Abstract
Discovered some 35 years ago, succinic semialdehyde dehydrogenase deficiency (SSADHD) represents a rare, autosomal recessively-inherited defect in the second step of the GABA degradative pathway. Some 200 patients have been reported, with broad phenotypic and genotypic heterogeneity. SSADHD represents an unusual neurometabolic disorder in which two neuromodulatory agents, GABA (and the GABA analogue, 4-hydroxybutyrate), accumulate to supraphysiological levels. The unexpected occurrence of epilepsy in several patients is counterintuitive in view of the hyperGABAergic state, in which sedation might be expected. However, the epileptic status of some patients is most likely represented by broader imbalances of GABAergic and glutamatergic neurotransmission. Cumulative research encompassing decades of basic and clinical study of SSADHD reveal a monogenic disease with broad pathophysiological and clinical phenotypes. Numerous metabolic perturbations unmasked in SSADHD include alterations in oxidative stress parameters, dysregulation of autophagy and mitophagy, dysregulation of both inhibitory and excitatory neurotransmitters and gene expression, and unique subsets of SNP alterations of the SSADH gene (so-called ALDH5A1, or aldehyde dehydrogenase 5A1 gene) on the 6p22 chromosomal arm. While seemingly difficult to collate and interpret, these anomalies have continued to open novel pathways for pharmacotherapeutic considerations. Here, we present an update on selected aspects of SSADHD, the ALDH5A1 gene, and future avenues for research on this rare disorder of GABA metabolism.
Keywords: GABA (4-aminobutyric acid), GHB (4-hydroxybutyric acid), knockout mouse model, neurological disease, pathophysiology, oxidative damage, crystal structure, autophagy, mitophagy, succinic semialdehyde dehydrogenase deficiency (SSADHD), pathogenic mutations, polymorphisms, SNP (single nucleotide polymorphism), multifactorial traits, GWAS, genome wide association study, GABAergic neurotransmission, pathophysiology
Introduction
It is widely accepted that monogenic disorders represent extreme examples of metabolic variations, the latter present to a much lower degree of severity in the majority of the population. Succinic semialdehyde dehydrogenase deficiency (SSADHD), a rare disease caused by mutations of the ALDH5A1 gene, pointedly reminds us of this issue. Compound heterozygous or homozygous pathogenic mutations associate with a debilitating disease, whereas functional single nucleotide polymorphisms (SNPs) associated with more common or milder neurological impairments which can also be identified in various populations. The focus of this review is to summarize selected aspects of our current knowledge of the role of the SSADH enzyme and its genetic counterpart, the ALDH5A1 gene, in human diseases, with an emphasis on the diverse pathophysiology of the disease linked to gene polymorphism and susceptibility to multifactorial traits.
Succinic semialdehyde dehydrogenase deficiency (SSADHD)
Succinic semialdehyde dehydrogenase deficiency (SSADHD; OMIM #271980) is an autosomal-recessively inherited disorder of GABA degradation caused by mutations in the ALDH5A1 gene on chromosome 6p22.3. The clinical features include global developmental delay, hypotonia, epilepsy, extrapyramidal manifestations, and hyporeflexia (Pearl et al 2003; 2003, 2005; 2009; Novotny et al 2003; Knerr et al 2007, 2008, 2010; Kim et al 2011; Vogel et al 2013). Diagnosis of autism spectrum disorder occurs disproportionately (Gibson et al 2003). Abnormalities in MRI signal involving the globus pallidus, subthalamic nucleus, and cerebellar dentate nuclei are frequently encountered, and all patients have elevated urine excretion of γ-hydroxybutyric acid (GHB), the biochemical hallmark of the disorder. The incidence is unknown, but has recently been estimated at 1:106 (unpublished). While the median age of diagnosis is less than 5 years, 10% of cases are diagnosed in adolescence or adulthood. Late and undiagnosed cases are suspected, although increased detection of SSADHD is anticipated with the increasing utility of next generation gene sequencing. The challenges inherent to late diagnosis is noteworthy. Not only do families undergo a diagnostic odyssey of clinician evaluation and metabolic testing (most likely associated with the non-specific neurological phenotype), but possible therapeutics accompanied by potentially beneficial physical/occupational/speech therapies remain uninitiated. Key landmarks in clinical and basic research of SSADHD are highlighted (Table 1).
Table 1.
Landmarks in SSADHD Research
| Year(s) | Achievement(s) | Citation(s) |
|---|---|---|
| 1981-1983 | Biochemical (GHB)/Enzymatic Identification of SSADHD | Jakobs et al 1981; Gibson et al 1983 |
| 1992 | Purification of Mammalian SSADHD | Chambliss and Gibson 1992 |
| 1993 | Prenatal Detection of SSADHD; Identification of CNS Enzyme Defect | Jakobs et al 1993; Chambliss et al 1993 |
| 1995-1998 | Molecular Cloning of ALDH5A1 Gene; Identification of Mutations | Chambliss et al 1995; 1998 |
| 1997 | Genotype/Phenotype Correlations in 23 Patients: Case Histories | Gibson et al 1997 |
| 2001-2002 | Development of Murine Knockout Mouse-Pharmacological Rescue | Hogema et al 2001; Gupta et al 2002 |
| 2003 | Compilation of 27 Disease-Associated Mutations in SSADHD | Akaboshi et al 2003 |
| 2004-2006 | Definition of KO Mouse Seizure Profile; GABABR/GABAAR Abnormalities |
Cortez et al 2004; Buzzi et al 2006; Wu et al 2006 |
| 2008 | Identification of Metabolic Disruptions in Embryonic KO Mice | Jansen et al 2008 |
| 2008 | Rescue of KO Mice with the Ketogenic Diet | Nylen et al 2008 |
| 2009 | Human GABAAR Dysfunction using 11C-Flumazenil Binding | Pearl et al 2009 |
| 2009 | Crystal Structure of Human SSADH; “Dynamic Catalytic Loop” | Kim et al 2009 |
| 2009 | Efficacy of SGS-742 (GABABR antagonist) in KO Mouse | Pearl et al 2011 |
| 2012 | Human GABABR Dysfunction using Transcranial Magnetic Stimulation | Reis et al 2012 |
| 2014 | Clinical Trial of Taurine Intervention in SSADHD (completed)a | Pearl et al 2014a |
| 2014 | Clinical Trial of SGS-742 Intervention in SSADHD (ongoing) | (unpublished) |
| 2014, 2015 | Identification of GABA Role in Autophagy; Rapalog Therapeutics | Lakhani et al 2014; Vogel et al 2015 |
Legend to Table: Abbreviations employed: GHB, gamma-hydroxybutyric acid; CNS, central nervous system; GABAAR, GABAA receptor; GABABR, GABAB receptor; ALDH5A1=aldehyde dehydrogenase 5a1=succinic semialdehyde dehydrogenase; KO=knockout.
Taurine is a non-physiological amino acid with pleiotropic GABAergic roles, and was effective in rescuing early lethality in KO mice (Gupta et al 2002).
Patient registries and associations
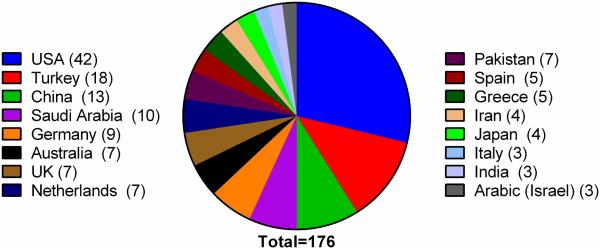
The SSADHD patient registry began with the goal of leveraging interdisciplinary scientific discussion on SSADHD, undertaking grassroot efforts to raise awareness, and raising much needed research funding for new studies. The patient registry has been maintained since 2003 with continuous IRB approval, and is currently located through the Boston Children’s Hospital. All enrolled patients have completed a detailed questionnaire which contains clinical history, developmental status, results of diagnostic testing, contact information for the family and primary care physician, and willingness to be recruited into additional studies. The information is password protected and has been instrumental in providing information on clinical trials thus far. There are currently 120 enrolled patients. The SSADH association (www.ssadh.net) also maintains a patient registry with 92 patients. The two registries are currently in the process of being merged. Since its first description in 1981, the literature reveals 176 patients presenting from 38 distinct countries, underscoring the panethnic nature of SSADHD (Fig. 1).
Fig. 1. Geographic distribution of SSADHD patients (n=176).
Afghanistan, Argentina, Taiwan, Canada, France, Lebanon, Bulgaria, Life (New Caledonia) (n=2 patients each). Malaysia, UAE (United Arab Emirates), Tunisia, Syria, Luxembourg, Inuit (Iceland), Uruguay, Yemen, Sweden, Denmark, Sicily, Albania, Algeria, Belgium (n=1 patient each; data taken from 36 publications).
The Primary Defect in SSADHD and Metabolic Perturbations in Patients and Knockout Mice
Jakobs and coworkers (Jakobs et al 1981) first reported increased excretion of GHB in humans. They examined the urine of three developmentally delayed pediatric patients with minimal language development using combined gas chromatography–mass spectrometry methodology. The mass spectrometric data aligned with library data indicating a fragmentation pattern consistent with GHB (gamma-hydroxybutyrate, a derivative of the inhibitory neurotransmitter GABA). The hypothesis developed to explain the presence of GHB was a block at the level of succinic semialdehyde dehydrogenase (SSADH; Fig. 2). In 1983, Gibson and coworkers presented enzymatic data that confirmed Jakob’s hypothesis, highlighting the first report of an inborn error of gamma-aminobutyric acid (GABA) metabolism (Gibson et al 1983). Autosomal-recessive inheritance was suspected since the original three patients were born to related parents, which was subsequently confirmed when the human gene was identified in 1998 (Chambliss et al 1998) and homozygously inherited mutations were identified. Obligate heterozygotes (parents) each carried the pathogenic allele on one copy each of chromosome 6, confirming the inheritance pattern.
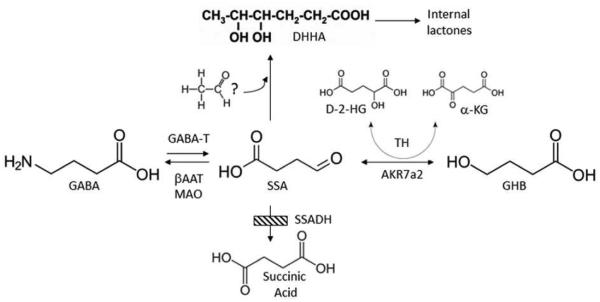
Fig. 2. GABA metabolism and SSADHD.
GABA normally interconverts to succinic semialdehyde via GABA-transaminase (GABA-T) activity, and subsequently forms succinic acid via succinic semialdehyde dehydrogenase (SSADH), the defect in SSADH deficiency (SSADHD; cross-hatched box). Gamma-hydroxybutyrate (GHB; whose formation is catalyzed by aldo-keto reductase 7a2, AKR7a2), D-2-hydroxyglutaric acid (D-2-HG; the formation of which is catalyzed by nicotinamide-independent D-2-hydroxyglutaric transhydrogenase (TH)), SSA and 4,5-dihydroxyhexanoic acid (DHHA; possibly derived from SSA condensation with an “activated” two carbon species, but unproven) are increased in patient body fluids. Monoamine oxidase (MAO) and β-alanine aminotransferase (βAAT) can also metabolize GABA.
