Abstract
Clonidine, an α2-adrenergic receptor agonist, decreases circulating norepinephrine and epinephrine, attenuating sympathetic activity. Although catechol-O-methyltransferase (COMT) metabolizes catecholamines, main effectors of sympathetic function, COMT genetic variation effects on clonidine treatment are unknown. Chronic fatigue syndrome (CFS) is hypothesized to result in part from dysregulated sympathetic function. A candidate gene analysis of COMT rs4680 effects on clinical outcomes in the Norwegian Study of Chronic Fatigue Syndrome in Adolescents: Pathophysiology and Intervention Trial (NorCAPITAL), a randomized double-blinded clonidine versus placebo trial, was conducted (N=104). Patients homozygous for rs4680 high-activity allele randomized to clonidine took 2,500 fewer steps compared to placebo (pinteraction=0.04). There were no differences between clonidine and placebo amongst patients with COMT low-activity alleles. Similar gene-drug interactions were observed for sleep (pint=0.003) and quality of life (pint=0.018). Detrimental effects of clonidine in the subset of CFS patients homozygous for COMT high-activity allele warrant investigation of potential clonidine-COMT interaction effects in other conditions.
Introduction
Genetic variation in COMT, the locus that encodes catechol-O-methyltransferase, an enzyme which metabolizes norepinephrine, epinephrine, dopamine, and catechol estrogens, has been implicated in a broad cross section of disease and conditions from hypertension (1, 2), preeclampsia (3), cardiovascular disease (4), psychiatric disorders (5), cancer (6, 7) and chronic fatigue syndrome (CFS) (8). Furthermore, COMT has also been shown to modify clinical response to both active drugs (4, 9–12) and placebo treatments (13, 14) in randomized clinical trials. Thus COMT is emerging as an important hub in pharmacogenomics and the development of precision medicines.
Chronic fatigue syndrome (CFS) is a disabling condition characterized by unexplained, long-lasting, fatigue and exertion intolerance, accompanied by pain, cognitive impairments and orthostatic intolerance (15–17). Elevated norepineprhine and epinephrine levels, as well as attenuated heart rate variability in adolescents with CFS (18), support the hypothesis that heightened sympathetic and decreased parasympathetic nervous system activity contribute to the disease process (19). This disabling condition affects 0.1–1.0% of children world-wide, but the pathophysiology is poorly understood and there are few treatment options.
The most well-studied single nucleotide polymorphism (SNP) in COMT, rs4680 (val158met), encodes a three- to four-fold difference in enzymatic activity between the valine high-activity and methionine low-activity forms of the enzyme (20). COMT enzymatic activity has been shown to be inversely related to catecholamine levels (21). Thus, individuals homozygous for the high-activity valine (val/val) form of the enzyme tend to have lower catecholamine levels compared to those homozygous for the low-activity methionine (met/met) form (22–24). Consistent with the observation that adolescents diagnosed with CFS have elevated levels of norepinephrine and epinephrine (25), the COMT low-activity met/met genotype appears to be more prevalent amongst adolescents with CFS (8).
Clonidine is a centrally acting α2-adrenergic receptor agonist which attenuates sympathetic nervous activity, decreasing circulating norepinephrine and epinephrine levels while increasing parasympathetic nervous activity (26). Clonidine has also been shown to inhibit dopamine activation in the brain (27, 28). The Norwegian Study of Chronic Fatigue Syndrome in Adolescents: Pathophysiology and Intervention Trial (NorCAPITAL), was a randomized placebo controlled trial (RCT) designed to study the effects of short-term low-dose clonidine treatment for CFS among adolescents (29). In NorCAPITAL low-dose clonidine was found to reduce catecholamine levels and attenuate markers of sympathetic outflow and systemic inflammation; however clonidine treatment failed to provide greater benefit than placebo (30).
To date, the effect of genetic variation in COMT on treatment response to clonidine has not been examined. Based on current evidence, we hypothesized that individuals homozygous for the low-activity met-allele form of COMT rs4680 might benefit more from the catecholamine reduction induced by clonidine treatment than individuals homozygous for the high-activity val allele form. Further, given the COMT rs4680 association with response to placebo treatment, and clonidine effects on dopamine activation in brain regions implicated in placebo response, the association between COMT and outcomes in individuals randomized to placebo treatment was examined.
Materials and Methods
Study population and intervention
Details of the Norwegian Study of Chronic Fatigue Syndrome in Adolescents: Pathophysiology and Intervention Trial (NorCAPITAL) trial have been published elsewhere (29). Briefly, between March 1, 2010, and October 15, 2012, 120 CFS patients aged 12–18 years with 3 months of unexplained disabling, chronic/relapsing fatigue of new onset were included in a randomized clinical trial conducted at Oslo University Hospital, Norway. Patients with depression as a primary cause of fatigue were excluded from the trial. The Norwegian National Committee for Ethics in Medical Research and the Norwegian Medicines Agency approved the study. Written informed consent was obtained from all participants or from parents or next of kin as required.
