Abstract
Contradictory reports on the effects of diabetes and hyperglycemia on myocardial infarction range from cytotoxicity to cytoprotection. The study was designed to investigate acute effects of high glucose-driven changes in mitochondrial metabolism and osmolarity on adaptive mechanisms and resistance to oxidative stress of isolated rat cardiomyocytes.
We examined the effects of high glucose on several parameters of mitochondrial bioenergetics, including changes in oxygen consumption, mitochondrial membrane potential and NAD(P)H fluorometry. Effects of high glucose on the endogenous cytoprotective mechanisms elicited by anesthetic preconditioning (APC) and the mediators of cell injury were also tested. These experiments included real-time measurements of reactive oxygen species (ROS) production and mitochondrial permeability transition pore (mPTP) opening in single cells by laser scanning fluorescence confocal microscopy, and cell survival assay.
High glucose rapidly enhanced mitochondrial energy metabolism, observed by increase in NAD(P)H fluorescence intensity, oxygen consumption and mitochondrial membrane potential. This substantially elevated production of ROS, accelerated opening of the mPTP and decreased survival of cells exposed to oxidative stress. Abrogation of high glucose-induced mitochondrial hyperpolarization with 2,4 dinitrophenol (DNP) significantly, but not completely, attenuated ROS production to a level similar to hyperosmotic mannitol control. DNP treatment reversed high glucose-induced cytotoxicity to cytoprotection. Hyperosmotic mannitol treatment also induced cytoprotection. High glucose abrogated APC-induced mitochondrial depolarization, delay in mPTP opening and cytoprotection.
In conclusion, high glucose-induced mitochondrial hyperpolarization abolishes APC and augments cell injury. Attenuation of high glucose-induced ROS production by eliminating mitochondrial hyperpolarization protects cardiomyocytes.
Keywords: high glucose, mitochondria, hyperosmolarity, anesthetic preconditioning, mitochondrial permeability transition pore, reactive oxygen species, cardiomyocytes
Introduction
The reports on the effects of diabetes mellitus and accompanying hyperglycemia on myocardial infarction are controversial. While some studies demonstrated increased sensitivity of the diabetic heart to ischemia-reperfusion injury (Paulson, 1997, Aronson et al., 1997), others reported cardioprotective effects of diabetes (Feuvray and Lopaschuk, 1997, Liu et al., 1993). High glucose aggravates ischemia-reperfusion injury and abolishes anesthetic preconditioning (APC) and other types of preconditioning (Di Filippo et al., 2005, Canfield et al., 2012, Baotic et al., 2013). However, it was also reported that high glucose itself can induce preconditioning (Schaffer et al., 2000). Glucose may enter cardiomyocytes independent of insulin by GLUT 12 (Waller et al., 2012), GLUT4, which is translocated to sarcolemma during stress, such as ischemia, and possibly by Na+/glucose cotransporter 1 that is highly expressed in these cells (Sun et al., 1994, Zhou et al., 2003). High glucose enhances production of reactive oxygen species (ROS) by mitochondria and other sources like NAD(P)H oxidase (Shen, 2010, Balteau et al., 2011, Yu et al., 2006). While excessive ROS generation appears critical for reperfusion injury, finite bursts of ROS production can induce preconditioning and protect cells from ischemia-reperfusion injury and oxidative stress (Vanden Hoek et al., 2003, Sepac et al., 2010). Excessive ROS generation triggers opening of the mitochondrial permeability transition pore (mPTP), which rapidly dissipates mitochondrial membrane potential (ΔΨm) initiating death pathways (Weiss et al., 2003).
A moderate decrease of ΔΨm is an important effector of cardioprotection by APC. In previous study, we showed that this partial decrease in ΔΨm attenuated excessive generation of ROS in injured cardiomyocytes that translated into attenuated calcium overload and delayed opening of the mPTP (Sedlic et al., 2010b). Interestingly, addition of pyruvate abolished cardioprotective effects of APC, which was caused by pyruvate-induced increase in ΔΨm. Cardioprotective properties of volatile anesthetics have been recognized by the clinicians and applied in specific clinical settings, which is described in the guidelines by the American Heart Association and the American College of Cardiology (Fleisher et al., 2007).
Using various live cell imaging and mitochondrial bioenergetics techniques, we designed this study to investigate the acute effects of high glucose, alone or in combination with APC, on the resistance to oxidative stress of isolated rat cardiomyocytes. The results indicate that ROS production driven by high glucose elicit opposite effects on the survival of cardiomyocytes, depending on the amount of ROS produced. The study is important as it identifies a mechanism by which high glucose acutely abolishes APC. Another important aspect of the manuscript is that it sheds light on a controversy pertaining to cardiotoxic and cardioprotective effects of hyperglycemia.
Materials and Methods
The use of animals and the experimental protocols in this study were approved by the Institutional Animal Use and Care Committee of the Medical College of Wisconsin, Milwaukee, WI, USA.
Isolation of cardiomyocytes
Ventricular myocytes were isolated from adult male Wistar rats (180–250 g), as we previously described (Sedlic et al., 2009). In brief, animals were anesthetized with an intraperitoneal injection of sodium thiobutabarbital (Inactin, USA; 150 mg/kg), hearts were excised and mounted on modified Langendorff apparatus for retrograde perfusion and enzymatic dissociation of cardiomyocytes with solution containing collagenase and protease. Dissociated cardiomyocytes were stored in the Tyrode’s solution (in mM: 132 NaCl, 10 HEPES, 5 glucose, 5 KCl, 1 CaCl2, 1.2 MgCl2, adjusted to pH of 7.4) and used for experiments within five hours after the isolation. Insulin was not present in the media to obtain a better control of external glucose concentration. All experiments were performed in Tyrode’s solution with or without addition of extra glucose, 2,4 dinitrophenol (DNP), anesthetic, mannitol or fluorescent indicators.
APC and experimental groups
APC was induced by exposing cells to 0.5 mM isoflurane for 20 min, followed by 10 min of isoflurane washout. After anesthetic washout cells were considered preconditioned and subjected to stress in cell survival and mPTP opening experiments or loaded with TMRE for determination of mitochondrial membrane potential. Isoflurane was dissolved in Tyrode’s solution by sonication. It was delivered to a recording chamber by an airtight glass superfusion system and its concentration was verified by gas chromatography. In the control group cells were exposed to 5 mM glucose. Cells were exposed to 20 mM glucose in high glucose group, while hyperosmolarity group included exposure of cells to 5 mM glucose and 15 mM mannitol. In groups APC + high glucose or APC + hyperosmolarity, high glucose or mannitol were started after anesthetic has been removed and were present throughout the rest of the experiment. We decided to apply high glucose after treating cells with anesthetic to avoid interference with the signal transduction cascade of APC and to investigate effects on APC-induced mitochondrial depolarization. DNP was applied simultaneously with high glucose. It is a protonophore that decreases ΔΨm.