Although SSADHD is considered a “neurometabolic” disorder, emerging data indicates a number of systemic disturbances (Fig. 2). A summary of metabolic data available in the literature from SSADHD patients (Table 2) and the corresponding murine knockout mouse model (Hogema et al 2001) highlights the extent of metabolic abnormalities. In addition to accumulation of GABA and GHB, we and others have found accumulation of a number of likely GABA-associated metabolites in tissues and body fluids derived from both patients and the knockout mouse model. These include D-2-hydroxyglutaric acid (D-2-HG), 4,5-dihydroxyhexanoic acid (DHHA), as well as accumulation of homocarnosine (a precursor dipeptide of GABA composed of L-histidine and GABA), guanidinobutyrate (derived from the substitution of GABA for glycine in the arginine-glycine amidinotransferase reaction involved in creatine synthesis) (Jansen et al 2006). Species-specific metabolite data provides complementary results, since adequate amounts of either urine or blood is almost impossible to obtain from the knockout mouse model (in volumes sufficient to facilitate metabolic studies; Gupta et al 2002, 2004), and organ tissues (or biopsies) are not ethically acquired from SSADHD patients.
Table 2.
Metabolites in Physiological Fluids from Patients with SSADHD
| Metabolite | Urine (mmol/mol creatinine) | Plasma (μM) | Cerebrospinal fluid (μM) |
|---|---|---|---|
| GHB | 34-514 (n=10) (nl < 10) |
26-533 (n=17) (nl < 3) |
116-1110 (n=4) (nl < 3) |
| D-2-HG | 22-102 (n=7) (nl < 18) |
3.1-7.6 (n=4) (nl < 1) |
0.4-4.7 (n=6) (nl < 0.3) |
| SSA | 3-20 (n=13) (nl < 2) |
-- | 1280-2570 (n=3; nmol/L) (nl < 10) |
| DHHA | -- | -- | -- |
| GABA | ND | -- | 13.6-22.4 (n=4; total GABA) (nl < 12) |
| Homocarnosine | -- | -- | 14.8-41 (n=9) (nl < 10) |
| GBA | 4.6-35 (n=7) (nl < 5.8) |
-- | 0.04-0.32 (nl < 0.03) |
Legend: Abbreviations: GHB, γ-hydroxybutyric acid; D-2-HG, D-2-hydroxyglutaric acid; SSA, succinic semialdehyde; DHHA, 4,5-dihydroxyhexanoic acid; GABA, γ-aminobutyric acid; GBA, guanidinobutyrate; nl,normal; ND, not done. SSA in cerebrospinal fluid is reported in units of nmol/L. Total GABA refers to free and esterified forms of GABA (including esterified forms such as homocarnosine). See Fig. 2 for metabolic interconversions.
Metabolite data for SSADHD patients is sparse and often incomplete (Table 2), quite possibly because most diagnostic studies today only include molecular analysis of the ALDH5A1 gene. For example, DHHA has been noted to be “elevated” in several case reports, but quantitation of this metabolite in human body fluids is lacking. Similarly, SSA has been quantified in urine and cerebrospinal fluid, but not plasma. Additionally, GABA has never been quantified in patient plasma. Turning attention to the knockout mouse model, central metabolite accumulations (brain: GHB, SSA, D-2-HG, DHHA) are manifest systemically, including liver and kidney. Some peripheral disturbances exceed the level of metabolic perturbations observed in CNS (DHHA, D-2-HG; Vogel et al, unpublished). Although the CNS GABA level far exceeds peripheral levels, GABA is still significantly increased in the periphery of the knockout mouse model (Vogel et al, unpublished). Following these metabolites longitudinally in a significant patient cohort would provide rich insights into metabolic and clinical variation, while simultaneously offering the potential to correlate metabolite levels across the developmental course of the disease.
Pathophysiological Mechanisms in SSADHD
There remains no consensus as to the predominant source of pathology in SSADHD, although elevated GABA and GHB likely play an important role. During early neurodevelopment, GABA(A) receptors, which mediate fast inhibitory neurotransmission, are excitatory. The switch to inhibitory neurotransmission occurs early in the postnatal period (Jansen et al 2008). Accordingly, if metabolic perturbations are present in the developing embryo, a heightened excitatory state is already underway which may lead to poor outcomes, possibly underscoring an early path toward patient developmental delays (Jansen et al, 2008). However, our recent understanding of the cellular and molecular features of SSADHD suggest that other mechanisms are at work and likely to contribute to the complex phenotype. These mechanisms include imbalances in GABAergic/glutamatergic neurotransmission, oxidative damage, and dysregulation of autophagic processes.
Imbalances in GABAergic/glutamatergic neurotransmission
Elevation of GHB and GABA in physiological fluids are the biochemical landmarks of SSADHD. GHB can reach concentrations approximating 1 mM in cerebrospinal fluid (CSF) of patients, while normal levels are < 3 µM. CSF GABA is also elevated in patients who have undergone diagnostic lumbar puncture (Gibson et al 1995), usually 2-4 times above the control range. GABA’s central role as inhibitory neurotransmitter is well established, predominantly working through GABA(A) receptors that are ion-channels mediating fast inhibitory neurotransmission, while GABA(B) receptors (metabotropic) mediate slower inhibitory neurotransmission. Further, GABA plays key developmental roles in neuronal migration, myelination and synaptogenesis (Bowery and Smart 2006; Waagepeterson, 1999). The exact function of GHB in CNS physiology remains to be defined (Bay et al 2014; Thiesen et al 2015; Tatar et al 2015). GHB is neuromodulatory in its ability to alter dopamine release and turnover in the CNS (Maitre, 1997; Maitre et al 2016; Kasten et al 2015). Importantly, GHB‘s neuropharmacological properties (Snead 1978; Snead and Morley 1981), including discrete spike-wave discharge patterns on the electroencephalogram (EEG) when administered to animals, are consistent with the neurological sequelae and EEG features in SSADHD. Finally, several studies have highlighted the finding that impaired GABAergic signaling is implicated in the pathogenesis of neurocognitive delay, seizures, obsessive compulsive disorders, anxiety, aggressiveness and sleep disorders (Wu et al 2006; Buzzi et al 2006; Möhler et al 2006; Szabadi et al 2006; Jacobson et al 2007; Galanopoulou et al 2008; Mûller et al 2009; Nikolaus et al 2010; Kleschevnikov et al 2012; Heaney and Kinney 2016), all of which represent typical clinical manifestations of SSADHD.
A potential pathophysiological role of GABA and GHB has been confirmed in the corresponding murine knockout model of SSADHD (Gupta et al 2002; 2004). In this model, tissue GHB and GABA concentrations are elevated and correlate with an progressive epileptic phenotype, including absence seizures by day of life 14 (DOL 14), generalized tonic-clonic seizures by DOL 21, and lethal status epilepticus leading to death by 1 month (Cortez et al 2004; Buzzi et al 2006; Wu et al 2006). Premature lethality suggests that the animal model disease course resides on the severe end of the human phenotypic spectrum. Consistent with this developmental course in the mouse model, instances of SUDEP (sudden unexplained death in epilepsy) have been observed in SSADHD (Knerr et al 2010; Gibson and Pearl, unpublished). The epileptic course in the knockout mouse correlates temporally with down-regulated GABAAR and GABABR function, likely the result of use-dependent down-regulation associated with elevated GABA and GHB (Drasbek et al 2008; Dosa et al 2010; Vardya et al 2010). Importantly, GABAergic neurotransmission deficits quantified in the mouse model have been verified in human patients, employing transcranial magnetic stimulation evaluations (Reis et al 2012), which assesses cortical GABA(B)ergic activity, as well as by [11C]flumazenil binding to assess GABA(A)ergic function (Pearl et al 2009). Overall, impaired GABAergic neurotransmission caused by dramatic GABA and GHB tissue accumulation, together with decreased GABA receptor expression (Wu et al 2006; Buzzi et al 2006) likely contributes a significant component of the neurological pathophysiology of SSADHD. This hypothesis is consistent with the description of other conditions featuring impaired GABA neurotransmission and developmental delay, including Down syndrome (Kleschnikov et al 2012).
Oxidative Stress and damage
Oxidative stress is implicated in the pathology of many inborn errors of metabolism (IEMs), especially those with neuropsychiatric involvement (Vaya 2013; Fransen et al 2012; Ng et al 2008). Oxidative damage is also purported to have a role in the pathophysiology of other neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression and anxiety, ADHD and autism (Ng et al 2008), and a recent report revealed elevated biomarkers of oxidative damage in physiological fluids derived from patients with autism (Pecorelli, 2012). Numerous investigators hold the hypothesis that oxidative damage in these disorders associates with accumulation of metabolites which represent mitochondrial toxins (Braconi et al 2010). This may apply to SSADHD as well since administration of GHB to rats induces oxidative damage in the cortex (Sgaravatti et al 2007), which raises the potential for oxidative damage since GHB accumulates to significant levels in both patients and the knockout mouse model (Gibson et al 2002; Gupta et al 2002, 2004).
More recently, various investigators have highlighted the capacity of other metabolites accumulating in SSADHD (e.g., D-2-HG, GHB) to induce oxidative stress when independently administered to rodents (Latini et al 2007; Sgaravatti et al 2006; Gibson et al 2006; da Rosa et al 2014; Latini et al 2003). Moreover, since succinic semialdehyde itself is an reactive aldehyde, any substantive accumulation of this intermediate would be predicted to induce oxidative damage, potentially including protein and DNA adducts. Additionally, Brown and colleagues, who first identified the presence of DHHA in the body fluids of patients with SSADHD (Brown et al 1987) hypothesized that this unusual metabolite derives from condensation of SSA with an “activated” 2-carbon species in the oxidative phosphorylation pathway (see Fig. 2). Murphy and colleagues (2003) demonstrated that SSADH was the enzyme actively engaged in the further metabolism of 4-hydroxy-2-nonenal (4-HNE), a highly reactive, α-β unsaturated aldehyde species that is produced during the breakdown of lipid bilayers undergoing oxidative degradation. Further, these investigators noted that SSADH was active in the further metabolism of 4-HNE in brain, but not in the liver. Confirming this hypothesis, Vogel and coworkers (unpublished) have recently verified accumulation of conjugated derivatives of 4-HNE in tissues of the murine knockout mouse. The cumulative effect of these multiple metabolites could be additive, or perhaps synergistic, in different regions of the brain of both patients and mice, dependent upon local tissues concentrations and transport.
Oxidative stress and related damage in SSADHD led to the prediction that there would be depletion of the major intracellular antioxidant, glutathione (GSH). This has been confirmed by a number of investigators in the knockout mouse model (Gibson et al 2006; Latini et al 2007; Vogel et al, in press). Moreover, Sauer and colleagues (2007) extended these findings to demonstrate significant disruptions of enzyme complexes involved in mitochondrial oxidative metabolism in brain tissues derived from SSADHD knockout mice, which lends additional credence to the potential disruptive nature of DHHA in mitochondrial metabolism. It must be emphasized, however, that the exact pathway by which DHHA is formed has not been absolutely identified.
