Abstract
Vasopressin V1a receptors (V1aR) are thought to contribute to the pathophysiology of psychiatric disorders such as anxiety and depression, sparking interest in V1aR as a therapeutic target. Although the global effects of V1aR have been documented, less is known about the specific neural circuits mediating these effects. Moreover, few studies have examined context-specific V1aR function in both males and females. By using the California mouse, we first studied the effects of sex and social defeat stress on V1aR binding in the forebrain. In females but not males, V1aR binding in the bed nucleus of the stria terminalis (BNST) was negatively correlated to social interaction behavior. In females, stress also increasesd V1aR binding in the nucleus accumbens (NAc). Infusions of V1aR antagonist in to the medioventral BNST (BNSTmv) had anxiogenic effects only in animals naïve to defeat. For males, inhibition of V1aR in BNSTmv had anxiogenic effects in social and nonsocial contexts, but for females, anxiogenic effects were limited to social contexts. In stressed females, inhibition of V1aR in the NAc shell had no effect on social interaction behavior, but had an anxiogenic effect in an open field test. These data suggest that V1aR in BNSTmv have anxiolytic and prosocial effects in males, and that in females, prosocial and anxiolytic effects of V1aR appear to be mediated independently by receptors in the BNSTmv and NAc shell, respectively. These findings suggest that males have more overlap in neural circuits modulating anxiety in social and nonsocial contexts than females.
Introduction
Vasopressin V1a receptors (V1aR) are key mediators of a myriad of social behaviors including social recognition (Bielsky et al., 2004), social communication (Albers et al., 1986; Lukas and Wohr, 2015), and social approach (Ramos et al., 2013). Acute pharmacological inhibition of V1aRs can produce anxiolytic effects while genetic deletion of V1aR produced both anxiolytic effects and reductions in social function in male mice (Bielsky et al., 2004). The modulation of both social behavior and anxiety by V1aR has sparked interest in its potential as a novel therapeutic target for stress-related psychiatric disorders, in which anxiety and social deficits can be comorbid (Lee et al., 2013; Meyer-Lindenberg and Tost, 2012). Currently it is unclear whether the effects of V1aR on social and anxiety-like behavior are mediated by independent or overlapping circuits. Although there are important species differences in V1aR binding (Bester-Meredith et al., 1999; Hammock and Young, 2006), dense V1aR binding in the bed nucleus of the stria terminalis (BNST) is observed in a diverse group of rodents (Bales et al., 2007; Bosch et al., 2010) and primates (Freeman et al., 2014a; Freeman et al., 2014b; Young et al., 1999). The BNST has been proposed to be a key nucleus for stress-induced psychiatric disorders (Lebow and Chen, 2016), as it is an important regulator of both social behavior (O’Connell and Hofmann, 2011) and affective states (Avery et al., 2015; Hammack et al., 2012). Thus, the BNST is well positioned to modulate effects of neuropeptides on anxiety and social behavior (Coria-Avila et al., 2014; Greenberg et al., 2014; Markham et al., 2009). The BNST is sexually dimorphic both at functional and anatomical levels (Campi et al., 2013), which is important because stress-induced psychiatric disorders are more common in women than men (Kessler et al., 1995). Here we examined the effects of social stress on V1aR binding in the BNST and the effects of V1aR inactivation on behavior using the California mouse model of social defeat.
The California mouse is a monogamous species in which both males and females aggressively defend a joint territory (Ribble and Salvioni, 1990). This social organization provides ethological validity to the use of protocols in which males and females experience social defeat (Trainor et al., 2013). Three episodes of social defeat reduce social interaction behavior in females but not males (Greenberg et al., 2015; Trainor et al., 2011). This effect is independent of gonadal steroids (Trainor et al., 2013) and can be reversed with chronic but not acute sertraline treatment (Greenberg et al., 2014). Male California mice exposed to defeat exhibit increased fear behavior in a resident-intruder test (Steinman et al., 2015), similar to the conditioned defeat phenotype described in Syrian hamsters (Clinard et al., 2015; Gray et al., 2015).
We first examined the effects of social defeat on V1aR binding in the hypothalamus, lateral septum, BNST, and nucleus accumbens (NAc). These nuclei are important regulators of social and affective behavior (Davis et al., 2010; Walker and Davis, 2008), and have strong connections with one another (O’Connell and Hofmann, 2011). These data showed that in females but not males, V1aR expression is significantly increased in NAc core and shell, and that in the medioventral BNST (BNSTmv), social interaction behavior is negatively correlated with V1aR expression. In a previous study we observed that defeat increases the activity of OT neurons in the BNSTmv of females but not males and that intranasal OT reduces social interaction behavior in females but not males (Steinman et al., 2016). Based on observations that V1aR can be activated by OT release (Song et al., 2014), we hypothesized that stress-induced increases in the activity of OT neurons in the BNSTmv reduce social interaction behavior through activation of V1aR. To test this hypothesis, we examined the effects of V1aR antagonist in either the NAc or BNSTmv on social interaction behavior in females. In addition we examined the effects of V1aR inhibition in BNSTmv in the resident intruder test in males, based on previous work showing V1aR regulation of aggression. Our results provide important insights into how V1aR modulates anxiety-like behavior in social and nonsocial contexts in males and females.
