1. Introduction
Fibromyalgia (FM) is characterized by widespread pain in the absence of identifiable peripheral pathology.13 Although there is no unanimity regarding the precise pathophysiology of fibromyalgia and related conditions, there is general agreement that this represents some type of central sensitization, and that most individuals with this condition display diffuse hyperalgesia/allodynia, identifiable via both quantitative sensory testing and functional neuroimaging.23; 51 A number of neurotransmitter systems could be partially responsible for this diffuse hyperalgesia/allodynia, including increases in excitatory neurotransmitters (e.g. glutamate29; 56 and Nerve Growth Factor22) and reduced levels of inhibitory neurotransmitters (e.g. GABA,19 norepinephrine55). The fact that these alterations are playing a critical role in fibromyalgia is supported by the fact that CNS-acting drugs that normalize these neurotransmitters are efficacious in subsets of individuals with fibromyalgia.31; 57 To date, the only neurotransmitter system studied in fibromyalgia that is not characterized by increased excitatory and decreased inhibitory influences on pain processing is the endogenous opioid system.
Binding of endogenous opioids to the μ-opioid receptor (MOR) can be inferred indirectly selective radiotracer [11C]-carfentanil using positron emission tomography (PET) 69 and reflects a combination of MOR density and MOR affinity. This technique has shown that increased MOR binding potential (BP) is associated with reduced pain sensitivity and more effective endogenous analgesia in healthy human subjects,28; 52 and we previously reported that regional MOR BP is reduced in the brains of chronic pain patients diagnosed with FM.30 These findings, coupled with data showing elevated endogenous opioid concentrations in the cerebrospinal fluid (CSF) of FM patients,5 has led to speculation that FM is characterized by 1) increased synaptic levels of opioids and fewer unoccupied MORs at rest, 2) down-regulation or decreased efficacy of postsynaptic membrane-embedded MORs, or 3) some combination of the two.30 However, the role that endogenous opioids play in promoting core symptoms of FM, including widespread clinical pain and hyperalgesia to experimental stimuli, has yet to be characterized. This knowledge deficit is particularly striking considering the continued use of opioids in the treatment of FM, despite poor evidence supporting opioid efficacy in this condition and good evidence showing that long-term opioid administration can lead to opioid-induced hyperalgesia (OIH).3; 63
To date, no studies have directly compared pain-evoked brain activity and MOR binding within the same chronic pain patients. To better characterize the relationship between endogenous opioids and neural correlates of pain processing in FM, we measured tonic, resting MOR availability using [11C]-carfentanil PET and the blood oxygenation level dependent (BOLD) effect during evoked pain assessed by functional magnetic resonance imaging (fMRI).14; 25; 30 This allowed us to explore the relationship between MOR availability and dynamic pain responses in patients with deficient MOR availability. This relationship was explored using whole-brain voxel-to-voxel comparisons of BOLD and MOR BP and a targeted approach using regions of interest exhibiting lower BP in FM patients relative to controls.30 To explore the clinical relevance of these findings, we investigated whether opioid availability and pain-evoked activity in FM were associated with self-reported clinical pain.
2. Methods
2.1. Participants and Study Design
18 right-handed female fibromyalgia patients (age 45.4+/− 13.0 years) were studied. These included the 17 patients studied in our 2007 report and an additional patient that lacked a matched control and was not included in that report.30 This study is a new investigation of the relationship between MOR BP and evoked pain brain activation using fMRI which was conducted concurrently but not previously reported. Each patient underwent a 60-minute fMRI scanning session with varying intensities of pressure pain applied to the left thumb as well as a separate 90-minute [11C]-carfentanil PET scan under resting conditions (both scans were conducted within 48 hours of each other). Participants gave written informed consent and all study protocols were approved by the University of Michigan Institutional Review Board and the Radioactive Drug Research Committee.
2.1.1 Inclusion and Exclusion Criteria
All participants (1) met clinical criteria for FM using the American College of Rheumatology Criteria65 with symptoms persisting for at least one year and current pain occurring on at least 50% of days, (2) agreed not to introduce any new medications or treatment modalities during the study, (3) were between 18 and 75 years of age, (4) female, (5) right handed, (6) abstained from alcohol for 48 hours prior to PET imaging and (7) provided written informed consent. Participants were excluded if they (1) used narcotic analgesics in the prior year or had a history of substance abuse, (2) had known coagulation abnormality, thrombocytopenia, or bleeding diathesis, (3) had autoimmune or inflammatory disease (e.g., rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease) known to cause pain, (3) were participating in therapeutic trials, (4) were pregnant or nursing, (5) had severe psychiatric illnesses (e.g., schizophrenia, major depression with suicidal ideation, within prior 2 years) or current major depression, or (6) had contraindications to PET. No participants were currently using or had a history of opioid use. Ten participants were using serotonin reuptake inhibitors or dual serotonin/norepinephrine reuptake inhibitors while eight were not.
2.2. Magnetic Resonance Imaging (MRI): Data Acquisition
Structural and pain-evoked fMRI scans were performed on a 3 tesla scanner (Signa LX; General Electric, Milwaukee, WI). T1-weighted spoiled gradient echo (SPGR) structural images were acquired for spatial registration of the functional data (TE=3.4ms, TR=10.5ms, FA=20°, FOV = 24 cm and 0.94×0.94×1.5 mm voxels) and were inspected revealing no gross morphological abnormalities for any patient.