These metabolic permutations pointing to oxidative damage are consistent with neuroimaging findings observed in SSADHD. A number of investigators have noted symmetrically increased T2 signals in the globus pallidi, subthalamic nuclei, and cerebellar dentate nuclei of patients (Pearl et al 2003; Pearl et al 2009; Acosta et al 2010; Lapalme-Remis et al 2015). These hyperintensities are not specific to SSADHD and likely reflect cytotoxic metabolic edema (Pearl et al 2009; Yalcinkaya et al 2000; Ziyeh et al 2002; Horino et al 2016; Gogou et al 2016; Wang et al 2015; Li et al 2015). The globus pallidus generates significant metabolic demands associated with a cellular environment especially prone to oxidative damage linked to its high iron content (Bartzokis et al 2007). Hence, the MRI findings noted in SSADHD may reflect CNS oxidative damage, and suggest that antioxidant interventions may be effective in SSADHD, as already shown for other rare disorders (Scaini et al 2012; Atkuri et al 2009; Biancini et al 2012; Perna et al 2012; Lopez-Erauskin et a; 2011; Minder et al 2009; Tinti et al 2010; Singh et al 1988).
The potential impact of oxidative damage in SSADHD is further underscored in view of the crystal structure of SSADH protein from both E. Coli and human (Kim et al 2009; Kim et al 2011; Tamazian et al 2015). Consistent with the aldehyde dehydrogenase superfamily of proteins, a catalytic cysteine is found in SSADH. In the mouse model, this active-site cysteine is located in exon 7 of the orthologue mouse gene, and this exon was removed when developing the knockout model (Hogema et al 2001). In their modeling of the SSADH active site, Kim and coworkers observed that the active site of the protein contained an additional cysteine that formed what was termed a “dynamic catalytic loop” that was potentially susceptible to large structural changes depending upon the cellular redox status. In vivo and in vitro studies confirmed that this loop was responsive to reactive oxygen species. In conditions which favored an oxidative milieu, the proximal cysteines formed an internal disulfide which prohibited the entry of substrate, SSA, into the active site. Conversely, intracellular conditions favoring a reducing environment facilitated reduction of these cysteines and entry of substrate for subsequent oxidation to succinic acid (Ilbert et al 2007). These data suggest that mutations affecting the catalytic loop of SSADH will alter the ability of the enzyme to conformationally cope with intracellular redox changes, and potentially direct the enzyme toward rapid proteasomal degradation during conditions of oxidative stress. Moreover, the catalytic loop might enhance the susceptibility of the enzyme to oxidative damage, further depleting residual activity even in the case of mutations that alter sequences not implicated in the redox loop of the protein. Based on the cumulative data, the potential benefits of prophylactic antioxidant therapy in SSADHD may be warranted.
Autophagy dysregulation
Autophagy (e.g., organelle recycling during nutrient depletion) represents an intricate signaling hub whose central component is the protein known as the molecular target of rapamycin (mTOR; Ainslie et al 2016). This pathway is a master regulator of growth, proliferation, and catabolic outcomes that includes autophagy and other components of cellular fate. Defects in the regulation of effectors upstream of mTOR forms a component of the pathophysiology of numerous conditions, e.g., metabolic diseases, neurological disorders, epilepsy, and autism. In terms of genetic conditions in which metabolic sequences are disrupted, the upstream signaling components impacting mTOR include primarily amino acid input on nutrient status (e.g., phenylalanine, tyrosine, leucine, glycine, GABA).
Autophagy itself is a major catabolic pathway involved in the degradation of intracellular proteins and organelles in the lysosome/vacuole (Yang et al 2009), and this pathway is conserved from yeast to humans (Meijer et al 2007). Defects in autophagy-related pathways have been implicated and therapeutically targeted in many diseases, ranging from cancer and neurodegenerative disorders (e.g., Huntington’s, Alzheimer’s and Parkinson’s diseases) to aging, underscoring the concept that autophagic processes play an essential role in cellular homeostasis and disease pathogenesis (Ravikumar, 2004; Chong, 2010; Mizushima, 2008).
Using yeast as a model system, Lakhani and colleagues (2014) documented that elevated GABA inhibits the selective autophagy pathways pexophagy and mitophagy, leading to oxidative stress (Lakhani et al 2014). Further studies by the same group of investigators revealed that elevated GABA activated mTOR and selectively inhibited the autophagy pathways for peroxisomes (pexophagy) and mitochondria (mitophagy) through Sch9, the homolog of the mammalian kinase, S6K1 (Lakhani et al 2014). This activation could be overridden by rapamycin, a pleiotropic bacteria-derived drug that is commonly used to trigger autophagy pathways via its ability to inhibit the TOR kinase (Raught et al 2001). This previously undocumented link between increased GABA and selective autophagy pathways provides novel insight into the function of GABA, its expanding role in the pathophysiology of SSADHD, and the therapeutic potential of inhibitors of mTOR in SSADHD. Along those lines, a variety of newer analogues of rapamycin are now available, including Torin 1 and 2. Early studies with these analogues are underway in the animal model of SSADHD (Vogel et al, unpublished).
Treatment of SSADHD
No successful targeted therapy has emerged for SSADHD, and treatment has remained symptomatic and non-specific (e.g., antiepileptics for seizures; SSRIs for OCD; Gropman et al; 2003; Parviz et al 2014; Lapalme-Remis et al 2015). A recent case report has identified magnesium valproate as an agent effective in ameliorating behavioral problems and refractory epilepsy (Vanadia et al 2013). This was an unexpected finding since earlier observations by Simler and colleagues (1981) had revealed that valproate is an inhibitor of SSADH.
Lowering GHB has been the focus of several therapeutic strategies. Vigabatrin (VGB; γ-vinyl GABA), an inhibitor of GABA-transaminase approved in the U.S. for use in patients with infantile spasms (Gaily et al 2012; Greiner et al 2012), as well as complex partial seizures, represents a logical choice in SSADHD because the drug prevents the conversion of GABA to γ-hydroxybutyrate via irreversible inhibition of GABA-transaminase (ABAT; aminobutyrate aminotransferase). However, treatment with VGB has resulted in mixed outcomes in SSADHD (Vogel et al 2013). Further, long-term treatment with VGB is contraindicated due to marked and permanent visual-field impairments (Singh et al 2013; Froger et al 2014; Good et al 2011; Pellock 2011; Escalera et al 2010; Casarano et al 2011; Matern et al 1996; Al-Essa et al 2000). Of further concern is the predicted expectation that VGB will augment brain GABA (Ergezinger et al 2003; Pearl et al 2014a; 2014b), which is a relevant observation in light of recent studies (described above) highlighting GABA-induced impairment of autophagic processes (Vogel et al 2015). The potential use of mTOR inhibitors (e.g., rapamycin, Torin 1 or 2) along with VGB might be an effective intervention, since this would both decrease production of GHB and clear the oxidative-damaging effects of further-enhanced GABA levels. Nonetheless, until the ocular toxicity component of VGB is mitigated, long-term use of this unique antiepileptic will remain unfeasible. Studies highlighting the rescue of the murine knockout model from premature lethality (Gupta et al 2002; Pearl et al 2009) using one of the earlier GABA(B) receptor prototypes, CGP 35348, served as the basis for an ongoing clinical trial in SSADHD with a newer generation GABA(B) receptor antagonist, SGS-742 (Froestl et al 2004; www.clinicaltrials.gov; NCT02019667).
A potentially more promising, or at a minimum complementary, therapeutic strategy would be based upon GHB receptor inhibition. One challenge with this approach remains the still undefined nature of the exact, high-affinity GHB receptor itself. An early report highlighting the molecular cloning of the GHB receptor was found to be incorrect (Andriamampandry et al 2007). One complicating factor in the search for the elusive GHB receptor is that GHB itself has weak affinity for GABA(B) receptors, although at concentration approximating mM levels, which are unlikely to be of physiological significance in the normal setting, but may be more relevant in the setting of SSADHD. Recently, Wellendorph and colleagues have performed in vitro studies suggesting that subtypes of the GABA(A) receptor display high-affinity GHB binding (Absalom et al 2012). Since then, other reports have highlighted a number of high-affinity ligands which will likely be valuable in the identification of the exact nature of the GHB receptor (Bay et al 2014). A broadly employed GHB receptor antagonist, NCS-382, is relatively selective for antagonism of GHB binding, and demonstrates an affinity for the GHB receptor approximately 10-15-fold stronger than that of the endogenous ligand GHB (Wellendorph et al 2009). NCS-382 has no affinity for GABA(A) or GABA(B) receptors (Castelli et al 2004). The use of NCS-382 in the murine knockout model demonstrated efficacy in rescuing the phenotype of premature lethality (Gupta et al 2002; Vogel et al 2013), although human studies have yet to be reported. Other GHB-receptor ligands (e.g., 3-hydroxycyclopent-1-enecarboxylic acid; HOCPCA) with even greater affinity for GHB receptor binding (~ 40-fold greater than GHB itself), as well as high blood-brain barrier penetration, might prove superior to NCS-382, but HOCPCA preclinical efficacy data in SSADHD, or other clinical settings of GHB accumulation, have yet to be presented (Thiesen et al 2015).
Additional therapeutic strategies in both murine and human SSADHD have centered on earlier evidence for oxidative damage (Gibson et al 2006; Latini et al 2007). One potential strategy involves the use of N-acetylcysteine (NAC). NAC is a pharmacotherapeutic prodrug which can deliver cysteine needed for the restoration of endogenous GSH (glutathione) levels. Widely employed as an antidotal therapeutic for NSAID (acetaminophen) overdose, the use of NAC would be rational in providing a source of cysteine needed for endogenous synthesis of GSH (the tripeptide of glycine-cysteine-glutamate). Several orally available forms of NAC are in use, although in the setting of acetaminophen toxicity this agent is applied intravenously to expedite absorption. In summary, multiple therapeutic strategies are under active investigation for SSADHD, using both clinical and preclinical strategies. As for many other disorders with complex pathophysiology, it is quite likely that multiple therapeutics will be required to leverage improvements in a variety of domains leading to incremental improvements in quality of life of patients.
