Abstract
Objective
This experiment tested the effects of an individualized risk-based online mammography decision intervention. The intervention employs exemplification theory and the Elaboration Likelihood Model of persuasion to improve the match between breast cancer risk and mammography intentions.
Methods
2,918 women ages 35-49 were stratified into two levels of 10-year breast cancer risk (< 1.5%; ≥ 1.5%) then randomly assigned to one of eight conditions: two comparison conditions and six risk-based intervention conditions that varied according to a 2 (amount of content: brief vs. extended) × 3 (format: expository vs. untailored exemplar [example case] vs. tailored exemplar) design. Outcomes included mammography intentions and accuracy of perceived breast cancer risk.
Results
Risk-based intervention conditions improved the match between objective risk estimates and perceived risk, especially for high-numeracy women with a 10-year breast cancer risk <1.5%. For women with a risk < 1.5%, exemplars improved accuracy of perceived risk and all risk-based interventions increased intentions to wait until age 50 to screen.
Conclusion
A risk-based mammography intervention improved accuracy of perceived risk and the match between objective risk estimates and mammography intentions.
Practice Implications
Interventions could be applied in online or clinical settings to help women understand risk and make mammography decisions.
Keywords: risk communication, communication intervention, decision aid, mammography, perceived risk, numeracy
1. Introduction
The U.S. Preventive Services Task Force (USPSTF) [1] recommends that women between the ages of 40 and 49 make a decision about mammography based on their evaluation of its risks and benefits. In 2015, the American Cancer Society (ACS) recommended that women begin routine mammography at age 45 [2]. Some medical organizations recommend that women start at age 40 [3,4]. This means that women below the age of 50 have an important health decision to make. However, many women are not equipped to make informed decisions because they have little knowledge of screening guidelines, the predictive validity of mammograms, or their personal risk for breast cancer (BC) [5-7]. In prior research, 44.1% of female participants overestimated the risk of having BC, and 68.1% overestimated the risk of dying from BC [6].
Poor understanding of objective estimates of BC risk contributes to a poor match between objective risk and screening. Though a positive link between perceived risk and mammography screening has been reported [7-10], the relationship between objective risk estimates and screening has been less well documented. In fact, research has shown that risk factors used to estimate objective risk do not predict screening behavior [11] and that perceived and objective risk are independent predictors of screening behavior [9], suggesting that the correlation between objective risk and screening is imperfect. Since perceived risk does predict mammography, increasing the match between perceived risk and objective estimates of risk should improve the match between estimated objective risk and mammography.
Communication interventions have shown some success in changing perceived risk and mammography behavior. In a study of the effects of a mammography decision tool on women in their 40s and 50s, Rimer et al. [12,13] demonstrated the effectiveness of a tailored booklet and a tailored booklet plus telephone counseling in changing perceived BC risk compared to a usual care condition. The counseling group also showed an increase in mammography at short-term and long-term follow-up. Additionally, a decision aid for young women [14] increased both the likelihood of making a decision about whether or not to screen and the likelihood of deciding to delay mammography. In both of these situations, becoming more informed about the risks and benefits of mammography helped women make screening decisions that were appropriate for their level of risk (based on age), though the authors of these studies did not comment explicitly on the match between estimates of objective risk and behavior. Based on prior literature, we predicted that, compared to comparison conditions, women receiving interventions with individualized risk information would experience improved accuracy of perceived risk, women with an estimated 10-year BC risk < 1.5% who received the intervention would report increased intentions to begin mammography at age 50, and women with an estimated 10-year BC risk ≥ 1.5% who received the intervention would report increased intentions to start or continue mammography in their 40s.i
Rimer et al. [12,13] and Mathieu et al. [14] did not report any moderators of effects, such as numeracy, which has been shown to predict ability to interpret representations of BC risk [17]. Prior research suggests that less numerate individuals are more influenced by the format in which risk information is presented and more influenced by nonnumerical information than more numerate individuals [18]. Therefore, we expected that numeracy would moderate the effects of providing risk information on perceived risk and mammography intentions; more numerate women who saw the numeric risk-based intervention should have more accurate perceived risk and a better match between estimates of objective risk and behavior than less numerate women.
Another aspect missing in prior studies is the experimental manipulation of communication elements shown to have an impact on perceived risk and behavior, specifically exemplars (example cases) and tailoring. Exemplification theory predicts that exemplars can be used to shape an individual's perceptions of a particular issue [19]. Zillmann found that exemplars presented as part of a message are often more memorable than numeric risk information presented in the message itself and have a stronger effect on perceived risk than numeric risk [19]. Because individuals with low numeracy tend to be more influenced by nonnumeric information [18], using exemplars to model risk-based decision-making may also improve the match between risk and intentions, even when numerical risk information is not well understood. Specifically, we predicted that exemplars (example women presented in the intervention making a decision to screen or delay mammography) would have a stronger effect on perceived risk and intentions than aggregate statistical information presented without exemplars.
We also proposed that tailoring the intervention would increase its effectiveness. Tailoring is “any combination of strategies and information intended to reach one specific person, based on characteristics that are unique to that person, related to the outcome of interest, and derived from an individual assessment” [20, p. 674]. The Elaboration Likelihood Model of persuasion [21] predicts that ability and motivation influence the amount of thinking about a message. According to Briñol and Petty, “attitudes based on high amounts of thinking are postulated to be stronger than attitudes based on little thought.” [22, p. S83] One way to increase motivation is by increasing relevance [22] through tailoring.[23] Rimer et al. [12,13] tested the effects of a tailored mammography decision tool compared to usual care, but, to our knowledge, no mammography decision interventions have compared materials that are tailored on participant characteristics to versions that are untailored on these characteristics. We predicted that tailoring exemplars to match participant characteristics, including objective BC risk estimates, age, race, family history of BC, and parity, would enhance the impact on perceived risk and screening behavior.
2. Method
2.1 Setting and sampling
This research protocol was approved by the Institutional Review Board at [removed]. Participants were contacted by Survey Sampling International (http://www.surveysampling.com/) via email in September 2013 and provided a link to the online experiment. Participants completed an online consent form and responded to screening items: age, BC history, and genetic mutation related to BC. Participants were eligible for inclusion if they were female and between the ages of 35 and 49 with no history of BC or BRCA1 or BRCA2 mutation.
2.2 Study design
Eligible participants completed measures (see section 2.4) that included items to estimate objective BC risk. Risk was estimated using the National Cancer Institute Breast Cancer Risk Assessment Tool (NCI BCRAT) [24], which is based on the Gail model [15]. Participants were stratified into two groups: participants with an estimated 10-year BC risk <1.5% and participants with an estimated 10-year BC risk ≥1.5%. After stratification, participants were randomly assigned to one of eight conditions within their level of BC risk (yielding 16 conditions; see Table 1 and section 2.3). Following the experimental manipulation, participants completed additional measures (see section 2.4).
