Abstract
Compared with men, women show enhanced responses to drugs of abuse, and consequently are thought to be more vulnerable to addiction. The ovarian hormone estradiol has emerged as a key facilitator in the heightened development of addiction in females. These actions of estradiol appear mediated by estrogen receptor (ER) activation of metabotropic glutamate receptor type 5 (mGluR5). However, the downstream effectors of this ER/mGluR5 signaling pathway are unknown. Here we investigate whether cannabinoid 1 receptor (CB1R) activation is a part of the mechanism whereby estradiol influences behavioral and synaptic correlates of addiction. Following repeated cocaine administration, estradiol-treated ovariectomized rats exhibited both sensitized locomotor responses and decreases in the dendritic spine density of nucleus accumbens core medium-spiny neurons in comparison to oil-treated controls. Both effects of estradiol were blocked by AM251, a CB1R inverse agonist. These results indicate that part of the signaling mechanism through which estradiol impacts behavioral and synaptic correlates of addiction in female rats requires activation of CB1Rs.
1. Introduction
There are sex differences in the etiology of drug addiction, with women progressing more rapidly to an addicted state. This is particularly true for psychostimulants, but applies to multiple classes of abused drugs (Becker and Hu, 2008; Carroll et al., 2004). The ovarian hormone estradiol is a key biological factor contributing to this vulnerability in females (Hedges et al., 2010). Clinical research demonstrates that women are more sensitive to the rewarding properties of psychostimulants when endogenous estradiol levels are elevated, and administration of exogenous estradiol further enhances these properties (Justice and de Wit, 1999; Maria et al., 2014; McCance-Katz et al., 2005). Animal models of addiction parallel human reports, in that high estradiol concentrations correspond with greater locomotor responses to psychostimulants, a more rapid acquisition of psychostimulant self-administration, enhanced motivation for psychostimulants, and increased total psychostimulant consumption (Anker and Carroll, 2011; Becker and Hu, 2008; Festa and Quinones-Jenab, 2004; Segarra et al., 2010) Yet, despite the abundance of behavioral observations of the effects of estradiol in females on responsiveness to psychostimulants, little is known regarding the cellular mechanisms underlying these phenomena.
While estradiol was once thought to act solely through a single nuclear estrogen receptor (Couse and Korach, 1999; Klinge, 2001), recent studies have established that estradiol can affect cellular function through multiple receptors, many localized to the surface membrane (Micevych and Kelly, 2012). In addition to GPER-1 (GPR30) and the putative ER-X, and the STX-sensitive receptors (Barton, 2016; Filardo and Thomas, 2012; Qiu et al., 2003; Toran-Allerand et al., 2002), palmitoylation of both ERα and ERβ promotes receptor trafficking to the surface membrane (Meitzen et al., 2013; Pedram et al., 2007). Within the nervous system, these two estrogen receptors functionally couple to metabotropic glutamate receptors (mGluRs) leading to glutamate-independent mGluR signaling (Boulware et al., 2007, 2005).
Recent work from our lab has identified a putative signaling mechanism through which estradiol can impact structural and behavioral substrates of drug addiction, which in turn may promote a greater vulnerability for female drug abuse. Within medium spiny neurons (MSNs) of the striatum and nucleus accumbens (NAc), estradiol activates ERα coupled to mGluR subtype 5 (mGluR5) (Grove-Strawser et al., 2010). Specific to the NAc core, estradiol induces synaptic plasticity via decreases in dendritic spine density through transactivation of mGluR5 (Peterson et al., 2014). In general, synaptic plasticity within the NAc core is thought to be a neurobiological substrate of drug addiction, and to underlie psychostimulant behavioral sensitization (Dumitriu et al., 2012; Li et al., 2004; Robinson and Kolb, 2004; Waselus et al., 2013). Related, estradiol enhances psychostimulant locomotor sensitization through membrane ER coupling to mGluR5 (Martinez et al., 2014).