Materials and Methods
Experiments
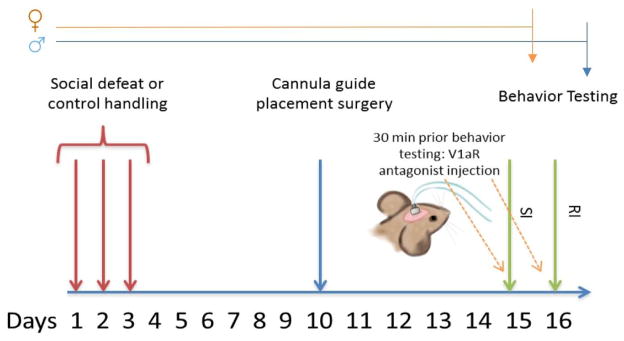
Four experiments were conducted. Experiment 1: Effects of social defeat stress on V1aR binding in males (naïve n=8, stress=7) and females (naïve n=11, stress=9); Experiment 2: effects of V1aR antagonist infusion into BNSTmv on social interaction behavior in naïve (artificial cerebrospinal fluid (aCSF) n=6, V1aR antagonist n=10) and stressed (aCSF n=8, V1aR antagonist n=8) females; Experiment 3: effects of V1aR antagonist infusion into BNSTmv on social interaction behavior and territorial aggression in naïve (aCSF n=8, V1aR antagonist n=10) and stressed (aCSF n=8, V1aR antagonist n=9) males; and Experiment 4: effects of V1aR antagonist infusion into NAc shell on social interaction behavior in stressed females (aCSF n=7, V1aR antagonist n=8). Procedures are detailed below (see Fig. 1 for timelines).
Fig. 1. Timeline for experiments 2–4.
Males and females were tested in social interaction test (SI) after one injection of V1a receptor antagonist or artificial cerebrospinal fluid into BNSTmv (exp. 2,3). Only males received a second injection before resident intruder test (RI) one day after SI (exp. 3). In experiment 4, only females were tested in SI after an injection of V1aR antagonist or saline into nucleus accumbens shell. Arrows represent end of study for females (orange) and males (blue).
Animals and housing conditions
Three-month old male and female California mice from our breeding colony at UC Davis were group housed (2–3 same-sex animals per cage). Those that underwent the cannula implantation surgery were singly housed immediately following the procedure. Animals were ear punched for identification and maintained in clear polypropylene cages in a room with controlled temperature (68–74 °F) and 16L:8D light:dark cycle (lights off at 1400). Humidity was maintained at ambient levels. Water and food (Harlan Teklad 2016, Madison, WI) were provided ad libitum. Each polycarbonate plastic cage was provided with Sanichip bedding and environmental enrichment consisting of nestlets and enviro-dri. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and conformed to NIH guidelines. All behavioral tests were conducted during lights out (1430–1730) under dim red light (3 lux).
Social defeat
Mice were randomly assigned to social defeat or control handling for 3 consecutive days. Mice assigned to social defeat were placed in the cage of an aggressive same-sex mouse on consecutive days (Trainor et al., 2013). Each episode lasted 7 min or until the resident attacked the focal mouse 7 times (whichever occurred first). Control mice were introduced into a clean cage for 7 min. Immediately after defeat/control, mice were returned to their home cage. All behavioral and receptor binding analyses were conducted two weeks after social defeat based on previous observations that the effects of defeat become stronger over time (Trainor et al. 2011).
Social interaction test (SI)
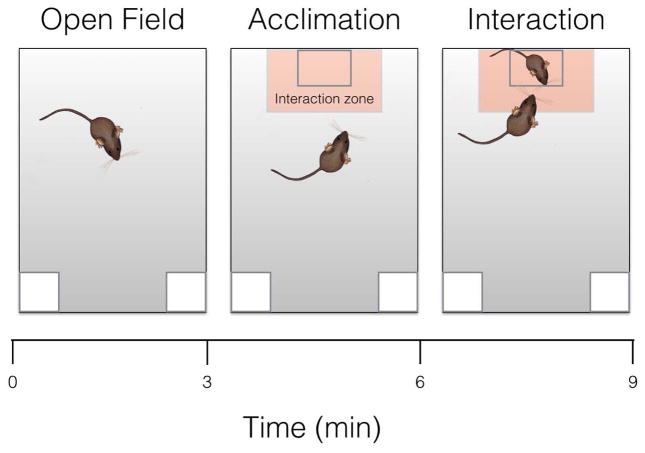
Two weeks after defeat or control conditions, all animals were tested in SI as previously described (Greenberg et al., 2014; Trainor et al., 2013). First, each focal mouse was introduced into the open field (89x63x60cm) for 3 min (open field phase). Total distance traveled (Anymaze, Stoelting) was recorded to assess locomotor behavior, and time spent in the center of the arena (within 8 cm of the sides and within a center zone located 14 cm from the sides) was recorded to assess anxiety-like behavior. Next, an empty wire cage was placed against one wall (acclimation phase). The amount of time that the focal mouse spent within 8 cm of the cage (interaction zone) and in the corners opposite to the wire cage was recorded for 3 min. Finally, an unfamiliar intact same-sex mouse was placed into the wire cage for 3 min (interaction phase) and the time spent in the interaction zone and corners was recorded (Fig. 2). Mice were returned to their home cage immediately after the end of SI.
Fig. 2. Diagram of social Interaction test.
(3 consecutive stages). Each phase of the social interaction tests, a) open field, b) acclimation, and c) interaction, lasted 3 minutes. Mouse in the small box represents novel conspecific target mouse. In light orange is represented the area considered as the interaction zone.