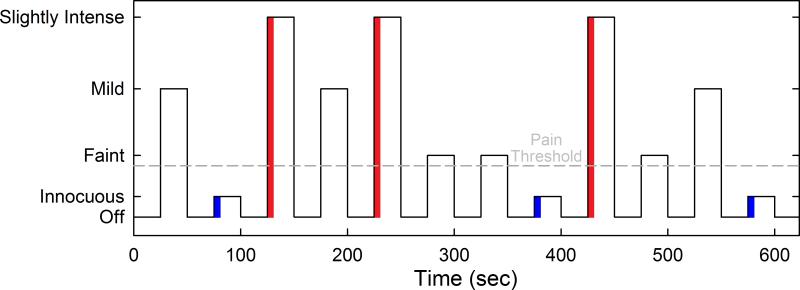
Evoked pain BOLD responses were collected using two identical block design fMRI scans, each lasting 640 sec. Functional MRI images were collected using a T2*-weighted spiral sequence (TR = 2.5 s, TE = 30 ms, FA = 90°, matrix size 64×64 with 48 slices, FOV = 22 cm and 3.44×3.44×3 mm voxels). Evoked pain levels were determined using the 0 -20 Gracely Box Scale (GBS) for pain intensity.51 Prior to scanning, pressure applied to the thumbnail that elicited “faint” (0.5 on the GBS) “mild-moderate” (7.5 on the GBS) and “slightly intense” (13.5 on the GBS) pain were determined for every subject using the multiple random staircase method.24 While FM is characterized by widespread pain, the thumbnail is a largely asymptomatic area in this condition. During the pain-evoked BOLD scanning session, pressure was applied with a computer-controlled pneumatic system to the left thumbnail, via a rubber-tipped piston with a contact surface area of 1 cm2. The three distinct, subject-specific amounts of pressure eliciting each of the three perceived pain levels, and an innocuous (touch) pressure (0.25 kg/cm2) were delivered in pseudo-random fashion and interleaved with a resting condition during which no pressure was applied. Each session included three 25-sec blocks of all pressure intensities. Therefore, all pain and innocuous stimuli were presented a total of six times (See Figure 1 for schematic of experimental design). This number of stimulus presentations has been shown to be sufficient to produce acceptably reliable BOLD signals.61
Figure 1.
Schematic of the fMRI experimental design. The portions of the stimuli used to assess pain related BOLD activity are indicated by red and blue shading.
2.3. Positron Emission Tomography (PET): Data Acquisition
Scans were acquired with a Siemens (Knoxville, TN) HR+ scanner in three-dimensional mode [reconstructed full width at half-maximum (FWHM) resolution, ~5.5 mm in-plane and 5.0 mm axially], with septa retracted and scatter correction. Participants were positioned in the PET scanner gantry, and an intravenous (antecubital) line was placed in the right arm. A light forehead restraint was used to eliminate intra-scan head movement. [11C]-carfentanil was synthesized at high specific activity by the reaction of [11C]-methyliodide and a nonmethyl precursor as described previously.37 15 +/− 1 mCi (555 +/− 55 MBq) were administered during the scan. Receptor occupancy by carfentanil was calculated to be between 0.2 and 0.6% for brain regions with low, intermediate, and high MOR concentrations, based on the mass of carfentanil administered and the known concentration of opioid receptors in the postmortem human brain.20; 27 Fifty percent of the [11C]-carfentanil dose was administered as a bolus, and the remaining 50% was administered by continuous infusion for the remainder of the study. Twenty-eight frames of images were acquired over 90 min with an increasing duration (30 seconds up to 10 minutes).
2.4. PET and fMRI Image Processing
PET images were reconstructed using iterative algorithms (brain mode; FORE/OSEM, four iterations, 16 subsets; no smoothing) into a 128×128 pixel matrix in a 28.8 cm diameter field of view. Attenuation correction was performed through a 6 min transmission scan (68Ge source) obtained before the PET study and with iterative reconstruction of the blank/transmission data followed by segmentation of the attenuation image. Small head motions during emission scans were corrected by an automated computer algorithm for each subject before analysis, and the images were coregistered to each other with the same software.48 Time points were then decay corrected during reconstruction of the PET data. Image data were transformed on a voxel-by-voxel basis into two sets of parametric maps: (1) a tracer transport measure (K1 ratio) and (2) a receptor-related measure (non-displaceable binding potential, BP). To avoid the need for arterial blood sampling, the tracer transport and binding measures were calculated using a modified Logan graphical analysis,42 using the occipital cortex (an area devoid of MORs) as the reference region. The slope of the Logan plot was used for the estimation of BP, a measure equal to the (f2Bmax/Kd) for this receptor site and radiotracer. f2Bmax/Kd is the receptor-related measure (BP or MOR availability). The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value. K1 and BP images for each experimental period were coregistered to each other and to the high resolution T1 MRI structural image. The T1 structural image was spatially normalized with the nonlinear warping algorithm in statistical parametric mapping (SPM5) to the standard Montreal Neurological Institute (MNI) space. BP images were then normalized to the same template for inter-subject comparisons. Functional MRI images went through slice timing correction and spatial realignment to the first image of the first run. These images were then coregistered to the T1 structural image obtained from MRI, and in turn were normalized to the standard T1 template in MNI space. The normalized BP and fMRI images both had a voxel size of 2×2×2 mm. The accuracy of coregistration and nonlinear warping algorithms were confirmed for each subject individually by comparing the transformed MRI and PET images to each other and the standard T1 template. The normalized fMRI images were further spatially smoothed with a 3 dimensional Gaussian kernel (full width half maximum of 8mm) to enhance the signal to noise ratio. Subject head motion was assessed by evaluating three translations and three rotations for each scan. Translational thresholds were set to ± 2 mm, while rotational thresholds were limited to ± 1°. No subjects were excluded for head motion.