Clonidine doses were based on a pilot study (31), and were designed to be in the lower range of what is considered clinically effective (25 μg or 50 μg for body weight <35 kg or >35 kg, respectively twice daily). The treatments were double-blinded. After 8 weeks of treatment, Steps Taken, the primary outcome, defined as mean number of steps per day, was monitored during 7 consecutive days with an accelerometer device. An incremental increase of 2,500 steps per day was considered clinically significant improvement.
Five validated inventories were selected a priori as secondary outcomes from the NorCAPITAL trial to assess the impact of clonidine versus placebo treatment on the patient’s symptoms and quality of life (29). Briefly, functional disability was measured using the Functional Disability Inventory (FDI). The FDI, designed as a global measure of children’s and adolescent’s physical and psychosocial functioning in everyday social roles, consists of 15 items, which are scored on a 0–4 Likert scale (32). The score represents the total sum across all items, hence the total range is from 0 to 60; higher scores indicate more severe disability. Fatigue was assessed using the Chalder Fatigue Questionnaire (CFQ) (33), a validated measure for assessing CFS in adolescents (34). The 11 CFQ items are scored on a 0–3 Likert scale. The range of the sum total score is from 0 to 33; higher scores indicate more severe fatigue. Sleep disturbances were assessed using the Karolinska Sleep Questionnaire (KSQ) (35). The KSQ sleepiness subscale averages four items addressing sleep problems during the previous month and is scored on a 1–6 Likert scale. The total ranges from 1 to 24 with a lower score indicating poorer sleep. The Pediatric Quality of Life (PedsQL) Inventory combines measures of physical, emotional, social and school functioning to assess the patient’s health-related quality of life (36). The Brief Pain Inventory (BPI) (37) consists of single items addressing different aspects of pain during the past week on 0–10 Likert scales; higher scores represent more severe pain.
Laboratory Analyses
Clonidine steady-state concentration
As previously described, the plasma clonidine concentration was measured 3 and 8 weeks after the initiation of therapy (29). The trough level was estimated as the plasma concentration 12 hours after the last dosage. The mean trough value was taken as an estimate of clonidine steady-state concentration during the intervention period.
Epinephrine and norepinephrine
Urine samples were processed as previously described (30). Briefly, urine was acidified immediately after collection and samples were analyzed using high-performance liquid chromatography (HPLC) with a reversed-phase column and glassy carbon electrochemical detector (Antec, Leyden Deacade II SCC, Zoeterwoude, The Netherlands) using a Chromsystems commercial kit (Chromsystems, München, Germany).
Blood Pressure
As previously described, a Task Force Monitor (TFM) (Model 3040i, CNSystems Medizintechnik, Graz, Austria), with combined hardware and software device for noninvasive recording of cardiovascular variables was used to measure blood pressure. Orthostatic tolerance was estimated as the difference between SBP supine and SBP standing.
Genotyping
Genotyping of the COMT SNP rs4680 was carried out by predesigned TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA), using the SDS 2.2 software (Applied Biosystems). Approximately 10% of the samples were re-genotyped, and the concordance rate was 100%.
Statistical Methods
For primary and secondary outcomes, linear regression models were used to carry out an a priori candidate gene analysis of COMT rs4680 on the outcomes at 8 weeks. Models were adjusted for baseline, age, sex and BMI (38). A per-protocol approach was adopted. The heterozygote genotype (val/met) was used as the reference, and individuals homozygous for the val (val/val) or met (met/met) alleles were assumed to be different from val/met in their response to drug and/or placebo treatment. Interaction terms for genotype by treatment allocation (drug vs. placebo) were included in the model. For each outcome, we tested the interaction of clonidine with COMT genotype using a conservative 2-degree of freedom test, creating multiplicative terms for the treatment by genotype interactions (in 3 categories). To test interactions on the non-linear scale, we used a COMT gene dosage model and included a quadratic term for genotype and the cross-product of treatment arm and the quadratic term. We tested the joint effect of both terms with a 2-degree of freedom test. For ease of interpretation, graphs of the change or difference in each outcome from baseline to 8 weeks of treatment (8 weeks – 0 weeks) jointly stratified by genotype and treatment allocation are presented here. Differences in clonidine levels in individuals randomized to clonidine treatment were analyzed by ANOVA. The normal distributions of all the outcomes or the residuals of week 8 compared to week 0 were confirmed using the Shapiro-Wilk Test.
Using Spearman correlations, we examined the correlation between Steps Taken and five subjective outcome measures at 8-weeks. As this was an exploratory analysis with deliberately correlated outcomes, we did not correct for multiple testing. Hardy–Weinberg Equilibrium (HWE) was calculated using the Online Encyclopedia for Genetic Epidemiology studies (39). Statistical analyses were performed using SAS 9.3 and JMP Pro 12.0.1 (SAS Institute Inc., Cary, NC).