Table 1.
Condition Components
| Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comparison conditions | Risk-based intervention conditions | |||||||
| Comparison: no information | Comparison: basic info | Brief intervention + expository | Brief intervention + untailored exemplars | Brief intervention + tailored exemplars | Extended intervention + expository | Extended intervention + untailored exemplars | Extended intervention + tailored exemplars | |
| Participants with 10-year breast cancer risk <1.5% | No information | Basic information | Brief intervention + Expository reasons women wait to have mammograms at 50 (3 for, 1 against waiting) | Brief intervention + Exemplar who is undecided, lists reason for and reason against waiting; Exemplar who is waiting, list 2 reasons for waiting | Brief intervention + Risk-, age-, race-matched exemplar who is undecided, lists reason for and reason against waiting; Risk-, age-, race-matched exemplar who is waiting, lists 2 reasons for waiting | Extended intervention + Expository reasons women wait to have mammograms at 50 (3 for, 1 against waiting) | Extended intervention + Exemplar who is undecided, lists reason for and reason against waiting; Exemplar who is waiting, lists 2 reasons for waiting | Extended intervention + Risk-, age-, race-matched exemplar who is undecided, lists reason for and reason against waiting; Risk-, age-, race-matched exemplar who is waiting, lists 2 reasons for waiting |
| Participants with 10-year breast cancer risk ≥ 1.5% | No information | Basic information | Brief intervention + Expository reasons women have mammograms at 40 (3 for, 1 against screening) | Brief intervention + Exemplar who is undecided, lists reason for and reason against screening; Exemplar who is screening, lists 2 reasons for screening | Brief intervention + Risk-, age-, race-matched exemplar who is undecided, lists reason for and reason against screening; Risk-, age-, race-matched exemplar who is screening, list 2 reasons for screening | Extended intervention + Expository reasons women have mammograms at 40 (3 for, 1 against screening) | Extended intervention + Exemplar who is undecided, lists reason for and reason against screening; Exemplar who is screening, lists 2 reasons for screening | Extended intervention + Risk-, age-, race-matched exemplar who is undecided, lists reason for and reason against screening; Risk-, age-, race-matched exemplar who is screening, list 2 reasons for screening |
2.3 Conditions
2.3.1 Comparison conditions
The first comparison condition provided no information; participants moved directly from the first set of measures to the next. The second, a “basic information comparison” condition, included the definition of mammography, a statement that women between the ages of 40 and 50 years old have a choice to make about when to begin mammography, screening options, and a table summarizing recommendations from the ACS and the USPSTF.
2.3.2 Intervention conditions: Brief vs. extended
In addition to the information presented in the “basic information” comparison condition, participants in the three “brief intervention” conditions received individualized 10-year and lifetime estimates of their objective risk for developing BC and the risk of an average-risk age-matched woman, all presented as both numeric frequencies and icon arrays. In the remaining three “extended intervention” conditions, participants saw all of the brief intervention information in addition to how their risk compared to a typical 50-year-old woman, statistics about outcomes of mammography (e.g., rates of false positives and mammogram-detected cancers), and information about effects of mammography on reducing BC mortality.
2.3.3 Intervention conditions: Expository vs. untailored exemplar vs. tailored exemplar
Participants in the six risk-based intervention conditions also received arguments for having mammograms in one's 40s or waiting until age 50. Arguments varied between risk levels and were presented in either expository or exemplar formats. In the expository conditions, arguments were didactic, with general statements about women making choices and no example case. In the untailored exemplar conditions, arguments were presented using exemplars—example women making decisions about mammography—and all participants in these conditions read the same text. In the tailored conditions, exemplars’ ages, BC risks, family history of BC, and parity were similar to the participant and exemplar names were chosen to reflect common names for women of the participant's race and age (see Appendix for examples).
2.4 Measures
Participants first responded to measures of mammography history (“Have you ever had a mammogram?”), education (“What is the highest level of education you have completed?”), insurance status (“What kind of health insurance do you have?” Options included private health insurance, Medicaid or medical assistance, Medicare, other, and no health insurance and this was recoded into a dichotomous insurance/no insurance response), and additional factors needed to estimate objective BC risk (age, age at menarche, parity, family history of BC, biopsy history, and race/ethnicity). Items used to estimate objective BC risk were adapted from the National Cancer Institute Breast Cancer Risk Assessment Tool [24]. Participants also estimated their 10-year and lifetime BC risk.
Following the experimental manipulation, participants completed items measuring perceived risk of BC, mammography intentions, and numeracy [25] (numeracy scale was 11 items; Cronbach's alpha = .79).
2.4.1 Accuracy of perceived risk of BC
Perceived risk of BC was measured two ways: by asking participants, “What is your chance of developing breast cancer in the next 10 years?” as a percentage (0% = no chance of breast cancer and 100% = I definitely will get breast cancer) and as a frequency out of 1000 (“X out of 1000”).ii Similar measures have been used previously to measure perceived BC risk [26, 27]. A measure of accuracy of perceived risk of BC was created by subtracting each woman's estimated objective risk for BC, estimated using the NCI BCRAT [24], from her perceived risk measurement.iii
2.4.2 Mammography intentions
Mammography intentions were measured by asking, “Which statement best describes how you feel?” Options included “I will start or continue to have mammograms in my 40s,” “I will have my first or next mammogram at age 50,” “I will never have a mammogram,” and “I am undecided.” The intention measure was recoded into a dichotomous measure. For women with an estimated 10-year BC risk <1.5%, the measure was coded “1” for women who intended to have their first or next mammogram at age 50 and “0” for other intentions. For women with an estimated 10-year BC risk ≥1.5%, it was coded “1” for women who intended to start or continue to have mammograms in their 40s and “0” for other intentions.
2.5 Analysis
Because arguments presented to participants above and below the 1.5% 10-year risk threshold varied, data from the two risk strata were analyzed separately. For the analysis of accuracy of risk, risk-based intervention conditions were compared to the two comparison conditions using Ordinary Least Squares (OLS) regression. For the analysis of mammography intentions, risk-based intervention conditions were compared to the comparison conditions using logistic regression. We performed an overall F test for each OLS regression and an overall chi-square test for logistic regressions. To guard against Type I error, individual conditions were only interpreted as being significantly different from the comparison condition if the overall test was significant (p < .10). Finally, post-hoc tests were used following each regression to compare brief to extended conditions, expository to exemplar conditions, and untailored exemplar to tailored exemplar conditions. To test for moderation by numeracy level, we repeated the analyses above, including a continuous measure of numeracy and an interaction between numeracy and condition in each of the regressions.