2.5. PET and fMRI Image Analysis
Initial statistical analysis of the fMRI images was performed with SPM5 and the biological parametric mapping (BPM) toolbox.8 Functional MRI images were analyzed with a general linear model at individual subject level. Innocuous, “faint” pain, “mild-moderate” pain, and “slightly intense” pain pressures were built in the model as experimental conditions. An inspection of the BOLD time series showed that pain induced BOLD peaks and tapers off within 5 seconds of the onset of the stimuli. With this observation in mind, we modeled the conditions using 5-sec duration rather than the entire 25-sec block. See supplemental Figure 1. This decision is consistent with recent work suggesting that the most of the BOLD signal response to some forms of continuous pain stimulation is due to the sharp rise in the signal at stimulus onset followed immediately afterwards by a sharp decay.34 The contrast images from the general linear model representing activation levels corresponding to the three pain conditions were collected for analysis with the BPM toolbox.8 The primary contrast of interest for fMRI analyses was “slightly intense” pain (13.5 on the GBS) vs. innocuous touch; this was because this contrast produced the largest (as expected) difference in BOLD signal, and because higher pressure pain ratings appear to be more strongly associated with clinical pain ratings.21
Correlations between fMRI contrast images and PET BP images were then performed. Activation clusters were defined based on a correlation coefficient of |R|>=0.6, a voxelwise P-value < 0.001, and cluster size > 10 voxels with cluster correction for multiple comparisons at P < 0.05. We performed a single small volume correction (SVC) for a cluster in the subgenual anterior cingulate cortex (sgACC) previously identified in a study of pain in FM (MNI coordinates = 8, 46, 4). A separate region of interest (ROI) analysis based on seven clusters previously identified in our 2007 report on differences in MOR BP between FM patients and healthy controls was also performed30 (see Table 2). Regions examined included ACC, nucleus accumbens (NAc), amygdala, and putamen. Mean voxel values of the differential images from each of the clusters were extracted for each subject and correlation coefficients across subjects were calculated for statistically significant clusters in SPSS Version 22.0. For our ROI-based analyses significance was set at P < 0.05. See supplemental material for evaluation of mean BOLD activation produced by the contrast of interest.
Table 2.
Regions of interest (ROIs) identified in Harris et al. 2007 28 that differed between fibromyalgia patients and healthy controls in μ-opioid receptor (MOR) availability. Bolded regions were significantly associated with the fMRI BOLD signal in the same region.
| ROI | MNI coordinates | size | r | P |
|---|---|---|---|---|
| dACC | 10, −11, 48 | 10mm | 0.35 | 0.155 |
| dACC | −4, −11, 43 | 10mm | 0.39 | 0.109 |
| ACC | −12, 38, 28 | 10mm | 0.67 | 0.002 |
| Putamen | −26, 4, −8 | 10mm | 0.01 | 0.971 |
| NAc | 9, −7, −11 | 4mm | 0.37 | 0.133 |
| NAc | −18, 6, −12 | 4mm | 0.47 | 0.050 |
| Amygdala | 29, −10, −13 | 4mm | 0.42 | 0.079 |
ACC= anterior cingulate cortex, d=dorsal, NAc= nucleus accumbens
2.6 Clinical outcomes
Clinical pain was assessed with the Short Form McGill Pain Questionnaire (SFMPQ).47 This questionnaire yields a total score (ranging from 0 to 45), comprised of both sensory (score of 0 to 33) and affective (score of 0 to 12) scores. Higher scores reflect greater clinical pain. We used a ratio of affective pain to sensory pain as our dependent variable following our previous analyses.30 Because opioids are believed to be more strongly related to the affective component of pain,39; 62 we used this ratio to determine how clinical affective pain changes in proportion to sensory pain. We conducted bivariate correlations (pearson r) with this measure, BOLD responses, and MOR BP. Depression was assessed with the Center for Epidemiologic Studies – Depression (CES-D) measure.54 We also conducted correlations of imaging findings with levels of depression to determine if imaging outcomes were associated with overall mood. Finally, we conducted an exploratory mediation analyses to investigate the possibility that clinical pain is influenced by MOR BP via evoked-pain brain activation.
3. Results
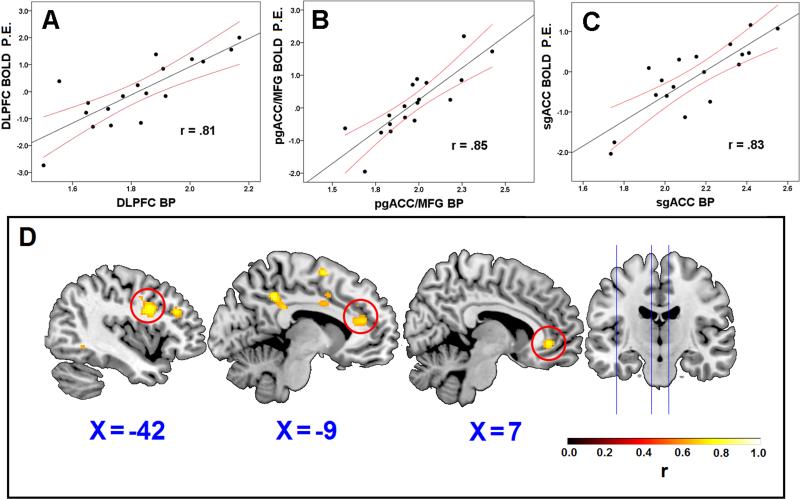
Within our whole brain search as well as well as our a priori regions of interest, we observed strong within-patient associations between neural activity in response to experimental pressure pain and MOR BP. In whole-brain analyses eight regions showed significant associations between fMRI BOLD and MOR BP, all in a positive direction (Table 1). Interestingly, some correlations were detected in regions considered to inhibit pain when activated, including the dorsolateral prefrontal cortex (DLPFC), and multiple regions within the cingulate cortex (Figure 2). Two of these regions were located in the rostral ACC (rACC), one in the perigenual ACC extending into the medial frontal gyrus (pgACC/MFG), and another in the subgenual ACC (sgACC). Our ROI-based approach from regions discovered in the 2007 report30 also revealed positive correlations between fMRI BOLD and MOR BP within the ACC and the nucleus accumbens (NAc), suggesting a patient-specific relationship (Table 2). For both our whole brain search and targeted ROI approach, no significant negative associations were detected.