Receptor Autoradiography
V1aR binding was measured using receptor autoradiography according to previously established methods (Perkeybile et al., 2015). Immediately following SI testing mice were euthanized and brains were frozen on dry ice. Brains were sliced at 20 μm using a cryostat and mounted on Super-frost plus slides. Slides were fixed in 0.1 % paraformaldehyde (Sigma Aldrich, St. Louis Missouri, USA) in phosphate buffered saline (PBS) for 2 min and then rinsed in two 10 min tris buffer washes 50 mM Trizma Base (Sigma, pH 7.4) followed by a 1 hr-room temperature incubation in 50 pM of radiotracer diluted in a tracer buffer (50 mM Trizma Base (pH 7.4), 10 mM MgCl2, 0.1% bovine serum albumin). We used 125I-lin-vasopressin [125I-phenylacetyl-D-Tyr(ME)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2] (NEN Nuclear) to detect V1aR. Non-specific binding was identified by immersing adjacent sections in buffer containing both the radioactive specific ligand and 50 μM of unlabeled competitor ligand, (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin (Bachem, King of Prussia, PA). Following a 60 min incubation, were washed four times in 50 mm Trizma Base (pH 7.4) with 10 mM MgCl2 at 4 °C, with a subsequent 30 min wash. Slides the n were rinsed in cold water and dried for 15 min. Sections were apposed to Kodak BioMaxMR film (Kodak, Rochester, NY, USA) with 125I microscale standards (Perkin-Elmer/NEN) for 72 h. Sections as well as 125I microscale standards (Perkin-Elmer/NEN) were placed on Kodak BioMaxMR film (Kodak, Rochester, NY, USA) for 96 h. ImageJ was used to quantify 125I-receptor binding. Uncalibrated optical density was converted disintegrations per minute (DPM) using the 125I standards. If available, six sections per area were quantified bilaterally and then averaged for analysis. In some animals, one or two sections were damaged, so only four or five were available for analysis. The damaged sections were distributed across all four groups. On each section we also measured a nearby white matter tract that did not show receptor binding and subtracted this from the measurements for the area of interest to account for background (Inoue et al., 2013; Laredo et al., 2015). There was no difference in signal in these regions and sections used to control for non-specific binding.
Cannula implantation surgery
Males and females were randomly assigned to control or defeat conditions as described above. Seven days after defeat or control handling, all mice were anesthetized with isoflurane (3–5%) and implanted with bilateral stainless steel guide cannula (Plastics One, C235G-2.2W 1 mm PROJ) aimed at the BNSTmv (AP=0.39 mm, LM=1.1 mm, DV=6.85, fig. 3). The guide cannulae (26 ga, o.d.=0.46 mm; i.d.=0.24 mm; length=5.85 mm), were lowered into burr holes (#105 dremel bit, 1/16″ tip) and attached to the skull with skull screws (plastics one, 00-96 X 1/16) and acrylic dental cement. Bilateral dummy caps (Plastics One, C235DC) were used to maintain patency. Carprofen (5mg/kg) was administered subcutaneously immediately before surgery and once a day during 3 consecutive days after surgery. Animals were given five to seven days for recovery, during which the mice were observed and handled daily. After this recovery period, each male and female received an infusion of V1aR antagonist or aCSF and was tested in the social interaction test as described above. One day later, males but not females were tested in the resident intruder test after receiving an injection of V1aR antagonist or aCSF (each male received same treatment as day before). An additional set of stressed females was implanted with guide cannulae aimed at the NAc shell as previously described (AP=0.51 mm, LM=1.1 mm, DV=6.85) (Campi et al., 2014). We decided to focus on NAc shell and no core because previous studies have implicated an important role of NAc shell on interaction behavior (Campi et al., 2014; Trainor et al., 2011). Seven days after recovery, each female received an infusion of V1aR antagonist or aCSF and was tested in the social interaction test. Males and control females were not included in this analysis due to very low V1aR binding levels.
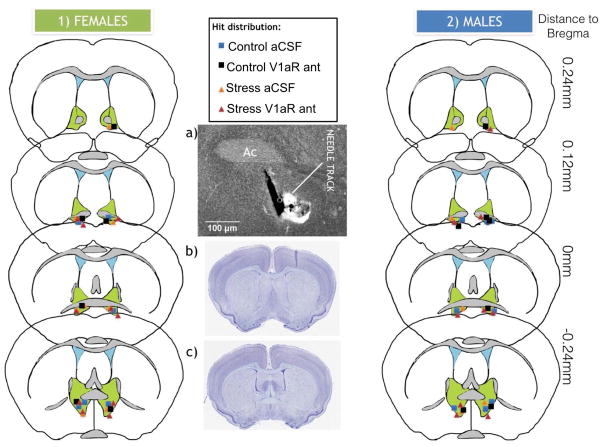
Fig.3. Distribution of cannula placement into bed nucleus of the stria terminalis medioventral considered as hits in males and females.
Injections that were misses are not shown. a) Representative picture of needle tracks used in order to determine hits. Ac= anterior commissure. b) California mouse coronal section from brainmaps.org showing most anterior section considered as hit and c) most posterior section considered as hit.
V1aR antagonist infusion
In males and females, infusions into BNSTmv were made using bilateral internal cannulae Plastics One, C235I/SPC; 33ga; o.d.=0.21 mm; i.d.=0.11 mm, that projected 1 mm past the cannula guide (6.85 mm total length). In stressed females, infusions into NAc shell were made using bilateral internal cannulae Plastics One, C235I/SPC; 26ga; o.d.=0.46 mm; i.d.=0.24 mm. For injections both in BNSTmv and NAc shell, the V1aR antagonist [β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-Vasopressin (Manning compound, Sigma catalog #:V2255) was dissolved in 2 ml aCSF, and frozen in 50 microliter aliquots at a dose of 0.5 mg/ml. Prior to daily injection, one aliquot was further dissolved in aCSF at a 1:20 dilution. Animals were randomly assigned to receive a 200 nl infusion per side containing either aCSF or 5 ng of V1aR antagonist. This dose was adjusted from dose previously shown to have behavioral effects when injected into BNST in rats (10 ng per side) (Gray et al., 2012; Veenema et al., 2010). Using a California mouse brain atlas (brainmaps.org) we estimated that the sizes of the BNST and NAc are about 50% smaller in California mice compared to rats. Hamilton syringes were attached to an automatic micropump delivery apparatus (PHD 2000, Harvard Apparatus, Cambridge, MA) set to deliver 0.1uL/min. Internal guides were kept in place for 1 min after injection. Both males and females were given one infusion, returned to the home cage, and then tested in SI after 30 min (Figs 1 and 2). Females in experiments 2 (injection in BNSTmv) and 4 (injection in NAc shell) were euthanized after testing to determine injection sites (for hits in BNSTmv, see Fig. 3. Hits in NAc shell are not shown). One day after SI, males were tested in the resident-intruder test (described below) 30 min after receiving a second infusion of the same treatment they had received the day before. Males where then euthanized after testing to determine injection sites (Fig. 3).