3. Results
3.1 Participants
Of 4,549 participants who consented to participate, we excluded those who were not women between 35 and 49 years old (n = 130) and those with a prior history of BC or a genetic mutation in BRCA1 or BRCA2 (n = 314). Fifty-nine were inadvertently terminated due to a programming error and 91 dropped out during the pre-test. The remaining 3,955 were stratified by estimated objective risk level (10-year BC risk <1.5% vs. 10-year BC risk ≥1.5% as determined using the NCI BCRAT [24]) and then randomized to condition. We excluded participants who left the survey or whose completion time was either >90 minutes or less than half of the mean time for participants who completed their condition. Exclusion did not vary by condition.
The final analytic sample included 2,918 participants (see Table 2). For most of the participant characteristics measured, distribution across conditions was relatively equal. However, for women with an estimated 10-year risk ≥1.5%, drop-out rates, percentage reporting insurance coverage, and percentage reporting prior mammograms differed significantly across conditions. Because insurance status and mammogram history were associated with both likelihood of dropping out of the study and intention, they were included as covariates in analyses of intention for women with an estimated risk ≥1.5%.
Table 2.
Participant Characteristics
| Characteristic | Mean (SD) or average % | |
|---|---|---|
| Estimated 10-Year Breast Cancer Risk <1.5% | Estimated 10-Year Breast Cancer Risk ≥1.5% | |
| N | 1227 | 1691 |
| Mean age (SD) | 38.9 (0.10) | 44.0 (0.09) |
| Estimate of 10-year BC riska, M (SD) | 1.08 (0.01) | 2.53 (0.04) |
| Race: | ||
| % White | 69.0 | 81.8 |
| % African American | 11.5 | 11.0 |
| % other | 19.5 | 7.2 |
| Education: | ||
| % Less than HS | 2.2 | 1.8 |
| % HS | 20.1 | 17.9 |
| % Some college | 37.7 | 36.2 |
| % College+ | 40.0 | 44.1 |
| % Have insurance | 79.9 | 82.7b |
| % Had prior mammogram | 40.0 | 74.1b |
| Mean numeracy (SD) | 6.44 (2.64) | 6.57 (2.73) |
Note. Participants are divided into two levels of risk based on estimates of their 10-year risk for developing breast cancer according to the NCI Breast Cancer Risk Assessment Tool [24]. SD = Standard Deviation. HS = high school.
“10-year BC risk” refers to the average estimated risk for developing breast cancer in the next 10 years for women in each condition.
Insurance status and history of prior mammograms varied significantly across conditions for women with an estimated 10-year breast cancer risk ≥ 1.5% and were also associated with likelihood of dropping out of the survey and with mammography intentions. These variables have been controlled for in analyses of mammography intentions for women with an estimated 10-year breast cancer risk ≥ 1.5%.
3.2 Accuracy of Perceived Risk
Results of the effects of the intervention on accuracy of perceived risk are presented in Table 3 and described in sections 3.2.1 and 3.2.2. In the presentations of results in Table 3, changes in accuracy are represented as changes in overestimation, which allows for preservation of directionality of the change in perceived risk.
Table 3.
Perceived Risk and Intentions to Have Mammogram, by Condition
| Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison: no information |
Comparison: basic info |
Brief intervention + expository |
Brief intervention + untailored exemplars |
Brief intervention + tailored exemplars |
Extended intervention + expository |
Extended intervention + untailored exemplars |
Extended intervention + tailored exemplars |
||
| Participants with an estimated 10-Year Breast Cancer Risk <1.5%a | |||||||||
| F (df) | |||||||||
| n | 194 | 150 | 141 | 169 | 121 | 160 | 154 | 138 | |
| Accuracy of risk (amount of overestimation): Risk measured as Mean (SD) | 17.4 (19.1) | 20.2 (19.4) | 16.7 (20.7) | 12.7 (17.0) | 13.8 (17.5) | 14.7 (19.2) | 13.8 (17.3) | 11.7 (16.1) | 3.39 (7, 1215)** |
| Beta | 0.048 | −0.012 | −0.087*‡‡‡ | −0.057†‡‡ | −0.048‡ | −0.063†‡‡ | −0.096**‡‡‡ | ||
| Accuracy of risk (amount of overestimation): Risk measured as frequency, Mean (SD) | 10.0 (16.7) | 10.5 (18.2) | 11.8 (26.2) | 7.5 (21.1) | 15.3 (32.2) | 9.8 (24.5) | 2.9 (14.3) | 8.8 (24.2) | 3.50 (7, 1212)*** |
| Beta | 0.007 | 0.025 | −0.038 | 0.071*b | −0.003 | −0.105**‡‡ | −0.017 | ||
| X2 (df) | |||||||||
| % intend to wait until age 50 to get mammogram, Mean (SD) | 7.2 (26.0) | 6.7 (25.0) | 18.4 (38.9) | 16.7 (37.4) | 14.0 (34.9) | 19.4 (39.6) | 24.2 (43.0) | 21.0 (40.9) | 31.02 (7)*** |
| OR | 0.91 | 2.89**‡‡ | 2.56**‡‡ | 2.09†‡ | 3.07**‡‡ | 4.08***‡‡‡ | 340***‡‡ | ||
| Participants with an estimated 10-Year Breast Cancer Risk ≥1.5%c | |||||||||
| F (df) | |||||||||
| N | 259 | 196 | 210 | 195 | 200 | 212 | 205 | 214 | |
| Accuracy of risk (amount of overestimation): Risk measured as %, Mean (SD) | 20.2 (21.2) | 23.2 (22.7) | 15.8 (18.4) | 16.0 (19.1) | 14.0 (17.0) | 14.3 (18.4) | 13.9 (18.1) | 13.9 (17.7) | 6.85 (7, 1671)*** |
| Beta | 0.051† | −0.074*‡‡‡ | 0.068*‡‡‡ | −0.102**‡‡‡ | −0.101**‡‡‡ | −0.105***‡‡‡ | −0.108***‡‡‡ | ||
| Accuracy of risk (amount of overestimation): Risk measured as frequency, Mean (SD) | 9.0 (18.7) | 10.6 (19.6) | 10.9 (24.7) | 11.6 (25.3) | 10.9 (25.2) | 9.6 (26.0) | 7.7 (23.7) | 7.9 (23.3) | 0.79 (7, 1666) |
| Beta | 0.022 | 0.026 | 0.035 | 0.026 | 0.008 | −0.018 | −0.015 | ||
| X2 (df) | |||||||||
| % intend to get mammogram at age 40, Mean (SD)d | 72.6 (44.7) | 73.0 (44.5) | 76.7 (42.4) | 77.9 (41.6) | 70.5 (45.7) | 75.9 (42.8) | 76.1 (42.8) | 68.7 (46.5) | 12.92 (7)† |
| OR f | 0.89 | 1.13 | 1.64*‡ | 1.07 | 1.32 | 1.12 | 0.71 | ||
Note. N = 2918. All risk-based intervention conditions are compared to the “Comparison: no information” condition and the “Comparison: basic info” condition using OLS regression to regress accuracy of perceived risk on condition and logistic regression to regress a dichotomous measure of mammography intention on condition. “Accuracy of risk (amount of overestimation): Risk as frequency” refers to the degree to which participants overestimated their objective risk for breast cancer when asked to report perceived risk as a frequency out of 1000. This number was then converted to a 0 to 100 scale so that it could be compared to “Accuracy of risk (amount of overestimation): Risk as %,” which is overestimation when risk was asked as a percentage. The Beta reported above is the standardized regression coefficient associated with each condition compared to “Comparison: no information.” The OR is the odds ratio (the ratio of the odds that the intention is present for participants in a given condition to the odds that the intention is present for those in the comparison condition). To guard against Type I error, individual conditions are only noted as being significantly different from the comparison if the overall test (the F test for OLS regression or the X2 test for logistic regression) for that variable is significant (p < .10). SD = Standard Deviation.