Results
Study Sample
Of the 120 participants included in the trial, data from 104 patients who were genotyped at baseline were available for primary end-point evaluation at week 8. There were no differences between the individuals who completed the trial and those who dropped out by COMT genotype (p=0.4), baseline severity for Steps Taken (p=0.7) or any of the other outcome measures examined. The rs4680 SNP was found to be in Hardy-Weinberg equilibrium (p=0.99), and the minor allele frequency of rs4680 (G or val) was 0.44. Patients did not vary by demographic or baseline measures as function of COMT rs4680 genotype (Table 1). There was no difference in the concentration of clonidine at steady state across the 3 COMT genotypes in the clonidine treatment arm (p=0.5), mean(SD) ug/L: met/met=0.25(0.09), val/met=0.23(0.11), val/val=0.23(0.08).
Table 1.
Baseline demographics and measures of all genotyped patients (N=104) and genotyped patients by COMT rs4680 genotype in the NorCAPITAL trial. Numbers are mean (SD) unless otherwise indicated.
| All | val/val | val/met | met/met | P | |
|---|---|---|---|---|---|
|
Demographics
|
|||||
| N | 104 | 20 | 51 | 33 | |
| Age (years) | 15.4 (1.6) | 15 (1.7) | 15.3 (1.6) | 15.8 (1.4) | 0.16 |
| Female (%) | 72 | 65 | 72 | 76 | 0.70 |
| BMI (kg/m2) | 21.5 (4.1) | 20.3 (4.3) | 22.1 (4.4) | 21.3 (3.5) | 0.24 |
|
| |||||
|
Baseline Measures
|
|||||
| Steps at Week 0 | 4630 (2380) | 4685 (1951) | 4947 (2588) | 4120 (2240) | 0.45 |
| KSQ | 3.7 (0.8) | 3.7 (0.8) | 3.7 (0.9) | 3.5 (0.8) | 0.54 |
| CFQ | 19 (6) | 18 (6) | 20 (6) | 20 (6) | 0.41 |
| FDI | 23 (9) | 20 (8) | 23 (9) | 25 (9) | 0.14 |
| PedsQL | 49 (13) | 53 (12) | 50 (13) | 45 (13) | 0.07 |
| BPI | 4.4 (2.2) | 3.8 (2.6) | 4.2 (2) | 5.1 (2.2) | 0.07 |
| Norepinephrine (nM) | 185 (86) | 159 (80) | 189 (80) | 195 (99) | 0.33 |
| Epinephrine (nM) | 20 (12) | 17 (8) | 22 (14) | 21 (11) | 0.31 |
| SBP supine (mmHg) | 113 (10) | 111 (11) | 114 (10) | 115 (9) | 0.35 |
| SBP standing (mmHg) | 116 (13) | 115 (14) | 115 (14) | 119 (11) | 0.35 |
| DBP supine (mmHg) | 64 (10) | 64 (11) | 64 (11) | 63 (7) | 0.93 |
| DBP standing (mmHg) | 75 (12) | 74 (12) | 75 (12) | 76 (10) | 0.82 |
Abbreviations in alphabetical order: BMI, body mass index; BPI, Brief Pain Inventory; CFQ, Chalder Fatigue Questionnaire; DBP, diastolic blood pressure; FDI, Functional Disability Inventory; KSQ, Karolinska Sleep Questionnaire; PedsQL, Pediatric Quality of Life Inventory; SBP, systolic blood pressure.
Outcomes
Steps Taken
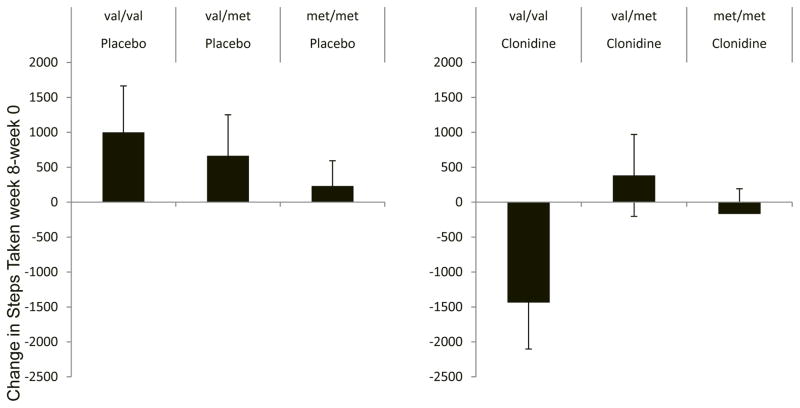
In the unadjusted model of the primary outcome Steps Taken, individuals homozygous for the val allele (val/val) randomly allocated to clonidine took approximately 1,500 fewer steps than baseline, in contrast those allocated to placebo took 1,000 more steps after 8 weeks of treatment (Figure 1A and B). This finding was consistent with a significant val/val by clonidine treatment interaction (beta[SE] = −2026[990]; pinteraction=0.043). In contrast, differences between clonidine and placebo in heterozygote individuals and individuals homozygous for the met allele were non-significant. In the placebo arm, individuals homozygous for the val allele had the greatest placebo response compared to those with at least one met allele (val/met and met/met), but this difference was non-significant (Figure 1A). The results were essentially equivalent when the models were adjusted for age, gender and BMI (beta[SE] = −2215[985]; pinteraction=0.027).