Activation of group I mGluRs leads to a variety of other cellular responses, one of which is the release of endogenous cannabinoids (endoCBs) (Alger and Kim, 2011; Wilson and Nicoll, 2002). Upon release from post-synaptic membranes, endoCBs within the CNS retrogradely bind to presynaptic type 1 cannabinoid receptors (CB1Rs). Interestingly, multiple lines of research demonstrate that estradiol enhances endoCB activity in the nervous system of both women and female rodents (Bradshaw et al., 2006; El-Talatini et al., 2010; Gorzalka and Dang, 2012; Huang and Woolley, 2012; Rodríguez de Fonseca et al., 1994; Scorticati et al., 2004). Moreover, similar to estradiol, both endoCBs and CB1Rs have emerged as key endogenous moderators of drug addiction (Olière et al., 2013). Although these lines of evidence were developed independently, an intriguing possibility is that within females, estradiol and endoCBs may act through a common pathway to exert effects on structural plasticity and addiction. At least in male animals, activation of mGluR5 leads to mobilization of endoCBs in the NAc (Jung et al., 2005; Robbe et al., 2002; Uchigashima et al., 2007). Based on these observations, we hypothesized that estradiol activates ERs to initiate mGluR5-dependent endoCB signaling, and that it is the endoCB system that is ultimately responsible for the effects of estradiol on structural and behavioral substrates of drug addiction.
2. Methods
2.1 Animals
Female Sprague Dawley rats 12 weeks of age (175–200g) were ovariectomized at Harlan labs (Indianapolis, IN). Upon arrival at our animal facility animals were housed in pairs, handled daily, and allowed to habituate for one week prior to experimentation. Animals were maintained on a 12 hr light-dark cycle (lights on at 6:00 am) with all behavioral testing occurring between 8:30 am and 1:30 pm. Food and water were available ad libitum. Animal procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of Minnesota.
2.2 Drugs and hormones
17β-estradiol (Sigma-Aldrich, St. Louis, MO) was dissolved in cottonseed oil (Sigma-Aldrich) and injected s.c. at 2 μg in 0.1 ml oil. Cottonseed oil (0.1 ml s.c.) was injected as the vehicle control. The type 1 cannabinoid receptor inverse agonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251; Tocris Biosciences, Minneapolis, MN) was dissolved in DMSO to make a 100 mM stock solution and stored at −20 °C. On the day of injections the stock solution was diluted in 0.5% methylcellulose (Sigma-Aldrich) in deionized water to a working solution of 1 mg/kg/ml. We chose a low dose of AM251 because it does not affect locomotor activity on its own (Shearman et al., 2003; Thiemann et al., 2008). This low dose of AM251 allowed us to examine the role of CB1R activation on estradiol-induced locomotor responses to cocaine, without compromising general locomotor responses.
Throughout the experiment, ovariectomized (OVX) females were injected s.c. with estradiol or oil on a 2 days on-2 days off schedule to mimic the estrous cycle of an intact female rat (Butcher et al., 1974). Thirty min prior to each hormone injection, females received an i.p. injection of AM251 or vehicle. Beginning on the third day of the experiment, females were injected i.p. with 15 mg/kg/ml cocaine (cocaine hydrochloride; Covidien, St. Louis, MO) in saline or a comparable volume of the saline vehicle for 5 consecutive days. This pattern of cocaine injections produces a modest (if any) sensitization in untreated OVX females (Segarra et al., 2010; Sircar and Kim, 1999), allowing us to more clearly isolate a synergistic effect of estradiol on cocaine-induced locomotor sensitization and test whether this effect is attenuated by AM251 pretreatment.
2.3 Locomotor Activity
Locomotor activity was assessed according to previously published methods (Martinez et al., 2014) with the following modifications: Locomotor chambers were placed inside a sensing frame (Kinder Scientific, Poway, CA) to measure activity in the XY plane (i.e. ambulations). Animals were in locomotor chambers for 2 hrs each day for the 7 days of the experiment. For the 5 consecutive days that females were injected with cocaine, animals were in the chamber 1 hr before (habituation session) and 1 hr following (test session) the injection.
2.4 Activity data analyses
For each animal, locomotor data were obtained for the entire 1 hr test period on the first and fifth test sessions. Depending on treatment condition, a final sample size of 14–20 animals was obtained for each treatment group (see Fig 2 for treatment group sample sizes). As an index of sensitization, locomotor activity was compared on first vs. fifth test sessions using paired-samples t-tests with Bonferroni correction for multiple comparisons (SPSS version 20.0, IBM Corp, Armonk, NY). For all statistical tests, results were considered to be statistically significant if p < 0.0125.
Fig. 2.