Molecular genetics of the ALDH5A1 gene encoding SSADH
Prior to development of a murine knockout model (Hogema et al 2001) for SSADHD, the relevant gene remained uncloned, and causative mutations remained unconfirmed. An early stumbling block in the gene-cloning process included the identification of the SSADH enzyme from human brain as a tetrameric structure of weight non-identical subunits (Ryzlak and Pietruszko 1988). This was the first evidence of an aldehyde dehydrogenase protein that was composed of non-identical subunits with potentially multiple protein-encoding genes (Sheikh et al 1997; Hempel et al 1993). However, Gibson and Chambliss (1992) undertook the purification of SSADH from rat brain and documented that the protein was composed of weight-identical subunits of molecular mass ~60kDa, giving a tetrameric MW of ~240,000. This was consistent with earlier reports on the aldehyde dehydrogenase subfamily (Hempel et al 1993). Rat brain SSADH was purified to homogeneity, the protein sequence was determined and degenerate oligonucleotide primer pools necessary for cDNA library screening were developed. Subsequent studies reported the isolation of three rat brain cDNA clones (3500, 1465, and 1135 bp) encoding SSADH (Chambliss et al 1998). Composite clones encoding the processed SSADH protein predicted a polypeptide with 488 amino acids (molecular mass 52,000 Da). The cDNA clones were confirmed by expression analysis and protein sequence data from the purified rat brain SSADH. Two human liver ALDH5A1 cDNA clones of 1091 and 899 bp were also isolated, but isolation of complete cDNA clones was hampered by the high GC content of the 5’-region of the human gene. Comparative analysis of human and rat brain SSADH proteins and genomic sequences revealed 83% and 91% identity in nucleotide and protein sequence, respectively. Northern blot analysis revealed two differentially expressed transcripts of ~2.0 and 6.0 kb in rat and human tissues, respectively. Southern blotting of the human gene revealed that both transcripts derived from a single copy gene >38kb. More recent examination of the GENE database (NCBI) reveals that the mean total gene size of ALDH5A1 encoding SSADH in homo sapiens is approximately 50-51 kB (www.ncbi.nlm.nih.gov).
With regard to pathogenic mutations, Akaboshi and coworkers (Akaboshi, 2003) presented the most up-to-date summary of pathogenic alleles. Of the approximate 30 alleles, there were missense, nonsense, gene deletions, and splicing errors, without a major mutation hotspot. Alterations of several well-conserved glycine residues, previously reported as critical for enzyme function in the aldehyde dehydrogenase superfamily, led to nearly complete ablation of enzyme activity (Akaboshi et al 2003). Since the report by Akaboshi and colleagues, only sporadic reports of inherited mutations have been presented (Lin et al 2015; Tay et al 2015; Jiang et al 2013; Puttman et al 2013; Kwok et al 2012; Lemes et al 2006; Bekri et al 2004). Many of these alleles are predicted to be pathogenic using SIFT analysis, but a comprehensive analysis of mutations has yet to be presented since that of Akaboshi in 2003. Many of the newly presented mutations are either missense alleles, or small-to-large deletions, in the ALDH5A1 gene encoding SSADH.
SSADH activity measured in human lymphoblasts or freshly isolated tissues shows significant inter-individual variability with an ~5-fold difference between the extremes of the range of enzyme activity (n=27; range 0.69-4.56 nmol/min/mg protein; Gibson et al 1991). To gain insight into this protein variation at the molecular level, the ALDH5A1 coding region was analyzed in a panel of about one hundred random healthy individuals. The analysis revealed eight missense variants and one same-sense variant (Blasi et al 2002), with five attaining polymorphic frequencies: c.106G>C (rs4646832, p.G36R), c.538C>T (rs2760118, p.H180Y), c.545C>T (rs3765310, p.P182L), c.709G>T (rs62621664, p.A237S) and c.1389T>C, rs24640371, p.D463D) (Table 3). All variants (except c.709) involved amino acids in the coding region of the ALDH5A1 gene that were not conserved across animal phyla. To assess the functional consequence of these alleles, expression analysis in cultured mammalian cells was undertaken. A wide spectrum of SSADH enzyme variation was found associated with each amino acid replacement, which ranged from 48-87% of the enzyme activity observed with respect to the wild-type gene expression (Blasi et al 2002).
Table 3.
Single nucleotide polymorphisms (SNPs) in SSADH gene associated with multifactorial traits
| dbSNP | Chr. 6 (bp)§ | Nucleotide variation |
Gene location |
Amino acid substitution |
MAF* (allele) |
In vitro
enzyme activity# |
Associated Multifactorial Trait |
References |
|---|---|---|---|---|---|---|---|---|
| rs1883415 | 24491247 | A>C | upstream | n.a. | 0.17-0.39 (C) | n.a | IGE and PPR** mTLE^ ALP, ALT, GGT plasma conc. |
Lorenz et al., 2006
Pernhorst et al., 2011 Chambers et al., 2011 |
| rs4646832 | 24495102 | c.106G>C | exon 1 | p.G36R | 0.02-0.11 (C) | 86.7% | ||
| rs2760118 | 24503362 | c.538C>T | exon 3 | p.H180Y | 0.17-0.47 (T) | 82.5% | Intelligence quotient (IQ) Aging: rate and quality General cognitive ability Response to methadone treatment |
Plomin et al., 2004
De Rango et al., 2008 Chabris et al., 2012 Fonseca et al., 2013 |
| rs3765310 | 24503369 | c.545C>T | exon 3 | p.P182L | 0.02-0.11 (T) | 46.7% | ||
| rs62621664 | 24504968 | c.709G>T | exon 4 | p.A237S | 0.00-0.02 (T) | 65.1% | ||
| rs1569579 | 24513408 | C>T | intron 5 | n.a. | 0.09-0.47 (C) | n.a. | Ocular phoria | Bosten et al., 2014 |
| rs807518 | 24526015 | C>A | intron 7 | n.a. | 0.01-0.51 (C) | n.a. | Age-related Macular Degeneration | SanGiovanni et al., 2014 |
| rs809419 | 24526762 | G>A | intron 7 | n.a. | 0.16-0.73 (A) | n.a. | Age-related Macular Degeneration | SanGiovanni et al., 2014 |
| rs2744594 | 24527605 | T>A | intron 7 | n.a. | 0.10-0.57 (T) | n.a. | Age-related Macular Degeneration | SanGiovanni et al., 2014 |
| rs12199955 | 24531222 | T>G | intron 8 | n.a. | 0.05-0.54 (G) | n.a. | Age-related Macular Degeneration | SanGiovanni et al., 2014 |
| rs61744005 | 24532164 | c.1389T>C | exon 9 | p.D463D | 0.00-0.01 (C) | n.a. |
Segregation analysis was employed to identify heterozygous individuals for all of the five polymorphic positions (c.106, c.538 c.545, c.709 and c.1389), which led to the identification of compound genotypes. The haplotype phase of these alleles was resolved with familial segregation analysis. Of the 32 haplotypes predicted to exist, only 5 haplotypes were actually observed, with complete linkage disequilibrium (LD) between c.106C, c.538T and c.545T alleles (Blasi et al 2006; Malaspina et al 2009). Haplotypes with multiple amino acid substitutions were also expressed in cultured cells, which resulted in a more significant alteration/reduction of enzyme activity (36% of wild-type). Accordingly, Blasi and coworkers postulated that at least some component of the variation in enzyme activity detected in the general population would likely be attributable to the occurrence of single or multiple polymorphic missense mutations.
Additional evidence for variation in both gene structure and eventual function was observed through the demonstration of SNPs in the 800 bp sequence upstream of the ATG start codon, and in close proximity to the presence of transcription control elements (Blasi et al 2002). Resequencing of 870 bp in 24 individuals of distinct geographical origin revealed polymorphisms at nine positions, eight of which had been reported in the SNP DataBank (Malaspina et al 2009). Haplotype reconstruction revealed six possible arrangements for these eight SNPs in the promoter region of the ALDH5A1 gene. Significant LD was detected within, and between, this region and the coding sequence, resulting in the potential for only limited promoter-coding arrangements. Constructs with the six different promoter haplotypes were cloned upstream of the luciferase gene and assayed to evaluate the corresponding functional effects. Considerable variation in the activity of the reporter gene was found, thus confirming that these variant positions contribute distinct effects on the transcriptional levels of the enzyme (Malaspina et al 2009). Perusal of the level of polymorphism for the eight SNPs that were identified in the 870 bp of the promoter region was gained through exploration of a recent DataBank release [dbSNP, Build 144]: four of the polymorphisms reside in the 24 -29% frequency range, while others are much lower, in the 1 - 5% range. The extensive variation in both the regulatory region of the ALDH5A1 gene, combined with the presence of multiple haplotype arrangements altering transcriptional activity, is expected to contribute substantively to the wide range of inter-individual enzyme activity observed in the general population.
Multifactorial traits and ALDH5A1 gene structure
Heterogeneity of the gene structure of ALDH5A1 has been associated with several multifactorial traits, including epilepsy, cognition and developmental delay, ocular and liver function, and response to therapeutics (Table 3). Dervent and colleagues (2004) reported a patient heterozygous for a pathological allele in the ALDH5A1 gene who presented with absence and myoclonic seizures, photoparoxysmal EEG and generalized epileptiform discharges. The homozygously affected proband of this patient manifested an SSADH activity in leucocytes that was 3% of control values, whereas the same activity for the heterozygously affected sib with epilepsy was 23% of the same controls, raising the possibility that reduced catalytic activity altered GABA catabolism in specific CNS regions which associated with neuronal excitability and eventual epileptic discharges. Lorenz and coworkers (2006) noted a significant association for SNP rs1883415 (A>C), located 3.7 kb upstream of the ALDH5A1 gene, with an excess of carriers with the A allele in individuals with IGE (idiopathic generalized epilepsy; Table 3). The potential role of this SNP was further examined in patients with mesial temporal lobe epilepsy (mTLE) (Pernhorst et al 2011). The genotype frequencies for rs1883415 differed significantly between patients with mTLE vs. healthy controls, with the C allele over-represented in the epileptic cohort. Additionally, expression analysis in hippocampal biopsy specimens of mTLE patients revealed higher ALDH5A1 mRNA expression in the C vs. A allele homozygotes. Lorenz and colleagues postulated that ALDH5A1 expression was dependent upon the allelic variant at rs1883415. Accordingly, an increased ALDH5A1 gene expression would be expected in the hippocampus of mTLE patients homozygous for the C allele, whereas the predominant frequency of the A allele detected in IGE patients (Lorenz et al 2006) would lead to lowered SSADH protein production in the brain, the latter supporting the hypothesis for reduced GABA turnover in IGE.
Multiple investigators (Blasi et al 2002; 2006; Plomin et al 2014) have demonstrated that the c.538C allele (p.180H) of the ALDH5A1 gene (see above), which results in the production of an SSADH enzyme with higher activity than that of the alternative T allele (p.180Y) (Table 3), associates with cognitive performance. Further, the homozygous T/T genotype for the c.538C>T polymorphism was over-represented in individuals with impaired cognitive function, suggesting that decreased SSADH enzyme activity associated with that genotype may induce cerebral oxidative stress while accelerating the rate of cerebral damage and decreasing the quality of life for patients as they age (De Rango et al 2008; Chabris et al 2012). Additionally, both hyper- and hypoactivity of the ALDH5A1 protein is associated with neurological sequelae and/or deficits. Siggberg and coworkers (2011) described a family with three patients manifesting mild developmental delay and atypical attacks of lowered consciousness (MDD/LC). Comparative genomic hybridization (CGH) analyses revealed a duplicated region of 0.7 Mb region in 6p22.2 in all three sibs, spanning ALDH5A1, DCDC2 (doublecortin domain-containing protein 2) and KIAA0319 (encoding a cell membrane protein involved in neuronal migration) genes, the latter two associated with dyslexia. All three probands carried a partial duplication of DCDC2 and a complete duplication of KIAA0319. Molecular analysis of the ALDH5A1 coding region excluded the occurrence of known pathological mutations. Lymphoblasts derived from the three sibling revealed an SSADH enzyme hyperactivity, suggesting that the most likely candidate gene associated with clinical symptoms (MDD/LC) was ALDH5A1.
