Abstract
Electrochemical monitoring-on-chip (E-MoC)-based approach for rapid assessment of human immunodeficiency virus (HIV)-infection in the presence of cocaine (Coc) and specific drugs namely i.e., tenofovir (Tef), rimcazole (RA) is demonstrated here, for the first time, using electrochemical impedance spectroscopy (EIS). An in-vitro primary human astrocytes (HA) model was developed using a cultureware chip (CC, used for E-MoC) for HIV-infection, Coc exposure and treatment with anti-HIV drug i.e., Tef, and Coc antagonist i.e., RA. The charge transfer resistance (Rct) value of each CC well varies with respect to infection and treatment demonstrated highly responsive sensitivity of developed chip. The results of E-MoC, a proof-of-the concept, suggested that HIV-infection progression due to Coc ingestion and therapeutic effects of highly specific drugs are measurable on the basis of cell electrophysiology. Though, this work needs various molecular biology-based optimizations to promote this technology as an analytical tool for the rapid assessment of HIV-infection in a patient to manage HIV diseases for timely diagnosis. The presented study is based on using CNS cells and efforts are being made to perform this method using peripheral cells such as monocytes derived dendritic cells.
Keywords: Monitoring-on-chip (MoC), Electrochemical sensing, HIV monitoring, Cocaine
1. Introduction
In-spite of significant advancements in anti-retroviral (ARV) therapeutics, health agencies declared human immunodeficiency virus (HIV-1) infections, a deadly viral infection leading to AIDS, as a serious threat to human health (Nair et al., 2016; Ruiz et al., 2015). Complete eradication of virus especially neuroAIDS is not achieved yet using state-of-the-art, highly active antiretroviral therapy (HAART). At early HIV-infection stage, virus penetrates the blood-brain-barrier (BBB) to enter central nervous system (CNS) to cause neuroAIDS and also develope latent HIV reservoirs (Jayant et al., 2015; Ruiz et al., 2015). The natural integrity of the BBB inhibits penetration of anti-HIV drugs to the brain and make neuroAIDS treatment challenging (Jayant et al., 2015). The recommended HAART exhibited various side-effects which further fuel the epidemic (Jayant et al., 2015). Besides this, the neurological disorder associated with HIV-infected patients becomes more severe on consuming substance of abuse, for example cocaine (Coc) (Dahal et al., 2015). Presently, this is a very serious on-going challenge for developing efficient therapeutics for HIV patients consuming Coc. In HIV patients, Coc alters neurobehavior, neurotransmitters secretion, speeds up cell-to-cell virus infection by 200-times, and helps HIV-infection penetrate the BBB resulting in increased neuroAIDS. Moreover, Coc is involved in inducing neuronal apoptosis via triggering viral products and potentiates astrocyte toxicity (Dahal et al., 2015). Reports confirmed that HIV-infected individuals consuming Coc showed increase severity and developed neurocognitive impairments (Yang et al., 2010). This phenomenon also plays a crucial role in drug adherence. Thus, a complete understanding of HIV-infection progression at cellular level, the potential effects of Coc in HIV patient brain, and the effects of drug resistance in Coc using HIV-infected patient are of great diagnostic significance. Such understanding is required to manage HIV disease of patient to decide timely treatment. The related side-effects of Coc in HIV patient and the need of its assessment is illustrated in Fig. 1(A).
Fig. 1.