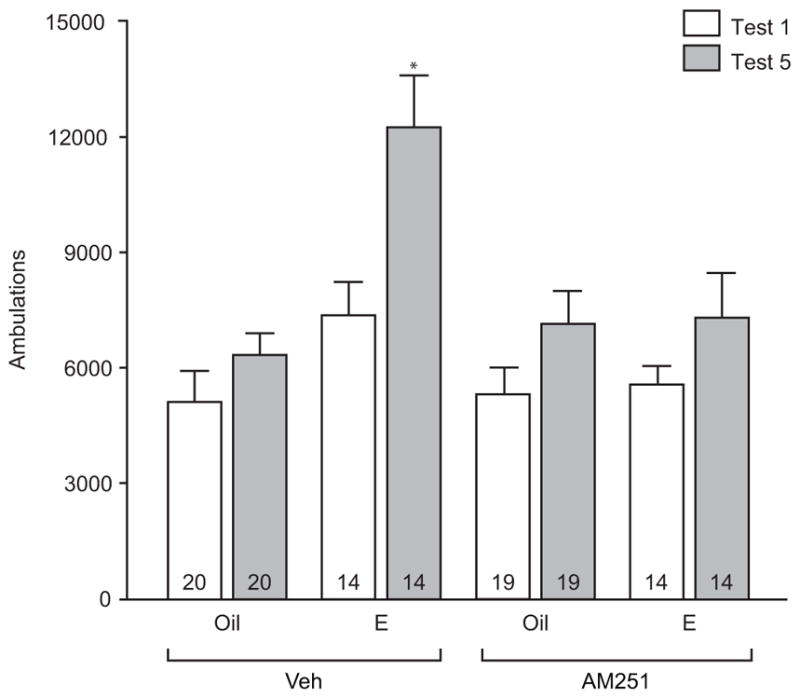
The estradiol-mediated increase in cocaine-induced ambulations depends on type 1 cannabinoid receptors. In estradiol (E) treated females, ambulations (mean + SEM) were higher in response to the fifth cocaine (Coc) vs. first Coc injection (Test 5 vs. Test 1). This effect of estradiol was not observed when females were treated with the cannabinoid type 1 receptor inverse agonist, AM251, 30 min prior to estradiol. Oil or AM251 treatment alone did not affect Coc-induced ambulations. The number inside of each bar represents the number of animals per treatment condition. * p < 0.0125 Veh+E Test 1 vs Veh+E Test 5
2.5 Tissue preparation for dendritic spine measurements
A subset of animals that completed the behavioral study was examined for neuronal morphology. Perfusions, DiI labeling, confocal imaging, quantification and data analysis followed previously published methods (Staffend and Meisel, 2011a) with some refinements (Peterson et al., 2015, 2014).
Twenty-four hrs following the final cocaine injection, animals were anesthetized using Beuthanasia-D (0.3 ml i.p./animal, Schering, Union, NJ), injected with 0.25 ml heparin into the left ventricle of the heart and intracardially perfused with 25 mM phosphate buffered saline (PBS, pH = 7.2) for 3 min at 25 ml/min followed by ice-cold 1.5% paraformaldehyde in 25 mM PBS for 20 min. Perfusion with 1.5% paraformaldehyde results in more complete and clearer visualization of DiI-labeled neurons when compared to perfusion with the standard 4% paraformaldehyde (Staffend and Meisel 2011b). Brains were removed and coronally blocked to allow for penetration of the post-perfusion fixative (1.5% paraformaldehyde for 1 hr) into the tissue blocks containing the striatum. Brains were sectioned at 300 μm in serial, coronal sections through the striatum using a Vibratome (Lancer Series 1000, St. Louis, MO) and placed in wells containing 25 mM PBS until DiI labeling.
2.6 DiI labeling
Instructions for DiI labeling are described in detail by Staffend and Meisel (2011b). Briefly, microcarriers containing DiI-coated tungsten particles (2 mg lipophilic carbocyanine DiI; Molecular Probes, Carlsbad, CA) dissolved in 100 μl of dichloromethane mixed with 90 mg of 1.3 μm tungsten particles (Biorad, Hercules, CA) were made in Tefzel tubing (Biorad) pre-coated with freshly prepared 10–15 mg/ml polyvinylpyrrolidone (PVP, Sigma-Aldrich) and cut into 1.3 mm segments. A Helios Gene Gun (BioRad) with a modified barrel, 40 mm spacer and 70 μm nylon mesh filter was used to deliver the microcarriers to the lightly fixed brain sections using helium gas at a pressure of 100 PSI. Prior to DiI delivery, PBS was removed from wells containing the brain sections. Immediately following DiI delivery, brain sections were re-submerged in PBS for 24 hrs in the dark at room temperature for diffusion of DiI. After 24 hrs, the PBS was removed and replaced with 4% paraformaldehyde in PBS for 1 hr at room temperature.