Variation of ALDH5A1 gene structure has been implicated in the etiology of ocular phoria, the latter representing a misalignment of the eyeballs that occurs sporadically, such as when the synchronization between the eyes is broken by covering one eye (Bosten et al 2014). In their study, Bosten and colleagues found that near horizontal phoria was strongly associated with the SNP rs1569579 T>C located in the fifth intron of the ALDH5A1 gene. Genotyping of individuals for rs1569579 identified the association of near horizontal phoria with TC and CC genotypes, and showed that the second copy of the C allele shifts phoria by 0.45 SD (standard deviation) in the direction of esophoria (a condition characterised by inward deviation of the eye, usually due to extra-ocular muscle imbalance). These findings are relevant to the identification of strabismus in SSADHD (Gibson et al 1997; Pearl et al 2003). Moreover, Chambers et al (2011) reported on the association of ALDH5A1 variation with levels of hepatic enzymes in plasma (e.g., alanine transaminase (ALT), alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT)). Employing functional genomic approaches, including gene expression and GRAIL (gene relationships across implicated loci) analyses, several metabolic pathways and interrelated genes were identified, among them the metabolic pathways associated with both GABA (and ALDH5A1) and glutathione homeostasis. Specifically, the rs1883415 SNP resulted in a “sentinel” non-coding SNP, localized upstream of the ALDH5A1 gene, which may have relevance to decreased glutathione levels in the brain of the murine model of SSADHD (see above) (Gibson et al 2006)
Fonseca and collaborators (2013) reported that ALDH5A1 gene variation can influence the response to methadone maintenance treatment (MMT) for opioid-dependent patients (Veilleux et al 2010). These authors found that individuals carrying the T allele of the c.538 C>T SNP have a higher risk to be non-responsive to methadone intervention. This finding might be correlated with reduced SSADH enzyme activity associated with TC and TT genotypes, potentially altering intracerebral GABA and GHB levels and a potentially enhanced metabolic interference with the role of methadone in blockade of opioid pathways (Antoniou and Tseng 2002). Further, SanGiovanni and colleagues (2014) identified a significant association between AMD (age-related macular degeneration) and ALDH5A1 gene structure. AMD patients showed different allele frequencies with respect to controls for three SNPs localized in intron 7 of ALDH5A1, and a second SNP in intron 8, pointing to an interaction of GABA metabolism with AMD. Of interest, the visual field toxicity associated with use of the antiepileptic agent vigabatrin, whose mode of action is irreversible inactivation of the ABAT protein (associated with GABA accumulation) would be further consistent with this role for GABA and its metabolism in visual field disorders such as AMD (Pellock 2011).
Concluding remarks
In over 35 years of investigation on SSADHD, it is abundantly clear that the more we seem to learn about this rare disorder, the more we realize there is so much to unravel. In other words, the onion is at best “partially peeled”. While initially thought to be a simple, monogenic disorder of GABA metabolism, current data reveals a disorder of broad phenotypic and genotypic heterogeneity with a surprisingly complex pattern of pathophysiology. Is GABA the culprit? Is GHB the culprit? How many other systems may be involved, metabolic pathways impacted or compromised, and would there ever be an approach to mitigate all of the potential neuropathology? Such observations again form the underpinning of a magic-bullet approach for a disease such as SSADHD, and such a “magic bullet” is highly unlikely. Rather, targeted approaches that lead to incremental improvements in neurological function and quality of life will certainly be the objective, with the ever present goal of minimizing adverse effects associated with polypharmacy.
As stated in the 1600s by the physician William Harvey, a pioneer in understanding human physiology who first documented the systemic circulation, nature reveals itself quite eloquently in rare disorders. The study of these “rare” disorders offers rich insight into the nature of normal human physiology. For example, the breadth of oxidative stress associated with SSADHD would not have been considered in the 1980s. Likewise, an effect of GABA in the pathways of autophagy and mitophagy would be completely unexpected, since GABA was only considered an inhibitory neurotransmitter (a fairly significant role in physiology in itself), but the more we learn about neuromodulators such as GABA and GHB, the more we find ourselves asking additional questions that can only serve to extend our knowledge base, both in genetics, medicine, and cell biology.
GWAS and metabolomic analyses have revealed the involvement of common variants in the ALDH5A1 gene which could well confer susceptibility to a number of multifactorial traits. Multiple common variants in the general population appear to represent moderate-risk alleles for multifactorial traits and would explain a component of the variability observed in complex phenotypes. With respect to SSADHD, these multifactorial linkages might provide a new range of insight into disease management. As an example, we can see the potential outcome of multifactorial linkages in the treatment of SSADHD with vigabatrin. Biochemical data in the cerebrospinal fluid of patients receiving high-dose vigabatrin supports the biochemical effect (lowered GHB, elevated GABA; Gibson et al 1995), yet the clinical response to vigabatrin is highly variable, with some patients showing benefit, others not showing benefit, and even some patients demonstrating clinical deterioration on this agent (Good 2011; Pellock 2011; Escalera et al 2010; Casarano et al 2011; Matern et al 1996; Al-Essa et al 2000). The recent identification of another aldehyde dehydrogenase in addition to ALDH5A1 (e.g., ALDH1A1) which mediates GABAergic neurotransmission pathway in non-GABAergic neurons of mammalian brain adds another layer to the complexity of understanding of the pathophysiology of SSADHD (Kim et al 2015).
Finally, association studies of multifactorial traits with ALDH5A1 gene structure holds additional promise to reveal biological pathways involved in complex traits. Those studies will likely lead investigators to a search for less frequent SNPs associated with greater effects on the phenotypes of patients with multifactor traits, as well as patients with SSADHD. The genomic approach also offers the potential to reveal new therapeutic targets and pathways that might mitigate the SSADHD neurological phenotype. At the end of the day, these convergent studies (genomic, pharmacogenomic, and metabolic) will provide the integrated therapeutic strategy for SSADHD that is the goal of researchers and families engaged in the study of this rare “monogenic” disorder of GABA metabolism.
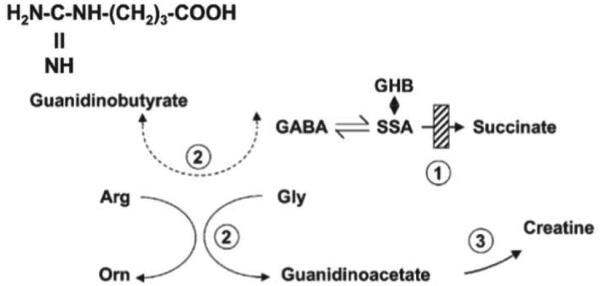
Fig. 3. Interrelationships of GABA and creatine metabolism.
Numbered enzymes include: 1, SSADH; 2, arginine-glycine amidinotransferase (AGAT); and 3, guanidinoacetate methyltransferase. The dashed line represents the probable conversion of accumulated GABA to guanidinobutyrate via the action of AGAT, as previously reported (Watanabe et al 1994). Abbreviations: SSA, succinate semialdehyde; GHB, gamma-hydroxybutyrate; GABA, gamma-aminobutyrate; Gly, glycine; Arg, arginine; Orn, ornithine. The structure of guanidinobutyrate is also shown.
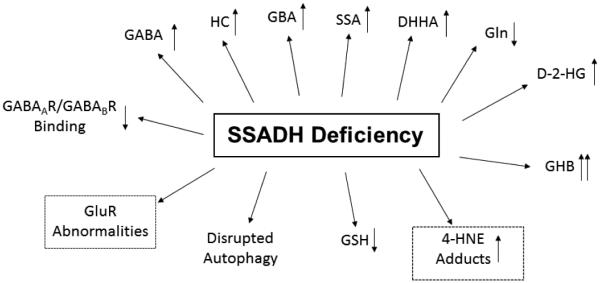
Fig. 4. Pathophysiological alterations in heritable SSADH deficiency.
Arrows indicate the direction and magnitude of metabolic disturbance. Abbreviations: 4-HNE, 4-hydroxyl-2-nonenal; GSH, glutathione; GluR, glutamate receptors; GABAAR/GABABR, GABAA and GABAB receptors; HC, homocarnosine (the dipeptide of GABA:L-histidine); GBA, guanidinobutyrate; Gln, glutamine. See Fig. 2 for other metabolic interconversions associated with GABA. 4-HNE is a major by-product of lipid peroxidation, and is metabolized by SSADH in brain. The dashed boxes indicate recent findings from the knockout mouse model which are not yet published, but are consistent with earlier reports in SSADHD (Pearl et al 2009; Reis et al 2012; Wu et al 2006; Buzzi et al 2006).
HIGHLIGHTS.