Table 1.
Regions showing correlations between μ-opioid receptor availability (MOR BP) and evoked pain brain activity (BOLD). Correlations between MOR BP and BOLD yielded multiple brain regions showing associations of lower MOR BP with lower brain activations. Of note the pgACC/MFG, sgACC, and DLPFC are regions commonly thought to be involved in pain inhibition.
| Brain region | MNI coordinates (X Y Z) | Z | Cluster size (mm3) | r value | Peak Voxel R Value | P |
|---|---|---|---|---|---|---|
| MFG | −12, 2, 62 | 3.89 | 3224 | .80 | .80 | <.001** |
| PCC | −8, −44, 40 | 4.25 | 888 | .80 | .84 | .036** |
| pgACC/MFG | −16, 40, 38 | 4.77 | 6176 | .85 | .89 | <.001** |
| Precentral | 32, −4, 34 | 4.17 | 1240 | .77 | .83 | .004** |
| DLPFC | −42, 8, 30 | 3.76 | 1760 | .81 | .79 | <.001** |
| MTG | −58, −58, 4 | 3.59 | 848 | .81 | .77 | .047** |
| sgACC | 10, 40, −2 | 4.02 | 728 | .83 | .82 | .017** |
| Cerebellum | 34, −60, −44 | 3.91 | 1144 | .81 | .81 | .008** |
Whole brain cluster corrected
Small volume correction
MFG=medial frontal gyrus, ACC= anterior cingulate cortex, PCC = posterior cingulate cortex, pg = perigenual, DLPFC = dorsolateral prefrontal cortex, sg= subgenual, MTG = middle temporal gyrus
Figure 2.
Scatterplots showing the association between brain activation (BOLD, Parametric Estimates [P.E.]) and μ-opioid receptor MOR availability (MOR BP) in the dorsolateral prefrontal cortex (DLPFC; panel A), perigenual ACC/medial frontal gyrus (pgACC/MFG; panel B), and the subgenual ACC (sgACC; panel C). 95% confidence intervals are displayed in red. Panel D shows each of the voxels listed in panels A-C where MOR BP and BOLD were associated.
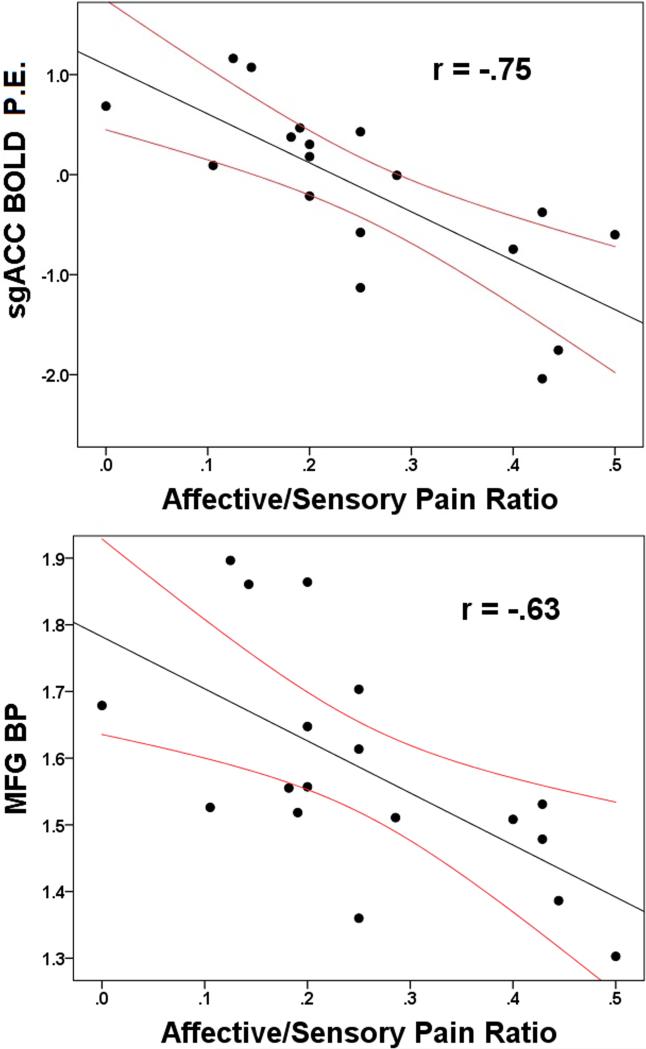
To determine if our neurobiological outcomes were related to clinical measures of pain or depression, we correlated BOLD and MOR BP from any significant region identified above with our mood and clinical pain outcomes. Bivariate analyses revealed that the affective/sensory pain ratio was negatively associated with a number of imaging outcomes, such that higher BOLD responses to evoked pain and higher MOR BP were associated with less affective pain proportional to sensory pain. Specifically, fMRI BOLD signals in multiple regions including the medial frontal gyrus (MFG), the posterior cingulate cortex (PCC), pgACC/MFG and sgACC were negatively associated with the affective/sensory ratio (all r < −.51, all P < .03). Similarly, higher MOR BP was negatively associated with the affective/sensory pain ratio in the MFG, PCC, pgACC, precentral gyrus, ACC, sgACC (all absolute r > .49, all p < .04). All correlations, including those that did not reach significance, were in a negative direction. No imaging outcome was associated with overall depression suggesting that the above associations are specific to pain (all p > .1; Figure 3 and Table 3). See supplemental material showing the mean BOLD activation for our contrast of interest (presented in the Supplemental Figure 1 legend) and exploratory mediation analyses demonstrating that clinical pain is influenced by MOR BP through evoked pain brain activation (Supplemental Figure 2).
Figure 3.
Scatterplots showing the association between brain activation (BOLD, Parametric Estimates [P.E.]) in the subgenual anterior cingulate cortex (agACC) and affective pain divided by sensory pain, and μ-opioid receptors availability (MOR BP) in the medial frontal gyrus (MFG) and affective pain divided by sensory pain. 95% confidence intervals are displayed in red.