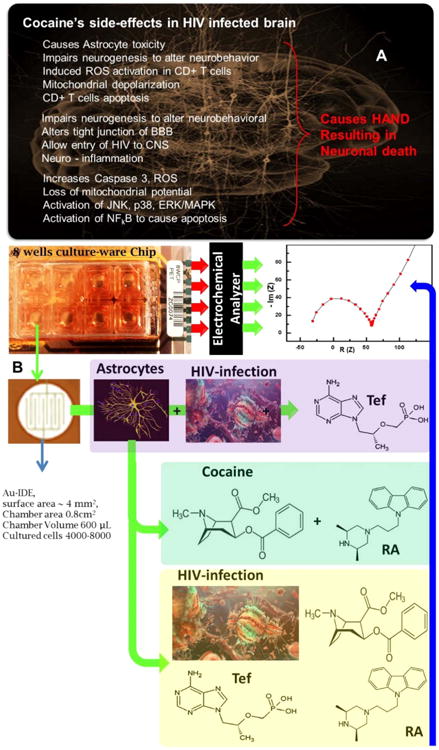
(A) Illustration of cocaine's side effects in HIV-infected patient. 2) IDE-Au containing chip based electrochemical protocol to monitor HIV-infection progression in presence of cocaine and drugs (Tef and RA). We hypothesized that HIV-infection variation on cocaine exposure and treatment with specific drug is responsive to EIS, as indicated by blue arrow. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To cure HIV, considerable efforts are being made to explore therapeutics for brain delivery (Kaushik et al., 2016a, 2014a). Significant efforts are also being made to explore analytical tools for monitoring HIV-infection in patients to decide therapeutics (Shafiee et al., 2015b; Yager et al., 2008). As-of-now, enzyme-linked immunosorbent immunoassay (ELISA), reverse transcriptase/quantitative polymerase chain reaction (RT/Q-PCR), and western blot are the most commonly used methods to detect HIV-infection (Shafiee et al., 2015b). Optical assays based surface plasmon resonance (SPR) system have also been reported to quantify CD4+ cells for HIV-infection progression (Shafiee et al., 2015b; Yager et al., 2008). Unfortunately, these methods are expensive, time consuming, and need high operation expertise. We believe that, time taking diagnosis may delay therapeutics needed to control HIV-infection. Thus, developing a rapid and sensitive analytical tool capable of detection and monitoring of HIV infection progression is very crucial (Shafiee et al., 2015b; Yager et al., 2008). Recently, electrochemical analytical systems of reduced form factors, which are miniaturized, nano-enabled, and paper based platforms integrated with fluidic systems, have emerged as sensitive, selective and stable analytical devices to detect and monitor target diseases (Cheng et al., 2007; Cruz et al., 2014; Kaushik et al., 2015; Luongo et al., 2013; Shafiee et al., 2015a; Shah et al., 2014; Tokel et al., 2015; Vasudev et al., 2013) like HIV-infection (Mahmoud and Luong, 2008; Shafiee et al., 2013a, 2013b, 2015a, 2014). Beside remarkable success with electrochemical systems, there is a considerable scope to promote these systems at point-of-care application (Kaushik et al., 2016a, 2016b, 2014b). Such investigated system under the criteria of ASSURED i.e. affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free and deliverable have been used for monitoring of HIV progression/variation in patients using Coc and drugs (Shafiee et al., 2015b).
Presented work explores, for the first time, an E-MoC-based on electrochemical impedance spectroscopy (EIS) for the assessment of HIV-infection in the presence of Coc and therapeutic drugs specific to HIV-infection and Coc, as illustrated in Fig. 1(B). Based on literature, tenofovir (Tef), and rimcazole antagonist (RA) are used as model drugs selected to eradicate HIV-infection and to block effects induced by Coc, respectively. In this research, we explored the electrophysiology of a cell on HIV-infection, Coc exposure and treatment using specific drugs. Therefore, HA was selected as a model cell type for this research because Coc affects physiology of HA rapidly. During therapeutics the secretion of various inflammatory agents, intra/extra cellular, environment changes and led to effect the electrophysiological nature of cells. The resulted HIV-infection variation is monitored herein using EIS as a function of charge transfer resistance (Rct). Such investigated E-MoC method is sensitive for HIV-infection monitoring and can be used as an analytical tool to generate useful bioinformatics and manage diseases for future diagnosis to decide therapeutics.
