Abstract
The range of therapeutic treatment options for central nervous system (CNS) diseases is greatly limited by the blood-brain barrier (BBB). While a variety of strategies to circumvent the blood-brain barrier for drug delivery have been investigated, little clinical success has been achieved. Focused ultrasound (FUS) is a unique approach whereby the transcranial application of acoustic energy to targeted brain areas causes a noninvasive, safe, transient, and targeted opening of the BBB, providing an avenue for the delivery of therapeutic agents from the systemic circulation into the brain. There is a great need for viable treatment strategies for CNS diseases, and we believe that the preclinical success of this technique should encourage a rapid movement towards clinical testing. In this review, we address the versatile applications of FUS-mediated BBB opening, the safety profile of the technique, and the physical and biological mechanisms that drive this process.
Keywords: Blood-brain barrier, Focused ultrasound, Microbubbles, Noninvasive surgery, Drug delivery, MRI
1. Structure and function of the blood-brain barrier
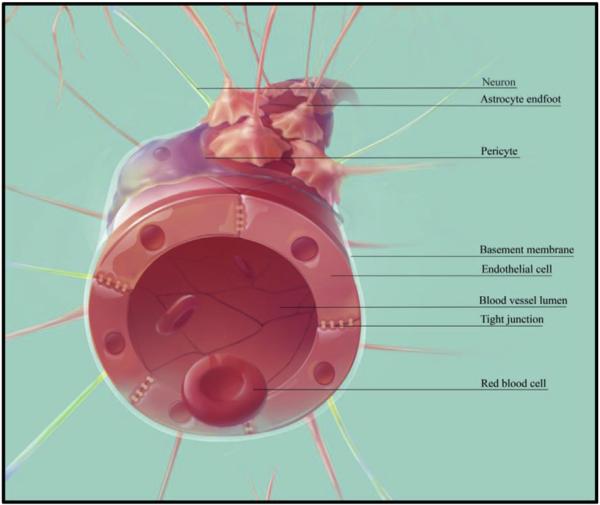
Proper cerebral function is dependent upon a tightly regulated extracellular milieu surrounding neurons and glia (De Bock et al., 2013). The blood-brain barrier (BBB) plays a major role in maintaining this environment by selectively isolating the parenchyma from the circulatory system. The major anatomical features of the BBB include a layer of specialized endothelial cells (ECs), a basement membrane, and a non-continuous layer of pericytes, which is surrounded by another basement membrane. An additional layer, composed of astrocytic endfeet, surrounds the second basement membrane (Hawkins and Davis, 2005) (Fig. 1). At the interface of adjacent ECs are adherens and tight junction (TJ) complexes, consisting of transmembrane proteins, which include various junctional adhesion molecules, claudins, and occludens. The intracellular domains of these proteins are anchored to the cytoskeleton of ECs, while the extracellular domains form homodimers with proteins on adjacent ECs (Abbott et al., 2006). Together, these bonds create a tight link between ECs, contributing to a ‘physical barrier’ which limits paracellular diffusion to small (<400–500 Da), water soluble molecules (Pardridge, 2005).
Fig. 1.
Components of the BBB. The BBB is composed of a layer of specialized vascular ECs, linked together by adherens and TJ proteins. This layer is surrounded by a basement membrane, then a non-continuous layer of pericytes. An additional basement membrane and astrocytic endfeet complete the major anatomical features of the BBB. This configuration contributes to the selective exclusion of a vast majority of therapeutic agents in circulation from entering the brain parenchyma. Abbreviations: BBB = blood-brain barrier, EC = endothelial cell, TJ = tight junction.
While small gaseous or lipophilic molecules can freely diffuse through the lipid membrane of ECs, the presence of specific transporter complexes in the luminal and abluminal surfaces strictly regulate the transcellular movement of larger polar and non-polar molecules (Abbott et al., 2006). These complexes contribute to a ‘transport barrier’ which facilitates the movement of select substances, such as glucose and essential amino acids, transported by specific carrier proteins (Abbott et al., 2006). Vascular ECs in the central nervous system (CNS) also make use of receptor-mediated transcytosis and adsorptive transcytosis to regulate the movement of large molecules. However, these cells have a much lower degree of endocytosis than in peripheral endothelia (Pardridge, 2002). While fewer fenestrations and vesicles result in reduced transcytosis, efflux pumps (e.g. P-glycoprotein and multidrug resistance-associated protein) and enzymes prevent toxic molecules from accumulating (Abbott et al., 2006).