Figure 1.
Differences in the primary outcome Steps Taken between 8-weeks of treatment and baseline as a function of treatment allocation and COMT genotype. pint = p value for the effect estimate of the interaction between val/val and clonidine versus placebo allocation.
Subjective outcomes
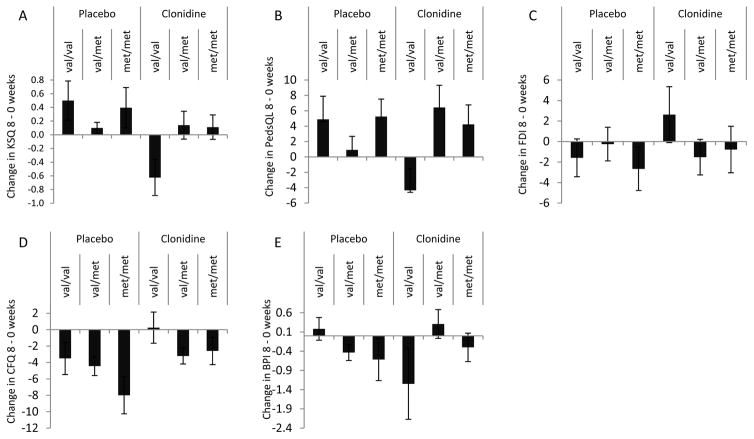
Table 2 shows the Spearman correlations amongst the five 8-week subjective outcomes and Steps Taken. Absolute correlations ranged from 0.11–0.74, suggesting these inventories represent complementary but incompletely overlapping domains, as expected. Consistent with the findings for Steps Taken, the unadjusted model of Karolinska Sleep Questionnaire (KSQ) also revealed a significant difference between val/val individuals treated with clonidine compared to placebo and heterozygous individuals (va/met) (beta[SE]= −1.25[0.42], pint=0.003), suggesting that the sleep quality of val/val adolescents worsened with 8-weeks of clonidine versus placebo treatment (Figure 2A). The similar pattern observed in the unadjusted model for PedsQL (beta[SE] = −14.2[5.9], pint =0.018), further reinforced the findings that val/val individuals had overall worse outcomes in the clonidine treatment arm compared to placebo (Figure 2B). The results were essentially equivalent when the models were adjusted for age, gender and BMI: KSQ (beta[SE] = −1.15[0.40]; pint=0.01) and PedsQL (beta[SE] = −12.1[5.7], pint =0.04). The direction of the COMT val/val by treatment interaction effects for CFQ (beta[SE]= 2.3[3.0], pint=0.43), FDI (beta[SE] = 5.5[4.4], pint=0.22) and BPI (beta[SE] = −0.18[1.00], pint=0.86), (Figure 2C, D and E) were qualitatively consistent with the other three outcomes, but these effects were non-significant. The strong positive response of val/val individuals randomized to placebo treatment was consistent across all the secondary outcomes, whereas there was more variability amongst individuals with at least one met allele.
Table 2.
Correlation between outcomes in the NorCAPITAL Trial at week 8 (R values and p values, unadjusted).
| Steps | KSQ | CFQ | FDI | PedsQL | BPI | |
|---|---|---|---|---|---|---|
| Steps | 1.00 | |||||
|
| ||||||
| KSQ | 0.14 * | 1.00 | ||||
| 0.15 † | ||||||
|
| ||||||
| CFQ | −0.13 | −0.31 | 1.00 | |||
| 0.19 | 0.002 | |||||
|
| ||||||
| FDI | −0.42 | −0.31 | 0.34 | 1.00 | ||
| <0.0001 | 0.001 | 0.001 | ||||
|
| ||||||
| PedsQL | 0.36 | 0.44 | −0.38 | −0.74 | 1.00 | |
| 0.0002 | <0.0001 | <0.0001 | <0.0001 | |||
|
| ||||||
| BPI | 0.11 | −0.34 | 0.19 | 0.36 | 0.42 | 1.00 |
| 0.26 | <0.001 | 0.06 | <0.001 | <0.0001 | ||
Abbreviations in alphabetical order: BPI, Brief Pain Inventory; CFQ, Chalder Fatigue Questionnaire; FDI, Functional Disability Inventory; KSQ, Karolinska Sleep Questionnaire; PedsQL, Pediatric Quality of Life Inventory; Steps, Steps Taken.
Correlation coefficients and
p-values
Figure 2.