2.7 Confocal Imaging
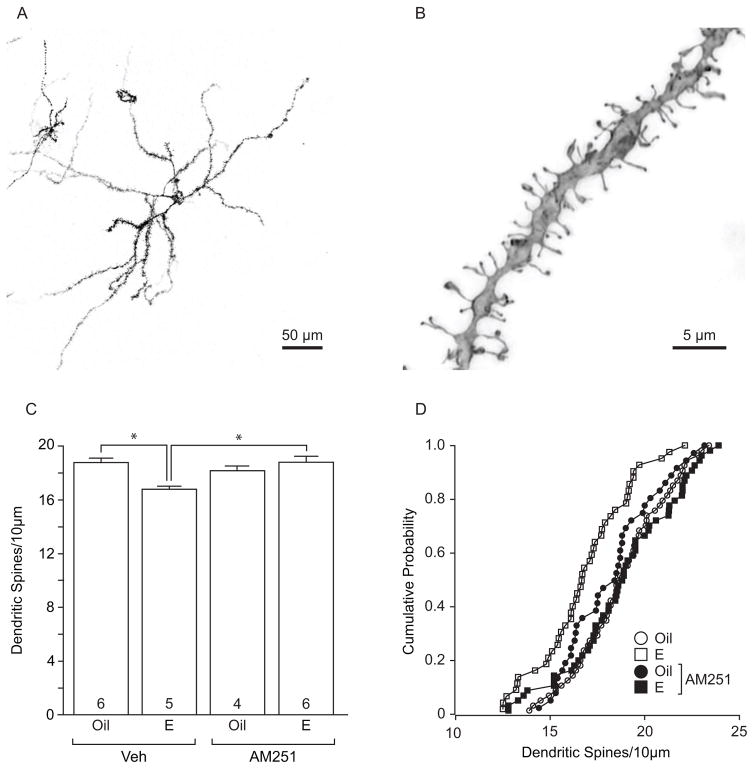
Following DiI labeling, coronal sections through the nucleus accumbens (NAc) were washed in PBS, mounted on Superfrost slides (Brain Research Laboratories, Newton, MA) and coverslipped with Fluorglo mounting medium for lipophilic dyes (Spectra Service, Ontario, NY). Dendritic segments were imaged using Type LDF immersion oil (refractive index: 1.515; Cargille, Cedar Grove, NJ) and a Leica PLAN APO 63X, 1.4 NA oil immersion objective (11506187; Leica, Mannheim, Germany). Based on our recent analyses, this immersion oil is critical to resolve dendritic spines in the axial plane when used with this objective lens (Peterson et al 2015). All z-stacks were maintained at an xy pixel distribution of 512 × 512 and scanned at a frequency of 400 Hz. Whole neurons were imaged with a 20x air objective at 1.0 μm (micron???) increments in the z-axis and reconstructed using Leica LAS AF software to measure the distance from the soma of target dendritic segments: 70–200 μm from soma in medium spiny neurons. Dendritic segments were imaged with a 63x oil immersion objective with a zoom of 5.61 and z-stacks were acquired in 0.12 μm increments. By adjusting the laser power and photomultiplier, each dendrite was imaged in its full dynamic range. Within the NAc core, 3 neurons and 3 dendritic segments per neuron were imaged to generate a total of 9 segments from every animal. Depending on the treatment conditions, a final sample size of 4–6 animals was obtained per treatment group (see Fig 3 for treatment group sample sizes).
Fig. 3.
Estradiol-mediated dendritic spine plasticity in the nucleus accumbens core depends on type 1 cannabinoid receptors. a Low power DiI-labeled nucleus accumbens medium spiny neuron, scale bar 50 μm. b High power dendritic segment from an NAc core medium spiny neuron, scale bar 5 μm. c In cocaine-treated females, estradiol treatment decreased dendritic spine density of medium spiny neurons in the NAc core, and this effect was attenuated by pretreatment with the cannabinoid type 1 receptor inverse agonist, AM251. Dendritic spine density in the NAc core of females treated with AM251 alone did not differ from estradiol or oil treatment alone. d Cumulative probability distribution plot of dendritic spine density within treatment groups for nucleus accumbens core highlights the effect of estradiol on dendritic spines. The number inside of each bar represents the number of animals per treatment condition. * p < 0.05 Veh+Oil vs Veh+E and Veh+E vs AM251+E
Confocal z-stacks of dendritic segments were first subjected to a 3D-deconvolution process using Autoquant X AutoDeblur Gold CF software (version 3.0; Media Cybernetics, Bethesda, MD). Deconvoluted images were then reconstructed in 3D using the Imaris software (version 7.7; Bitplane Inc., St. Paul, MN).