These participants are classified as being at a low risk for breast cancer because they have an estimated 10-year risk for developing breast cancer that is <1.5%, according to the NCI BCRAT [24].
Though significant, this finding is in the unexpected direction, as it represents reduced accuracy (i.e., higher overestimation).
These participants have an estimated 10-year risk for developing breast cancer that is ≥1.5%, according to the NCI Breast Cancer Risk Assessment Tool [24].
Analyses of mammography intentions for women with an estimated 10-year risk ≥1.5% include prior history of mammogram and insurance status as comparison variables.
p < .10
p < .05
p < .01
p < .001 when compared to “Comparison: no information” condition or overall tests (e.g., X2)
†† p < .10
p < .05
p < .01
p < .001 when compared to “Comparison: basic info” condition
3.2.1 Effects of condition on accuracy of perceived risk for women with a risk <l.5%
For women with an estimated 10-year BC risk <1.5%, when perceived risk was measured as a percentage, five of the six risk-based intervention conditions led to significant improvements in accuracy of perceived risk (represented as decreases in overestimation). Exemplar conditions led to greater improvements than expository conditions, F(1, 1215) = 4.12, p = .043. There were no significant differences between the brief and extended conditions or between untailored and tailored exemplar conditions.
When perceived risk was measured as a frequency, only the condition in which participants saw the extended intervention with untailored exemplars resulted in increased accuracy. There were no significant differences between expository conditions and exemplar conditions. When compared to the brief intervention conditions, extended intervention conditions led to significantly greater accuracy, F(1, 1212) = 8.35, p = .004. When conditions with tailored exemplars were compared to those with untailored exemplars, untailored exemplars resulted in larger increases in accuracy of perceived risk measured as a frequency, F(1, 1212) = 13.45, p < .001. The brief intervention with tailored exemplars led to a significant decrease in accuracy.
3.2.2 Effects of condition on accuracy of perceived risk for women with a risk ≥1.5%
For participants with an estimated 10-year BC risk ≥ 1.5%, when perceived BC risk was measured as a percentage, all risk-based intervention conditions led to a significant increase in accuracy of perceived risk (versus comparison condition). There were no significant differences between brief and extended conditions, between expository and exemplar conditions, or between untailored and tailored conditions. When perceived BC risk was measured as a frequency, there were no differences in accuracy among conditions.
3.2.3 Moderation of effects on accuracy of perceived risk
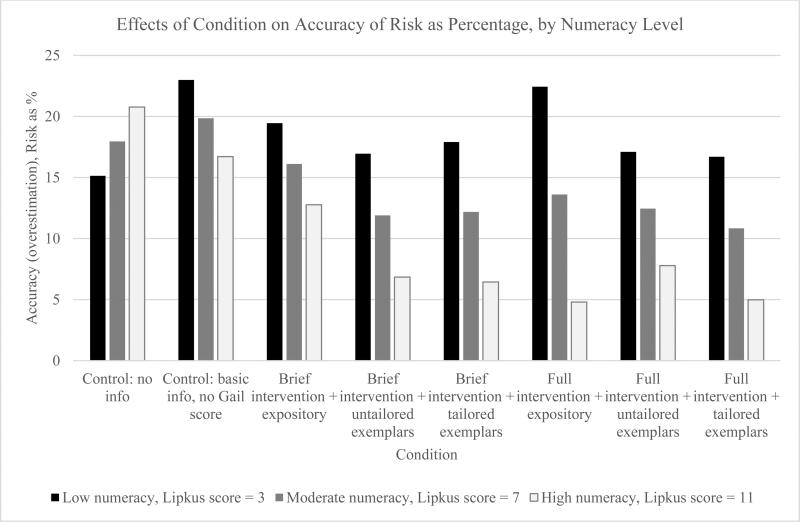
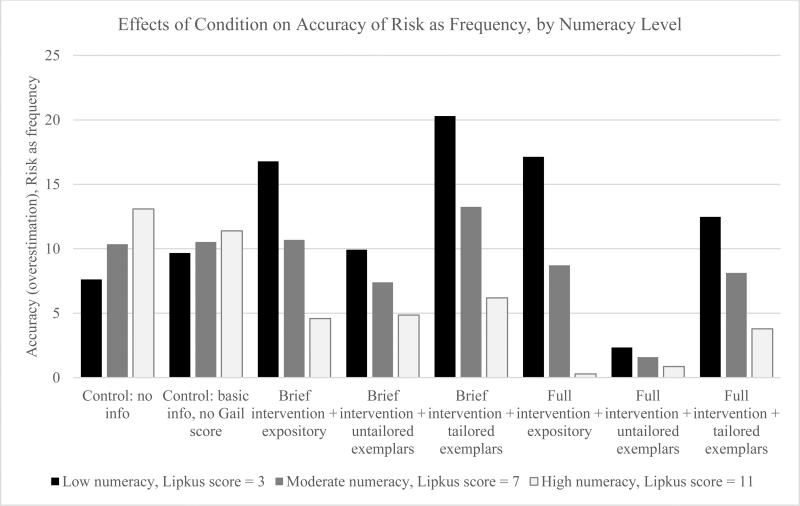
The effects of conditions on accuracy of perceived risk when risk was measured as a percentage varied significantly by participant numeracy score for women with an estimated risk <1.5%, F(7, 1164) = 2.67, p = .01; women with high numeracy experienced greater increases in accuracy of perceived risk than women with low numeracy. This moderation effect is shown in Figure 1. A similar pattern is shown in Figure 2 for women with an estimated risk <1.5% when risk was asked as a frequency, and the overall moderation effect by numeracy was significant, F (7, 1162) = 2.24, p = .03. There were no significant moderation effects of numeracy for accuracy of perceived risk for women with an estimated risk ≥1.5%.