Table 3.
Associations between brain responses to evoked pain (BOLD), MOR availability (MOR BP), and McGill affective/ sensory pain ratios. Bolded regions were significantly associated with clinical pain.
| Brain region | MNI co-ordinates (X Y Z) | Affective/Sensory Pain Ratio | |
|---|---|---|---|
| r | P | ||
| Whole Brain | |||
| MFG (BOLD) | −12, 2, 62 | −.518 | .027 |
| MOR BP | −.629 | .005 | |
| PCC (BOLD) | −8, −44, 40 | −.593 | .010 |
| MOR BP | −.497 | .036 | |
| pgACC/MFG (BOLD) | −16, 40, 38 | −.581 | .012 |
| MOR BP | −.571 | .013 | |
| Precentral (BOLD) | 32, −4, 34 | −.436 | .070 |
| MOR BP | −.507 | .032 | |
| DLPFC (BOLD) | −42, 8, 28 | −.331 | .179 |
| MOR BP | −.246 | .324 | |
| MTG (BOLD) | −58, −58, 4 | −.408 | .093 |
| MOR BP | −.410 | .091 | |
| sgACC (BOLD) | 8, 38, −2 | −.754 | <.001 |
| MOR BP | −.545 | .019 | |
| NAc (BOLD) | −18, 6, −12 | −.425 | .079 |
| MOR BP | −.301 | .224 | |
| Cerebellum (BOLD) | 28, −58, −40 | −.294 | .236 |
| MOR BP | −.318 | .198 | |
| ROI | |||
| ACC (BOLD) | −12, 38, 28 | −.378 | .122 |
| MOR BP | −.552 | .018 | |
| NAc (BOLD) | −18, 6, −12 | −.425 | .079 |
| MOR BP | −.301 | .224 |
MFG=medial frontal gyrus, BOLD = blood oxygen level-dependent, MOR= μ opioid receptor, BP= binding potential, ACC= anterior cingulate cortex, PCC = posterior cingulate cortex, pg = perigenual, d= dorsal, DLPFC = dorsolateral prefrontal cortex, MTG = middle temporal gyrus, sg = subgenual, ROI = region of interest, NAc= nucleus accumbens
4. Discussion
Our study has shown, for the first time, robust associations between evoked pain brain activity and MOR availability within the same brain regions and the same individuals with chronic pain that have already been shown to display MOR availability deficiencies in pain-processing brain regions.30 The only other pain studies we are aware of that employed PET and fMRI together were performed in healthy individuals and used the non-selective radioligand ([11C]diprenorphine).16; 64 Our study is also the first to perform a whole brain search specifically correlating fMRI BOLD responses to pain with resting MOR BP. Our findings suggest that the dysregulation of the endogenous opioid system we previously reported in these FM patients is strongly related to an objective neural marker of their pain sensitivity. In particular, we observed a strong coupling of evoked-pain brain activity and MOR availability in two regions – the DLPFC and rACC – known to play an important role in anti-nociception.7; 32; 43 These findings extend to the clinical pain experience, as increased brain activation and MOR availability was also associated with lower ratings of pain affect. These findings may therefore contribute to the mechanistic understanding of experimental pain sensitivity and clinical pain report in FM.
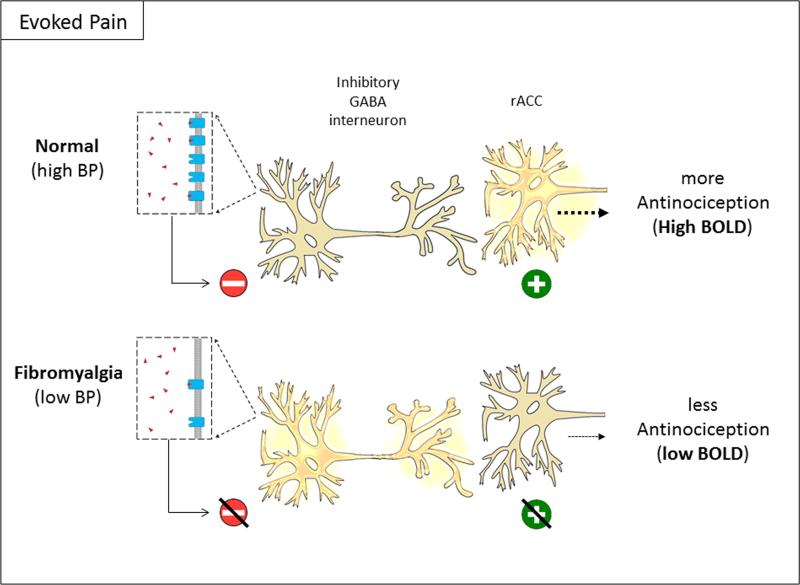
We propose a conceptual model of affective pain dysregulation secondary to endogenous opioid system dysregulation in FM (Fig. 4 and 5). In a healthy individual, GABAergic interneurons tonically inhibit neurons in regions such as the ACC, PFC, hippocampus and periaqueductal gray (PAG).1; 10; 11; 53 These GABAergic interneurons become inhibited when bound by opioids resulting in disinhibition and excitation of antinociceptive neurons such as those found in the rACC that project to descending inhibitory pain structures such as the PAG.7 In FM, however, high levels of tonic endogenous opioids, lead to downregulation or reduced affinity of MORs on GABAergic interneurons in antinociceptive brain regions. Consequently, phasic endogenous opioid release as occurs during noxious stimulation does not produce appropriate inhibition of GABAergic interneurons, and antinociceptive neurons fail to activate. High levels of endogenous opioid are attested to by our previous report showing their elevation in the CSF of FM patients4 and our previous report comparing MOR BP between these FM participants and healthy controls demonstrates a loss of MOR number or affinity.30 That high endogenous opioid tone likely translates to loss of MOR availability is supported by pre-clinical studies demonstrating that chronically increasing synaptic endogenous opioid levels (by preventing their degradation) leads to a decrease in MOR density,17 while chronically decreasing endogenous opioid neurotransmission (by blocking MOR receptors) leads to an increase in MOR density.60 Our current findings now demonstrate a direct association between the endogenous opioid system and antinociceptive brain responses. It is possible that MOR availability and evoked pain BOLD responses are also associated in healthy individuals, which would suggest that we are observing a normal mechanism that is simply shifted in FM. Alternatively, the same level of MOR BP in a healthy individual might produce a more pronounced BOLD response in antinociceptive regions. These are important distinctions that need to be addressed in future studies.