Together, the unique combination of features that make up the BBB act to maintain homeostasis and protect the brain from infection. Its importance is exemplified by several neurodegenerative diseases where aberrant BBB function has been observed. In humans, a variant of the APOE gene is strongly associated with Alzheimer's disease (AD; Genin et al., 2011). In mice, this protein variant triggers an inflammatory response in pericytes, which is thought to cause chronic BBB dysfunction and contribute to neurodegenerative changes (Bell et al., 2012). Other neurodegenerative disorders in which BBB dysfunction has been observed include Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) (Zlokovic, 2008). While further work may be necessary to determine whether BBB dysfunction is causative or a result of the underlying neuropathology, the links established thus far highlight the essential role of the BBB in normal brain function.
2. Approaches for drug delivery to the brain
The limited pharmacological success in treating diseases of the CNS is not due to the lack of candidate drugs, but due to the highly selective nature of barriers that protect the CNS, namely the BBB and the blood-spinal cord barrier. The BBB limits almost all large-molecule neurotherapeutics and over 98% of small-molecule drugs from passing from the systemic circulation to the brain parenchyma (Pardridge, 2005). The majority of current techniques designed to enhance delivery of therapeutics to the brain do so in a non-targeted manner (for extensive review, see Chen and Liu, 2012). Methods of circumventing the BBB can be categorized as (i) chemical, (ii) biological, or (iii) physical stimuli. This section will briefly summarize the techniques and limitations of each category.
2.1. Chemical stimuli to enhance blood-brain barrier permeability
Several chemical stimuli bypass the BBB by acting on the TJs between ECs, thereby increasing paracellular permeability. Arterial injection of hyperosmotic solutions, such as mannitol (Nagy et al., 1979) or arabinose (Dorovini-Zis et al., 1984), into the systemic circulation causes ECs to shrink, thereby stretching TJs and creating a leaky BBB. Other substances that alter TJ integrity include oleic acid (Sztriha and Betz, 1991) and lysophosphatidic acid (On et al., 2013). However, some of these techniques, such as the administration of hyperosmolar solutions, have been found to cause structural changes to the vasculature and cellular damage in the brain parenchyma (Salahuddin et al., 1988).
Solvents such as dimethyl sulfoxide (DMSO) (Broadwell et al., 1982), ethanol (Hanig et al., 1972), sodium dodecyl sulphate (SDS) (Saija et al., 1997), or other surfactants (Pardridge, 2005) can also cause BBB opening (BBBO) by solubilizing the lipid bilayer. Solvent-mediated BBBO is common for the delivery of drugs, as small amounts are often sufficient. Additionally, immune adjuvants can recruit endogenous antibodies, induce an inflammatory response, and open the BBB; however this BBBO lasts for weeks (Rabchevsky et al., 1999).
The field of bioengineering has also contributed various techniques to circumvent the BBB. Examples include BBB-targeted nanocarriers, which target brain capillary ECs (Fornaguera et al., 2015), and hydrogels containing hyaluronan and methylcellulose (Tuladhar et al., 2015).
Intranasal injections can also bypass the BBB, but the drug type and delivered volume may be limited. In addition, the route of delivery from nose to brain remains controversial (Kristensson and Olsson, 1971; Thorne et al., 2004; Pardridge, 2005; Lochhead and Thorne, 2012). Intranasally delivered drugs must first bypass the nasal epithelial cells, which are also connected by TJs. Drugs that successfully pass through the nasal epithelia are delivered to the cerebrospinal fluid (CSF) of the subarachnoid space and the systemic circulation. Thus, drug effects in other organs and vascular beds must also be considered for intranasal injections. Finally, the volume of drug that can be delivered to the CNS without causing damage to the olfactory system by intranasal injection is approximately 25–200 μL, which is less than the minimum therapeutic volume required for most drugs, even at high concentrations. Repeated drug administration would be necessary, which would render the olfactory system at risk of damage (Lochhead and Thorne, 2012).