Oxygen consumption measurements
Prior to each experiment, the percentage of viable, rod-shaped cardiomyocytes was determined and only solutions containing 70% of such cells were used for the measurement. Non-permeabilized cardiomyocytes suspended in Tyrode’s solution were placed in previously calibrated oxygen electrode (Hansatech Instruments, Norfolk, UK) and the rate of oxygen consumption was recorded at 37°C, as we previously described (Sedlic et al., 2010a). The rate of oxygen consumption was recorded in 5 mM glucose (baseline) and in 20 mM glucose (high glucose). Unlike isolated mitochondria, respiratory states do not strictly exist in intact cells, such as cardiomyocytes tested here. It is considered that intact cell respire somewhere between states 3 and 4 (Brand et al., 2011). Antimycin A (40 µM), was used to inhibit mitochondrial respiratory chain and thereby investigate whether high glucose affects oxygen consumption by the respiratory chain. In this experiment antimycin A was added 5 minutes prior recording cellular respiration and was present throughout the experiment.
NAD(P)H fluorometry
NAD(P)H fluorescence intensity was measured with a laser-scanning confocal microscope (Leica TCS SP5, Mannheim, Germany) to assess changes in NADH concentration in cardiomyocytes, as we previously described (Sedlic et al., 2010a). Fluorescence intensities of NADPH and NADH endogenous fluorophores is proportional to their concentration. NAD(P)H fluorometry is dominated by changes in NADH/NAD+ redox couple (Klingenberg et al., 1959, Estabrook, 1962). In brief, the fluorophore was excited at 730 nm using a two-photon titanium:sapphire laser (SpectraPhysics, Mountain View, USA) and a 8000 Hz resonant scanner. Each data point represents an average of six scans. The excitation power was set at 12.5% of maximum using an AOTF. Photodamage was not observed, which would be indicated by a change in cell behavior or significant fluctuation of fluorescence intensity during prolonged scanning. The emitted light was collected at the 390–490 nm wavelength range. Data were processed with LAS AF software (Leica) and normalized to baseline. After establishing baseline NAD(P)H fluorescence intensity in non-permeabilized cardiomyocytes placed in control glucose, cells were exposed to high glucose. The average fluorescence intensities of baseline and of plateau observed following glucose application were analyzed statistically.
Measurements of ΔΨm
ΔΨm was assessed in intact cardiomyocytes using the laser-scanning confocal microscope (Eclipse TE2000-U; Nikon, Tokyo, Japan) and the fluorescence indicator tetramethylrhodamine ethyl ester (TMRE, 100 nM, Molecular Probes, USA), as we previously described (Sedlic et al., 2010b). Excitation/emission wavelengths, λex/λem, for TMRE were 543/560–610 nm, respectively. TMRE was loaded for 20 min, followed by dye washout and dana acquisition. Data were analyzed with MetaMorph 6.1 software (Universal Imaging, USA). High glucose, DNP or mannitol were present during the dye loading and throughout recording. In groups with APC, TMRE was loaded into cells after the application of isoflurane for 20 min and its washout for 10 min.
Measurements of ROS production
ROS production in cardiomyocytes was analyzed using the confocal microscope and the fluorescence indicator 5-(and- 6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Molecular Probes) as we previously described (Sedlic et al., 2009). In brief, the 2 µM of the dye was loaded into the cells for 20 min. The λex/λem was 488/500–550 nm wavelength, respectively. Data were analyzed with MetaMorph 6.1 software. The average fluorescence intensity of baseline was compared to the last data point of each treatment for statistical analyses.
mPTP opening experiments
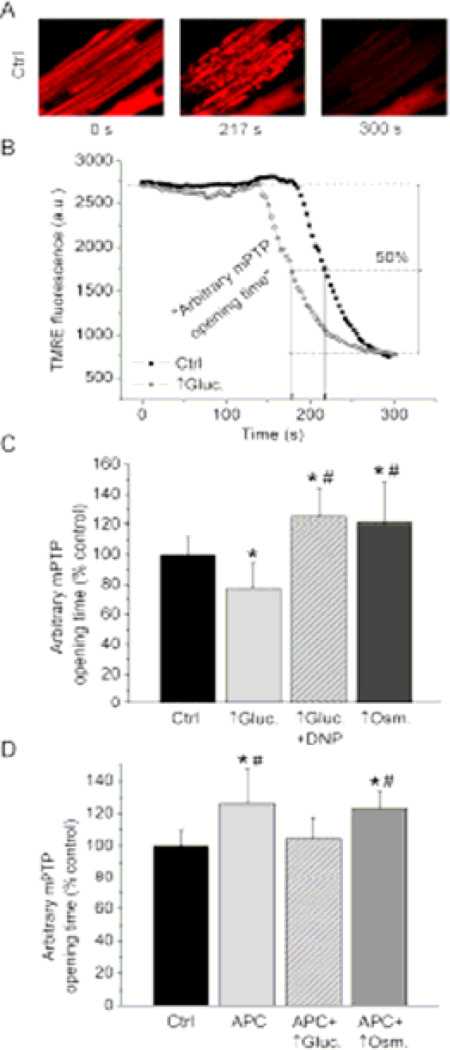
mPTP opening in isolated cardiomyocytes was recorded following our previously published protocol using the confocal microscope (Sedlic et al., 2010b). In brief, strictly controlled photoexcitation of TMRE loaded into cells produced oxidative stress within the region of interest (30 × 30 µm) that triggered mPTP opening. Rapid and complete dissipation of ΔΨm indicates mPTP opening, which is sensitive to mPTP inhibitors and coincides with a calcein release, as we previously demonstrated (Pravdic et al., 2009). The time point at which TMRE fluorescence intensity decreased 50% between baseline and residual value, termed “arbitrary mPTP opening time”, was compared among experimental groups (Figure 4 B). The TMRE was loaded into the cells similar to the protocol for the measurements of ΔΨm, with a slight adjustment of TMRE loading time. This change enabled equal delivery of TMRE at the end of dye loading in all experimental groups, despite differences in ΔΨm. Only the cells with equal TMRE fluorescence intensity were included in the study.
Figure 4. High glucose differentially affects mPTP opening depending on the ΔΨm, and blocks effects of APC.