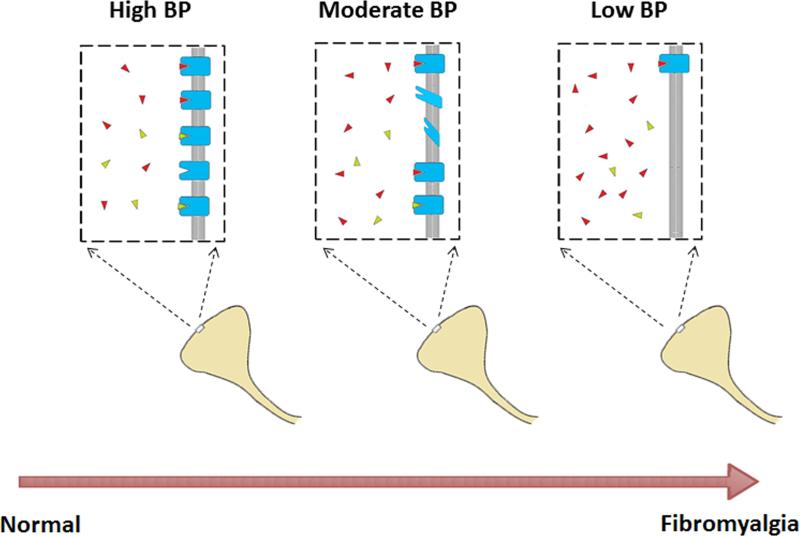
Figure 4.
Conceptual model of reduced of μ-opioid receptor (MOR) binding potential (BP) in fibromyalgia. In healthy individuals, MORs are available for binding by endogenous opioids (red) and [11C]-carfentanil (green) expressed as high BP during PET imaging (left panel). In individuals with FM, higher levels of tonic endogenous opioids lead to MOR downregulation and/or loss of affinity (middle and right panel). This results in a lower BP as fewer MORs remain available for [11C]-carfentanil binding. Images modified from open source material.
Figure 5.
Reduced antinociceptive activity in FM due to reduced binding potential (BP) of μ-opioid receptors. At rest, GABAergic interneurons tonically inhibit antinociceptive neurons in regions such as the rACC by releasing GABA (not shown). In normal individuals, phasic release of endogenous opioids due to evoked pain inhibits GABAergic interneurons. This leads to disinhibition and subsequent activation of antinociceptive neurons in the rACC (top). In fibromyalgia patients, reduced BP leads to ineffective inhibition of GABAergic interneurons, reducing activation of antinociceptive neurons in the rACC (bottom). Red minus signs indicate neuronal inhibition, green plus signs indicate neuronal excitation. Images modified from open source material.
Clinical and pre-clinical studies using low dose naltrexone, an opioid receptor antagonist with strong affinity for the μ subtype, provide additional inferential evidence for the role of the endogenous opioid system in pain sensitization processes. In a series of studies, Younger et al. demonstrated analgesic effects of low dose naltrexone treatment in opioid-naïve FM patients.66; 67 While these authors propose a different mechanism whereby low dose naltrexone acts to inhibit glial activation, we propose that low dose naltrexone could also increase MOR BP and consequently increase brain responses to endogenous opioid release. This latter hypothesis is consistent with early observations by Levine et al.41 wherein low dose naltrexone was shown to be analgesic only in responders to placebo, a process linked to increased MOR binding,68 but not in placebo non-responders. In preclinical studies, naltrexone has also been shown to block hyperalgesia evoked by repeated stress.40
Activation in two regions where MOR availability and fMRI BOLD responses to evoked pain were strongly associated in our study – the DLPFC and rACC –have previously been associated with reduced pain in healthy subjects.7; 43 In FM patients, the rACC exhibits reduced activation to evoked pain,35 and reduced connectivity to other pain inhibitory regions when compared to healthy controls.36 Additionally, enhanced rACC activation is associated with better long-term pain habituation – a critical finding as FM is a chronic disease with progressive elements.6; 7 Furthermore, these antinociceptive structures were linked to the endogenous opioid system in both preclinical and clinical studies. A recent study employing transcranial direct current stimulation (tDCS) indicates that this stimulation produces an analgesic effect concurrent with increased endogenous opioid binding (measured by [11C]-carfentanil) in the left PFC.15 Animal models demonstrate that opioid receptor blockade in the rACC inhibits NAc dopamine release and alters conditioned place preference following non-opioid pain treatments – a result the authors posit may help explain the preferential action of opioids in attenuating the affective reaction to pain.49 Clinical studies indicate that the extent of binding by endogenous opioids in response to placebo in the rACC and NAc predicts the magnitude of pain relief experienced in healthy volunteers.59; 68 It is important to note that both greater MOR BP and BOLD from clusters in the rACC identified in our whole brain search were strongly associated with lower pain affect.