Differences in subjective outcome measures between 8-weeks of treatment and baseline as a function of treatment allocation and COMT genotype. (A) Karolinska Sleep Questionnaire (KSQ), (B) Pediatric Quality of Life (PedsQL), (C) Functional Disability Inventory (FDI), (D) Chalder Fatigue Questionnaire (CFQ) and (E) Brief Pain Inventory (BPI). pint = p value for the effect estimate of the interaction between val/val and clonidine versus placebo allocation.
We explored the possibility of non-linear interaction of genotype with treatment allocation across the primary and secondary endpoints. Although we observed no such interaction for CFQ (p=0.4), FDI (p=0.4) or BPI (p=0.7), we found borderline or significant effects for the primary outcome Steps Taken (p=0.06), KSQ (p=0.03), the PedsQL (p=0.07) suggesting that heterozygotes may differ from both val and met homozygotes in their responses to clonidine or placebo.
Catecholamines and orthostatic tolerance
Norepinephrine
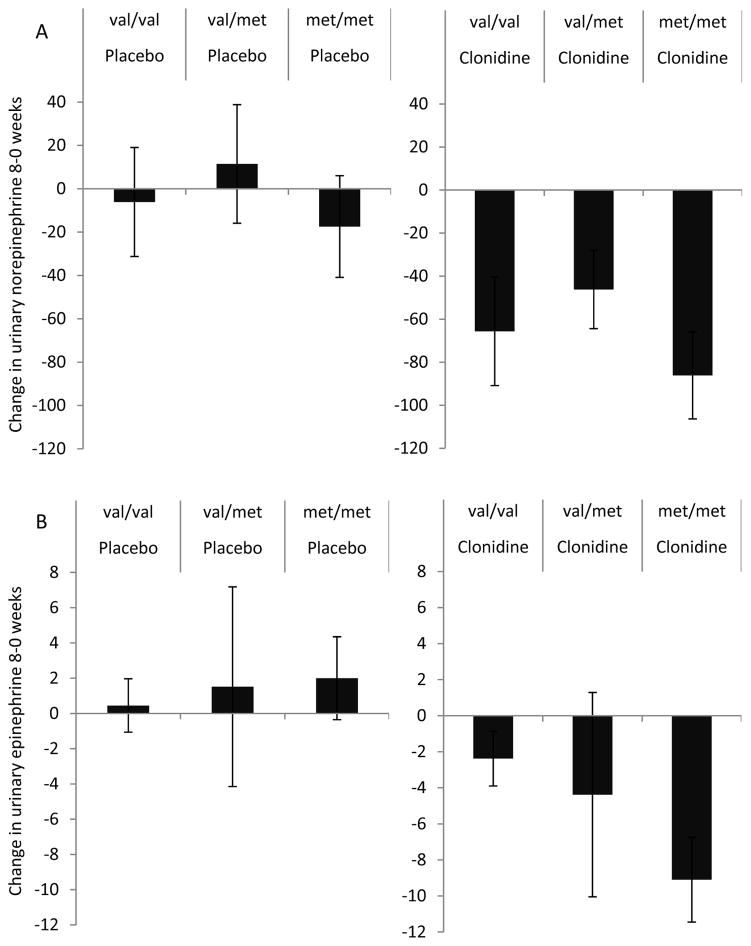
Clonidine treatment effectively reduced norepinephrine levels across all COMT genotypes compared to placebo (beta[SE]= −61[18], p=0.001), (Figure 3A). Val/val individuals randomized to clonidine treatment had the lowest levels of norepinephrine (101±40 nmol) and met/met individuals the highest (126±87 nmol). The val/val by treatment interaction effect was non-significant (beta[SE]= −0.64[47.2], pint=0.99).
Figure 3.
Change from baseline in norepinephrine (A) and epinephrine (B) levels (nM) after 8-weeks of treatment as a function of COMT genotype and treatment allocation. pint = p value for the effect estimate of the interaction between val/val and clonidine versus placebo allocation.
Epinephrine
Clonidine treatment reduced epinephrine levels in all three genotype subsets (beta [SE]= −6.3[2.7], p=0.024) relative to placebo treatment (Figure 3B). Again the val/val by treatment interaction effect was non-significant (beta[SE]= 4.2 [7.2], pint=0.6).
Orthostatic tolerance
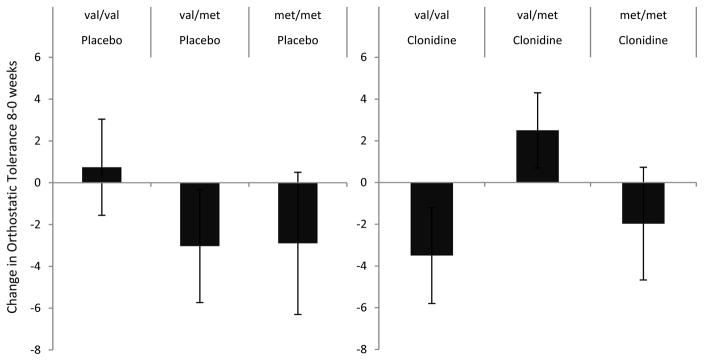
Individuals homozygous for the val allele in the clonidine treatment arm had a decrease in orthostatic tolerance compared to those in the placebo arm (beta[SE]= −11.5[5.0], p=0.02) (Figure 4). The test for a non-linear interaction of genotype with treatment allocation for orthostatic tolerance was significant (p=0.02) suggesting that heterozygotes differed from both val and met homozygotes in their responses to clonidine or placebo. Individuals heterozygous for the val/met alleles had improved orthostatic tolerance, whereas there was no change in individuals homozygous for the met allele in the clonidine arm compared to placebo treatment.