(A) Representative confocal images of cardiomyocytes loaded with ΔΨm-sensitive fluorophore TMRE. Oxidative stress-induced opening of mPTP was monitored by occurrence of rapid ΔΨm dissipation. (B) Representative signal traces and arbitrary mPTP opening time used for statistical analyses. (C) Summarized data are means ± SD. Compared to control (Ctrl.), high glucose (↑Gluc.) decreased arbitrary mPTP opening time. Combination of high glucose and DNP, and hyperosmolarity (↑Osm.) delayed mPTP opening compared to Ctrl. *P < 0.05 vs. control (Ctrl.); #P < 0.05 vs. ↑Gluc. (D) Summarized data show that APC increased arbitrary mPTP opening time compared to Ctrl, while addition of ↑Gluc. abrogated this effect. ↑Osm. had no effect on APC-induced delay in mPTP opening. *P < 0.05 vs. control (Ctrl.); #P < 0.05 vs. APC + ↑Gluc.
Cardiomyocyte survival experiments
Sensitivity of cardiomyocytes to oxidative stress was tested with the cell survival experiment, as we previously described (Sedlic et al., 2009). Oxidative stress was induced by application of 250 µM of H2O2 for 30 min followed by 15 min H2O2 washout. The number of live cells (rod-shaped cells without membrane blebs that excluded Trypan blue) was determined at the beginning and at the end of the experimental protocol. Cell death was normalized to control. APC was induced prior to treatment with H2O2, while high glucose, DNP or mannitol were present during H2O2 exposure and second cell count.
Statistical analyses
Data are presented as means ± SD, where n indicates number of experiments. Comparisons were performed with one-way or repeated-measures analysis of variance with pair-vise comparisons against control group with Tukey post hoc test for experiments presented in Figures 2–5. or paired samples t-test for data presented in Figure 1. Differences at P < 0.05 were considered significant.
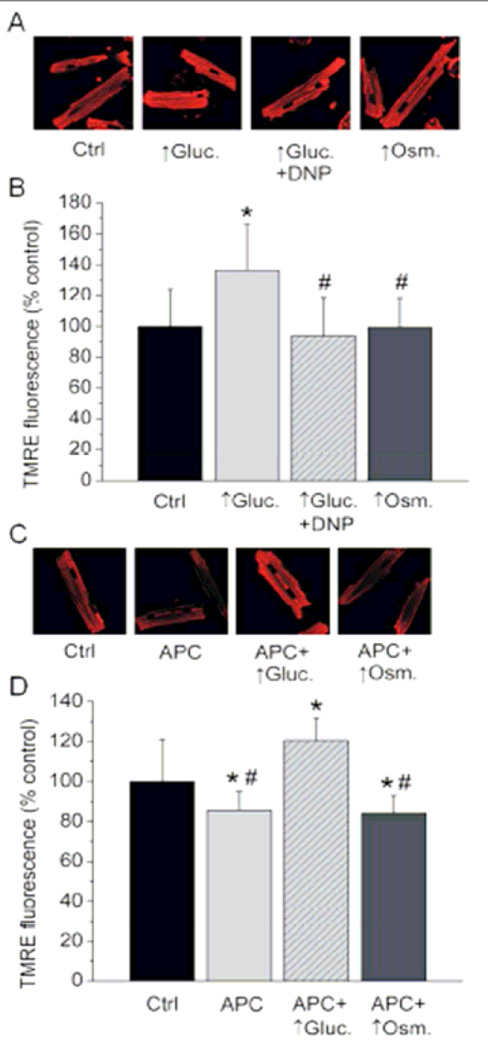
Figure 2. High glucose hyperpolarizes mitochondria and overrides APC-induced mitochondrial depolarization.
(A&C) Representative confocal images of cardiomyocytes loaded with ΔΨm-sensitive fluorophore TMRE. (B) Compared to control (Ctrl), treatment with high glucose (↑Gluc.) increased TMRE fluorescence intensity indicating an increase in ΔΨm. DNP reversed mitochondrial hyperpolarization caused by ↑Gluc. Application of hyperosmotic solution (↑Osm.) did not alter mitochondrial membrane potential compared to control. Summarized data are means ± SD. *P<0.05 vs. Ctrl; #P < 0.05 vs. ↑Gluc. (D) Summarized dana (means ± SD) show that APC-induced mitochondrial depolarization is reversed by ↑Gluc. And not affected by ↑Osm. *P < 0.05 vs. control (Ctrl.); #P < 0.05 vs. APC + ↑Gluc.
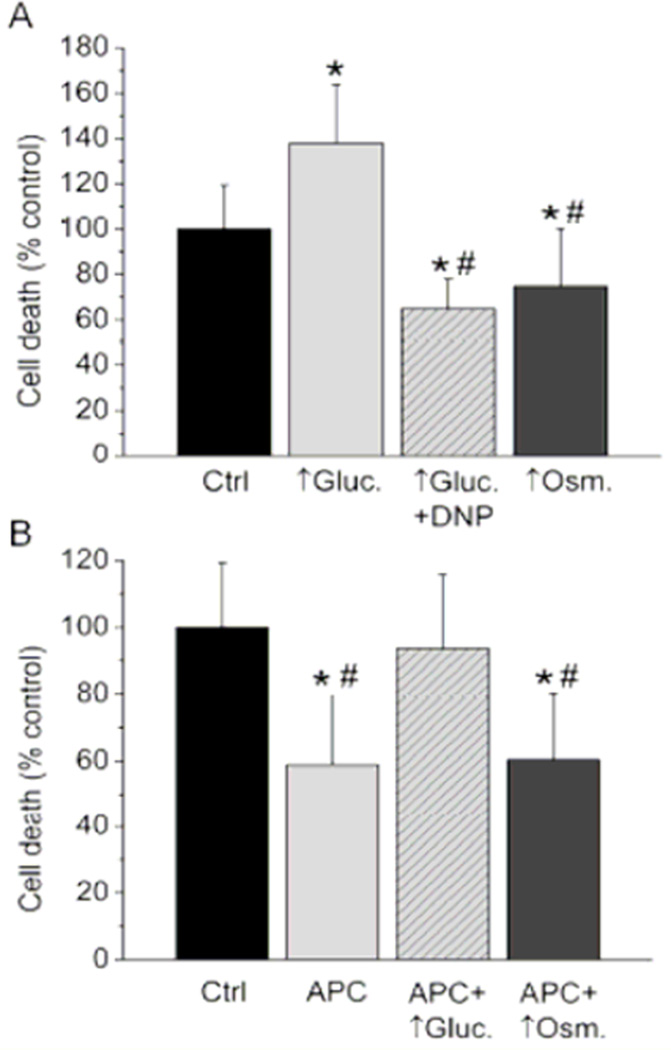
Figure 5. High glucose differentially affects cell survival depending on the ΔΨm, and blocks APC.