These findings are relevant to the large body of preclinical work demonstrating a distinct alteration of the opioid system in response to chronic administration of opioids.3 In preclinical pain models, OIH is elicited following increased exposure to opioids resulting in reduced experimental pain thresholds, an effect that has been shown to be dose-dependent46 and independent of withdrawal.45 While this mechanism is considerably less well characterized in humans, OIH has been demonstrated by reductions in experimental pain thresholds in drug addicts taking methadone, patients receiving high doses of exogenous opioids during surgery, and community dwelling adults with chronic pain using opioids long term.2; 33; 63 While short-term use of opioids may not exacerbate experimental pain in human chronic pain patients,12 long-term exposure, particularly at higher doses, may well have different and more deleterious effects.9; 63 It is therefore possible that the relationship between low MOR BP and reduced anti-nociceptive brain responses to pain is an endogenous process analogous to OIH. This may help explain why recent prospective long-term studies of opioid use in FM do not generally support their efficacy.18; 50
Our study has limitations. Though designed to address clinical aspects of pain in FM, we did not have a healthy control group. Our analyses were cross-sectional and consisted only of women. We examined a small number of participants so our findings need to be replicated with larger trials. While we evaluated clinical pain outcomes in this study we did not examine other important aspects of FM symptomology including fatigue and cognitive dysfunction. Our paradigm also produced some activation in expected pain-processing regions (i.e., insula)26 but did not produce robust activation in the antinociceptive regions subsequently identified in the whole brain correlation between MOR BP and evoked pain BOLD. Interestingly, these regions have not been shown to be robustly activated by evoked pain in other FM samples26 suggesting that they may be modulatory in nature. Future studies might employ different paradigms that have been shown to produce more antinociceptive brain activation. Finally, the number of stimulations used in this study (six) may not be optimal for pain fMRI research.
Our findings provide important mechanistic information that may help identify weaknesses in the current treatment of FM and other chronic pain patients. Our study seems to provide evidence that the endogenous opioid system is involved in particular aspects of the pain experience in FM. Pain affect and expectancy/placebo effects appear to be most strongly related to this system in FM, at least as it relates to evoked pain brain responses. We think it is notable that affective pain is strongly associated with the identified regions, especially given the lack of an association of MOR availability and BOLD with overall levels of depression; this suggests an important degree of specificity to pain. Despite current trends in opioid consumption there is little evidence supporting the long-term use of opioid medications in chronic non-cancer pain.44 Our current findings strengthen our contention that opioids are unlikely to be efficacious in FM due to already reduced numbers of MORs or receptor affinity,30 and actually support a plausible mechanism by which long term opioid use could worsen pain outcomes by exacerbating an existing vulnerability in the endogenous opioid system. These hypotheses can be tested by examining basal endogenous opioid system tone in FM and determining if this measure predicts a reduced response to exogenous opioids or an increase in their deleterious side effects. Future research is needed to determine if these findings may be generalizable to other pain states displaying enhanced brain responses to pain58 and reduced opioid receptor binding.38
Supplementary Material
Acknowledgements
This work was funded by Department of Army grants DAMD (17-002-0018 to DJC and W81XWH-07-2-0050 to REH) and National Institutes of Health (M01-RR000042, K01 AT01111-01, and R01 AT007550-01 to REH; R01 DA 016423 to JKZ; K12 DE023574-03 to DJC). The authors declare no conflict of interest and no competing financial interests with this work. Dr. Harte has received research funding from Cerephex, Forest Laboratories, and Merck; and served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics. Dr. Clauw has received research funding from Cerephex, Forest, Merck, and Pfizer, and serves as a consultant for Tonix, Theravance, Cerephex, Pfizer, Abbott, Merck, Eli Lilly, UCB, Johnson & Johnson, Forest Laboratories, and Purdue Pharma. Dr. Harris consults for and has received grant support from Pfizer.
Footnotes
All other authors have no competing financial interests to disclose.
References
- 1.Akaishi T, Saito H, Ito Y, Ishige K, Ikegaya Y. Morphine augments excitatory synaptic transmission in the dentate gyrus through GABAergic disinhibition. Neuroscience Research. 2000;38:357–363. doi: 10.1016/s0168-0102(00)00177-2. [DOI] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. The New England journal of medicine. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Baraniuk JN, Casado B, Maibach H, Clauw DJ, Pannell LK, Hess SS. A Chronic Fatigue Syndrome - related proteome in human cerebrospinal fluid. BMC neurology. 2005;5:22. doi: 10.1186/1471-2377-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC musculoskeletal disorders. 2004;5:48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingel U, Herken W, Teutsch S, May A. Habituation to painful stimulation involves the antinociceptive system--a 1-year follow-up of 10 participants. Pain. 2008;140:393–394. doi: 10.1016/j.pain.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143:65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen TC, Cheng YY, Sun WZ, Shyu BC. Differential regulation of morphine antinociceptive effects by endogenous enkephalinergic system in the forebrain of mice. Mol Pain. 2008;4:41. doi: 10.1186/1744-8069-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiou LC, Huang LY. Mechanism underlying increased neuronal activity in the rat ventrolateral periaqueductal grey by a mu-opioid. J Physiol. 1999;518(Pt 2):551–559. doi: 10.1111/j.1469-7793.1999.0551p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu LF, D'Arcy N, Brady C, Zamora AK, Young CA, Kim JE, Clemenson AM, Angst MS, Clark JD. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153:1583–1592. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Clauw DJ. Fibromyalgia: a clinical review. Jama. 2014;311:1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 14.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 15.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, Bikson M, Zubieta JK, DaSilva AF. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9:e102350. doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty DD, Kong J, Webb M, Bonab AA, Fischman AJ, Gollub RL. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res. 2008;193:63–68. doi: 10.1016/j.bbr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer HS, Zernig G, Schuligoi R, Miczek KA, Hauser KF, Gerard C, Saria A. Alterations within the endogenous opioid system in mice with targeted deletion of the neutral endopeptidase ('enkephalinase') gene. Regul Pept. 2000;96:53–58. doi: 10.1016/s0167-0115(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 18.Fitzcharles M-A, Faregh N, Ste-Marie PA, Shir Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain research and treatment. 