Aspects of the current knowledge of the role of the SSADH enzyme are summarized Pathological and polymorphic variations are responsible for SSADH enzyme variability Integrated genomics and metabolomic approaches for treatment of SSADHD patients
Acknowledgements
The authors acknowledge the support of NIH NS 82286 and NS 85369, the SSADH Association (www.ssadh.net), and the assistance of Speragen, Inc., during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absalom N, Eghorn LF, Villumsen IS, Karim N, Bay T, Olsen JV, Knudsen GM, Bräuner-Osborne H, Frølund B, Clausen RP, Chebib M, Wellendorph P. α4βδ GABA(A) receptors are high-affinity targets for γ-hydroxybutyric acid (GHB) Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13404–9. doi: 10.1073/pnas.1204376109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MT, Munasinghe J, Pearl PL, et al. Cerebellar atrophy in human and murine succinic semialdehyde dehydrogenase deficiency. J Child Neurol. 2010;25:1457–61. doi: 10.1177/0883073810368137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Essa MA, Bakheet SM, Patay ZJ, Powe JE, Ozand PT. Clinical, fluorine-18 labeled 2-fluoro-2-deoxyglucose positron emission tomography (FDG PET) MRI of the brain and biochemical observations in a patient with 4-hydroxybutyric aciduria; a progressive neurometabolic disease. Brain Dev. 2000;22:127–31. doi: 10.1016/s0387-7604(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Ainslie GR, Gibson KM, Vogel KR. Maiese K, editor. mTOR, autophagy, aminoacidopathies, and human genetic disorders. Molecules to Medicine with mTOR, II. mTOR in Genetic Disorders and Neurodegenerative Disease. 2016:143–166. chap. 9. [Google Scholar]
- Akaboshi S, Hogema BM, Novelletto A, Malaspina P, Salomons GS, Maropoulos GD, Jakobs C, Grompe M, Gibson KM. Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease-causing mutations in patients with SSADH deficiency. Hum. Mutat. 2003;22:442–450. doi: 10.1002/humu.10288. [DOI] [PubMed] [Google Scholar]
- Andriamampandry C1, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB J. 2007 Mar;21(3):885–95. doi: 10.1096/fj.06-6509com. [DOI] [PubMed] [Google Scholar]
- Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002 Oct;36(10):1598–613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007 Mar;28(3):414–23. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bay T, Eghorn LF, Klein AB, Wellendorph P. GHB receptor targets in the CNS: Focus on high-affinity binding sites. Biochem Pharmacol. 2014;87(2):220–8. doi: 10.1016/j.bcp.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Bekri S, Fossoud C, Plaza G, Guenne A, Salomons GS, Jakobs C, Van Obberghen E. The molecular basis of succinic semialdehyde dehydrogenase deficiency in one family. Mol Genet Metab. 2004 Apr;81(4):347–51. doi: 10.1016/j.ymgme.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Blasi P, Palmerio F, Aiello A, Rocchi M, Malaspina P, Novelletto A. SSADH variation in primates: intra- and interspecific data on a gene with a potential role in human cognitive functions. J. Mol. Evol. 2006;63:54–68. doi: 10.1007/s00239-005-0154-8. [DOI] [PubMed] [Google Scholar]
- Blasi P, Pilo-Boyl P, Ledda M, Novelletto A, Gibson KM, Jakobs C, Hogema B, Akaboshi S, Loreni F, Malaspina P. Structure of human succinic semialdehyde dehydrogenase gene: identification of promoter region and alternatively processed isoforms. Mol. Genet. Metab. 2002;76:348–362. doi: 10.1016/s1096-7192(02)00105-1. [DOI] [PubMed] [Google Scholar]
- Bosten JM, Hogg RE, Bargary G, Goodbourn PT, Lawrance-Owen AJ, Mollon JD. Suggestive association with ocular phoria at chromosome 6p22. Invest. Ophthalmol. Vis. Sci. 2014;55:345–352. doi: 10.1167/iovs.13-12879. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br. J. Pharmacol. 2006;147:S109–S119. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi D, Laschi M, Amato L, Bernardini G, Millucci L, Marcolongo R, Cavallo G, Spreafico A, Santucci A. Evaluation of anti-oxidant treatments in an in vitro model of alkaptonuric ochronosis. Rheumatology (Oxford) 2010;49:1975–83. doi: 10.1093/rheumatology/keq175. [DOI] [PubMed] [Google Scholar]
- Brown GK, Cromby CH, Manning NJ, Pollitt RJ. Urinary organic acids in succinic semialdehyde dehydrogenase deficiency: evidence of alpha-oxidation of 4-hydroxybutyric acid, interaction of succinic semialdehyde with pyruvate dehydrogenase and possible secondary inhibition of mitochondrial beta-oxidation. J Inherit Metab Dis. 1987;10(4):367–75. doi: 10.1007/BF01799979. [DOI] [PubMed] [Google Scholar]
- Buzzi A, Wu Y, Frantseva MV, Velazquez JLP, Cortez MA, Liu CC, Shen LQ, Gibson KM, Snead OC., II Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Casarano M, Alessandri MG, Salomons GS, et al. Efficacy of vigabatrin intervention in a mild phenotypic expression of succinic semialdehyde dehydrogenase deficiency. JIMD Rep. 2011;2:119–123. doi: 10.1007/8904_2011_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Pibiri F, Carboni G, Piras AP. A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor. CNS Drug Rev. 2004;10(3):243–260. doi: 10.1111/j.1527-3458.2004.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris CF, Hebert BM, Benjamin DJ, Beauchamp J, Cesarini D, van der Loos M, Johannesson M, Magnusson PK, Lichtenstein P, Atwood CS, Freese J, Hauser TS, Hauser RM, Christakis N, Laibson D. Most reported genetic associations with general intelligence are probably false positives. Psychol. Sci. 2012;23:1314–1323. doi: 10.1177/0956797611435528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, Holm H, Sanna S, Kavousi M, Baumeister SE, Coin LJ, Deng G, Gieger C, Heard-Costa NL, Hottenga JJ, Kühnel B, Kumar V, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Gibson KM. Succinic semialdehyde dehydrogenase from mammalian brain: subunit analysis using polyclonal antiserum. Int J Biochem. 1992 Sep;24(9):1493–9. doi: 10.1016/0020-711x(92)90077-e. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Lee CF, Ogier H, Rabier D, Jakobs C, Gibson KM. Enzymatic and immunological demonstration of normal and defective succinic semialdehyde dehydrogenase activity in fetal brain, liver and kidney. J Inherit Metab Dis. 1993;16(3):523–6. doi: 10.1007/BF00711671. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Zhang YA, Rossier E, Vollmer B, Gibson KM. Enzymatic and immunologic identification of succinic semialdehyde dehydrogenase in rat and human neural and nonneural tissues. J Neurochem. 1995 Aug;65(2):851–5. doi: 10.1046/j.1471-4159.1995.65020851.x. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Hinson DD, Trettel F, Malaspina P, Novelletto A, Jakobs C, Gibson KM. Two exon-skipping mutations as the molecular basis of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria) Am. J. Hum. Genet. 1998;63:399–408. doi: 10.1086/301964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Wu Y, Gibson KM, Snead OC. Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–53. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- da Rosa MS, Seminotti B, Amaral AU, Parmeggiani B, de Oliveira FH, Leipnitz G, Wajner M. Disruption of redox homeostasis and histopathological alterations caused by in vivo intrastriatal administration of D-2-hydroxyglutaric acid to young rats. Neuroscience. 2014 Sep 26;277:281–93. doi: 10.1016/j.neuroscience.2014.07.011. [DOI] [PubMed] [Google Scholar]
- De Rango F, Leone O, Dato S, Novelletto A, Bruni AC, Berardelli M, Mari V, Feraco E, Passarino G, De Benedictis G. Cognitive functioning and survival in the elderly: the SSADH C538T polymorphism. Ann. Hum. Genet. 2008;72:630–635. doi: 10.1111/j.1469-1809.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- Dervent A, Gibson KM, Pearl PL, Salomons GS, Jakobs C, Yalcinkaya C. Photosensitive absence epilepsy with myoclonias and heterozygosity for succinic semialdehyde dehydrogenase (SSADH) deficiency. Clin. Neurophysiol. 2004;115:1417–1422. doi: 10.1016/j.clinph.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Dósa Z, Nieto-Gonzalez JL, Korshoej AR, Gibson KM, Jensen K. Effect of gene dosage on single-cell hippocampal electrophysiology in a murine model of SSADH deficiency (gamma-hydroxybutyric aciduria) Epilepsy Res. 2010 Jun;90(1-2):39–46. doi: 10.1016/j.eplepsyres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Vardya I, Delenclos M, Gibson KM, Jensen K. SSADH deficiency leads to elevated extracellular GABA levels and increased GABAergic neurotransmission in the mouse cerebral cortex. J Inherit Metab Dis. 2008 Dec;31(6):662–8. doi: 10.1007/s10545-008-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenzinger K, Jeschke R, Frauendienst-Egger G, Korall H, Gibson KM, Schuster VH. Monitoring of 40hydroxybutyric acid levels in body fluids during vigabatrin treatment in succinic semialdehyde dehydrogenase deficiency. Ann. Neurol. 2003;54:686–689. doi: 10.1002/ana.10752. [DOI] [PubMed] [Google Scholar]
- Escalera GI, Ferrer I, Marina LC, et al. Succinic semialdehyde dehydrogenase deficiency: decrease in 4-OH-butyric acid levels with low doses of vigabatrin. An Pediatr (Barc) 2010;72:128–32. doi: 10.1016/j.anpedi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Fonseca F, Gratacòs M, Escaramís G, De Cid R, Martín-Santos R, Farré M, Estivill X, Torrens M. ALDH5A1 variability in opioid dependent patients could influence response to methadone treatment. Eur. Neuropsychopharmacol. 2014;24:420–424. doi: 10.1016/j.euroneuro.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Froestl W, Gallagher M, Jenkins H, et al. SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem Pharmacol. 2004;68:1479–87. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Froger N, Moutsimilli L, Cadetti L, Jammoul F, Wang QP, Fan Y, Gaucher D, Rosolen SG, Neveux N, Cynober L, Sahel JA, Picaud S. Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog Retin Eye Res. 2014 Jul;41:44–63. doi: 10.1016/j.preteyeres.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Gaily E. Vigabatrin monotherapy for infantile spasms. Expert Rev Neurother. 2012;12:275–86. doi: 10.1586/ern.12.3. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. GABAA receptors in normal development and seizures: friends or foes? Curr. Neuropharmacol. 2008;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Sweetman L, Nyhan WL, et al. Succinic semialdehyde dehydrogenase deficiency: an inborn error of gamma-aminobutyric acid metabolism. Clin Chim Acta. 1983;133:33–42. doi: 10.1016/0009-8981(83)90018-9. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Christensen E, Jakobs C, Fowler B, Clarke MA, Hammersen G, Raab K, Kobori J, Moosa A, Vollmer B, Rossier E, Iafolla AK, Matern D, Brouwer OF, Finkelstein J, Aksu F, Weber HP, Bakkeren JA, Gabreels FJ, Bluestone D, Barron TF, Beauvais P, Rabier D, Santos C, Lehnert W, et al. The clinical phenotype of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria): case reports of 23 new patients. Pediatrics. 1997;99:567–574. doi: 10.1542/peds.99.4.567. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Lee CF, Chambliss KL, Kamali V, Francois B, Jaeken J, Jakobs C. 4-Hydroxybutyric aciduria: application of a fluorometric assay to the determination of succinic semialdehyde dehydrogenase activity in extracts of cultured human lymphoblasts. Clin. Chim. Acta. 1991;196:219–221. doi: 10.1016/0009-8981(91)90076-o. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Jakobs C, Ogier H, et al. Vigabatrin therapy in six patients with succinic semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 1995;18:143–6. doi: 10.1007/BF00711750. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Schor DS, Gupta M, Guerand WS, Senephansiri H, Burlingame TG, Bartels H, Hogema BM, Bottiglieri T, Froestl W, Snead OC, Grompe M, Jakobs C. Focal neurometabolic alterations in mice deficient for succinate semialdehyde dehydrogenase. J Neurochem. 2002;81:71–9. doi: 10.1046/j.1471-4159.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Gupta M, Pearl PL, Tuchman M, Vezina LG, Snead OC, 3rd, Smit LM, Jakobs C. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (gamma-hydroxybutyric aciduria) Biol Psychiatry. 2003;54:763–768. doi: 10.1016/s0006-3223(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Gupta M, Senephansiri H, Jansen EEW, Montine TJ, Hyland K, Switzer RC, Snead OC, Jakobs C. Oxidant stress and neurodegeneration in murine succinic semialdehyde dehydrogenase (SSADH) deficiency. In: Hoffmann GF, editor. Diseases of Neurotransmission-from bench to bed. SPS Verlagsgessellschaft mbH; Heilbronn, Germany: 2006. pp. 199–212. Symposia Proceedings. [Google Scholar]