(A) Oxidative stress-induced cell death was significantly greater in high glucose (↑Gluc.) than in control (Ctrl.). Addition of DNP reversed this effect to cytoprotection, which was also observed in hyperosmotic group (↑Osm.). Data are means ± SD. *P < 0.05 vs. Ctrl; #P < 0.05 vs. ↑Gluc. (B) Compared to control, APC attenuated cell death, but failed to do so in ↑Gluc. ↑Osm. did not abolish APC-induced cytoprotection. *P < 0.05 vs. Ctrl; #P < 0.05 vs. APC + ↑Gluc.
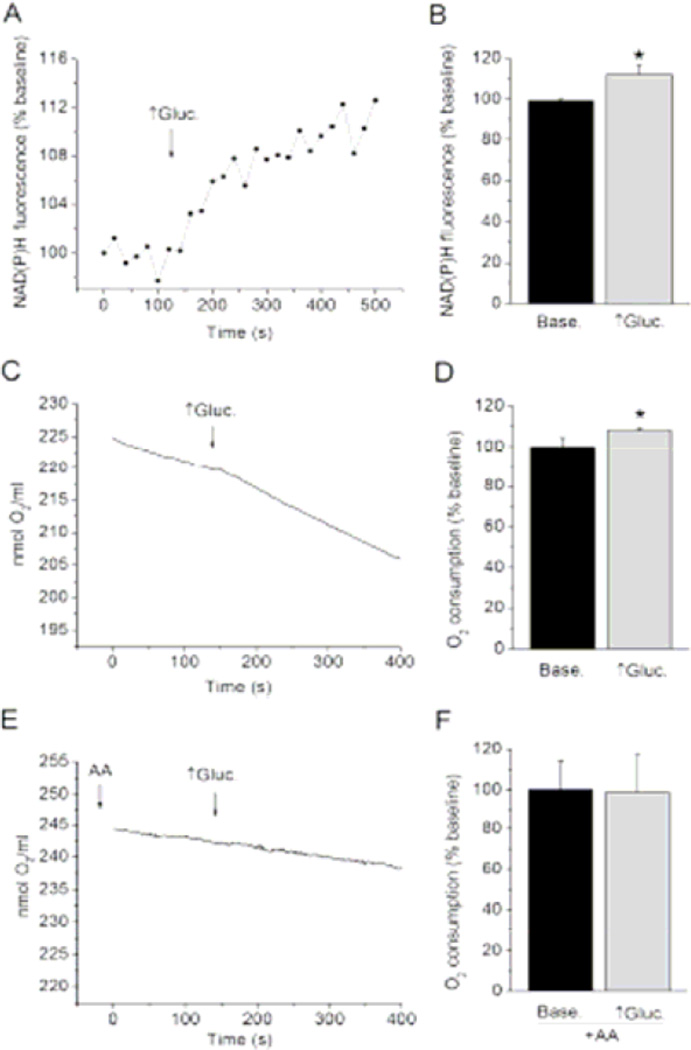
Figure 1. High glucose accelerates mitochondrial metabolism in isolated cardiomyocytes.
(A) NAD(P)H fluorometry was used to assess changes in NADH concentration in cardiomyocytes. Representative signal trace shows a rapid increase in signal following transition from control to high glucose (↑Gluc.; marked with an arrow) indicating increase in cellular NADH. (B) Summarized data of NAD(P)H fluorescence intensity before and after exposure to high glucose. (C) Oxygen consumption measurements. Representative signal trace shows a rapid increase in the rate of oxygen consumption by isolated cardiomyocytes following transition from control to high glucose (arrow). (D) Summarized data of average oxygen consumption rates in control and high glucose. (E) Oxygen consumption measurements. Representative signal trace shows that addition of antimycin A prevents high glucose induced-increase in oxygen consumption. (F) Summarized data of average oxygen consumption rates before and after high glucose in the presence of AA. Data are means ± SD. P < 0.05 vs. baseline (Base.)
Results
High glucose fuels mitochondria in isolated cardiomyocytes
Changes in NADH levels by NAD(P)H fluorometry and mitochondrial respiration by oxygen consumption measurements in isolated cardiomyocytes were undertaken to verify that glucose enters cardiomyocytes and is metabolized in the absence of insulin. It can be observed in Figure 1 C that isolated cardiomyocytes consume oxygen in baseline conditions when they are supplied only with 5 mM glucose as substrate. A switch from 5 (control) to 20 mM (high) glucose induced rapid and significant increase in NAD(P)H fluorescence intensity in isolated cardiomyocytes, suggesting an increase in cellular NADH (Figure 1 A, B). The same increase in glucose concentration acutely and significantly elevated the rate of oxygen consumption by isolated cardiomyocytes (Figure 1 C, D). However, 20 mM glucose did not increase the rate of cellular oxygen consumption in the presence of antimycin A, indicating that increase in oxygen consumption by high glucose involved mitochondrial respiratory chain (Figure 1 E, F). All these results suggest that excess glucose is taken up by cardiomyocytes, fueling mitochondrial metabolism and increasing electron flux along the respiratory chain.
High glucose increases ΔΨm and abolishes APC-induced decrease in ΔΨm
Next we tested whether fueling mitochondria with excess glucose increases ΔΨm. It can be observed in Figure 2 A, B that TMRE fluorescence intensity was significantly greater in cells exposed to high glucose (136.3 ± 29.5% of control; n = 14) then in control cells (100 ± 24.0%; n=14). This indicates that high glucose increases ΔΨm in isolated cardiomyocytes. Hyperosmotic mannitol solution was used to mimic hyperosmolarity exerted by high glucose. Compared to control, hyperosmolarity did not alter significantly TMRE fluorescence intensity (99.4 ± 19.0% of control, n = 14). Application of mitochondrial uncoupling agent 2,4 dintrophenol (DNP; 1 µM) together with the high glucose reversed ΔΨm to control levels (93.8 ± 24.8% of control; n = 14). Thus, the DNP treatment and the hyperosmotic control allowed us to specifically examine and distinguish effects of mitochondrial hyperpolarization and hyperosmolarity induced by high glucose.
We also tested whether high glucose-induced increase in ΔΨm abolishes APC-induced mitochondrial depolarization, a crucial effector of cardioprotection by APC. Compared to control cardiomyocytes exhibiting TMRE fluorescence intensity of 100 ± 21.2% (n = 13), cells treated with APC showed a significant decrease in TMRE fluorescence intensity of 85.5 ± 9.4% of control (n = 13), indicating partial mitochondrial depolarization (Figure 2 C, D). Cells treated with APC and exposed to high glucose after anesthetic removal exhibited a significant increase in TMRE fluorescence intensity to 120.5 ± 11.2% of control (n = 13). This indicated that hyperpolarizing effect of high glucose on mitochondria abolished APC-induced mitochondrial depolarization. Hyperosmotic solution applied after APC did not alter APC-induced mitochondrial depolarization, as TMRE fluorescence intensity in this group was 84.2 ± 8.7% of control (n = 13), which was similar to the effect of APC alone. These experiments indicate that acute high glucose abolishes APC-induced mitochondrial depolarization by directly increasing ΔΨm.