Figure 4.
Change from baseline in orthostatic tolerance after 8-weeks of treatment as a function of treatment allocation and COMT genotype. pint = p value for the effect estimate of the interaction between val/val and clonidine versus placebo allocation.
Discussion
To our knowledge, this is the first report to examine the effect of genetic variation in COMT on clonidine treatment outcomes. Furthermore, this is the first study to explore genetic effects on both drug and placebo responses. Here, we demonstrate that genetic variation at COMT rs4680 modified outcomes in both the clonidine and placebo treatment arms of the NorCAPITAL trial. Specifically with respect to the primary outcome, Steps Taken, individuals homozygous for the COMT rs4680 high-activity val-allele took on average 1,500 fewer steps in the clonidine treatment arm versus 1,000 more steps in the placebo arm after 8-weeks of treatment. In addition, val/val subjective outcomes were consistently inferior in individuals randomized to clonidine treatment and superior in those randomized to placebo, compared to all the other participants across treatment arms and across the broad range of clinical measures assessed. This robust relationship between val/val genotype and poor clonidine versus beneficial placebo outcomes remained after controlling for age, sex and BMI. Taken together these findings suggest that for adolescents with the COMT val/val genotype, treatment with clonidine for CFS would not be recommended, although the possibility of modest benefit among individuals with other genotypes remains open.
As a centrally acting α2-adrenergic receptor agonist, clonidine is broadly used in the treatment of hypertension and often in the treatment of children with ADHD and as a mild sedative (40). In NorCAPITAL, the expectation was that the enhanced sympathetic nervous activity reported in adolescents with CFS (18), and associated higher levels of the catecholamines norepinephrine and epinephrine compared to healthy individuals, would be restored to normal levels by the sympatholytic effects of clonidine. However, despite the reduction in catecholamines observed in individuals treated with clonidine, orthostatic tolerance in individuals homozygous for the val allele worsened. Clonidine induced decrease, in the already lower epinephrine and norepinephrine levels of individuals homozygous for the COMT high activity val-allele, could mechanistically explain its detrimental effects on symptoms of CFS in these individuals. Alternatively, the potent negative effects of clonidine could be attributed to reduced “competition” for the alpha-2 adrenergic receptor by endogenous norepinephrine in individuals homozygous for the COMT high activity val-allele. In contrast, met/met and val/met adolescents did not display this negative effect, perhaps due to their higher baseline sympathetic activity. Thus, these data suggest that high levels of catecholamines might be compensatory rather than disease-promoting in CFS pathophysiology.
The possibility exists that the effects observed here might be attributed to other genetic loci. Clonidine treatment effects in irritable bowel syndrome and hypertension studies were shown to be modified by genetic variation in two other genes involved in adrenergic signaling, the α2A-adrenoreceptor (ADRA2A) (41), and the beta subunit of guanine nucleotide-binding protein (GNBR) (42) which regulates signal transduction between receptors like ADRA2A. Given that COMT is located on chromosome 22 and ADRA2A and GNBR are on chromosomes 10 and 12 respectively, the clonidine effect modification reported here is not likely related to these genes. Our results suggest that a broader examination of the effects of COMT genetic variation on clonidine in other conditions, such as hypertension, pain, or attention deficit hyperactivity disorder is warranted.
Few studies have examined drug-placebo interaction effects (43). Here, in the absence of a no-treatment control, interpretation of the placebo response is limited. Still, it is important to consider the mechanism underlying the consistently robust response of individuals with the val/val genotype randomized to placebo, compared to the negative responses of those randomized to clonidine. Neuroimaging studies have demonstrated that dopamine signaling is activated in response to placebo treatment (44), and there is evidence that genetic variation in COMT modifies individual response to placebo treatment (13, 14). Clonidine has been shown to inhibit dopamine activation in brain regions implicated in placebo response (27, 28); therefore we hypothesized that clonidine might also modify the placebo response. The negative clonidine and positive placebo responses observed in val/val homozygotes in NorCAPITAL, support a potential drug-placebo interaction. Thus, the findings reported here, suggest exploring drug-placebo interactions and their modification by genetic variation may indeed be important to developing precision medicines.