- Good WV. Measuring field loss in children administered vigabatrin: a problem in search of a solution. J AAPOS. 2011;15:411–2. doi: 10.1016/j.jaapos.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Greiner HM, Lynch ER, Fordyce S, et al. Vigabatrin for childhood partial-onset epilepsies. Pediatr Neurol. 2012;46:83–8. doi: 10.1016/j.pediatrneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Gropman A. Vigabatrin and newer interventions in succinic semialdehyde dehydrogenase deficiency. Ann Neurol. 2003;54(Suppl 6):S66–72. doi: 10.1002/ana.10626. [DOI] [PubMed] [Google Scholar]
- Gogou M, Spilioti M, Tramma D, Papadopoulou-Alataki E, Evangeliou A. Succinic Semialdehyde Dehydrogenase Deficiency Presenting as Autism Spectrum Disorder. Indian J Pediatr. 2016 Jan 25; doi: 10.1007/s12098-015-2003-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gupta M, Greven R, Jansen EE, Jakobs C, Hogema BM, Froestl W, Snead OC, Bartels H, Grompe M, Gibson KM. Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutyric aciduria) J Pharmacol Exp Ther. 2002;302:180–7. doi: 10.1124/jpet.302.1.180. [DOI] [PubMed] [Google Scholar]
- Gupta M, Polinsky M, Senephansiri H, Snead OC, Jansen EE, Jakobs C, Gibson KM. Seizure evolution and amino acid imbalances in murine succinate semialdehyde dehydrogenase (SSADH) deficiency. Neurobiol Dis. 2004;16(3):556–62. doi: 10.1016/j.nbd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Heaney CF, Kinney JW. Role of GABAB receptors in learning and memory and neurological disorders. Neurosci Biobehav Rev. 2016 Apr;63:1–28. doi: 10.1016/j.neubiorev.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Hempel J, Nicholas H, Lindahl R. Aldehyde dehydrogenases: widespread structural and functional diversity within a shared framework. Protein Sci. 1993 Nov;2(11):1890–900. doi: 10.1002/pro.5560021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogema BM, Gupta M, Senephansiri H, et al. Pharmacologic rescue of lethal seizures in mice deficient in succinate semialdehyde dehydrogenase. Nat Genet. 2001;29:212–6. doi: 10.1038/ng727. [DOI] [PubMed] [Google Scholar]
- Horino A, Kawawaki H, Fukuoka M, Tsuji H, Hattori Y, Inoue T, Nukui M, Kuki I, Okazaki S, Tomiwa K, Hirose S. A case of succinic semialdehyde dehydrogenase deficiency with status epilepticus and rapid regression. Brain Dev. 2016 Apr 22; doi: 10.1016/j.braindev.2016.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ilbert M, Horst J, Ahrens S, Winter J, Graf PC, Lilie H, Jakob U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat Struct Mol Biol. 2007;14:556–63. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. Specific roles of GABA(B(1)) receptor isoforms in cognition. Behav Brain Res. 2007 Jul 19;181(1):158–62. doi: 10.1016/j.bbr.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs C, Bojasch M, Mönch E, Rating D, Siemes H, Hanefeld F. Urinary excretion of γ-hydroxybutyric acid in a patient with neurological abnormalities. The probability of a new inborn error of metabolism. Clin Chim Acta. 1981;111:169–178. doi: 10.1016/0009-8981(81)90184-4. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Verhoeven NM, Jakobs C, Schulze A, Senephansiri H, Gupta M, Snead OC, Gibson KM. Increased guanidino species in murine and human succinate semialdehyde dehydrogenase (SSADH) deficiency. Biochim Biophys Acta. 2006 Apr;1762(4):494–8. doi: 10.1016/j.bbadis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Struys E, Jakobs C, Hager E, Snead OC, Gibson KM. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. BMC Dev Biol. 2008 Nov 28;8:112. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SZ, Shu JB, Zhang YQ, Fan WX, Meng YT, Song L. Analysis of ALDH5A1 gene mutation in a Chinese Han family with succinic semialdehyde dehydrogenase deficiency. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013 Aug;30(4):389–93. doi: 10.3760/cma.j.issn.1003-9406.2013.04.002. Chinese. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Boehm SL., 2nd Identifying the role of pre-and postsynaptic GABA(B) receptors in behavior. Neurosci Biobehav Rev. 2015 Oct;57:70–87. doi: 10.1016/j.neubiorev.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Pearl PL, Jensen K, Snead OC, Malaspina P, Jakobs C, Gibson KM. Succinic semialdehyde dehydrogenase: biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance. Antioxid. Redox. Signal. 2011;15:691–718. doi: 10.1089/ars.2010.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Lee S, Kwon OS, Park SY, Lee SJ, Park BJ, Kim KJ. Redox-switch modulation of human SSADH by dynamic catalytic loop. EMBO J. 2009;28:959–968. doi: 10.1038/emboj.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Chen L, Ding JB. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015 Oct 2;350(6256):102–6. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, Mobley WC. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci. 2012 Jul 4;32(27):9217–27. doi: 10.1523/JNEUROSCI.1673-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr I, Gibson KM, Ganesh J, Bennett MJ, Salomons GS, Jakobs C, Myers SM. Diagnostic challenges in a severely delayed infant with hypersomnolence, failure to thrive and arteriopathy: a unique case of gamma-hydroxybutyric aciduria and Williams syndrome. Am J Med Genet B Neuropsychiatr Genet. 2007 Oct 5;144B(7):946–8. doi: 10.1002/ajmg.b.30553. [DOI] [PubMed] [Google Scholar]
- Knerr I, Gibson KM, Jakobs C, Pearl PL. Neuropsychiatric morbidity in adolescent and adult succinic semialdehyde dehydrogenase deficiency patients. CNS Spectr. 2008 Jul;13(7):598–605. doi: 10.1017/s1092852900016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr I, Gibson KM, Murdoch G, Salomons GS, Jakobs C, Combs S, Pearl PL. Neuropathology in succinic semialdehyde dehydrogenase deficiency. Pediatr Neurol. 2010 Apr;42(4):255–8. doi: 10.1016/j.pediatrneurol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JS, Yuen CL, Law LK, Tang NL, Cherk SW, Yuen YP. A novel ALDH5A1 mutation in a patient with succinic semialdehyde dehydrogenase deficiency. Pathology. 2012 Apr;44(3):280–2. doi: 10.1097/PAT.0b013e32835140c2. [DOI] [PubMed] [Google Scholar]
- Lakhani R, Vogel KR, Till A, Liu J, Burnett SF, Gibson KM, Subramani S. Defects in GABA metabolism affect selective autophagy pathways and are alleviated by mTOR inhibition. EMBO Mol Med. 2014 Apr;6(4):551–66. doi: 10.1002/emmm.201303356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalme-Remis S, Lewis EC, De Meulemeester C, Chakraborty P, Gibson KM, Torres C, Guberman A, Salomons GS, Jakobs C, Ali-Ridha A, Parviz M, Pearl PL. Natural history of succinic semialdehyde dehydrogenase deficiency through adulthood. Neurology. 2015 Sep 8;85(10):861–5. doi: 10.1212/WNL.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini A, Scussiato K, Rosa RB, Llesuy S, Belló-Klein A, Dutra-Filho CS, Wajner M. D-2-hydroxyglutaric acid induces oxidative stress in cerebral cortex of young rats. Eur J Neurosci. 2003;17:2017–22. doi: 10.1046/j.1460-9568.2003.02639.x. [DOI] [PubMed] [Google Scholar]
- Latini A, Scussiato K, Leipnitz G, Gibson KM, Wajner M. Evidence for oxidative stress in tissues derived from succinate semialdehyde dehydrogenase-deficient mice. J Inherit Metab Dis. 2007;30:800–10. doi: 10.1007/s10545-007-0599-6. [DOI] [PubMed] [Google Scholar]
- Lemes A1, Blasi P, Gonzales G, Russi ME, Quadrelli R, Novelletto A, Malaspina P. Succinic semialdehyde dehydrogenase (SSADH) deficiency: Molecular analysis in a South American family. J Inherit Metab Dis. 2006 Aug;29(4):587. doi: 10.1007/s10545-006-0277-0. Epub 2006 Jun 19. [DOI] [PubMed] [Google Scholar]
- Li X, Ding Y, Liu Y, Zhang Y, Song J, Wang Q, Li M, Qin Y, Huang S, Yang Y. Succinic semialdehyde dehydrogenase deficiency of four Chinese patients and prenatal diagnosis for three fetuses. Gene. 2015 Dec 10;574(1):41–7. doi: 10.1016/j.gene.2015.07.078. [DOI] [PubMed] [Google Scholar]
- Lin CY, Weng WC, Lee WT. A novel mutation of ALDH5A1 gene associated with succinic semialdehyde dehydrogenase deficiency. J Child Neurol. 2015 Mar;30(4):486–9. doi: 10.1177/0883073814544365. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Taylor KP, Gehrmann A, Muhle H, Gresch M, Becker T, Tauer U, Stephani U, Sander T. Candidate gene analysis of the succinic semialdehyde dehydrogenase gene (ALDH5A1) in patients with idiopathic generalized epilepsy and photosensitivity. Neurosci. Lett. 2006;397:234–239. doi: 10.1016/j.neulet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signaling system in brain: organization and functional implications. Prog. Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Maitre M, Klein C, Mensah-Nyagan AG. Mechanisms for the Specific Properties of γ-Hydroxybutyrate in Brain. Med Res Rev. 2016 Apr;36(3):363–88. doi: 10.1002/med.21382. [DOI] [PubMed] [Google Scholar]
- Malaspina P, Picklo MJ, Jakobs C, Snead OC, Gibson KM. Comparative genomics of aldehyde dehydrogenase 5a1 (succinate semialdehyde dehydrogenase) and accumulation of gamma-hydroxybutyrate associated with its deficiency. Hum. Genomics. 2009;3:106–120. doi: 10.1186/1479-7364-3-2-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern D, Lehnert W, Gibson KM, Korinthenberg R. Seizures in a boy with succinic semialdehyde dehydrogenase deficiency treated with vigabatrin (gamma-vinyl-GABA) J Inherit Metab Dis. 1996;19:313–8. doi: 10.1007/BF01799261. [DOI] [PubMed] [Google Scholar]
- Meijer WH, van der Klei IJ, Veenhuis M, Kiel JA. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy. 2007;3:106–116. doi: 10.4161/auto.3595. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H. GABAA receptors in central nervous system disease: anxiety, epilpepsy, and insomnia. J. Recept. Signal Transduct. Res. 2006;26:731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Müller H. Role of GABAA receptors in cognition. Biochem. Soc. Trans. 2009;37:1328–1333. doi: 10.1042/BST0371328. [DOI] [PubMed] [Google Scholar]