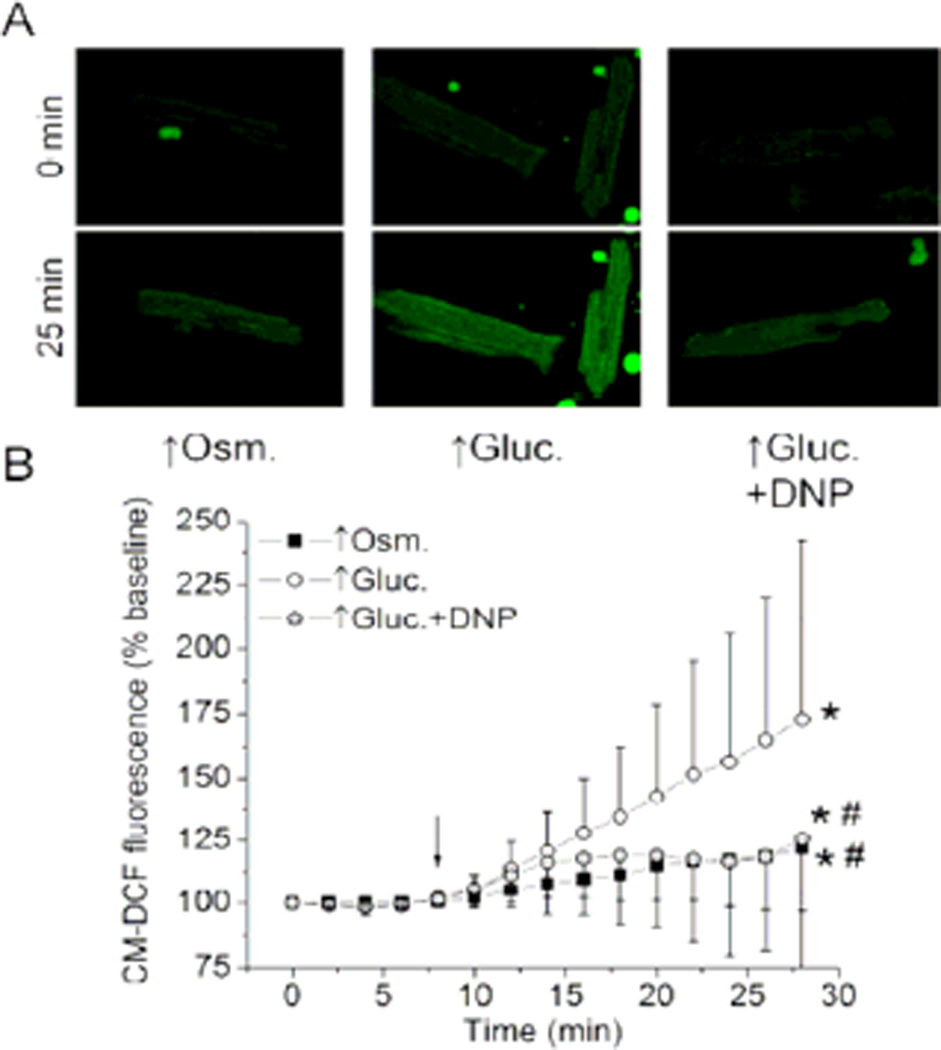
High glucose enhances ROS formation by increasing ΔΨm and osmolarity
Since mitochondria generate more ROS at higher ΔΨm, we tested whether high glucose-induced mitochondrial hyperpolarization enhances ROS production in cardiomyocytes (Lambert and Brand, 2004, Starkov and Fiskum, 2003). Superfusion with high glucose significantly increased CM-DCF fluorescence intensity to 172.8 ± 69.4% of baseline (n = 17), indicating a substantial increase in ROS formation (Figure 3). Addition of DNP to the high glucose solution significantly attenuated the increase in CM-DCF fluorescence to 125.6 ± 57.7% of baseline (n = 14), which was still significantly greater than the baseline. Interestingly, superfusion with the hyperosmotic solution significantly increased CM-DCF fluorescence intensity to 121.9 ± 25.1% of baseline (n = 15). Quantitatively, this increase in ROS production was similar to the increase produced by high glucose + DNP. This suggests that high glucose acutely induces ROS production in lesser part by increasing osmolarity and in greater part by increasing ΔΨm.
Figure 3. High glucose enhances production of ROS in ΔΨm- and osmolaritydependent manner.
(A) Representative confocal images of cardiomyocytes where CMDCF fluorescence intensity was used to monitor ROS production. (B) Summarized data (means ± SD) represent time course of changes in CM-DCF fluorescence intensity before and during (arrow) the specific treatment. Application of high glucose (↑Gluc.) substantially increased ROS production compared to baseline, which was attenuated in the presence of DNP. Mannitol solution with identical osmolarity as high glucose solution (↑Osm.) increased ROS production, but to a lesser extent than the ↑Gluc alone. *P < 0.05 vs. baseline; #P < 0.05 vs. ↑Gluc.
Hyperosmolarity by high glucose delays mPTP opening while accompanying increase in ΔΨm accelerates it and abolishes effects of APC
Since excessive ROS production can induce mPTP opening, we examined effects of high glucose on mPTP opening. Oxidative stress caused abrupt dissipation of ΔΨm in mitochondria within the selected region of the cardiomyocyte, indicating mPTP opening (Figure 4 A, B). Arbitrary mPTP opening time was reduced in high glucose group, 77.4 ± 16.7% of control (n = 14), compared to control cells (100.0 ± 11.9%, n = 14), which is a decrease by 23% (Figure 4 C). This indicated accelerated opening of mPTP in cardiomyocytes acutely exposed to high glucose. Conversely, combination of high glucose and DNP, and hyperosmolarity by mannitol exhibited a significant increase in arbitrary mPTP opening time to 125.5 ±19.1% of control (n = 13) and 120.4 ± 28.0% of control (n = 14), respectively. This suggests that hyperpolarization of mitochondria by high glucose accelerates mPTP opening, while hyperosmolarity alone (high glucose + DNP or mannitol) exhibits cytoprotective delay in mPTP opening.
