Abstract
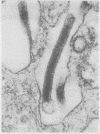
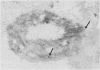
A filovirus, serologically related to Ebola virus, was detected by "post-embedment" immunoelectron microscopical examination of MA-104 cells. These had been infected by inoculation with serum samples obtained during the 1989 epizootic in cynomolgus monkeys (Macaca fascicularis), imported from the Philippines and maintained at Reston, Virginia, USA, a primate holding facility. The immunoelectron microscopy method, when used in conjunction with standard transmission electron microscopy (TEM) of infected cells, provided consistent results and was simple to perform in this epizootic. It is concluded that immunoelectron microscopy is potentially useful in the direct immunological diagnosis of Ebola and related filoviral infections (such as Marburg) in clinical samples obtained from those with acute infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellis D. S., Simpson I. H., Francis D. P., Knobloch J., Bowen E. T., Lolik P., Deng I. M. Ultrastructure of Ebola virus particles in human liver. J Clin Pathol. 1978 Mar;31(3):201–208. doi: 10.1136/jcp.31.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. S., Stamford S., Lloyd G., Bowen E. T., Platt G. S., Way H., Simpson D. I. Ebola and Marburg viruses: I. Some ultrastructural differences between strains when grown in Vero cells. J Med Virol. 1979;4(3):201–211. doi: 10.1002/jmv.1890040306. [DOI] [PubMed] [Google Scholar]
- Ellis D. S., Stamford S., Tvoey D. G., Lloyd G., Bowen E. T., Platt G. S., Way H., Simpson D. I. Ebola and Marburg viruses: II. Thier development within Vero cells and the extra-cellular formation of branched and torus forms. J Med Virol. 1979;4(3):213–225. doi: 10.1002/jmv.1890040307. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Geisbert T. W., Dalgard D. W., Johnson E. D., Ksiazek T. G., Hall W. C., Peters C. J. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet. 1990 Mar 3;335(8688):502–505. doi: 10.1016/0140-6736(90)90737-p. [DOI] [PubMed] [Google Scholar]
- Kelen A. E., Hathaway A. E., McLeod D. A. Rapid detection of Australia-SH antigen and antibody by a simple and sensitive technique of immunoelectronmicroscopy. Can J Microbiol. 1971 Jul;17(7):993–1000. doi: 10.1139/m71-157. [DOI] [PubMed] [Google Scholar]
- Palmer E., Sporborg C., Harrison A., Martin M. L., Feorino P. Morphology and immunoelectron microscopy of AIDS virus. Arch Virol. 1985;85(3-4):189–196. doi: 10.1007/BF01314230. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]