Figure 1.
Effects of condition on accuracy of perceived risk (overestimation) as a percentage for women with an estimated 10-year breast cancer risk <1.5%, by numeracy level. This figure illustrates the moderating effect of numeracy level on the accuracy of risk depending on condition when perceived risk is measured as a percentage. To create a measure of risk accuracy, the participant's 10-year estimated objective risk for BC (or NCI BCRAT score) was converted to a percentage and subtracted from the participant's perceived risk reported as a percentage. Thus, the numbers presented represent the difference between perceived and estimated objective BC risk (e.g., a woman who thinks her risk is 20% when it is actually 5% would have an accuracy [overestimation] score of 15).
Figure 2.
Effects of condition on accuracy of perceived risk (overestimation) as a frequency for women with an estimated 10-year BC risk <1.5%, by numeracy level. This figure illustrates the moderating effect of numeracy level on the accuracy of risk depending on condition when perceived risk is measured as a frequency out of 1000. To create a measure of risk accuracy, the participant's 10-year estimated objective risk for BC as a frequency out of 1000 was subtracted from the participant's perceived risk reported as a frequency out of 1000, and the measure was divided by 10 to put it on the same scale as perceived risk reported as a percentage. Thus, the numbers presented represent the difference between perceived and estimated objective BC risk divided by 10 (e.g., a woman who thinks her risk is 200 out of 1000 when it is actually 50 out of 1000 would have an accuracy [overestimation] score of 15).
3.3 Mammography Intentions
Results of the effects of the intervention on mammography intentions are presented in Table 3 and described in sections 3.3.1 through 3.3.3.
3.3.1 Effects of condition on mammography intentions for women with a risk <1.5%
For women with an estimated 10-year BC risk of <1.5%, all of the risk-based intervention conditions resulted in a significant or marginally significant increase in the percentage of women planning to wait until age 50 to have a mammogram, but there were no significant differences by type of condition.
3.3.2 Effects of condition on mammography intentions for women with a risk ≥1.5%
For women with an estimated 10-year BC risk ≥1.5%, only the brief intervention with untailored exemplars condition significantly increased the percentage of women who planned to have a mammogram at age 40. Untailored exemplar conditions led to a greater increase in intentions to have a mammogram at age 40 than tailored exemplar conditions, Χ2 (1, n = 814) = 6.02, p = .01. There were no significant differences between brief and extended conditions or expository and exemplar conditions.
3.3.3 Moderation of effects on mammography intentions
There were no significant moderation effects of numeracy for mammography intentions.
4. Discussion and Conclusion
4.1 Discussion
In summary, the risk-based interventions had some, but not all, of the predicted effects on accuracy of perceived risk and mammography intentions. First, the accuracy of perceived risk consistently improved only when risk was measured as a percentage, conditions with exemplars only improved accuracy for women with an estimated 10-year BC risk <1.5%, and tailoring did not result in improved accuracy of perceived risk. For women with an estimated 10-year risk <1.5%, the risk-based interventions increased their intentions to wait until age 50 to begin screening, but only the brief intervention with untailored exemplars condition increased intentions to begin screening at age 40 for women with an estimated 10-year risk ≥1.5%. Importantly, no intervention condition significantly decreased intentions to begin having mammograms at age 40 for women with an estimated 10-year risk ≥1.5%. The presence of exemplars did not alter mammography intentions, nor did the presence of tailored exemplars. Finally, there was a moderating effect of numeracy on the relationship between condition and accuracy of perceived risk for women with an estimated 10-year risk <1.5%, but the effect of the risk-based interventions on intentions was not moderated by numeracy.
The observed effects of presenting individualized objective risk information on perceived BC risk add to a small body of literature showing improvements in the accuracy of perceived risk following personalized risk communication [28]. It is also consistent with research on mammography decision interventions that included accuracy of perceived risk as an outcome [12,13]. Though the effect of exemplars on improved accuracy of perceived risk was only present for women with an estimated 10-year risk <1.5%, this may have been due to the smaller difference between perceived and objective risk estimates for women with an estimated risk ≥1.5%. Also, the lack of effects of tailoring could be due to a true absence of effects or a weak manipulation, since tailored conditions were quite similar to untailored conditions. This could be addressed in future research.
Additionally, the finding that effects of the risk-based intervention on accuracy of perceived risk varied with participant numeracy deserves further attention. This finding is consistent with research demonstrating that more numerate patients with BC were better able to use personalized survival estimates [29]. More numerate persons are also better able to communicate perceived risk using percentage and frequency formats [27]. Because more numerate women tend to benefit more from interventions that present objective risk information in numerical formats, these types of interventions may unintentionally increase disparities. We also report that our findings regarding the efficacy of the risk-based intervention in improving accuracy of perceived risk varied depending on how perceived risk was measured. This finding highlights the importance not only of the format used to communicate risk but the format used to assess perceived risk. The cognitive phenomenon of denominator neglect may have contributed to this finding. A decrease of 1 point using a percentage format conveys a greater drop in risk magnitude than a decrease of 1 in the numerator when using an X/1000 format. Although the use of icon arrays (such as those used in the intervention) can reduce denominator neglect [30], this bias may have contributed to our findings.
The effects of the intervention on mammography intentions were somewhat as predicted. Improving accuracy of perceived risk has been associated with an increase in screening uptake for cancers for which people underestimate their risk [28]. However, many women overestimate their risk for BC [6]. Because the goal of our research was to improve the accuracy of perceived risk and improve the match between objective risk estimates and behavioral intentions, we recognized that intentions to screen at age 40 could (and did) decrease for women with an estimated 10-year risk <1.5%. The mammography decision aid used by Mathieu et al. [14] also reduced women's intentions to screen. Finally, as with the effects on perceived risk, a lack of expected effects of exemplars or tailoring on mammography intentions may have been due to an absence of effects or limited exposure.