Resident-Intruder test (RI)
One day after SI, males were tested in RI as previously described (Steinman et al., 2015). Cage bedding had not been changed for three days, which has been shown to increase aggressive behavior (Bester-Meredith et al., 1999). Thirty minutes after the infusion of the V1aR antagonist or aCSF, a group housed, an unfamiliar same-sex intruder was introduced into the home cage of the resident. Tests lasted for 7 min after which the intruder was removed. Tests were video recorded and bouts of aggression, social investigation, and defensive behaviors (freezing, escape) were quantified as previously described (Steinman et al., 2015; Trainor et al., 2010) (Table 1).
Table 1. Ethogram used for behavior scoring during resident intruder test.
Behavioral observations were always focused on resident. Each activity was recorded in seconds of duration and frequency for a total of 7 minutes.
| Behavior | Description |
|---|---|
| 1. Environment investigation | |
| Rearing | Resident rears into hind legs and sniffs environment |
| Non-social explore | Resident sniffs bedding |
| 2. Social investigation | |
| Social exploration | Resident sniffs intruder in any body area but anogenital |
| Anogenital sniffing | Residents’ nose in immediate proximity to the anogenital region of intruder |
| Move towards | Resident approaches intruder. Usually precedes social investigation |
| 3. Aggressive behaviors | |
| Attack latency | Time until resident first attacks intruder |
| Chase | Resident follows intruder in a chasing manner |
| Bite | Resident bites the intruder |
| Keep down | Resident pushes intruder to stay on his back |
| 4. Defensive behavior | |
| Submission latency | Time until resident assumes a submissive posture |
| Submissive posture | Resident lays down on his back in a submissive posture |
| Boxing | Resident stands on its hind legs and moves forepaws towards the intruder |
| Move away | Resident moves away from intruder |
| 5. Anxiety related behaviors | |
| Freezing | Resident standing on four paws and does not move any part of its body for >2 s |
| Escaping | Resident stands along the edge of the home cage and jumps side to side |
| Self-grooming | Resident grooms himself |
| 6. Rest or inactivity (I) | Resident not active behavior but not freezing |
Histology
Brains were fixed overnight in 5% acrolein in PBS, after which they were immersed in 20% sucrose in PBS for a day, frozen and sectioned coronally at 40 μm. In order to confirm needle placement, sections were stained using NeuroTrace® 435/455 Blue Fluorescent Nissl Stain (Life Technologies). Mice with injection sites outside of the BNSTmv for experiments 2 and 3 and NAc shell for experiment 4 were excluded from statistical analysis.
Estrous cycle assessment
Conducting vaginal lavage before testing disrupts behavior in California mouse (Silva et al., 2010), so estrous cycle was assessed post mortem. There was no systematic bias in the distribution of estrous stage across treatment groups.
Statistical analysis
All the statistical analyses were performed with R software. For receptor autoradiography data we used two-way ANOVA (sex and stress). We also performed regression analyses of time spent engaged in social interaction using sex, V1aR binding, and their interaction as parameters (package “ggplot”). Two-way ANOVA was used to analyze behaviors in the SI and RI tests (stress and treatment). For two-way ANOVA analyses that showed a significant interaction between stress and treatment, planned comparisons were used to detect differences between groups (package “lsmeans”, Bonferroni, 0.95 confidence interval). Cohen’s d is used to report effect size in this case. Finally, we also performed a 3-way ANOVA to assess possible effects of estrous cycle (stress, treatment and estrous cycle) in the social interaction test.
RESULTS
Experiment 1:Effect of stress on V1aR binding in males and females
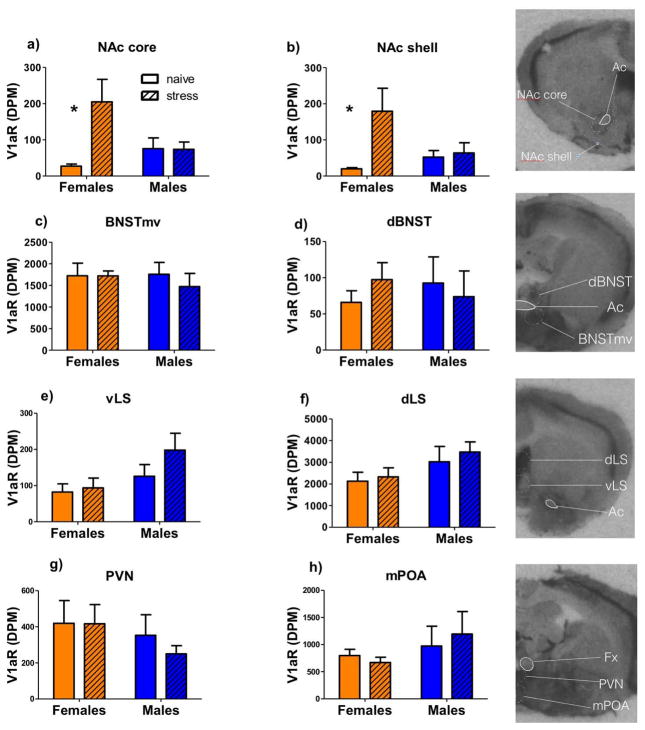
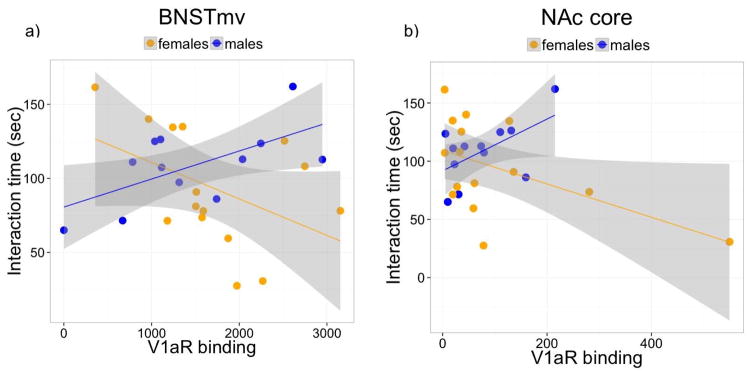
There was a stress*sex interaction on V1aR expression in NAc core (Fig. 4a, F1,24=6.39, p<0.05) and shell (Fig. 4b, F1,24=4.33, p<0.05). In males, there was no effect of defeat stress on V1aR expression in NAc core (p=0.9, Cohen’s d (d)=0.15) or shell (p=0.83, d=0.05), while in females, defeat stress significantly increased expression of V1aR in NAc core (Fig. 4a, F1,13=8.42, p<0.05, d= 1.17) and shell (Fig. 4b, F1,13=6.42, p<0.05, d=1.34). In the BNSTmv, dorsal BNST, lateral septum (LS) dorsal, LS ventral, paraventricular nucleus (PVN), and medial preoptic area (mPOA), there were no main effects of sex or stress (Fig. 4c–h). However, regression modeling of interaction time including the parameters of sex, V1aR binding, treatment, and the interaction between sex and V1aR binding revealed a significant difference between the slopes of V1aR binding in BNSTmv and interaction time in males and females (fig. 5a, regression model F4,22=5.21, sex * V1aR p=0.004). We also found a significant sex difference between slopes of V1aR binding in NAc core and interaction time (fig. 5b, regression model F4,22=3.41, sex * V1aR p=0.043), but this was mainly driven by an outlier. Regression models did not indicate any significant interaction between slopes for males and females for any other of the areas analyzed (all p’s > 0.1, table 2).