Possibly because of the higher prevalence of the met/met genotype amongst adolescents with CFS (8), val/val subjects may have been slightly underrepresented in this study. The number of val/val subjects in the clonidine treatment arm was particularly small and therefore posed a limitation to this study. Despite this, we found clear and consistent indication for an association between the COMT val allele and reduced benefit or even side effects inherent to treatment with clonidine. By testing for clonidine levels in participants at the end of the trial, we were able to confirm that differences in outcomes were not attributed to differential adherence to treatment. The use of quantitative, physiological and subjective measures in this study make our finding that COMT is associated with clinical outcomes more robust and speak to the broad effects on symptoms and functioning of clonidine treatment in these val/val adolescents.
The findings here add to a growing list of drugs and diseases that are affected by genetic variation in COMT for benefit and potentially harm. This study further demonstrates how genetic analysis can influence clinical trial findings and highlights the important role of pharmacogenetics in gaining insight into treatment response and precision medicine, as well as shedding light on disease mechanisms. Future studies should investigate the generalizability of these findings to other treatments that involve clonidine and other drugs that may likely be influenced by genetic variation in COMT such as drugs that target α- and β-adrenergic receptors or signaling in placebo related pathways.
Acknowledgments
KTH supported by T32AT000051, K01HL130625 and Harvard Catalyst Faculty Fellowship. JK supported by Grant P300P1_158427 from Swiss National Science Foundation and NLM grant # T15LM007092. TJK supported by NIH/NCCIH grant 2K24 AT004095. We thank Valerie Stone, Leigh Simmons and Irving Kirsch for helpful discussions, Kristin Godang for catecholamine analyses and Kari Gjersum for practical assistance.
Footnotes
Conflict of interest statement: The authors disclose no conflicts of interest.
References
- 1.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism: clinical and experimental. 2008;57(5):708–11. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-O-methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. American journal of hypertension. 2011;24(9):1022–6. doi: 10.1038/ajh.2011.93. [DOI] [PubMed] [Google Scholar]
- 3.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 4.Hall KT, Nelson CP, Davis RB, Buring JE, Kirsch I, Mittleman MA, et al. Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34(9):2160–7. doi: 10.1161/ATVBAHA.114.303845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacerda-Pinheiro SF, Pinheiro RF, Junior, Pereira de Lima MA, Lima da Silva CG, do Vieira dos Santos MS, Teixeira AG, Junior, et al. Are there depression and anxiety genetic markers and mutations? A systematic review. Journal of affective disorders. 2014;168:387–98. doi: 10.1016/j.jad.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Tian C, Liu L, Yang X, Wu H, Ouyang Q. The Val158Met polymorphism in the COMT gene is associated with increased cancer risks in Chinese population. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(4):3003–8. doi: 10.1007/s13277-013-1387-6. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, Olson J, Zhang J, Hildebrandt M, Wang L, Ingle J, et al. Breast cancer risk reduction and membrane-bound catechol O-methyltransferase genetic polymorphisms. Cancer research. 2008;68(14):5997–6005. doi: 10.1158/0008-5472.CAN-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommerfeldt L, Portilla H, Jacobsen L, Gjerstad J, Wyller VB. Polymorphisms of adrenergic cardiovascular control genes are associated with adolescent chronic fatigue syndrome. Acta Paediatr. 2011;100(2):293–8. doi: 10.1111/j.1651-2227.2010.02072.x. [DOI] [PubMed] [Google Scholar]
- 9.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(5):1011–20. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 10.Bitsios P, Roussos P. Tolcapone, COMT polymorphisms and pharmacogenomic treatment of schizophrenia. Pharmacogenomics. 2011;12(4):559–66. doi: 10.2217/pgs.10.206. [DOI] [PubMed] [Google Scholar]
- 11.Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biological psychiatry. 2012;71(6):538–44. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biological psychiatry. 2009;66(11):997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, et al. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PloS one. 2012;7(10):e48135. doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Human brain mapping. 2014;35(9):4583–93. doi: 10.1002/hbm.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health RCoPaC, editor. Evidence Based Guidelines for the Management of CFS/ME (Chronic Fatigue Syndrome/Myalgic Encephalopathy) in CHildren and Young Adults. London, England: Royal College of Paediatrics and Child Health; 2004. [Google Scholar]
- 16.Jordan KM, Landis DA, Downey MC, Osterman SL, Thurm AE, Jason LA. Chronic fatigue syndrome in children and adolescents: a review. J Adolesc Health. 1998;22(1):4–18. doi: 10.1016/S1054-139X(97)00212-7. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine (U.S.). Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Institute of Medicine (U.S.) Board on the Health of Select Populations. Beyond myalgic encephalomyelitis/chronic fatigue syndrome : redefining an illness. :xxi, 282. [Google Scholar]