Though this research demonstrates intended effects on intentions and perceived risk, our research has limitations. First, even when significant differences were present, there were no substantial differences between expository, untailored exemplar, and tailored exemplar conditions. However, those elements represented only a small part of the extended intervention and were preceded by large amounts of information. Also, this was not a pure test of the effects of tailoring in a decision intervention; all participants in risk-based intervention conditions received individualized risk information. Additionally, because participants were stratified by objective risk estimates, this research may not generalize to interventions in which participants of all risk levels receive the same message. Finally, we used the NCI BCRAT [24] to estimate objective BC risk. Though widely used, this model does not take into account all BC risk factors (e.g., breastfeeding), and participants may have determined their perceived risk based on additional risk factors.
4.2 Conclusion
In contrast to comparison conditions, among women with an estimated 10-year BC risk <1.5%, all of the risk-based intervention conditions improved accuracy of perceived BC risk and increased intentions to wait until age 50 to begin mammography, and the inclusion of exemplars improved accuracy of perceived risk. Thus, interventions can be designed to improve the accuracy of perceived BC risk and the match between objective estimates of BC risk and mammography behavior. Finally, effects may vary with numeracy; the effects of the risk-based intervention on increasing accuracy of perceived risk were greater for women with high numeracy than for women with low numeracy.
4.3 Practice Implications
For women at a lower risk of developing BC, providing personalized risk information can significantly improve the accuracy of perceived risk. Further, providing different decision interventions for women at different risk levels can improve the match between objective estimates of BC risk and screening intentions. The interventions used in this research could be applied in online or clinical settings to help women between the ages of 40 and 49 years understand their objective BC risk estimates and make informed mammography decisions.
Highlights.
We developed and tested a risk-based mammography decision intervention.
Match between objective estimates of BC risk and mammography intentions improved.
Accuracy of breast cancer risk perceptions increased.
Effects on risk perceptions were more pronounced for high-numeracy women.
Acknowledgments
Funding
This project was made possible by Penn Center for Innovation in Personalized Breast Cancer Screening (PCIPS) Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) Grant 1U54CA163313 from the National Cancer Institute. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. The National Cancer Institute was not involved in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Appendix
Sample Expository Text, Untailored Exemplars, and Tailored Exemplars
The following is an example of how the text appended to a decision aid varied based on expository, untailored exemplar, or tailored exemplar condition. Information in brackets is tailored to the participant. These examples are from conditions for women with a low risk for developing BC (10-year BC risk < 1.5%).
|
Expository Text for
Low-Risk Women |
| Every woman faces a decision about when to start screening for breast cancer. Other women like you have had to make the same decision that you are now facing. Their experiences may help you make your choice. Remember, for women with your risk factors, [Risk out of 1000] out of 1000 women will develop breast cancer in the next 10 years. |
| Women who used the same mammography decision aid that you just completed filled out our surveys as well. No matter what they decided, the women reported that staying healthy is what they care about most. Here is what we learned. |
| The women who completed the decision aid learned their personal risk of developing breast cancer in the next 10 years. Some women have a personal breast cancer risk that is higher than average. Other women have a risk that is lower than average. Each woman is different, and women with similar risk levels may even choose to begin screening at different ages. There isn't necessarily one right answer for all women. |
| One important risk factor is family history of breast cancer. While an important risk factor, it's not the only one that matters. When it comes to deciding when to start having mammograms, women also need to think about their age, race, and reproductive history, like how old they were when they had children. This decision aid uses these risk factors to estimate each woman's personal risk level. |
| It is important for women to think about personal breast cancer risk factors and to weigh the pros and cons of screening. As you think about when to start getting mammograms, you may decide to begin screening in your 40s or to wait until you turn 50. You may also want to talk to your doctor at your next visit about the best screening decision for you. |
| Here are some of the reasons why women get mammograms in their 40s or why they are waiting until age 50 to start screening: |
| • Some said that they get mammograms because mammograms help find breast cancer early, before symptoms like lumps are showing. When cancer is found before it grows or spreads, it is easier to treat. |
| ○ However, in addition to finding breast cancer, mammograms can sometimes draw attention to suspicious cells that would never have spread or become life-threatening. Doctors can't always tell the difference between suspicious cells that will and won't spread, which may lead to unnecessary treatment. |
| • Some women said that they'll wait until 50 to get mammograms because mammograms are less accurate for younger women. Mammograms sometimes produce false-positives, results that look like cancer but are not. False-positives are more common in younger women and can cause anxiety and lead to unnecessary biopsies, and painful surgeries. |
| • Some women said that they wait to get mammograms because more lives are saved when mammograms are started at an older, rather than younger, age. For younger women, mammograms reduce the number of deaths from breast cancer by only a tiny amount. |
| • Some women said that they'll wait to get mammograms because mammograms expose women to a small amount of radiation. Women who start mammograms later will be exposed to less radiation throughout their lives. |
| Overall, women who completed the decision aid were glad they had information about their personal risk. Knowing your risk level and thinking through your options can help you make your decision. |
| Talk to your doctor about your breast cancer risk and when you should start screening. For more information about breast cancer and screening options, visit www.cancer.gov. |
|
Untailored Exemplars for
Low-Risk Women |
| Every woman faces a decision about when to start screening for breast cancer. Other women like you have had to make the same decision that you are now facing. Their experiences may help you make your choice. Remember, for women with your risk factors, [Risk out of 1000] out of1000 women will develop breast cancer in the next 10 years. |
| Women who used the same mammography decision aid you just completed also talked with us about when they will start getting screened for breast cancer. No matter what they decided, staying healthy was what they cared about most. As Jennifer Johnson, age 43, said, “I just want to be able to keep doing the things I love, like spending time with my friends.” Here are a couple of their stories. |
| Jennifer used the same mammography decision aid you just completed and learned that her personal risk of developing breast cancer in the next 10 years is 16 out of 1000. That means that for every 1000 women with her risk factors, 16 will get breast cancer in next 10 years. |
| Michelle Smith, age 42, also shared thoughts about her decision. “I hadn't really thought about all of my risk factors, but I knew that I don't have breast cancer in my family.” |
| One important risk factor is family history of breast cancer. While an important risk factor, it's not the only one that matters. When it comes to deciding when to start having mammograms, women also need to think about their age, race, and reproductive history, like how old they were when they had children. This decision aid uses these risk factors to estimate each woman's personal risk level. When Michelle used the mammography decision aid, she learned that her risk of getting breast cancer over the next 10 years is 15 out of 1000. This is lower than the risk of a typical 50-year-old woman--and 50 is the age when all guidelines recommend screening. |
| Jennifer's Choice |