2.8 Dendritic spine data analysis
All analyses were completed by an experimenter blinded to the treatment conditions. Nine dendritic segments (from 3 separate neurons) were averaged to generate a single dendritic spine density value for each animal. In cocaine-treated females, a two-way ANOVA with subsequent Tukey HSD post hoc tests were used to evaluate the effects of drug, hormone and drug-hormone interactions among treatment groups in the NAc core using SPSS software (version 20.0, IBM Corp). For all statistical tests, results were considered to be statistically significant if p < 0.05.
3. Results
Estradiol-facilitated psychostimulant locomotor sensitization depends on CB1R
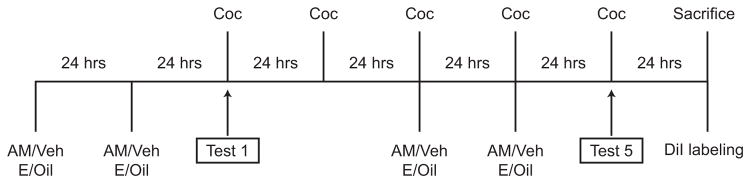
Several laboratories have demonstrated that estradiol treatment in females facilitates cocaine-induced sensitization of locomotor responses (Segarra et al., 2010; Sircar and Kim, 1999) More recently, we demonstrated that this effect of estradiol is dependent on activation of mGluR5 (Martinez et al., 2014). As the endoCB system lies downstream of mGluR5 signaling (Jung et al., 2005; Robbe et al., 2002; Uchigashima et al., 2007), we sought to determine whether this effect of estradiol depends on endoCB signaling, specifically through activation of CB1R. To examine this question, females received the CB1R inverse agonist, AM251 (or vehicle) 30 min prior to estradiol (or oil) treatment. This treatment regimen occurred for two consecutive days before the first and fifth cocaine locomotor test days (Fig 1). Importantly, on each locomotor test day cocaine was administered 24 hrs after other drug and hormone manipulations, to eliminate direct effects of these agents on locomotor activity following cocaine. In accordance with previous studies (Martinez et al., 2014; Segarra et al., 2010; Sircar and Kim, 1999), females treated with estradiol exhibited robust locomotor sensitization to cocaine when comparing ambulations after the fifth cocaine injection to the first cocaine injection (t(13) = 3.043, p < 0.0125; Fig 2). Pretreatment with AM251 blocked the effect of estradiol. AM251 or vehicle treatment in the absence of estradiol did not significantly affect ambulatory movement responses to repeated cocaine injections.
Fig. 1.
Timeline of experimental manipulations. Ovariectomized female rats were injected with the cannabinoid type 1 receptor inverse agonist, AM251, or vehicle (veh) followed by estradiol (E) or oil, on days 1, 2, 5, and 6 (n = 14–19 per group). Cocaine (Coc) was injected on days 3–7 and locomotor activity was assessed on day of the first and fifth Coc injections (Test 1 and Test 5 respectively). 24 hrs after the final Coc injection a subset of females that were tested for behavior were sacrificed for DiI labeling.
Estradiol-induced dendritic spine plasticity depends on CB1R
As synaptic plasticity within the NAc core is thought to form a neurobiological basis for pyschostimulant-induced locomotor sensitization (Dumitriu et al., 2012; Li et al., 2004; Robinson and Kolb, 2004; Waselus et al., 2013), we examined the effect of AM251 and estradiol treatment on dendritic spine plasticity of NAc core MSNs in cocaine-treated females. Twenty-four hrs after the fifth and final cocaine injection, a subset of females from each treatment group was arbitrarily selected for dendritic spine analyses. Representative images of a DiI-labeled MSN and dendritic segment used to quantify dendritic spine densities are shown in Fig 3a and Fig 3b, respectively. The effects of estradiol and AM251 differed across treatment groups (two-way ANOVA: hormone x drug interaction: F(1,21) = 10.6, p < 0.05). Estradiol treatment produced a significant reduction in dendritic spine density in the NAc core (p < 0.05, Tukey HSD post hoc test). In contrast, estradiol-treated females that received AM251 did not exhibit this change in the density of dendritic spines (Fig 3c,d). Compared with estradiol or oil treatment, females that received AM251 alone did not significantly differ in dendritic spine density within the NAc core.