Fig.4. V1aR binding in naïve and stressed males and females.
Mean + SEM of V1aR binding in a) NAc core, b) NAc shell, c) BNSTmv, d) dorsal BNST (BNSTd), e) ventrolateral septum (vLS), f) dorsolateral septum (dLS), g) paraventricular nucleus (PVN), and h) medial preoptic area (mPOA).*P<0.05 effect of stress. Ac= anterior commissure, Fx=fornix, V1aR= V1a receptor, DPM=disintegration per minute
Fig.5. Relationship between V1aR binding and social interaction behavior.
Fitted regression lines for V1aR binding on Interaction time in males and females in a) BNSTmv and b) NAc core
Table 2.
P values of linear regression model of V1aR binding and interaction time in males and females for all areas analyzed.
| Sex | V1aR | Stress | Sex* V1aR | |
|---|---|---|---|---|
| NAc core | 0.54 | 0.06 | 0.11 | 0.04* |
| NAc shell | 0.77 | 0.48 | 0.08 | 0.37 |
| BNSTmv | 0.03* | 0.00* | 0.00* | 0.00* |
| dorsal BNST | 0.61 | 0.12 | 0.02* | 0.08 |
| LS dorsal | 0.85 | 0.23 | 0.00* | 0.37 |
| LS ventral | 0.93 | 0.17 | 0.00* | 0.15 |
| PVN | 0.93 | 0.49 | 0.00* | 0.23 |
| mPOA | 0.74 | 0.38 | 0.00* | 0.35 |
p <0.05
Experiment 2: Effects of V1aR antagonist in BNSTmv on female behavior
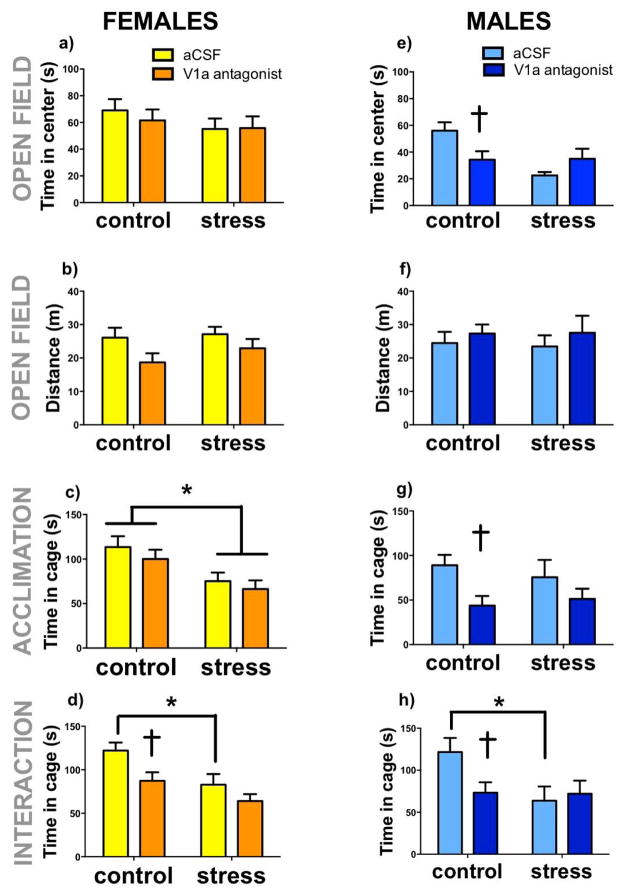
During the social interaction phase, both defeat (Fig. 6d, F1,28=5.74 p= 0.02) and V1aR antagonist (Fig. 6d, F1,28=5.08, p=0.03) reduced time spent in the interaction zone. Although the stress*antagonist interaction was not significant (Fig. 6d, F1,28=0.84, p=0.36), planned comparisons revealed that V1aR antagonist reduced social interaction in control females (Fig. 6d, p=0.01 d=1.29) but not in stressed females (Fig. 6d, p=0.21d=0.86). This is likely due to a floor effect, because stressed females treated with aCSF had significantly reduced social interaction compared to control females treated with aCSF (Fig. 6d, p=0.02, d=1.33). Interestingly, the effect size of V1aR antagonist in control females is similar to the effect size of social defeat in aCSF treated females. In females the effect of the V1aR antagonist in the BNSTmv appeared specific to social contexts, as there was no effect of V1aR antagonist in the acclimation phase on time in the interaction zone (Fig. 6c). However, defeat stress reduced time spent in the interaction zone during the acclimation phase (F1,29=12.06, p=0.001, Fig 6c). There were no differences in time spent in the center (fig. 6a) or locomotor behavior (fig. 6b) during the open field phase. When estrous phase was included in the ANOVA, there was no effect of cycle on time spent in the cage during acclimation or time spent in open field (Table 3, all p’s > 0.1).