2. Materials and methods
2.1. Chip for cell culture and electrochemical measurement
The CC, procured from Applied biophysics (# 8WCP PET arrays), containing eight wells, was used to grow HA for HIV-infection and various other planned treatments. Each well (substrate area of 0.8 cm2 and maximum volume of 600 μL) of CC consisted of an interdigitated electrode of gold (IDE-Au) (3.985 mm2) and approximately 4000 to 8000 cells were grown for electrochemical measurement. In a well, IDE-Au array was useful to grow large number of cells (8000) and designed specially to perform electrochemical measurements without fluctuation. The EIS measurement was conducted in 400 μL of 5 mM PBS (pH 7.4) containing 5 mM Fe (II)/Fe(III), and used as redox moieties using a VMP3, a multi-channel potentiostat/galvanostats of BioLogic instruments. Each measurement was made in triplet set and an average was used for analytical interpretation. Before processing chip for cell culture, EIS measurement was made on every well. The results of EIS study (data not shown) confirmed that each well of CC exhibited repeatable, reproducible, and identical electrochemical response within 2–3% of variation.
2.2. HIV-1 infection in HA and treatment with Tef, Coc, and RA
Primary HA were purchased from ScienCell Research Laboratories (Carlsbad, CA; Cat. # 1800-5) and grown in astrocyte medium purchased from ScienCell Laboratories (Cat. # 1801) containing 2% of fetal bovine serum (ScienCell Cat. # 0010), astrocyte growth supplement (ScienCell Cat. # 1852) and penicillin/streptomycin (ScienCell Cat. # 0503). HIV-1Ba-L (clade B) (NIH AIDS Reagent Program Cat. # 510) was obtained through AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The Cocaine hydrochloride-Sigma (Cat# C5776), rimcazole dihydrochloride-Tocris (1497, CAS# 75,859-03-9), and tenofovir disoproxil fumarate-Sigma (1,643,656; CAS # 202,138-50-9) was used for this research.
Primary HA were grown in eight-well CC plate at a concentration of 8000 cells and infected with different concentrations (10–30 ng/mL) of HIV-1 using a previously described protocol (Atluri et al., 2013; Eugenin and Berman, 2007). Each well was used for an individual treatment. After overnight infection, un-absorbed virus was washed away using PBS (pH 7.4), and cells were cultured for 5 days in the presence or absence of Coc (1 μM). RA (2 μM) was added to the wells (specific to Coc) 2 h before the treatment with Coc, dose response optimized previously in our laboratory (Roy et al., 2015). On the 5th day, Tef, 100 μg (100 μL of 1 mg/mL), dose optimized by (Jayant et al., 2015), was added in wells specific to HIV and incubated for 7 days in total. On every second day, fresh medium was added and after 7 days of HIV-infection, culture supernatants were collected for the p24 antigen estimation using ELISA kit (ZeptoMetrix Corp. Cat # 801,200).
2.3. MTT assay for cell viability and p24 assays
The MTT cell viability assay was performed using a modified assay method as described by Rao et al. (Kurapati et al., 2012). The p24 assay was performed to quantify the production of p24 antigen during HIV-infection, Coc exposure, and treatments via adopting a standard protocol used in our laboratory (Yndart et al., 2015).
All data are presented as the mean ± the standard deviation of the mean (S. D). The results were analyzed using ANOVA (Kruskal-Wallis test) followed by Dunn's Multiple Comparison post-test to determine statistical significance (Graph Pad 5 Software, Inc., La Jolla, CA, USA). Significance was considered to be p < 0.05.