4. Discussion
For women, estradiol is a key biological factor contributing to the etiology of addiction. Yet, our understanding of the underlying neural mechanisms through which estradiol acts is still in its infancy. Recent findings indicate that estradiol, through activation of metabotropic glutamate receptor type 5 (mGluR5), structurally remodels nucleus accumbens (NAc) reward circuits (Peterson et al., 2014) and imparts a greater sensitivity to psychostimulants (Martinez et al., 2014). Here we extend these findings by demonstrating that activation of the endogenous cannabinoid (endoCB) system is necessary for these effects. Specifically, CB1R signaling is required for estradiol potentiation of cocaine-induced locomotor responses and alterations to the dendritic spine density of NAc core medium spiny neurons (MSNs). While it is currently unknown whether these results generalize beyond psychostimulant use, they are relevant, as recent reports indicate ~50% more women than men receive treatment for psychostimulant abuse (SAMHSA, 2014).
The interplay between the estradiol and the endoCB system in the context of repeated exposure to cocaine complement and extend a growing body of work that in the absence of drugs of abuse, ovarian hormones, particularly estradiol, influence the endoCB system. Initial observations found that across the estrous cycle, CB1R density and ligand-binding affinity fluctuate in multiple brain regions; effects that are sensitive to ovariectomy and restored following hormone replacement (Rodriguez de Fonseca et al., 1994). In parallel, CNS levels of 2-arachidonly glycerol (2-AG) and anandamide (AEA) fluctuate in concert with ovarian hormones (Bradshaw et al., 2006) and evidence from in vitro (Huang and Woolley, 2012) and in vivo (Scorticati et al., 2004) studies suggests that AEA levels are increased in the CNS following treatment with estradiol. The activity of the endoCB system appears to increase with the initial rise in plasma estradiol during the estrous cycle (Gorzalka and Dang, 2012). Similarly, plasma endoCB levels are highest with rising estradiol in the follicular phase of the menstrual cycle (El-Talatini et al., 2010).
While estradiol can act through nuclear or membrane-localized estrogen receptors (ERs) to influence the endoCB system, membrane ER activation of mGluR signaling appears to be principally important. Physiological concentrations of estradiol rapidly stimulate AEA release from cultured human endothelial cells through surface membrane estrogen receptors (Maccarrone et al., 2002). Furthermore, in female rat hippocampal neurons membrane-localized ERα couples to mGluR1a, promoting AEA activation of CB1R, and leading to an attenuation of GABAergic neurotransmission (Huang and Woolley, 2012; Tabatadze et al., 2015). Coupling of ERs to mGluRs is in fact observed throughout the female nervous system, though the exact pairing of ERs and mGluRs is brain-region dependent. For example, whereas in CA1 hippocampal pyramidal neurons ERα activates mGluR1a (Boulware et al., 2005), in MSNs of the caudate and NAc core, ERα couples to mGluR5 (Grove-Strawser et al., 2010; Martinez et al., 2014; Peterson et al., 2014).
While ER/mGluR signaling plays an essential role in the regulation of endoCBs, other forms of estrogen signaling also appear to regulate this neurotransmitter system. For example, ERs down-regulate the expression of fatty acid amide hydroxylase (FAAH), the enzyme responsible for the degradation of AEA (Maccarrone et al., 2000; Waleh et al., 2002). Transcriptional repression of FAAH through ER binding traditional estrogen response elements results in elevated AEA tone. Presumably it is through this mechanism that estradiol elevates AEA to elicit anxiolytic effects in female rats, which are blocked with a CB1R antagonist (Hill et al., 2007). Moreover, this nuclear action of estradiol converges with membrane actions at the same functional endpoint to increase AEA (Huang and Woolley, 2012; Tabatadze et al., 2015). These findings parallel other biological systems, where both membrane and nuclear ERs work cooperatively to control various physiological processes (Levin, 2014, 2005; Pedram et al., 2016).
Estradiol regulation of AEA release and metabolism provide evidence that this endoCB is critical for the effects of estradiol; however, as mentioned previously, 2-AG is also regulated by ovarian hormones (Bradshaw et al., 2006). Intriguingly, at least in male subjects, the effects of mGluR5 activation in the NAc are primarily dependent on 2-AG (Jung et al., 2005; Seif et al., 2011), a more potent activator of the CB1R (Sugiura et al., 2006). Because the vast majority of studies utilize only male subjects, it would be of interest to determine if there is a sex difference between mGluR regulation of 2-AG and AEA signaling.