Fig.6. Effects of V1aR inhibition in the BNSTmv in naïve and stressed males and females on behavior in the social interaction test.
Control and stressed females and males receiving injections of artificial cerebrospinal fluid (aCSF) or V1aR antagonist into BNSTmv. Mean+ SEM time in center (a,e) and distance traveled (b,f) during open field. Time spent in proximity to the empty cage (c,g) during acclimation trial, and time spent in proximity to a cage containing a novel same sex individual (d,h) during interaction trial. *p<0.05 effect of stress, † p < 0.05 effect of V1aR antagonist.
Table 3. Experiment 2: Effects of estrous phase on behavior during social interaction test.
P values of 3 way ANOVA analysis for dependent variables (a) time in social interaction, (b) time in cage during acclimation, and (c) time in open field including independent variables estrous phase, treatment (trt), stress, and their interaction.
| Estrous phase | Treatment* estrous | Stress* estrous | Treatment* Stress*estrous | |
|---|---|---|---|---|
| Time in proximity to cage during social interaction | 0.85 | 0.12 | 0.65 | 0.73 |
| Time in proximity to cage during acclimation | 0.67 | 0.57 | 0.22 | 0.11 |
| Time in open field | 0.84 | 0.84 | 0.48 | 0.26 |
Experiment 3: Effects of V1aR antagonist in BNSTmv on male behavior
During open field testing, there was a significant stress*treatment interaction on time spent in center (F1,31= 7.66, p=0.009, fig. 6e). V1aR antagonist significantly reduced time spent in the center only in naïve animals (p=0.02, d=1.14), but not in stressed animals (p=0.16, d=0.74). There were no differences in total distance traveled (Fig. 6f). During the social interaction test, there were no main effects of stress or treatment on time spent in the interaction area, but there was a trend for a stress*treatment interaction (F2,34=3.64, p=0.06). Planned comparisons revealed that V1aR antagonist significantly reduced interaction time in naïve (p=0.03, d=1.11) but not stressed males (p=0.7, d=0.18) (Fig. 6h). During acclimation phase, there was a main effect of V1aR to reduce time spent in proximity to the empty cage (Fig. 6g, F1,31=6.78, p= 0.01). However planned comparisons detected an effect of V1aR antagonist in control males (p= 0.02, Cohen’s d=1.35) but not stressed males (0.21, d=0.52).
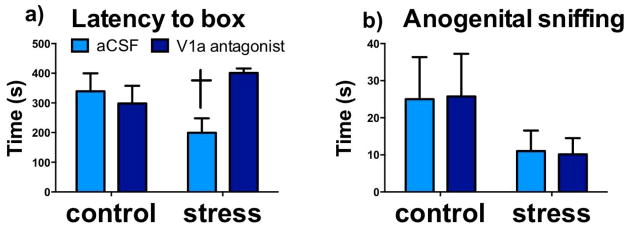
In the RI test there was a significant stress*treatment interaction on latency to box (Fig 7a, F1,31=7.36, p=0.01), with V1aR antagonist significantly increasing latency in stressed animals (p=0.04, d=0.94), and with no effect in naive animals (p=0.1, d=0.82). No significant differences were observed in other behaviors quantified during the resident-intruder tests.
Fig. 7. Effects of V1aR inhibition in the BNSTmv in naïve and stressed males on behavior in the resident intruder test.
Mean + SEM time spent showing Latency to box (a), and Anogenital sniffing (b), during resident intruder test in control and stressed males receiving injections of aCSF or V1aR antagonist into BNSTmv. † p < 0.05 effect of V1aR antagonist.
Experiment 4: Effects of V1aR antagonist in NAc shell on female behavior
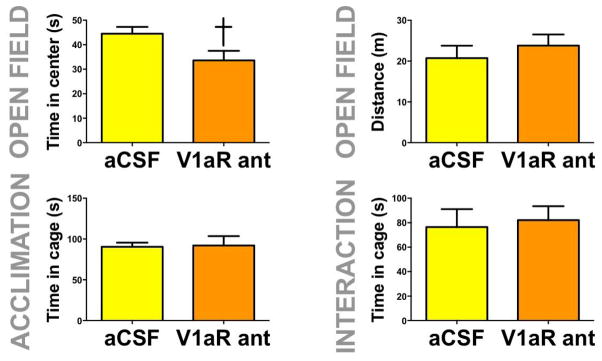
The V1aR antagonist reduced time spent in the center during open field testing in stressed females (fig. 8a, F1,13=4.90, p=0.04). There was no effect of V1aR on social interaction time, time spent in the cage zone during acclimation or distance traveled during open field (fig. 8b,c, and d).
Fig. 8. Effects of V1aR inhibition in the NAc shell in stressed females on behavior in the social interaction test.
Mean+SEM time in center (a) and distance traveled (b) during open field phase; time spent in proximity to the empty cage during acclimation phase (c) and time spent in proximity to a cage containing a novel same sex individual during interaction phase (d) in stressed females receiving injections of aCSF or V1aR antagonist into NAc shell. † p < 0.05 effect of V1aR antagonist.
DISCUSSION
Our results suggest that in both males and females, V1aR acting in BNSTmv facilitates social approach in naïve but not stressed animals. Interestingly, while V1aR inhibition in the BNSTmv had anxiogenic effects in both social and nonsocial contexts in males, the effects of V1aR inhibition in the BNSTmv in females were limited to social contexts. Infusions of the V1aR antagonist in the NAc shell, an area which is proximal anatomically to BNSTmv, had an anxiogenic effect in a non-social context in stressed females. Together, these data suggest that effects of V1aR on anxiety-like in males is less context-dependent in males and that in females different populations of V1aR modulate anxiety-like behaviors in social and non-social contexts.