3. Results and discussion
3.1. Electrochemical response evaluation
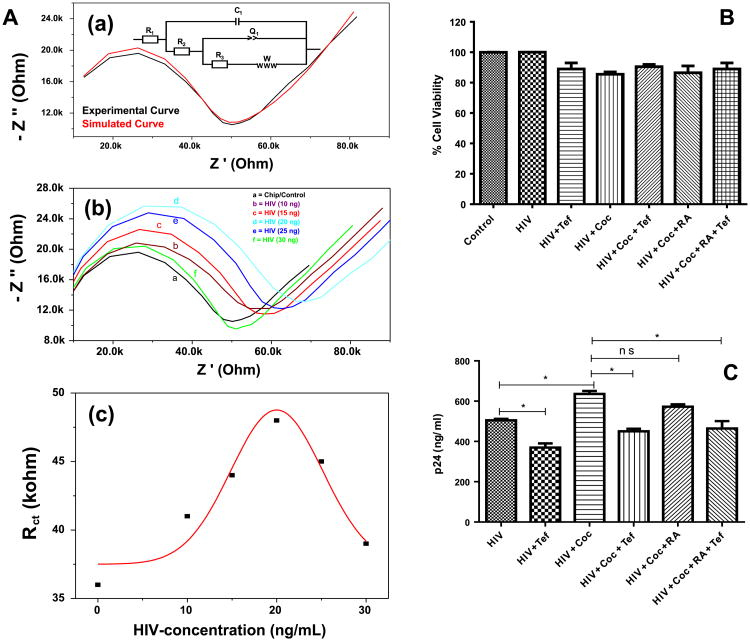
The EIS of IDE-Au exhibited a Nyquist plot [black curve, Fig. 2.A, (a)], wherein the and experimentally obtained data found in best match through simulation [red curve, Fig. 2. A, (a)] using an electronic circuit (inset, Fig. 2(A), (a)). The Nyquist plot of IDE-Au exhibited a semicircle part, observed at higher frequency, and corresponds to the electron-transfer limited process. The radius of this semicircle represents Rct of the system. The semicircle is followed up by a linear curve (45° to the axes) corresponding to the diffusion controlled electron transfer process (Kaushik et al., 2010). For a biological system, the Rct value varies on a small change in intra/extracellular cell properties. Thus we selected Rct as an analytical parameter for analysis during HIV-infections and treatment planned for this work. The HIV-infection and further treatments affected intracellular, extracellular, physical and electrochemical properties of HA that led to changes in the impedimetric parameters at interface with IDE-Au to affect Rct value. Therefore, the variation in Rct values were analyzed for the assessment of HIV-infection in HA and on treatment with Coc and drugs specific to both. The EIS was performed on HA as a function of HIV-infection doses which varies from 10 to 30 ng/mL [Fig. 2A, (b)]. In comparison to HA grown onto IDE-Au, the semicircle corresponds to Rct and Warburg impedance parameters of the chip were found responsive to HIV-infection doses. The results of the studies showed that the value of Rct increases from 36 to 48 KΩ on treating with HIV infection from 10 to 20 ng/mL and then decreases on increasing infection level i.e. 25–30 ng/mL [Fig. 2A, (c)]. The increment in Rct value at early stage infection might be due to the presence of virus in cellular environment which later attached to mannose receptors (Barre-Sinoussi et al., 2013). However, Rct value of chip decreases from 44 to 39 KΩ on HIV-infection using doses of more than 20 ng/mL. This might be due to internalization or fusion of virus genome into the cell. Wherein virus genome on multiplication by reverse transcription replicates DNA which integrate with genome of HA and also induce production of various cytokines, chemokines, neurotoxic factor, and extracellular toxic viral proteins (Barre-Sinoussi et al., 2013). These alterations in cell physiology ultimately make cell weak and electroactive.
Fig. 2.
(A) EIS measurement of a HA grown in well of a chip (a) using 400 μL of 5 mM PBS (pH 7.4) containing 5 mM Fe (II)/Fe(III), used as redox moieties, the experimentally obtained data was simulated using an electronic circuit (inset, image a), the EIS measurement of CC as a function of HIV concentrations (b), and variation in charge transfer resistance (Rct) of chip as a function of HIV-infection. B) HA cell viability evaluation on various treatment using MTT-assay. C) The estimation of p24 variation in HA on treatment with HIV-infection and treatment with cocaine and drugs. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Cell-viability and p24 production evaluation on infection and treatment
Considering cell viability as a major factor, we evaluated HA toxicity in terms of cell viability as a function of HIV-infection levels from 10 to 30 ng/mL using MTT assay. The results of this study confirmed that cells are viable ∼ 90–95% using doses ranging from 10 to 20 ng/mL (data not shown). However, HA cell with infection of more than 20 ng/mL showed cell viability to be less than 80%. Thus, we selected HIV-infection using 20 ng/mL for further experiments. The cell viability of HA, evaluated using MTT-assay, on infection with HIV and Coc followed by treatment using Tef and RA is shown in Fig. 2(B). The HA on infection and treatment showed cell viability in the range of 90–96% confirming that each treatment is not affecting health of the cells. However, the cell viability on HA on treatment with Coc is lower (∼ 85%) due to rapid effects of Coc on HA causing impairment, in comparison to other cells.