| Jennifer and Michelle have similar risk levels, but that doesn't mean they will start getting mammograms at the same age. Women under 50 have a decision to make when it comes to mammograms, and there isn't necessarily one right answer. Jennifer said, “I'm trying to figure out when I should start getting mammograms. I am weighing both sides.” |
| One of the reasons women say they get mammograms in their 40s is because mammograms can find cancer early, when it may be easier to treat. “One of my very best friends had a mammogram that found her cancer early,” Jennifer said. “She didn't have a lump or anything, and she felt fine.” Because doctors found her friend's cancer before it grew or spread, it was easier to treat. But, in addition to finding breast cancer, mammograms can sometimes draw attention to suspicious cells that would never have spread or become life-threatening. Since doctors can't always tell the difference between suspicious cells that will and won't spread, her friend's treatment may have been unnecessary. |
| Some women who wait until they are 50 to start mammograms say that it's because younger women are more likely to have screening results that look like cancer but aren't. “When my neighbor had an abnormal mammogram result at age 44, she had to go back for more tests,” Jennifer said. It turns out that Jennifer's neighbor didn't have cancer. This is called a “false-positive” result. According to Jennifer, “She said it was one of the scariest times of her life, and she still has a scar from the biopsy.” |
| When it comes to her own decision, Jennifer is still weighing the pros and cons of starting mammograms this year or waiting until she turns 50. She said, “I want all the information I can get, but I'm still planning to talk to my doctor at my next visit about what will work best for me.” |
| Michelle's Choice |
| Michelle also weighed the risks and benefits of screening after learning her risk for developing breast cancer. |
| After learning that mammograms reduce the number of deaths from breast cancer by only a tiny amount for women younger than 50, Michelle is planning to wait until she turns 50 to start getting regular mammograms. She said, “I was surprised to find out that mammograms for younger women don't really save many lives. If they save lives for women over 50 then that's when I'm going to start—if my doctor agrees.” |
| She's also concerned about being exposed to small amounts of radiation from mammograms unnecessarily. “Getting radiation that you don't need can't do any good!” Michelle said. If she waits until 50 to start getting mammograms, she will be exposed to less radiation throughout her life than if she had started at a younger age. |
| Overall, the women we talked to were glad they had information about their personal risk. “Knowing my risk level and thinking through all of my options gives me what I need to talk through this decision with my doctor. I know I'll feel a lot better about what to do,” Michelle said. |
| Talk to your doctor about your breast cancer risk and when you should start screening. For more information about breast cancer and screening options, visit www.cancer.gov. |
|
Tailored Exemplars for
Low-Risk Women |
| Every woman faces a decision about when to start screening for breast cancer. Other women like you have had to make the same decision that you are now facing. Their experiences may help you make your choice. Remember, for women with your risk factors, [Risk out of 1000] out of 1000 women will develop breast cancer in the next 10 years. |
| Women who used the same mammography decision aid you just completed also talked with us about when they will start getting screened for breast cancer. No matter what they decided, staying healthy was what they cared about most. As [namel], age [narr_agel], said, “I just want to be able to keep doing the things I love, like spending time with my [role: friends/kids].” Here are a couple of their stories. |
| [firstname1] used the same mammography decision aid you just completed and learned that her personal risk of developing breast cancer in the next 10 years is [BCRATfreq1] out of 1000. That means that for every 1000 women with her risk factors, [BCRATfreq1] will get breast cancer in next 10 years. |
| [name2], age [narr_age2], also shared thoughts about her decision. “I hadn't really thought about all of my risk factors, but I knew that I [history: have/don't have] breast cancer in my family.” |
| One important risk factor is family history of breast cancer. While an important risk factor, it's not the only one that matters. When it comes to deciding when to start having mammograms, women also need to think about their age, race, and reproductive history, like how old they were when they had children. This decision aid uses these risk factors to estimate each woman's personal risk level. |
| When [firstname2] used the mammography decision aid, she learned that her risk of getting breast cancer over the next 10 years is [BCRATfreq2] out of 1000. This is lower than the risk of a typical 50-year-old woman—and 50 is the age when all guidelines recommend screening. |
| [firstname1]'s Choice |
| [firstnamel] and [firstname2] have similar risk levels, but that doesn't mean they will start getting mammograms at the same age. Women under 50 have a decision to make when it comes to mammograms, and there isn't necessarily one right answer. [firstnamel] said, “I'm trying to figure out when I should start getting mammograms. I am weighing both sides.” |
| One of the reasons women say they get mammograms in their 40s is because mammograms can find cancer early, when it may be easier to treat. “One of my very best friends had a mammogram that found her cancer early,” [firstnamel] said. “She didn't have a lump or anything, and she felt fine.” Because doctors found her friend's cancer before it grew or spread, it was easier to treat. But, in addition to finding breast cancer, mammograms can sometimes draw attention to suspicious cells that would never have spread or become life-threatening. Since doctors can't always tell the difference between suspicious cells that will and won't spread, her friend's treatment may have been unnecessary. |
| Some women who wait until they are 50 to start mammograms say that it's because younger women are more likely to have screening results that look like cancer but aren't. “When my neighbor had an abnormal mammogram result at age 44, she had to go back for more tests,” [firstnamel] said. It turns out that [firstname1]'s neighbor didn't have cancer. This is called a “false-positive” result. According to [firstnamel], “She said it was one of the scariest times of her life, and she still has a scar from the biopsy.” |
| When it comes to her own decision, [firstname1] is still weighing the pros and cons of starting mammograms [whenscreening1: when she turns 40/this year] or waiting until she turns 50. She said, “I want all the information I can get, but I'm still planning to talk to my doctor at my next visit about what will work best for me.” |
| [firstname2]'s Choice |
| [firstname2] also weighed the risks and benefits of screening after learning her risk for developing breast cancer. |
| After learning that mammograms reduce the number of deaths from breast cancer by only a tiny amount for women younger than 50, [firstname2] is planning to wait until she turns 50 to start getting regular mammograms. She said, “I was surprised to find out that mammograms for younger women don't really save many lives. If they save lives for women over 50 then that's when I'm going to start—If my doctor agrees.” |
| She's also concerned about being exposed to small amounts of radiation from mammograms unnecessarily. “Getting radiation that you don't need can't do any good!” [firstname2] said. If she waits until 50 to start getting mammograms, she will be exposed to less radiation throughout her life than if she had started at a younger age. |
| Overall, the women we talked to were glad they had information about their personal risk. “Knowing my risk level and thinking through all of my options gives me what I need to talk through this decision with my doctor. I know I'll feel a lot better about what to do,” [firstname2] said. |
| Talk to your doctor about your breast cancer risk and when you should start screening. For more information about breast cancer and screening options, visit www.cancer.gov. |
In the tailored exemplar conditions, exemplar ages, breast cancer risks, family history of breast cancer, and parental status were made similar to the participant. Exemplar names were chosen to reflect names that are common for women of the participant's race and age, based on U.S. Census Bureau and U.S. Social Security Administration data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Section 2.2 for a description of how participants were divided into two strata of risk. The risk threshold of 1.5% used in this research was chosen to reflect the 90th percentile of 10-year risk for 50-year-old women, calculated using the Gail model [15] and data from NHANES III [16].
