [Google Scholar]
- 40.Diaz-Asper CM, Weinberger DR, Goldberg TE. Catechol-O-methyltransferase polymorphisms and some implications for cognitive therapeutics. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2006;3(1):97–105. doi: 10.1016/j.nurx.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev. 2013;37(9 Pt A):1976–84. doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1011–23. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakkarainen JJ, Jalkanen AJ, Kaariainen TM, Keski-Rahkonen P, Venalainen T, Hokkanen J, et al. Comparison of in vitro cell models in predicting in vivo brain entry of drugs. Int J Pharm. 2010;402(1–2):27–36. doi: 10.1016/j.ijpharm.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamidovic A, Dlugos A, Palmer AA, de Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20(3):85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38(5):802–16. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wardle MC, Hart AB, Palmer AA, de Wit H. Does COMT genotype influence the effects of d-amphetamine on executive functioning? Genes Brain Behav. 2013;12(1):13–20. doi: 10.1111/gbb.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheon KA, Jun JY, Cho DY. Association of the catechol-O-methyltransferase polymorphism with methylphenidate response in a classroom setting in children with attention-deficit hyperactivity disorder. Int Clin Psychopharmacol. 2008;23(5):291–8. doi: 10.1097/YIC.0b013e328306a977. [DOI] [PubMed] [Google Scholar]
- 49.Froehlich TE, Epstein JN, Nick TG, Melguizo Castro MS, Stein MA, Brinkman WB, et al. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1129–39. e2. doi: 10.1016/j.jaac.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kereszturi E, Tarnok Z, Bognar E, Lakatos K, Farkas L, Gadoros J, et al. Catechol-O-methyltransferase Val158Met polymorphism is associated with methylphenidate response in ADHD children. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1431–5. doi: 10.1002/ajmg.b.30704. [DOI] [PubMed] [Google Scholar]
- 51.Park S, Kim JW, Kim BN, Shin MS, Yoo HJ, Cho SC. Catechol-O-methyltransferase Val158-Met polymorphism and a response of hyperactive-impulsive symptoms to methylphenidate: A replication study from South Korea. J Psychopharmacol. 2014;28(7):671–6. doi: 10.1177/0269881114527654. [DOI] [PubMed] [Google Scholar]
- 52.McGough JJ, McCracken JT, Loo SK, Manganiello M, Leung MC, Tietjens JR, et al. A candidate gene analysis of methylphenidate response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1155–64. doi: 10.1097/CHI.0b013e3181bc72e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sengupta S, Grizenko N, Schmitz N, Schwartz G, Bellingham J, Polotskaia A, et al. COMT Val108/158Met polymorphism and the modulation of task-oriented behavior in children with ADHD. Neuropsychopharmacology. 2008;33(13):3069–77. doi: 10.1038/npp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salatino-Oliveira A, Genro JP, Zeni C, Polanczyk GV, Chazan R, Guimaraes AP, et al. Catechol-O-methyltransferase valine158methionine polymorphism moderates methylphenidate effects on oppositional symptoms in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70(3):216–21. doi: 10.1016/j.biopsych.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Contini V, Victor MM, Bertuzzi GP, Salgado CA, Picon FA, Grevet EH, et al. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol. 2012;32(6):820–3. doi: 10.1097/JCP.0b013e318270e727. [DOI] [PubMed] [Google Scholar]
- 56.McCracken JT, Badashova KK, Posey DJ, Aman MG, Scahill L, Tierney E, et al. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J. 2014;14(3):295–302. doi: 10.1038/tpj.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–20. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 58.Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–44. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giakoumaki SG, Roussos P, Bitsios P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology. 2008;33(13):3058–68. doi: 10.1038/npp.2008.82. [DOI] [PubMed] [Google Scholar]
- 60.Ashare RL, Wileyto EP, Ruparel K, Goelz PM, Hopson RD, Valdez JN, et al. Effects of tolcapone on working memory and brain activity in abstinent smokers: a proof-of-concept study. Drug Alcohol Depend. 2013;133(3):852–6. doi: 10.1016/j.drugalcdep.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20(1):15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20(6):612–27. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 63.Weickert TW, Goldberg TE, Mishara A, Apud JA, Kolachana BS, Egan MF, et al. Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol Psychiatry. 2004;56(9):677–82. doi: 10.1016/j.biopsych.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161(10):1798–805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 65.Bosia M, Zanoletti A, Spangaro M, Buonocore M, Bechi M, Cocchi F, et al. Factors affecting cognitive remediation response in schizophrenia: the role of COMT gene and antipsychotic treatment. Psychiatry Res. 2014;217(1–2):9–14. doi: 10.1016/j.psychres.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Woodward ND, Jayathilake K, Meltzer HY. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr Res. 2007;90(1–3):86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Mata I, Arranz MJ, Staddon S, Lopez-Ilundain JM, Tabares-Seisdedos R, Murray RM. The high-activity Val allele of the catechol-O-methyltransferase gene predicts greater cognitive deterioration in patients with psychosis. Psychiatr Genet. 2006;16(5):213–6. doi: 10.1097/01.ypg.0000218626.26622.a2. [DOI] [PubMed] [Google Scholar]
- 68.Rebollo-Mesa I, Picchioni M, Shaikh M, Bramon E, Murray R, Toulopoulou T. COMT (Val(158/108)Met) genotype moderates the impact of antipsychotic medication on verbal IQ in twins with schizophrenia. Psychiatr Genet. 2011;21(2):98–105. doi: 10.1097/YPG.0b013e32834371a7. [DOI] [PubMed] [Google Scholar]
- 69.Arts B, Simons CJ, Drukker M, van Os J. Antipsychotic medications and cognitive functioning in bipolar disorder: moderating effects of COMT Val108/158 Met genotype. BMC psychiatry. 2013;13:63. doi: 10.1186/1471-244X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mueller EM, Makeig S, Stemmler G, Hennig J, Wacker J. Dopamine effects on human error processing depend on catechol-O-methyltransferase VAL158MET genotype. J Neurosci. 2011;31(44):15818–25. doi: 10.1523/JNEUROSCI.2103-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32(21):7316–24. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 74.Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32(27):9402–9. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21(7):535–57. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- 76.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–6. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 78.Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Frontiers in neuroscience. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryan SG. Regression to the truth: replication of association in pharmacogenetic studies. Pharmacogenomics. 2003;4(2):201–7. doi: 10.1517/phgs.4.2.201.22631. [DOI] [PubMed] [Google Scholar]
- 80.Bakker PR, van Harten PN, van Os J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry. 2008;13(5):544–56. doi: 10.1038/sj.mp.4002142. [DOI] [PubMed] [Google Scholar]
- 81.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314(5807):1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 82.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biol Psychiatry. 2009;66(11):997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Zhao QZ, Liu BC, Zhang J, Wang L, Li XW, Wang Y, et al. Association between a COMT polymorphism and clinical response to risperidone treatment: a pharmacogenetic study. Psychiatr Genet. 2012;22(6):298–9. doi: 10.1097/YPG.0b013e328358629a. [DOI] [PubMed] [Google Scholar]
- 84.Fijal BA, Kinon BJ, Kapur S, Stauffer VL, Conley RR, Jamal HH, et al. Candidate-gene association analysis of response to risperidone in African-American and white patients with schizophrenia. Pharmacogenomics J. 2009;9(5):311–8. doi: 10.1038/tpj.2009.24. [DOI] [PubMed] [Google Scholar]