The assessment of HIV-infection level in HA on using Coc and drugs was also evaluated by estimating p24 antigen levels using ELISA techniques (Fig. 2C). A significant increment in HIV-infection was observed in the presence of Coc (p < 0.05) when compared to only HIV-infected cells (Fig. 2C). This is accepted because of a well-known fact that Coc consumption facilitates HIV-infection progression (Dahal et al., 2015). On treatment with Tef, a significant reduction of HIV-infection levels was observed in comparison of HIV infected cells alone, confirming therapeutic effects of Tef. Considering the synergistic effects, HA infected with HIV and Coc were treated with Tef and RA together. A significant reduction of HIV-infection level was observed which reveals that both RA and Tef can be used together to control HIV-infection and block the effects induced by Coc. Although we have seen reduced infection levels in the presence of Coc and RA in comparison with cells treated with HIV and Coc, it is not statistically significant. As we know, ELISA is a time consuming conventional reliable method to detect HIV-infection level. To make this monitoring rapid (1 min), we performed EIS to evaluate HIV-infection progression in HA infected with HIV in presence of Coc and treatment with drugs specific to HIV and Coc.
3.3. E-MoC for HIV-infection detection in presence of cocaine and drugs
To evaluate HIV-infection variation as a function of change in Rct value (Fig. 3)-based on EIS, an experiment was planned in 9 wells named as 1st –well HA=A, 2nd –well HA & HIV = B, 3rd – well HA & HIV + Tef = C, 4th –well HA & Coc =D, 5th –well HA & Coc + RA = E, 6th –well HA & HIV & Coc = F, 7th –well HA & HIV & Coc + RA = G, 8th -well HA & HIV & Coc + Tef. = H, 9th –well HA & HIV & Coc + Tef +RA =I. We observed that the value of Rct of CC increases from 48 to 210 kΩ on HIV-infection (20 ng/mL) in HA [Fig. 3(a)]. This suggests that the presence of virus in the cellular environment led to hinder the electron transport from mediator to IDE-Au. On treating HIV-infected HA with Tef, the value of Rct further decreases from 210 to 150 kΩ, confirming that Anti-HIV drugs eradicated virus levels in cellular environment thus HA behave as normal HA due to therapeutic effect. This also confirmed the sensitivity of chip for understanding drug resistance towards virus progression. The treatment of Coc to HA decreased Rct value significantly to 21 kΩ in comparison to normal HA. This might be due to the rapid effect of Coc on chemical and biological activities of HA. The Coc is known to cause HA toxicity via impairment rapidly. Beside this, Coc also facilitates the secretion of various electro-active inflammatory agents resulting in easier electron transfer from medium to IDE-Au. These secreted agents alter chemical, physical, and biological features of HA making it conducting in nature. This could facilitate the electron transfer from the medium to IDE-Au resulting in a low Rct value than normal HA. As expected, on treating HA-Coc with RA, the value Rct further increases to 34 kΩ [Fig. 3, (b)]. This suggests that the therapeutic action of RA is to block the effects induced by Coc impairment in HA.
Fig. 3.