Anxiogenic effects of social defeat stress and V1aR antagonist
We previously showed in the California mouse that three episodes of social defeat stress induce social withdrawal in females but not males. In addition stress increased the activity of OT neurons in the BNSTmv (Steinman et al., 2016). When we observed that females with more V1aR binding in the BNSTmv engaged in less social interaction, we hypothesized that increased OT release might reduce social interaction through V1aR (Song et al., 2014). However, the results from our experiments using V1aR infusions do not support this hypothesis. V1aR antagonist in the BNSTmv had no effect in stressed females and actually reduced social interaction behavior in both males and females naïve to defeat. Previous work has demonstrated that global V1aR inhibition in male rodents also reduces social interaction. For example, systemic injection of OT or AVP in adult male rats increased social interaction and these effects were blocked by systemic injections of the V1aR antagonist SR49059 (Ramos et al., 2013). Similarly, male V1aR knockout mice exhibited reduced social interaction behavior compared to wildtype littermates (Egashira et al., 2007). Our results suggest that these effects of V1aR observed in male rodents generalize to females, and may be mediated in part by V1aR in the BNSTmv. Although we did not specifically include an anatomical control in this study, we observed different effects of V1aR antagonist infused in the NAc (see below). Our results highlight the sex- and experience-dependent effects of central V1aR and leave open the question of whether V1aR expressing regions adjacent to BNSTmv may modulate social behaviors.
Autoradiography data also showed that stress increased V1aR binding in the NAc shell and NAc core in females but not males. Previous autoradiography studies in rodents have reported low levels of V1aR binding in the NAc (Johnson et al., 1993), so this stress-induced effect is a novel finding. The NAc shell has been previously implicated in sex specific effects of social defeat in California mouse (Campi et al, 2014; Trainor et al, 2011), so we next hypothesized that stress-induced increases in OT neural activity in females might reduce social interaction behavior by activating V1aR in the NAc shell. However, V1aR antagonist infusions into the NAc shell in stressed females had no effect on social interaction behavior. It’s possible that V1aR in NAc core may work together with V1aR in NAc shell to inhibit social interaction behavior and that more global inhibition of V1aR in NAc might be necessary to see an effect on this behavior. Still, our results do not support the hypothesis that increased V1aR activity contributes to stress-induced decreases in social interaction behavior in females.
Effects of V1aR inhibition on behavior: interaction with social defeat stress
While V1aR inhibition within the BNSTmv reduced social interaction behavior males and females naïve to defeat, there were no effects in stressed mice. Intriguingly, the effects of V1aR antagonist on social interaction in control mice were often similar to effects of social defeat in mice treated with aCSF. It’s possible that effects of defeat stress on male behavior could be mediated through decreased availability of AVP. Defeat stress reduces AVP immunoreactivity in both the PVN and BNST while lowering also Avp mRNA expression in the PVN of males (Steinman et al., 2015). Similar effects have been observed in male Mus musculus (Reber et al., 2007). However in our previous studies, social defeat alone was insufficient to reduce social interaction in males (Greenberg et al., 2014; Greenberg et al., 2015; Trainor et al., 2011; Trainor et al., 2013). Only when stressed male California mice receive an acute stressor such as an intranasal infusion (Steinman et al., 2016) or intracranial infusion (this paper) is reduced social interaction observed. Handling or immobilization increases the activity of locus coeruleus (LC) neurons (Kawahara et al., 2000) and norepinephrine release (Smagin et al., 1997), which in turn can reduce social interaction in male rats (Cecchi et al., 2002; Varlinskaya and Spear, 2012). It’s possible that social defeat exaggerates these responses. For example social defeat increased corticotropin releasing hormone (CRH) in the LC (Reyes et al., 2015) and CRH is known to increase neural discharge rates of LC neurons (Curtis et al., 1997; Jedema and Grace, 2004). If stressed male California mice had increased noradrenergic responses to handling, this might account for decreased social interaction following infusions.
Context-dependent effects of V1aR inhibition on behavior
There were also sex differences in how V1aR modulated behavior in social and nonsocial contexts. For males V1aR inhibition in the BNSTmv had anxiogenic effects in social and nonsocial contexts, but in females V1aR inhibition only affected behavior in social contexts. Thus it appears that in males the effects of V1aR in the BNSTmv on anxiety-like behavior are no dependent on social context. For females V1aR receptors in BNSTmv affect behavior in social contexts but not non-social contexts. Interestingly we found an anxiolytic role for V1aR in NAc shell in females. To our knowledge anxiolytic effects of V1aR in the NAc shell have not been reported. The 3-fold increase in V1aR binding in stressed females but not males is particularly interesting in the context of the open field test. Defeat reduced time in the center of the open field in males but not females. These results suggest that, in socially stressed females, V1aR could be exerting a ‘compensatory’ effect, reducing the effects of stress on exploration in an unfamiliar, non-social environment.
Intriguingly, while V1aR inhibition had no effect on behavior in stressed males in an unfamiliar environment, it did affect behavior in stressed males tested in the resident-intruder test. When focal males were tested as residents in a familiar home cage, stress significantly decreased latency to box in males, which is considered a defensive behavior (Linfoot et al., 2009; Litvin et al., 2007). These effects were partially reversed by the injection of V1aR antagonist into BNSTmv. Previous work in males has shown that the effects of V1aR are stronger in familiar environments compared to novel environments (Bester-Meredith and Marler, 2001). For the RI test, it’s important to consider V1aR antagonist treated mice were receiving a second injection. Thus it’s possible that prolonged inhibition of V1aR might have a different behavioral effect than acute inhibition.