2013:2013. doi: 10.1155/2013/898493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis and rheumatism. 2012;64:579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- 21.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis and rheumatism. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 22.Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- 23.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain : a journal of neurology. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 24.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and rheumatism. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 26.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 27.Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- 28.Hagelberg N, Aalto S, Tuominen L, Pesonen U, Nagren K, Hietala J, Scheinin H, Pertovaara A, Martikainen IK. Striatal mu-opioid receptor availability predicts cold pressor pain threshold in healthy human subjects. Neuroscience letters. 2012;521:11–14. doi: 10.1016/j.neulet.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neuroscience letters. 2012;520:192–196. doi: 10.1016/j.neulet.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 32.Harte SE, Spuz CA, Borszcz GS. Functional Interaction between Medial Thalamus and Rostral Anterior Cingulate Cortex in the Suppression of Pain Affect. Neuroscience. 2011;172:460–473. doi: 10.1016/j.neuroscience.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooten WM, Lamer TJ, Twyner C. Opioid-induced hyperalgesia in community-dwelling adults with chronic pain. Pain. 2015;156:1145–1152. doi: 10.1097/j.pain.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibinson JW, Vogt KM. Pain does not follow the boxcar model: temporal dynamics of the BOLD fMRI signal during constant current painful electric nerve stimulation. The journal of pain : official journal of the American Pain Society. 2013;14:1611–1619. doi: 10.1016/j.jpain.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jewett DM. A simple synthesis of [C-11]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28:733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 38.Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, Derbyshire S, Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- 39.Kupers RC, Konings H, Adriaensen H, Gybels JM. Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain. 1991;47:5–12. doi: 10.1016/0304-3959(91)90004-H. [DOI] [PubMed] [Google Scholar]
- 40.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain. 2011;12:1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Levine JD, Gordon NC, Bornstein JC, Fields HL. Role of pain in placebo analgesia. Proc Natl Acad Sci U S A. 1979;76:3528–3531. doi: 10.1073/pnas.76.7.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cerebr Blood F Met. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain : a journal of neurology. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 44.Manchikanti L, Singh A. Therapeutic Opioids: A Ten-Year Perspective on the Complexities and Complications of the Escalating Use, Abuse, and Nonmedical Use of Opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- 45.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 46.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 47.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 48.Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- 49.Navratilova E, Xie JY, Meske D, Qu CL, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous Opioid Activity in the Anterior Cingulate Cortex Is Required for Relief of Pain. Journal of Neuroscience. 2015;35:7264–7271. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng X, Robinson RL, Mease P, Kroenke K, Williams DA, Chen Y, Faries D, Wohlreich M, McCarberg B, Hann D. Long-term evaluation of opioid treatment in fibromyalgia. Clin J Pain. 2015;31:7–13. doi: 10.1097/AJP.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 51.Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain. 2005;9:325–335. doi: 10.1016/j.ejpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Piche M, Watanabe N, Sakata M, Oda K, Toyohara J, Ishii K, Ishiwata K, Hotta H. Basal mu-opioid receptor availability in the amygdala predicts the inhibition of pain-related brain activity during heterotopic noxious counter-stimulation. Neurosci Res. 2014;81-82:78–84. doi: 10.1016/j.neures.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Qu CL, Huo FQ, Huang FS, Tang JS. Activation of mu-opioid receptors in the ventrolateral orbital cortex inhibits the GABAergic miniature inhibitory postsynaptic currents in rats. Neuroscience letters. 2015;592:64–69. doi: 10.1016/j.neulet.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 54.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 55.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis and rheumatism. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 56.Sarchielli P, Di Filippo M, Nardi K, Calabresi P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr Pain Headache Rep. 2007;11:343–351. doi: 10.1007/s11916-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011;7:518–527. doi: 10.1038/nrrheum.2011.98. [DOI] [PubMed] [Google Scholar]
- 58.Schweinhardt P, Bountra C, Tracey I. Pharmacological FMRI in the development of new analgesic compounds. NMR Biomed. 2006;19:702–711. doi: 10.1002/nbm.1076. [DOI] [PubMed] [Google Scholar]
- 59.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 60.Unterwald EM, Rubenfeld JM, Imai Y, Wang JB, Uhl GR, Kreek MJ. Chronic opioid antagonist administration upregulates mu opioid receptor binding without altering mu opioid receptor mRNA levels. Brain research Molecular brain research. 1995;33:351–355. doi: 10.1016/0169-328x(95)00143-g. [DOI] [PubMed] [Google Scholar]
- 61.Upadhyay J, Lemme J, Anderson J, Bleakman D, Large T, Evelhoch JL, Hargreaves R, Borsook D, Becerra L. Test-retest reliability of evoked heat stimulation BOLD fMRI. Journal of neuroscience methods. 2015;253:38–46. doi: 10.1016/j.jneumeth.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 62.van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136:373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 63.Wasserman RA, Hassett AL, Harte SE, Goesling J, Malinoff HL, Berland DW, Zollars J, Moser SE, Brummett CM. Pressure Pain Sensitivity in Patients With Suspected Opioid-Induced Hyperalgesia. Reg Anesth Pain Med. 2015;40:687–693. doi: 10.1097/AAP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wey HY, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, Chonde DB, Rosen BR, Gollub RL, Kong J. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage. 2014;102(Pt 2):275–282. doi: 10.1016/j.neuroimage.2014.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 66.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis and rheumatism. 2013;65:529–538. doi: 10.1002/art.37734. [DOI] [PubMed] [Google Scholar]
- 67.Younger JW, Zautra AJ, Cummins ET. Effects of Naltrexone on Pain Sensitivity and Mood in Fibromyalgia: No Evidence for Endogenous Opioid Pathophysiology. Plos One. 2009;4:e5180. doi: 10.1371/journal.pone.0005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.