- 18.Wyller VB, Eriksen HR, Malterud K. Can sustained arousal explain the Chronic Fatigue Syndrome? Behav Brain Funct. 2009;5:10. doi: 10.1186/1744-9081-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyller VB, Helland IB. Relationship between autonomic cardiovascular control, case definition, clinical symptoms, and functional disability in adolescent chronic fatigue syndrome: an exploratory study. Biopsychosoc Med. 2013;7(1):5. doi: 10.1186/1751-0759-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghimire LV, Kohli U, Li C, Sofowora GG, Muszkat M, Friedman EA, et al. Catecholamine pathway gene variation is associated with norepinephrine and epinephrine concentrations at rest and after exercise. Pharmacogenet Genomics. 2012;22(4):254–60. doi: 10.1097/FPC.0b013e328350a274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase-Fielitz A, Haase M, Bellomo R, Lambert G, Matalanis G, Story D, et al. Decreased catecholamine degradation associates with shock and kidney injury after cardiac surgery. J Am Soc Nephrol. 2009;20(6):1393–403. doi: 10.1681/ASN.2008080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyller VB, Godang K, Morkrid L, Saul JP, Thaulow E, Walloe L. Abnormal thermoregulatory responses in adolescents with chronic fatigue syndrome: relation to clinical symptoms. Pediatrics. 2007;120(1):e129–37. doi: 10.1542/peds.2006-2759. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PA, De Quattro V, Foti A, Curzon G. Effects of clonidine on central and peripheral nerve tone in primary hypertension. Hypertension. 1986;8(7):611–7. doi: 10.1161/01.hyp.8.7.611. [DOI] [PubMed] [Google Scholar]
- 27.Murai T, Yoshida Y, Koide S, Takada K, Misaki T, Koshikawa N, et al. Clonidine reduces dopamine and increases GABA in the nucleus accumbens: an in vivo microdialysis study. Pharmacology, biochemistry, and behavior. 1998;60(3):695–701. doi: 10.1016/s0091-3057(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 28.Montes DR, Stopper CM, Floresco SB. Noradrenergic modulation of risk/reward decision making. Psychopharmacology. 2015;232(15):2681–96. doi: 10.1007/s00213-015-3904-3. [DOI] [PubMed] [Google Scholar]
- 29.Sulheim D, Fagermoen E, Winger A, Andersen AM, Godang K, Muller F, et al. Disease mechanisms and clonidine treatment in adolescent chronic fatigue syndrome: a combined cross-sectional and randomized clinical trial. JAMA Pediatr. 2014;168(4):351–60. doi: 10.1001/jamapediatrics.2013.4647. [DOI] [PubMed] [Google Scholar]
- 30.Fagermoen E, Sulheim D, Winger A, Andersen A, Gjerstad J, Godan K, et al. Effects of low-dose clonidine on cardiovascular and autonomic variables in adolescents with chronic fatigue: a randomized controlled trial. BMC. 2015 doi: 10.1186/s12887-015-0428-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagermoen E, Sulheim D, Winger A, Andersen AM, Vethe NT, Saul JP, et al. Clonidine in the treatment of adolescent chronic fatigue syndrome: a pilot study for the NorCAPITAL trial. BMC research notes. 2012;5:418. doi: 10.1186/1756-0500-5-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Fukuda S, Mizuno K, Imai-Matsumura K, Jodoi T, Kawatani J, et al. Reliability and validity of the Japanese version of the Chalder Fatigue Scale among youth in Japan. Psychol Rep. 2008;103(3):682–90. doi: 10.2466/pr0.103.3.682-690. [DOI] [PubMed] [Google Scholar]
- 35.Kecklund G, Akerstedt A. The psychometric properties of the Karolinska Sleep Questionnaire. Journal of sleep research. 1992;6:9. [Google Scholar]
- 36.Varni JW, Wilcox KT, Hanson V, Brik R. Chronic musculoskeletal pain and functional status in juvenile rheumatoid arthritis: an empirical model. Pain. 1988;32(1):1–7. doi: 10.1016/0304-3959(88)90016-4. [DOI] [PubMed] [Google Scholar]
- 37.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 38.Saquib N, Saquib J, Ioannidis JP. Practices and impact of primary outcome adjustment in randomized controlled trials: meta-epidemiologic study. Bmj. 2013;347:f4313. doi: 10.1136/bmj.f4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American journal of epidemiology. 2009;169(4):505–14. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesthesia progress. 2015;62(1):31–9. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camilleri M, Busciglio I, Carlson P, McKinzie S, Burton D, Baxter K, et al. Pharmacogenetics of low dose clonidine in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(4):399–410. doi: 10.1111/j.1365-2982.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurnberger J, Dammer S, Mitchell A, Siffert W, Wenzel RR, Gossl M, et al. Effect of the C825T polymorphism of the G protein beta 3 subunit on the systolic blood pressure-lowering effect of clonidine in young, healthy male subjects. Clinical pharmacology and therapeutics. 2003;74(1):53–60. doi: 10.1016/S0009-9236(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 43.Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends in molecular medicine. 2015;21(5):285–94. doi: 10.1016/j.molmed.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray D, Stoessl AJ. Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacology & therapeutics. 2013;140(3):306–18. doi: 10.1016/j.pharmthera.2013.07.009. [DOI] [PubMed] [Google Scholar]