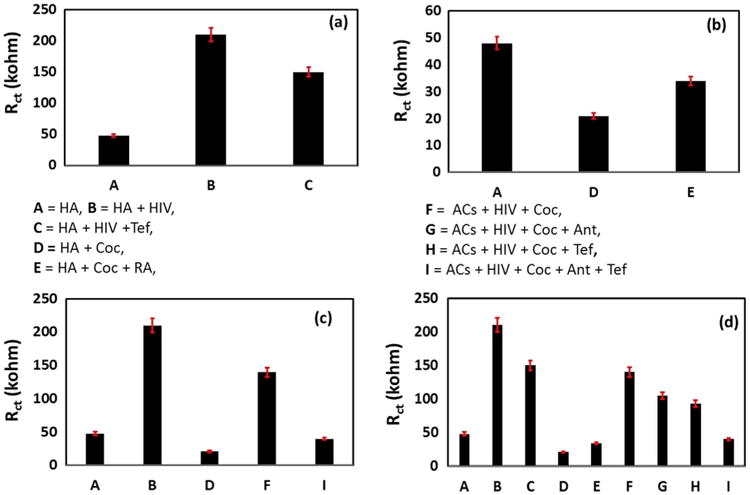
EIS measurement of CC treated with HA, HIV, Coc, Tef, RA and all together. 1st –well HA=A, 2nd –well HA & HIV = B, 3rd –well HA & HIV + Tef = C, 4th –well HA & Coc =D, 5th –well HA & Coc + RA = E, 6th –well HA & HIV & Coc = F, 7th –well HA & HIV & Coc + RA = G, 8th -well HA & HIV & Coc + Tef. = H, 9th –well HA & HIV & Coc + Tef +RA =I. (a) HA, HA & HIV-infection, HA & HIV-infection + Tef. (b) HA & Coc, HA & Coc + RA, (c) HA, HA & HIV-infection, HA & Coc, HA & HIV & Coc, HA & HIV-infection & Coc + RA + Tef. (d) Rct variation as function of all treatment. The EIS measurements were performed in 400 μL of 5 mM PBS (pH 7.4) containing 5 mM Fe (II)/Fe(III), as redox moieties.
We also explored the effect of Coc on HIV-infection levels in HA cells-infected with HIV [Fig. 3, (c)]. The Rct value of HIV-infected HA treated with Coc was found higher (140 kΩ) than HA & Coc and HA & Coc+RA i.e., 34 kΩ. This confirms that Coc facilitate HIV-infection in HA cells. This excess virus in cellular media hinders electro transport to IDE-Au and increases the Rct value. To explore the effects of Tef and RA synergistically, an EIS study was performed in HA treated with HIV and Coc together [Fig. 3, (d)]. The Rct value of HA infected with HIV in the presence of Coc decreases to 105 kΩ on treatment with RA suggesting that RA again blocks the effects caused by Coc. The further decrease in Rct of HA & HIV-infection + Coc to 93 kΩ on treatment with Tef confirmed the therapeutic action. Moreover, HA treated with HIV and Coc together on treatment with Tef and RA also decreases Rct to 40 kΩ, again confirming the therapeutic action of both drugs. During this phenomenon of Tef and RA participating in therapeutic actions, Tef eradicates HIV-infection and RA eliminates impairment caused by Coc consumption. The therapeutic action of drugs makes infected cells behave as normal HA. This affected electron transport and can be monitored via analysis of Rct variation. This suggests that FDA approved drugs and antagonist may work together to eradicate HIV-infection to block the effects induced by Coc in a cell culture. The results of the EIS study (Fig. 3) were found similar as obtained using CV techniques (Supplementary Fig. 1) as a function electrical current with respect to infections and treatments.
4. Conclusions
E-MoC for rapid HIV-infection monitoring using HA cells in the presence of Coc and treatment with specific drugs is demonstrated here successfully. We observed that on HIV-infection, the electrical characteristics changes at interface of CC and HA changes and further affected on Coc ingestion and treating with Tef and RA. Using E-MoC, HIV infection can successfully be monitored as a function Rct, as correlated with CV and ELISA p24 studies. Our investigated E-MoC can be used as an analytical tool to monitor HIV-infection during therapeutics along with patient activities such as substance of abuse, psychological stress, etc. Thus collected bio-informatics could be very useful for HIV management and to decide timely therapeutics.