We also tested the effects of high glucose on APC-induced delay in mPTP. Arbitrary mPTP opening time was greater in APC group (126.3 ± 21.0% of control, n = 11) that in control (100.0 ± 9.6%, n = 11), which was statistically significant (Figure 4 D). Addition of high glucose following anesthetic removal (APC + ↑Gluc.) abrogated the increase of arbitrary mPTP opening time (104.7 ± 12.8% of control, n = 11). Hyperosmotic solution did not attenuate APC-induced increase in mPTP opening time (123.4 ± 10.3% of control, n = 11), which was significantly more than in control. These results indicate that high glucose blocked APC by abolishing APC-induced partial mitochondrial depolarization.
Hyperosmolarity by high glucose decreases cardiomyocyte death while increase in ΔΨm enhances it and abolishes APC
Cell survival experiments were conducted to investigate whether shifts in mPTP opening time translate into changes in cardiomyocyte resistance to oxidative stress. Compared to control, where cell death was normalized to 100.0 ± 19.4% (n = 11), the cell death in high glucose group significantly increased to 137.9 ± 26.0% of control (n = 11) (Figure 5 A). Similar to mPTP opening experiments, application of DNP together with high glucose reversed the effect and significantly decreased the cell death to 64.9 ± 13.1% of control (n = 11). Again, significant cytoprotection was also observed in hyperosmolarity group where cell death was 74.9 ± 25.3% of control (n = 11). These results are in correlation with the mPTP experiments, showing that mitochondrial hyperpolarization by high glucose exacerbates cell injury during oxidative stress, while hyperosmolarity alone has opposite, protective effects.
Effects of high glucose on cytoprotection by APC were also tested by cell survival experiments. Compared to control, where normalized cell death induced by H2O2 was 100.0 ± 19.3% (n = 7), APC protected cells and significantly reduced cell death to 59.0 ± 20.5% of control (n = 7) as shown in Figure 5 B. In the presence of high glucose, applied following APC the cell death was 93.8 ± 22.0% of control (n = 7), which was significantly greater than in APC group alone indicating the loss of cytoprotection (Figure 5 B). Hyperosmolarity by mannitol did not alter cytoprotection by APC and the cell death in that group was 60.5 ± 19.6% of control (n = 7), which was significantly less than in control. This correlates well with the observation that high glucose abrogates APC-induced delay in mPTP opening by overriding APC-induced mitochondrial depolarization.
Discussion
Data presented here show that high glucose was rapidly metabolized in isolated rat cardiomyocytes, which stimulated generation of NADH and mitochondrial respiration, and led to an increase in ΔΨm. DNP annulled mitochondrial hyperpolarization induced by high glucose. High glucose acutely enhanced ROS generation, which was partly lowered with DNP to a level similar to hyperosmotic mannitol solution. APC-induced mitochondrial depolarization was abolished by application of high glucose, but not by hyperosmotic solution. High glucose alone accelerated mPTP opening and decreased survival of stressed cells. Completely opposite effects of high glucose were obtained with co-application of DNP. mPTP opening was delayed and cell survival was greater than in control, which was also observed with hyperosmotic solution. High glucose, but not hyperosmotic solution, also blocked APC-induced delay in mPTP opening and increased cell survival.
Contradictory reports on association between diabetes and hyperglycemia on one hand and myocardial infarction on the other may suggest existence of complex and opposing processes in the pathogenesis. Our previous work showed that hyperglycemia abrogated cardioprotective pathways elicited by APC (Baotic et al., 2013, Muravyeva M et al., 2014) and increased myocardial infarction (Muravyeva M et al., 2014). Treatment with antioxidant N-acetylcisteine abrogated deleterious effects of hyperglycemia on myocardial infarction, indicating involvement of oxidative stress (Muravyeva M et al., 2014). This is in agreement with other reports showing that high glucose blocks preconditioning and directly augments cell injury (Canfield et al., 2012, Schenning et al., 2015, Zhong et al., 2015). Conversely, a study showed that high glucose induced preconditioning of isolated neonatal cardiomyocytes, while other study demonstrated that diabetic and hyperglycemic rats were protected from myocardial infarction (Pastukh et al., 2005). Our previous study showed that pyruvate may also protect cardiomyocytes from oxidative stress (Sedlic et al., 2010b). Results from clinical trials are also contradictory, ranging from increased to decreased sensitivity of diabetic heart to ischemia-reperfusion injury (Paulson, 1997). Data from the ACCORD trial show that diabetic patients receiving standard antidiabetic treatment exhibited lower risk of all-cause mortality when their HbA1c was slightly greater than normal, suggesting protective effects of mild hyperglycemia (Riddle et al., 2010). While in diabetic subjects myocardial infarction is not solely affected by hyperglycemia, but also by other accompanying disorders like dyslipidemia, in vitro data from isolated cells confer that high glucose may exert opposite effects (Lee et al., 2012). Such opposing results may indicate dose-dependent, non-linear response to high glucose as discussed below.
Data presented here offer potential explanation for this controversy, at least in part, suggesting that the amount of ROS generated by high glucose treatment dictates the fate of stressed cardiomyocytes. Excessive production of ROS resulting from combined effects of hyperosmolarity and mitochondrial hyperpolarization by high glucose increased cardiomyocyte injury. Conversely, attenuation of high glucose-induced ROS production, by eliminating ROS stemming from mitochondrial hyperpolarization, decreased cell injury. It has been shown that ROS production steeply increases with increase in ΔΨm (Starkov and Fiskum, 2003, Lambert and Brand, 2004). This occurs presumably due to impeded proton pumping and obstructed electron flow, which favors electron “leak” and incomplete oxygen reduction. Therefore, even subtle elevation of oxygen consumption (electron flow along respiratory chain) combined with more pronounced increase in mitochondrial membrane potential by acute high glucose may have substantial effect on ROS production, especially in stressed cells. Our result support these observations as they show that the reversal of ΔΨm to normal by DNP acutely reduced ROS production by high glucose.
Here we also showed that high glucose rapidly increased NAD(P)H fluorescence intensity and increased the rate of oxygen consumption in cardiomyocytes. Increased NAD(P)H production following high glucose may reflect several events in the cell. However, in the context of other results obtained in response to high glucose, increased mitochondrial membrane potential and increased oxygen consumption by the respiratory chain, NAD(P)H fluorometry results are indicating, at least in part, increased oxidation of excess glucose and generation of NADH in mitochondria. This does not exclude occurrence of increased generation of NADH in glycolysis, which probably also takes place. In addition, our previous studies characterized changes in NAD(P)H and flavoprotein fluorescence after several perturbations of electron pathways within the respiratory chain and showed that the redox fluorometry is highly sensitive to either increased or decreased oxidation of substrates (Sedlic et al., 2010a, Sedlic et al., 2010b). Thus, a rapid metabolism of excess glucose likely provides extra substrates for mitochondria, increasing activity of respiratory chain and the ΔΨm. In the presence of antimycin A high glucose failed to increase oxygen consumption by cardiomyocytes. This demonstrates that increase in oxygen consumption occurred at the respiratory chain, reflecting increased oxidation of glucose and electron flux along the respiratory chain.