- Murphy TC, Amarnath V, Gibson KM, Picklo MJ., Sr Oxidation of 4-hydroxy-2-nonenal by succinic semialdehyde dehydrogenase (ALDH5A) J Neurochem. 2003;86:298–305. doi: 10.1046/j.1471-4159.2003.01839.x. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Müller HW. Cortiocal GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders – results from in vivo imaging studies. Rev. Neurosci. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Novotny EJ, Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54(Suppl 6):S25–31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JL, Likhodii SS, Cortez MA, Shen L, Leshchenko Y, Adeli K, Gibson KM, Burnham WM, Snead OC., 3rd A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008 Apr;210(2):449–57. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviz M, Vogel K, Gibson KM, Pearl PL. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy. 2014 Nov 25;3(4):217–227. doi: 10.3233/PEP-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Novotny EJ, Acosta MT, Jakobs C, Gibson KM. Succinic semialdehyde dehydrogenase deficiency in children and adults. Ann. Neurol. 2003;54(Suppl 6):S73–80. doi: 10.1002/ana.10629. [DOI] [PubMed] [Google Scholar]
- Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005;38:1051–8. doi: 10.1016/j.clinbiochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Cortez MA, Wu Y, Carter Snead O, 3rd, Knerr I, Forester K, Pettiford JM, Jakobs C, Theodore WH. Succinic semialdehyde dehydrogenase deficiency: lessons from mice and men. J Inherit Metab Dis. 2009;32(3):343–52. doi: 10.1007/s10545-009-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Shukla L, Theodore WH, Jakobs C, Gibson KM. Epilepsy in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. Brain Dev. 2011 Oct;33(9):796–805. doi: 10.1016/j.braindev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Parviz M, Vogel K, Schreiber J, Theodore WH, Gibson KM. Inherited disorders of gamma-aminobutyric acid metabolism and advances in ALDH5A1 mutation identification. Dev Med Child Neurol. 2014a Dec 29; doi: 10.1111/dmcn.12668. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Schreiber J, Theodore WH, McCarter R, Barrios ES, Yu J, Wiggs E, He J, Gibson KM. Taurine trial in succinic semialdehyde dehydrogenase deficiency and elevated CNS GABA. Neurology. 2014b;82(11):940–4. doi: 10.1212/WNL.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A, Leoncini S, De Felice C, Signorini C, Cerrone C, Valacchi G, Ciccoli L, Hayek J. Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev. 2013;35(2):146–54. doi: 10.1016/j.braindev.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Pellock JM. Balancing clinical benefits of vigabatrin with its associated risk of vision loss. Acta Neurol Scand Suppl. 2011;192:83–91. doi: 10.1111/j.1600-0404.2011.01603.x. [DOI] [PubMed] [Google Scholar]
- Pernhorst K, Raabe A, Niehusmann P, van Loo KM, Grote A, Hoffmann P, Cichon S, Sander T, Schoch S, Becker AJ. Promoter variants determine γ-aminobutyric acid homeostasis-related gene transcription in human epileptic hippocampi. J. Neuropathol. Exp. Neurol. 2011;70:1080–1088. doi: 10.1097/NEN.0b013e318238b9af. [DOI] [PubMed] [Google Scholar]
- Plomin R, Turic DM, Hill L, Turic DE, Stephens M, Williams J, Owen MJ, O'Donovan MC. A functional polymorphism in the succinate-semialdehyde dehydrogenase (aldehyde dehydrogenase 5 family, member A1) gene is associated with cognitive ability. Mol. Psychiatry. 2004;9:582–586. doi: 10.1038/sj.mp.4001441. [DOI] [PubMed] [Google Scholar]
- Püttmann L, Stehr H, Garshasbi M, Hu H, Kahrizi K, Lipkowitz B, Jamali P, Tzschach A, Najmabadi H, Ropers HH, Musante L, Kuss AW. A novel ALDH5A1 mutation is associated with succinic semialdehyde dehydrogenase deficiency and severe intellectual disability in an Iranian family. Am J Med Genet A. 2013 Aug;161A(8):1915–22. doi: 10.1002/ajmg.a.36030. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Reis J, Cohen LG, Pearl PL, Fritsch B, Jung NH, Dustin I, Theodore WH. GABAB-ergic motor cortex dysfunction in SSADH deficiency. Neurology. 2012 Jul 3;79(1):47–54. doi: 10.1212/WNL.0b013e31825dcf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzlak MT, Pietruszko R. Human brain "high Km" aldehyde dehydrogenase: purification, characterization, and identification as NAD+ -dependent succinic semialdehyde dehydrogenase. Arch Biochem Biophys. 1988 Nov 1;266(2):386–96. doi: 10.1016/0003-9861(88)90270-6. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. Clinical applications of age-related macular degeneration genetics. Cold Spring Harb Perspect Med. 2014 Aug 14;4(10):a017228. doi: 10.1101/cshperspect.a017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer SW, Kölker S, Hoffmann GF, Ten Brink HJ, Jakobs C, Gibson KM, Okun JG. Enzymatic and metabolic evidence for a region specific mitochondrial dysfunction in brains of murine succinic semialdehyde dehydrogenase deficiency (Aldh5a1−/− mice) Neurochem Int. 2007;50:653–9. doi: 10.1016/j.neuint.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Sgaravatti AM, Sgarbi MB, Testa CG, Durigon K, Pederzolli CD, Prestes CC, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS. Gamma-hydroxybutyric acid induces oxidative stress in cerebral cortex of young rats. Neurochem Int. 2007;50:564–70. doi: 10.1016/j.neuint.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sheikh S, Ni L, Hurley TD, Weiner H. The potential roles of the conserved amino acids in human liver mitochondrial aldehyde dehydrogenase. J Biol Chem. 1997 Jul 25;272(30):18817–22. doi: 10.1074/jbc.272.30.18817. [DOI] [PubMed] [Google Scholar]
- Siggberg L, Mustonen A, Schuit R, Salomons GS, Roos B, Gibson KM, Jakobs C, Ignatius J, Knuutila S. Familial 6p22.2 duplication associates with mild developmental delay and increased SSADH activity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:448–453. doi: 10.1002/ajmg.b.31180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simler S, Ciesielski L, Klein M, Gobaille S, Mandel P. Mechanism of action of an anticonvulsant, sodium dipropylacetate. C R Seances Soc Biol Fil. 1981;175(1):114–9. French. [PubMed] [Google Scholar]
- Singh D, Jethani SL, Dubey A. Vigabatrin induced Cell loss in the Cerebellar Cortex of Albino Rats. J Clin Diagn Res. 2013 Nov;7(11):2555–8. doi: 10.7860/JCDR/2013/6187.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC., 3rd Gamma hydroxybutyrate in the monkey. IV. Dopaminergic mechanisms. Neurology. 1978 Nov;28(11):1179–82. doi: 10.1212/wnl.28.11.1179. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Morley BJ. Ontogeny of gamma-hydroxybutyric acid. I. Regional concentration in developing rat, monkey and human brain. Brain Res. 1981;227:579–89. doi: 10.1016/0165-3806(81)90010-9. [DOI] [PubMed] [Google Scholar]
- Szabadi E. Drugs for sleep disorders: mechanisms and therapeutic prospects. Br. J. Clin. Pharmacol. 2006;61:761–766. doi: 10.1111/j.1365-2125.2006.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamazian G, Ho Chang J, Knyazev S, Stepanov E, Kim KJ, Porozov Y. Modeling conformational redox-switch modulation of human succinic semialdehyde dehydrogenase. Proteins. 2015 Dec;83(12):2217–29. doi: 10.1002/prot.24937. doi: 10.1002/prot.24937. Epub 2015 Oct 27. [DOI] [PubMed] [Google Scholar]
- Tay CG, Ariffin H, Yap S, Rahmat K, Sthaneshwar P, Ong LC. Succinic Semialdehyde Dehydrogenase Deficiency in a Chinese Boy: A Novel ALDH5A1 Mutation With Severe Phenotype. J Child Neurol. 2015 Jun;30(7):927–31. doi: 10.1177/0883073814540523. [DOI] [PubMed] [Google Scholar]
- Thiesen L, Kehler J, Clausen RP, Frølund B, Bundgaard C, Wellendorph P. In Vitro and In Vivo Evidence for Active Brain Uptake of the GHB Analog HOCPCA by the Monocarboxylate Transporter Subtype 1. J Pharmacol Exp Ther. 2015 Aug;354(2):166–74. doi: 10.1124/jpet.115.224543. [DOI] [PubMed] [Google Scholar]
- Vanadia E, Gibson KM, Pearl PL, Trapolino E, Mangano S, Vanadia F. Therapeutic efficacy of magnesium valproate in succinic semialdehyde dehydrogenase deficiency. JIMD Rep. 2013;8:133–7. doi: 10.1007/8904_2012_170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardya I, Drasbek KR, Gibson KM, Jensen K. Plasticity of postsynaptic, but not presynaptic, GABAB receptors in SSADH deficient mice. Exp Neurol. 2010 Sep;225(1):114–22. doi: 10.1016/j.expneurol.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaya J. Exogenous markers for the characterization of human diseases associated with oxidative stress. Biochimie. 2013 Mar;95(3):578–84. doi: 10.1016/j.biochi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin. Psychol. Rev. 2010;30:155–166. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Pearl PL, Theodore WH, McCarter RC, Jakobs C, Gibson KM. Thirty years beyond discovery-Clinical trials in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. J Inherit Metab Dis. 2013;36:401–410. doi: 10.1007/s10545-012-9499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Jansen EE, Salomons GS, Gibson KM. Torin 1 partially corrects vigabatrin-induced mitochondrial increase in mouse. Ann Clin Transl Neurol. 2015 Jun;2(6):699–706. doi: 10.1002/acn3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. The GABA paradox: multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J. Neurochem. 1999;73:1335–1342. doi: 10.1046/j.1471-4159.1999.0731335.x. [DOI] [PubMed] [Google Scholar]
- Wang KY, Barker PB, Lin DD. A case of acute onset succinic semialdehyde dehydrogenase deficiency: neuroimaging findings and literature review. Childs Nerv Syst. 2015 Oct 24; doi: 10.1007/s00381-015-2942-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Van Pilsum JF, Yokoi I, Mori A. Synthesis of neuroactive guanidino compounds by rat kidney L-arginine: glycine amidinotransferase. Life Sci. 1994;55(5):351–8. doi: 10.1016/0024-3205(94)00645-8. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Høg S, Skonberg C, Bräuner-Osborne H. Phenylacetic acids and the structurally related non-steroidal anti-inflammatory drug diclofenac bind to specific gamma-hydroxybutyric acid sites in rat brain. Fundam Clin Pharmacol. 2009 Apr;23(2):207–13. doi: 10.1111/j.1472-8206.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Buzzi A, Frantseva M, Velazquez JPL, Cortez M, Liu C, Shen L, Gibson KM, Snead OC., III Status epilepticus in mice deficient for succinate semialdehyde dehydrogenase: GABAA receptor-mediated mechanisms. Ann. Neurol. 2006;59:42–52. doi: 10.1002/ana.20686. [DOI] [PubMed] [Google Scholar]
- Yalçinkaya C, Gibson KM, Gündüz E, Koçer N, Fiçicioğlu C, Küçükercan I. MRI findings in succinic semialdehyde dehydrogenase deficiency. Neuropediatrics. 2000;31:45–6. doi: 10.1055/s-2000-15298. [DOI] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyeh S, Berlis A, Korinthenberg R, Spreer J, Schumacher M. Selective involvement of the globus pallidus and dentate nucleus in succinic semialdehyde dehydrogenase deficiency. Pediatr Radiol. 2002;32:598–600. doi: 10.1007/s00247-002-0717-4. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Gibson KM. mTOR inhibitors rescue premature lethality and attenuate dysregulation of GABAergic/glutamatergic transcription in Murine Succinate Semialdehyde Dehydrogenase Deficiency (SSADHD), a disorder of GABA metabolism. J. Inherit. Metab. Dis. doi: 10.1007/s10545-016-9959-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]