Supplementary Material
Acknowledgments
Authors acknowledge National Institutes of Health (NIH) grants RO1-DA027049, RO1-DA034547, R01-DA037838, R01-DA-040537, and RO1DA042706A.
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bios.2016.06.086.
Contributor Information
Ajeet Kaushik, Email: ajeet.npl@gmail.com.
Madhavan Nair, Email: nairm@fiu.edu.
References
- Atluri V, et al. PLoS One. 2013;8(4):e61399. doi: 10.1371/journal.pone.0061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre-Sinoussi F, et al. Nat Rev Microbiol. 2013;11(12):877–883. doi: 10.1038/nrmicro3132. [DOI] [PubMed] [Google Scholar]
- Cheng X, et al. Lab Chip. 2007;7(6):746–755. doi: 10.1039/b705082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AFD, et al. Biosens Bioelectron. 2014;62:249–254. doi: 10.1016/j.bios.2014.06.053. [DOI] [PubMed] [Google Scholar]
- Dahal S, et al. Front Microbiol. 2015;6:931. doi: 10.3389/fmicb.2015.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. J Neurosci. 2007;27(47):12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayant R, et al. Int J Nanomed. 2015;10:1077–1093. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, et al. Sci Rep. 2016a;6:25309. doi: 10.1038/srep25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, et al. Expert Opin Drug Deliv. 2014a;11(10):1635–1646. doi: 10.1517/17425247.2014.933803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, et al. Biosens Bioelectron. 2016a;80:273–287. doi: 10.1016/j.bios.2016.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, et al. Electroanalysis. 2010;22(10):1045–1055. [Google Scholar]
- Kaushik A, et al. Biosens Bioelectron. 2016b;75:254–272. doi: 10.1016/j.bios.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, et al. Biosens Bioelectron. 2014b;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Kaushik A, et al. Int J Nanomed. 2015;10(10):677–685. doi: 10.2147/IJN.S75514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurapati KRV, et al. J Basic Clin Physiol Pharmacol. 2012;23(4):139–146. doi: 10.1515/jbcpp-2012-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo K, et al. Biomicrofluidics. 2013;7(3):034108. doi: 10.1063/1.4809590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud KA, Luong JH. Anal Chem. 2008;80(18):7056–7062. doi: 10.1021/ac801174r. [DOI] [PubMed] [Google Scholar]
- Nair M, et al. Adv Drug Deliv Rev. 2016;103:202–217. doi: 10.1016/j.addr.2016.02.008. http://dx.doi.org/10.1016/j.addr.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy U, et al. Front Microbiol. 2015:6. doi: 10.3389/fmicb.2015.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, et al. Sci Lett J. 2015;4:172. [Google Scholar]
- Shafiee H, et al. Sci Rep. 2015a:5. [Google Scholar]
- Shafiee H, et al. Small. 2013a;9(15):2553–2563. doi: 10.1002/smll.201202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee H, et al. Transducers & Eurosensors XXVII. Proceedings of the 17th International Conference on IEEE. 2013b:2141–2144. [Google Scholar]
- Shafiee H, et al. Sci Rep. 2015a:5. [Google Scholar]
- Shafiee H, et al. Sci Rep. 2014;4:4116. doi: 10.1038/srep04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee H, et al. Ann Rev Med. 2015b;66:387–405. doi: 10.1146/annurev-med-092112-143017. [DOI] [PubMed] [Google Scholar]
- Shah P, et al. Analyst. 2014;139(9):2088–2098. doi: 10.1039/c3an02280c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokel O, et al. Sci Rep. 2015:5. [Google Scholar]
- Vasudev A, et al. Sens Actuators B. 2013;182:139–146. [Google Scholar]
- Yager P, et al. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. PLoS One. 2010;5(10):e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yndart A, et al. ACS Chem Neurosci. 2015;6(9):1600–1612. doi: 10.1021/acschemneuro.5b00156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.