It is well established that the majority of glucose extracted from the blood by beating heart is stored as glycogen. However, it has been demonstrated that acute hyperglycemia substantially increases cardiac glucose extraction and oxidation (Wisneski et al., 1990). This study employing healthy human subjects showed that the oxidation of glucose increased from 9% of extracted glucose in euglycemic state to 34% of extracted glucose in acute hyperglycemic state when glucose concentration increased twofold. At the same time, glycogen deposition presumably decreased from 78% to 57% of extracted glucose. A study using isolated hearts of transgenic mice with cardiac-specific overexpression of the insulin-independent glucose transporter GLUT1, showed that these hearts exhibit increased glucose uptake and oxidation (Yan et al., 2009). These studies are in agreement with our results that a significant proportion of excess glucose is acutely oxidized by cardiac mitochondria.
A recent study further supports our findings by showing that uncoupling protein-2, which mimics the effects of DNP, attenuates high glucose-induced ROS production in human endothelial cells (Koziel et al., 2015). We and others previously showed that low concentrations of uncoupling agents, such as DNP or FCCP, may induce cardioprotection when applied alone (Sedlic et al., 2010b, Brennan et al., 2006). Increased CM-DCF fluorescence intensity following high glucose exposure reflects increased bioavailability of ROS, stemming either from increased production or decreased elimination. However, considering that a small concentration of uncoupling agent DNP substantially attenuated ROS production and that glucose significantly increased oxygen consumption and mitochondrial membrane potential, it can be concluded that increased CM-DCF fluorescence intensity reports increased mitochondrial production of ROS by high glucose.
The DNP treatment and the hyperosmotic control allowed us to specifically examine and distinguish effects of mitochondrial hyperpolarization and hyperosmolarity induced by high glucose. The proportion of elevated ROS production unaffected by DNP most likely resulted from hyperosmolarity induced by high glucose. This is supported by observation that hyperosmotic mannitol treatment, induced quantitatively similar increase in ROS production. Hyperosmotic solution can increase ROS production by activating NADPH oxidase, which was shown to be overactive in diabetes (Ikari et al., 2013, Gray et al., 2013, Avogaro et al., 2003). A moderate increase in ROS production by high glucose, after annulling contribution from the mitochondrial hyperpolarization, may activate cytoprotective pathways (Kevin et al., 2003). This is corroborated by our and previous reports that application of small amounts of H2O2 elicits preconditioning (Furuichi et al., 2005, Sepac et al., 2010). ROS may activate at least two important mediators of cytoprotection, protein kinase C and hypoxia inducible factor 1α, which recruit effectors of cardioprotection, including those that inhibit mPTP opening (Pravdic et al., 2009, Finkel, 2011, Otani, 2004, Ke and Costa, 2006). The observation that quantitatively similar increase in ROS production by hyperosmotic solution and high glucose + DNP treatments translated into quantitatively similar delay in mPTP opening and reduction of cell death further supports importance of cytoprotective signaling by ROS.
Attenuation of excessive ROS production in stressed cardiomyocytes by partial mitochondrial depolarization, as an adaptive response triggered by APC, is demonstrated in our previous study (Sedlic et al., 2010b). The rate of ROS overproduction in stressed cells correlated with mPTP opening time and the extent of cell death in that study. mPTP opening initiates death pathways by releasing pro-apoptotic factors, calcium or by halting ATP production due to rapid loss of ΔΨm (Weiss et al., 2003, Hausenloy et al., 2003, Halestrap et al., 2004, Hausenloy et al., 2009). Attenuation of key triggers of mPTP opening, ROS and mitochondrial calcium overload, delays mPTP opening which translates into improved survival of cardiomyocytes in in vivo and in vitro experiments (Skyschally et al., 2010, Sedlic et al., 2010b). This is in agreement with the results presented here that the excessive ROS production by high glucose treatment accelerated mPTP opening and reduced cell death, which was not observed when ROS production was reduced by DNP. Moreover, high glucose-induced mitochondrial hyperpolarization overrode APC-induced mitochondrial depolarization and abrogated mPTP opening delay and cytoprotection by APC. This is in line with our previous study that showed similar negative effects of pyruvate on APC (Sedlic et al., 2010b).
Cardioprotection by volatile anesthetics is applied in clinical medicine (Fleisher et al., 2007). High glucose may impair APC by several mechanisms, including dysregulation of nitric oxide production (Cheng et al., 2011, Vladic et al., 2011, Baotic et al., 2013). This study was designed to apply high glucose after treating cells with anesthetic to avoid interference with the signal transduction cascade of APC and to focus on the mitochondrial depolarization as one of the effectors of cardioprotection. Relatively brief exposure to high glucose was also selected to delineate acute effects of high glucose that likely differ from the chronic effects. Thus, our study indicates importance of studying acute and intermittent increases in glycemia in diabetic patients, since these may be particularly detrimental due to substantial increase in ROS production, as opposed to chronic hyperglycemia that may decrease glucose oxidation (Aas et al., 2011). Although DNP is not suitable for clinical use, development of safer agents aimed at preventing mitochondrial hyperpolarization may be beneficial additional therapy for hyperglycemic conditions.
In conclusion, our results suggest that controversies regarding the effects of hyperglycemia on the cardiac ischemic injury are, at least in part, related to the extent of ROS production induced by high glucose. Excessive ROS production driven by combined effects of high ΔΨm and hyperosmolarity acutely exacerbates cell injury. Moderate ROS production driven only by hyperosmolarity, with abrogated mitochondrial hyperpolarization, triggers adaptive response and protects cardiomyocytes. High glucoseinduced increase in ΔΨm overrides APC-induced mitochondrial depolarization and blocks cytoprotection. Targeted modification of high ΔΨm in hyperglycemic conditions may not only prevent cardiotoxic effects of high glucose, but also protect the heart.
Acknowledgments
Contract grant sponsor: National Institutes of Health (Bethesda, Maryland, USA); contract grant numbers: P01GM066730 and R01HL034708; Contract grant sponsor: Operational Programme Human Resources Development, European Social Fund (EU) contract grant number HR.3.2.01-0182.
Footnotes
This work has been performed at the Department of Anesthesiology, Medical College of Wisconsin, Milwaukee Wisconsin, USA.