Conclusions
Our results suggest that a specific population of V1aR in BNSTmv exerts anxiolytic effects in both social and nonsocial contexts for males but that for females the anxiolytic effects of these receptors is limited to social contexts. While prosocial effects of V1aR have been previously reported (Pitkow et al., 2001; Ramos et al., 2013), most studies have found anxiogenic effects of systemic V1aR inactivation in non-social contexts (Bielsky et al., 2004; Mak et al., 2012). Interestingly, overexpression of V1aR in the ventral pallidum of male prairie voles had anxiogenic effects in the elevated plus maze (Pitkow et al., 2001), suggesting that effects of V1aR on anxiety-like behavior are site specific. This is similar to the behavioral effects of brain derived neurotrophic factor which has antidepressant effects in hippocampus (Autry et al., 2011), but pro-depressive effects in NAc or BNST (Berton et al., 2006; Greenberg et al., 2014). Thus it appears that specific populations of V1aR have different effects on anxiety depending on neuroanatomical location, context, and sex. This means that any investigation of potential therapeutic use of V1aR targeting drugs needs to take these factors in to account. Continued study of the effects of V1aR on neural circuits of anxiety in rodents and humans should provide important insights in the potential of V1aR as a novel therapeutic target.
Highlights.
Social defeat stress increased V1aR expression in nucleus accumbens of females
Inhibition of V1aR in the medioventral bed nucleus of the stria terminalis was anxiogenic in males and females naïve to defeat
Inhibition of V1aR in the nucleus accumbens was anxiogenic in stressed females in the open field test
Acknowledgments
Special thanks to Sae Yokoyama who helped with experiments, and to Emilio Ferrer for suggestions on statistical analyses. Supported by NIH R01 MH103322 to BCT, F31 MH095253 to MQS, NSF GRF to SAL, Becas Chile CONICYT to NDW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6:2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Horm Behav. 2001;40:51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Hormones and Behavior. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22:420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014;77:208–216. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson C, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus) Brain Behav Evol. 2013;81:236–249. doi: 10.1159/000353260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Clinard CT, Bader LR, Sullivan MA, Cooper MA. Activation of 5-HT2a receptors in the basolateral amygdala promotes defeat-induced anxiety and the acquisition of conditioned defeat in Syrian hamsters. Neuropharmacology. 2015;90:102–112. doi: 10.1016/j.neuropharm.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria-Avila GA, Manzo J, Garcia LI, Carrillo P, Miguel M, Pfaus JG. Neurobiology of social attachments. Neuroscience and Biobehavioral Reviews. 2014;43:173–182. doi: 10.1016/j.neubiorev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014a;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014b;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CL, Krebs-Kraft DL, Solomon MB, Norvelle A, Parent MB, Huhman KL. Immediate post-defeat infusions of the noradrenergic receptor antagonist propranolol impair the consolidation of conditioned defeat in male Syrian hamsters. Physiol Behav. 2015;152:56–61. doi: 10.1016/j.physbeh.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Innala L, Viau V. Central vasopressin V1A receptor blockade impedes hypothalamic-pituitary-adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology. 2012;37:2712–2719. doi: 10.1038/npp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC. Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci. 2015;42:3081–94. doi: 10.1111/ejn.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cooper MA, Lezak KR. Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology. 2012;62:565–575. doi: 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Burkett JP, Young LJ. Neuroanatomical distribution of mu-opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non-monogamous meadow voles (Microtus pennsylvanicus) Neuroscience. 2013;244:122–133. doi: 10.1016/j.neuroscience.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Audigier S, Rossi F, Jard S, Tribollet E, Barberis C. Localization and characterization of vasopressin binding sites in the rat brain using an iodinated linear AVP antagonist. Brain Res. 1993;622:9–16. doi: 10.1016/0006-8993(93)90795-o. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur J Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Coccaro EF, Cremers H, McCarron R, Lu SF, Brownstein MJ, Simon NG. A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Front Syst Neurosci. 2013;7:100. doi: 10.3389/fnsys.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linfoot I, Gray M, Bingham B, Williamson M, Pinel JP, Viau V. Naturally occurring variations in defensive burying behavior are associated with differences in vasopressin, oxytocin, and androgen receptors in the male rat. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1129–1140. doi: 10.1016/j.pnpbp.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Pentkowski NS, Blanchard RJ. A pinch or a lesion: a reconceptualization of biting consequences in mice. Aggress Behav. 2007;33:545–551. doi: 10.1002/ab.20222. [DOI] [PubMed] [Google Scholar]
- Lukas M, Wohr M. Endogenous vasopressin, innate anxiety, and the emission of pro-social 50-kHz ultrasonic vocalizations during social play behavior in juvenile rats. Psychoneuroendocrinology. 2015;56:35–44. doi: 10.1016/j.psyneuen.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Mak P, Broussard C, Vacy K, Broadbear JH. Modulation of anxiety behavior in the elevated plus maze using peptidic oxytocin and vasopressin receptor ligands in the rat. J Psychopharmacol. 2012;26:532–542. doi: 10.1177/0269881111416687. [DOI] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL. Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front Behav Neurosci. 2015;9:191. doi: 10.3389/fnbeh.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148:670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Zitnik G, Foster C, Van Bockstaele EJ, Valentino RJ. Social Stress Engages Neurochemically-Distinct Afferents to the Rat Locus Coeruleus Depending on Coping Strategy(1,2,3) eNeuro. 2015:2. doi: 10.1523/ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble DO, Salvioni M. Social-Organization and Nest Co-Occupancy in Peromyscus-Californicus, a Monogamous Rodent. Behavioral Ecology and Sociobiology. 1990;26:9–15. [Google Scholar]
- Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Zhou J, Harris RB, Ryan DH. CRF receptor antagonist attenuates immobilization stress-induced norepinephrine release in the prefrontal cortex in rats. Brain Res Bull. 1997;42:431–434. doi: 10.1016/s0361-9230(96)00368-1. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JKt, Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, Trainor BC. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WHD, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–336. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLOS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]