Though perceived risk can be characterized as a combination of perceived susceptibility and severity, in the present research, it is limited to susceptibility so that it can be compared the participant's objective estimate of risk.
Because estimated objective risk is subtracted from perceived risk, the measure represents the extent to which a participant overestimates risk. Thus, zero indicates perfect accuracy, positive numbers indicate overestimation, and negative numbers indicate underestimation.
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
References
- 1.U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Fontham ETH, Etzioni R, Herzig A, Michaelson JS, Shih Y-CT, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015 Oct 20. 314:1599. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians-Gynecologists Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011 Aug;118:372–82. doi: 10.1097/AOG.0b013e31822c98e5. [DOI] [PubMed] [Google Scholar]
- 4.Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol. 2013 Jan;10:11–4. doi: 10.1016/j.jacr.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Allen SV, Solberg Nes L, Marnach ML, Polga K, Jenkins SM, Files JA, Croghan IT, Ghosh K, Pruthi S. Patient understanding of the revised USPSTF screening mammogram guidelines: need for development of patient decision aids. BMC Womens Health. 2012;12:36. doi: 10.1186/1472-6874-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman RM, Lewis CL, Pignone MP, Couper MP, Barry MJ, Elmore JG, Levin CA, Van Hoewyk J, Zikmund-Fisher BJ. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Making. 2010;30:53S–64S. doi: 10.1177/0272989X10378701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Walker MJ, Chiarelli AM, Knight JA, Mirea L, Glendon G, Ritvo P. Perceived risk and adherence to breast cancer screening guidelines among women with a familial history of breast cancer: a review of the literature. Breast. 2013;22:395–404. doi: 10.1016/j.breast.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Gross CP, Filardo G, Singh HS, Freedman AN, Farrell MH. The relation between projected breast cancer risk, perceived cancer risk, and mammography use. Results from the National Health Interview Survey. J Gen Intern Med. 2006;21:158–64. doi: 10.1111/j.1525-1497.2005.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15:423–9. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 11.Daly MB, Lerman CL, Ross E, Schwartz MD, Sands CB, Masny A. Gail model breast cancer risk components are poor predictors of risk perception and screening behavior. Breast Cancer Res Treat. 1996;41:59–70. doi: 10.1007/BF01807037. [DOI] [PubMed] [Google Scholar]
- 12.Rimer BK, Halabi S, Sugg Skinner C, Kaplan EB, Crawford Y, Samsa GP, Strigo TS, Lipkus IM. The short-term impact of tailored mammography decision-making interventions. Patient Educ Couns. 2001;43:269–85. doi: 10.1016/s0738-3991(00)00172-5. [DOI] [PubMed] [Google Scholar]
- 13.Rimer BK, Halabi S, Sugg Skinner C, Lipkus IM, Strigo TS, Kaplan EB, Samsa GP. Effects of a mammography decision-making intervention at 12 and 24 months. Am J Prev Med. 2002;22:247–57. doi: 10.1016/s0749-3797(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 14.Mathieu E, Barratt AL, McGeechan K, Davey HM, Howard K, Houssami N. Helping women make choices about mammography screening: An online randomized trial of a decision aid for 40-year-old women. Patient Educ Couns. 2010;81:63–72. doi: 10.1016/j.pec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Brown SM, Culver JO, Osann KE, MacDonald DJ, Sand S, Thornton AA, Grant M, Bowen DJ, Metcalfe KA, Burke HB, Robson ME, Friedman S, Weitzel JN. Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Educ Couns. 2011;83:92–8. doi: 10.1016/j.pec.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters E. Beyond comprehension: The role of numeracy in judgments and decisions. Curr Dir Psychol Sci. 2012;21:31–5. [Google Scholar]
- 17.Zillmann D. Exemplification effects in the promotion of safety and health. J Commun. 2006;56:S221–37. [Google Scholar]
- 18.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133:673–93. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 19.Petty RE, Cacioppo JT. Communication and persuasion: Central and peripheral routes to attitude change. Springer-Verlag; New York: 1986. [Google Scholar]
- 20.Briñol P, Petty RE. Fundamental processes leading to attitude change: Implications for cancer prevention communications. J Commun. 2006;56:S81–104. [Google Scholar]
- 21.Kreuter MW, Wray RJ. Tailored and targeted health communication: Strategies for enhancing information relevance. Am J Health Behav. 2003;27:S227–232. doi: 10.5993/ajhb.27.1.s3.6. [DOI] [PubMed] [Google Scholar]
- 22.Breast Cancer Risk Assessment Tool Version 3.0 [internet] National Cancer Institute; Bethesda, MD: [2016 January 11]. [updated 2011 May 16]. Available from: http://www.cancer.gov/bcrisktool/ [Google Scholar]
- 23.Gail MH, Costantino JP. Validating and improving models for projecting the absolute risk of breast cancer. JNCI J Natl Cancer Inst. 2001;93:334–5. doi: 10.1093/jnci/93.5.334. [DOI] [PubMed] [Google Scholar]
- 24.NHANES III Series 11 Data Files [internet] National Center for Health Statistics; Hyattsville, MD: [2016 January 11]. [updated 2015 May 10]. Available from: http://www.cdc.gov/nchs/nhanes/nh3data.htm. [Google Scholar]
- 25.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 26.McQueen A, Swank PR, Bastian LA, Vernon SW. Predictors of perceived susceptibility of breast cancer and changes over time: A mixed modeling approach. Health Psychol. 2008;27:68–77. doi: 10.1037/0278-6133.27.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schapira MM, Davids SL, McAuliffe TL, Nattinger AB. Agreement between scales in the measurement of breast cancer risk perceptions. Risk Anal. 2004 Jun;24:665–73. doi: 10.1111/j.0272-4332.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, Playle R. Personalised risk communication for informed decision making about taking screening tests. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet] John Wiley & Sons, Ltd; Chichester, UK: 2013. [2014 Oct 28]. Available from: http://doi.wiley.com/10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipkus IM, Peters E, Kimmick G, Liotcheva V, Marcom P. Breast cancer patients’ treatment expectations after exposure to the decision aid program Adjuvant Online: The influence of numeracy. Med Decis Making. 2010;30:464–73. doi: 10.1177/0272989X09360371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Retamero R, Galesic M, Gigerenzer G. Do icon arrays help reduce denominator neglect? Med Decis Making. 2010 Nov 1;30:672–84. doi: 10.1177/0272989X10369000. [DOI] [PubMed] [Google Scholar]