Authors declare no conflict of interest.
References
- AaS V, Hessvik NP, Wettergreen M, Hvammen AW, Hallen S, Thoresen GH, Rustan AC. Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes. Biochim Biophys Acta. 2011;1812:94–105. doi: 10.1016/j.bbadis.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Pagnin E, Calo L. Monocyte NADPH oxidase subunit p22(phox) and inducible hemeoxygenase-1 gene expressions are increased in type II diabetic patients: relationship with oxidative stress. J Clin Endocrinol Metab. 2003;88:1753–1759. doi: 10.1210/jc.2002-021025. [DOI] [PubMed] [Google Scholar]
- Balteau M, Tajeddine N, de Meester C, Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S, Vanoverschelde JL, Gailly P, Hue L, Bertrand L, Beauloye C. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res. 2011;92:237–246. doi: 10.1093/cvr/cvr230. [DOI] [PubMed] [Google Scholar]
- Baotic I, Ge ZD, Sedlic F, Coon A, Weihrauch D, Warltier DC, Kersten JR. Apolipoprotein A-1 mimetic D-4F enhances isoflurane-induced eNOS signaling and cardioprotection during acute hyperglycemia. Am J Physiol Heart Circ Physiol. 2013;305:H219–H227. doi: 10.1152/ajpheart.00850.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JP, Berry RG, BaghaiI M, Duchen MR, Shattock MJ. FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarisation. Cardiovasc Res. 2006;72:322–330. doi: 10.1016/j.cardiores.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai X, Bosnjak ZJ. Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cellderived cardiomyocytes. Anesthesiology. 2012;117:735–744. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Sedlic F, Pravdic D, Bosnjak ZJ, Kwok WM. Biphasic effect of nitric oxide on the cardiac voltage-dependent anion channel. FEBS Lett. 2011;585:328–334. doi: 10.1016/j.febslet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–810. doi: 10.2337/diabetes.54.3.803. [DOI] [PubMed] [Google Scholar]
- Estabrook RW. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal Biochem. 1962;4:231–245. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: the sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res. 1997;34:113–120. doi: 10.1016/s0008-6363(97)00037-0. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- Ikari A, Atomi K, Yamazaki Y, Sakai H, Hayashi H, Yamaguchi M, Sugatani J. Hyperosmolarity-induced up-regulation of claudin-4 mediated by NADPH oxidase-dependent H2O2 production and Sp1/c-Jun cooperation. Biochim Biophys Acta. 2013;1833:2617–2627. doi: 10.1016/j.bbamcr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- Klingenberg M, Slenczka W, Ritt E. Comparative biochemistry of the pyridine nucleotide system in the mitochondria of various organs. Biochem Z. 1959;332:47–66. [PubMed] [Google Scholar]
- Koziel A, Sobieraj I, Jarmuszkiewicz W. Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels. Am J Physiol Heart Circ Physiol. 2015;309:H147–H156. doi: 10.1152/ajpheart.00759.2014. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Wang GJ, Chan HC, Chen FY, Chang CM, Yang CY, Lee YT, Chang KC, Chen CH. Electronegative low-density lipoprotein induces cardiomyocyte apoptosis indirectly through endothelial cell-released chemokines. Apoptosis. 2012;17:1009–1018. doi: 10.1007/s10495-012-0726-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW. Streptozotocininduced non-insulin-dependent diabetes protects the heart from infarction. Circulation. 1993;88:1273–1278. doi: 10.1161/01.cir.88.3.1273. [DOI] [PubMed] [Google Scholar]
- Muravyeva M, Baotic I, Bienengraeber M, Lazar J, Bosnjak ZJ, Sedlic F, Warltier DC, Kersten JR. Cardioprotection during diabetes: the role of mitochondrial DNA. Anesthesiology. 2014;120:870–879. doi: 10.1097/ALN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- Pastukh V, Wu S, Ricci C, Mozaffari M, Schaffer S. Reversal of hyperglycemic preconditioning by angiotensin II: role of calcium transport. Am J Physiol Heart Circ Physiol. 2005;288:H1965–H1975. doi: 10.1152/ajpheart.00855.2004. [DOI] [PubMed] [Google Scholar]
- Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34:104–112. doi: 10.1016/s0008-6363(97)00018-7. [DOI] [PubMed] [Google Scholar]
- Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anestheticinduced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111:267–274. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, Aambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, JR, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000;278:H1948–H1954. doi: 10.1152/ajpheart.2000.278.6.H1948. [DOI] [PubMed] [Google Scholar]
- Schenning KJ, Anderson S, Alkayed NJ, Hutchens MP. Hyperglycemia abolishes the protective effect of ischemic preconditioning in glomerular endothelial cells in vitro. Physiol Rep. 2015;3 doi: 10.14814/phy2.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlic F, Pravdic D, Hirata N, Mio Y, Sepac A, Camara AK, Wakatsuki T, Bosnjak ZJ, Bienengraeber M. Monitoring mitochondrial electron fluxes using NAD(P)Hflavoprotein fluorometry reveals complex action of isoflurane on cardiomyocytes. Biochim Biophys Acta. 2010a;1797:1749–1758. doi: 10.1016/j.bbabio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. 2009;109:405–411. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+ Am J Physiol Cell Physiol. 2010b;299:C506–C515. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010;88:241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 2010;24:85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Sun D, Nguyen N, Degrado TR, Schwaiger M, Brosius FC., 3RD Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;284:H141–H150. doi: 10.1152/ajpheart.00132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladic N, Ge ZD, Leucker T, Brzezinska AK, Du JH, Shi Y, Warltier DC, Pratt PF, Jr, Kersten JR. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2011;301:H2130–H2139. doi: 10.1152/ajpheart.01078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller AP, George M, Kalyanasundaram A, Kang C, Periasamy M, Hu K, Lacombe VA. GLUT12 functions as a basal and insulin-independent glucose transporter in the heart. Biochim Biophys Acta. 2012;1832:121–127. doi: 10.1016/j.bbadis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- Wisneski JA, Stanely WC, Neese RA, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J Clin Invest. 1990;85:1648–1656. doi: 10.1172/JCI114616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wu L, Qian Y, Fang Q, Liang D, Wang J, Zeng C, Wang Y, Liang G. Blockage of ROS and NF-kappaB-mediated inflammation by a new chalcone L6H9 protects cardiomyocytes from hyperglycemia-induced injuries. Biochim Biophys Acta. 2015;1852:1230–1241. doi: 10.1016/j.bbadis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Zhou L, Cryan EV, D'Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1) J Cell Biochem. 2003;90:339–346. doi: 10.1002/jcb.10631. [DOI] [PubMed] [Google Scholar]