Notwithstanding which endoCB is (primarily) activating CB1Rs in response to estradiol, converging lines of evidence begin to outline how estradiol acts at a circuit level to enhance female sensitivity to cocaine. In their pioneering study, Woolley and colleagues determined that estradiol mobilization of endoCBs activated CB1R on GABAergic terminals to inhibit presynaptic GABA release, and thus indirectly increase post-synaptic pyramidal neuron excitability (Huang and Woolley, 2012; Tabatadze et al., 2015). While these data were from the hippocampus, CB1Rs are also primarily localized on GABAergic terminals in the NAc (Winters et al., 2012), suggesting a similar effect may occur here. Moreover, activation of CB1Rs in the NAc locally disinhibits dopamine release in this region (Cheer et al., 2004; Chen et al., 1993; Sperlágh et al., 2009) as does estradiol in the female NAc and caudate resulting in increased locomotor responses to psychostimulants (Becker and Rudick, 1999; Schultz et al., 2009; Thompson and Moss, 1994). Sustained enhancement of dopamine neurotransmission in the NAc and changes in MSN excitability are recognized as a key factors to the onset of drug addiction through structural remodeling of reward circuits leading to enhanced behavioral responses to psychostimulants (Golden and Russo, 2012; Kalivas and Duffy, 1990; Kourrich and Thomas, 2009).
In contrast to the long-lasting effects on NAc structure and function imposed by repeated exposure to drugs of abuse, the effects of estradiol are both more transient and cyclical. This is true not only for the effects of estradiol on neurotransmission and excitability, but for structural plasticity as well. We observe decreased dendritic spine density in the NAc core 24–48 hours after estradiol (Peterson et al., 2014; Staffend et al., 2011). These effects reverse over several days in the absence of further hormone treatment (Woolley, 1998). Regardless of duration, group I mGluRs and CB1R are necessary for the effects of estradiol on structural plasticity (Christensen et al., 2011; Peterson et al., 2014;). In addition, activation of group I mGluRs or CB1Rs alone are sufficient to decrease dendritic spine density in the NAc core (Gross et al., 2015; Carvalho et al., 2014). Thus group I mGluRs and CB1R are both necessary and sufficient to produce dendritic spine plasticity in the NAc core.
At the same time that the effects of estradiol on dendritic spine plasticity in the NAc that have received little attention (Peterson et al., 2014; Staffend et al., 2011), numerous studies have investigated the effect of psychostimulants. Although initial studies indicated that repeated exposure to psychostimulants increased dendritic spine density within the NAc core (e.g., Li et al., 2004; Norrholm et al., 2003), more recent studies using advanced staining techniques and 3-D imaging, thought to more accurately represent neuroanatomical structure (Golden and Russo, 2012), have observed a decrease in spine density within this brain region following repeated cocaine administration (Dumitriu et al., 2012; Waselus et al., 2013). Independent of methodological issues, dendritic spine plasticity of MSNs in the NAc are associated with enhanced behavioral responsiveness to psychostimulants. While changes in dendritic spines may be necessary for behavioral sensitization, our findings suggest that alone they are not sufficient as estradiol enhances the effects of psychostimulants, but does not typically produce sensitization on its own.
In summary, it has been previously postulated that the endoCB system is an intermediary between estradiol and drug addiction (Maccarrone, 2004). Here, we outline a putative mechanism linking estradiol to the endoCB system in remodeling reward circuits, imparting a greater vulnerability for drug abuse in females. Whereas endoCB signaling via group I mGluR occurs in both males and females, the coupling of ERs to group I mGluRs appears relatively exclusive to the female nervous system. This provides a sex-specific mechanism that may account for the accelerated onset of female drug addiction. Hence, decoupling ERs to mGluR activation of endoCB in the reward system may be considered as a potential therapeutic means to mitigate hormonal influences on female vulnerability to addiction.
Highlights.
Estradiol facilitated sensitization depends on cannabinoid type 1 receptors.
Estradiol reduces dendritic spine density via cannabinoid type 1 receptors.
Endocannabinoids mediate estradiol potentiation of drug addiction.
Acknowledgments
This material is based upon work supported by the National Institutes of Health DA035008 (PGM and RLM) and DA040345-01 (BMP) a National Science Foundation Grant No. 00006595 (BMP). We would like to thank Ambrosia Smith, Sonal Napgal and Holly Korthas for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brittni M. Peterson, Email: bmpete@umn.edu.
Luis A. Martinez, Email: lamartin@umn.edu.
Robert L. Meisel, Email: meisel@umn.edu.
References
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Barton M. Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids. 2016 doi: 10.1016/j.steroids.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin Proteins Are Essential for Distinct Effects of Membrane Estrogen Receptors in Neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol - Regul Integr Comp Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Reyes BAS, Ramalhosa F, Sousa N, Bockstaele EJV. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct. 2014:1–13. doi: 10.1007/s00429-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PEM, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Marmur R, Pulles A, Paredes W, Gardner EL. Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana’s psychoactive ingredient. Brain Res. 1993;621:65–70. doi: 10.1016/0006-8993(93)90298-2. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Ann Endocrinol. 1999;60:143–148. [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. 2010;93:1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Dang SS. Minireview: Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- Gross K, Meisel RL, Mermelstein PG. Opposing effects of group I mGluRs on dendritic spine density in the rat nucleus accumbens. 2015 Neuroscience Meeting Planner; Chicago IL: Society for Neuroscience; Program No. 322.14. 2015 Online. [Google Scholar]
- Hedges VL, Staffend NA, Meisel RL. Neural mechanisms of reproduction in females as a predisposing factor for drug addiction. Front Neuroendocrinol. 2010;31:217–231. doi: 10.1016/j.yfrne.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Extranuclear estrogen receptor’s roles in physiology: lessons from mouse models. Am J Physiol - Endocrinol Metab. 2014;307:E133–E140. doi: 10.1152/ajpendo.00626.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M. Sex and drug abuse: a role for retrograde endocannabinoids? Trends Pharmacol Sci. 2004;25:455–456. doi: 10.1016/j.tips.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Battista N, Finazzi-Agrò A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, De Felici M, Bari M, Klinger F, Siracusa G, Finazzi-Agrò A. Down-regulation of anandamide hydrolase in mouse uterus by sex hormones. Eur J Biochem. 2000;267:2991–2997. doi: 10.1046/j.1432-1033.2000.01316.x. [DOI] [PubMed] [Google Scholar]
- Maria MMMS, Flanagan J, Brady K. Ovarian hormones and drug abuse. Curr Psychiatry Rep. 2014;16:1–8. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Thomas T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olière S, Joliette-Riopel A, Potvin S, Jutras-Aswad D. Modulation of the endocannabinoid system: vulnerability factor and new treatment target for stimulant addiction. Front Psychiatry. 2013;4:109. doi: 10.3389/fpsyt.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Blumberg B, Levin ER. Membrane and nuclear estrogen receptor α collaborate to suppress adipogenesis but not triglyceride content. FASEB J. 2016;30:230–240. doi: 10.1096/fj.15-274878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. A Conserved Mechanism for Steroid Receptor Translocation to the Plasma Membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Impact of immersion oils and mounting media on the confocal imaging of dendritic spines. J Neurosci Methods. 2015;242:106–111. doi: 10.1016/j.jneumeth.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2014;220:2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorticati C, Fernández-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A, Billi S, Franchi A, McCann SM, Rettori V. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci U S A. 2004;101:11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menéndez-Delmestre R, Puig-Ramos A, Torres-Diaz YM. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Makriyannis A, Kunos G, Bonci A, Hopf FW. The endocannabinoid 2-arachidonoylglycerol mediates D1 and D2 receptor cooperative enhancement of rat nucleus accumbens core neuron firing. Neuroscience. 2011;193:21–33. doi: 10.1016/j.neuroscience.2011.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Sperlágh B, Windisch K, Andó RD, Sylvester Vizi E. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int. 2009;54:452–457. doi: 10.1016/j.neuint.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling in fixed brain slices: phenotype, morphology and dendritic spines. Curr Protoc Neurosci. 2011a;59:2.13.1–2.13.15. doi: 10.1002/0471142301.ns0213s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. Diolistic labeling of neurons in tissue slices: a qualitative and quantitative analysis of methodological variations. Front Neuroanat. 2011b:5. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann G, Di Marzo V, Molleman A, Hasenöhrl RU. The CB1 cannabinoid receptor antagonist AM251 attenuates amphetamine-induced behavioural sensitization while causing monoamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 2008;89:384–391. doi: 10.1016/j.pbb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic-and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Nethrapalli IS, Tinnikov AA. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291:203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- Waselus M, Flagel SB, Jedynak JP, Akil H, Robinson TE, Watson SJ., Jr Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats. Neuroscience. 2013;248:571–584. doi: 10.1016/j.neuroscience.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Winters BD, Krüger JM, Huang X, Gallaher ZR, Ishikawa M, Czaja K, Krueger JM, Huang YH, Schlüter OM, Dong Y. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109:E2717–2725. doi: 10.1073/pnas.1206303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]