The major limitations of bypassing the BBB via chemical stimuli are that substances necessarily have a global effect across the brain and peripheral tissues, and cannot be targeted to specific brain regions. Not only is this a restricting factor for neurological diseases in which progression is not homogeneous across all brain areas (e.g. hippocampus in AD, substantia nigra in PD, stroke, glioblastoma), but there is also a potential risk of toxicity in unaffected tissue.
2.2. Biological stimuli to enhance blood-brain barrier permeability
Biological stimuli designed to enhance BBB permeability have mostly been limited to in vitro studies (Chen and Liu, 2012). Similar to chemical stimuli, some biological agents act on TJs to enhance BBB permeability (Abbott, 2000). Examples include zonula occludens (ZO) toxin (Karyekar et al., 2003) and vasoactive and inflammatory stimuli, such as histamine (Schilling and Wahl, 1994), bradykinin (Unterberg et al.,1984), and vascular endothelial growth factor (VEGF) (Roberts and Palade, 1995). Compounds can also be modified to increase their ability to bypass the BBB (e.g. Cereport, an analog of bradykinin) (Sanovich et al., 1995).
Viruses can cause BBBO by recruiting chemokines, thereby upregulating the infiltration of inflammatory cells and causing BBBO by disrupting TJs (Kuang et al., 2009; Nakamuta et al., 2008; Man et al., 2007). Other viruses, such as the West Nile virus (Verma et al., 2009), can cross the BBB without BBBO by upregulating cell adhesion molecules which facilitate the infiltration of infected immune cells. Intravenous injection of adeno-associated virus-9 (AAV9) has been shown to target various cell populations of the CNS without BBBO (Foust et al., 2009). The widespread delivery and side effects of using viral vectors must be carefully considered due to the genetic alterations that can result.
The use of carrier molecules and novel drug design have also been extensively investigated to deliver therapeutics to the brain parenchyma in clinically relevant doses. Carrier molecules that have been shown to bypass the BBB include nanocarriers (Bhaskar et al., 2010), liposomes (Afergan et al., 2008), and amphiphilic aggregates (Chen et al., 2011). It is also possible to tag drugs with various molecular groups to inhibit endogenous efflux pumps (Batrakova and Kabanov, 2008), or to take advantage of innate biological processes, such as receptor-mediated transcytosis (Pardridge, 2005) and cell-mediated transport mechanisms (Jain et al., 2003).
Similar to chemical stimuli, the major limitation to these biological modifications is the lack of targeting. Even if drug carriers and vectors can be delivered in clinically relevant quantities, it is currently impossible to selectively deliver these substances to specific locations in the brain. Receptor mediated transport and transport vectors allows some degree of specificity of drug delivery, but currently only low percentages (1–4%) of the injected dose can be successfully delivered to the relevant areas in the brain (Jones and Shusta, 2007).
2.3. Physical stimuli to enhance blood-brain barrier permeability
Although delivery of therapeutics via invasive methods, such as stereotaxic intracranial injections, have been shown to result in functional (Maddison, 1977; Suzuki et al., 1982) and beneficial (Frisella et al., 2001; Lee et al., 2010) effects and allow some degree of targeting, deeper structures such as the brainstem are difficult to reach. In addition, seizures and intracranial bleeding are often surgical risks (Muir et al., 2011).
Besides ultrasound, several techniques have been proposed to disrupt the BBB in a thermally-dependent manner. Low-level microwave energy has been used to open the BBB by increasing the temperature in the brain by at least 1 °C (Kiyatkin and Sharma, 2009). Other groups have reported that BBB opening only occurs if the brain is heated to at least 40 °C (Moriyama et al., 1991). Although purportedly a noninvasive strategy, it is clear that heating the brain to such a degree is likely detrimental and may increase the risk of infection (Lange and Sedmak, 1991). Other examples of thermally-dependent BBBO include using electromagnetic pulses to disrupt TJs (Qiu et al., 2010) and using a low radiofrequency field to heat magnetic nanoparticles (Tabatabaei et al., 2015).
These alternatives require further investigation, and although they can be considered for global BBBO, noninvasive and targeted specificity would be difficult to achieve. In contrast, focused ultrasound (FUS), used in conjunction with intravenous microbubble (MB) administration, is a noninvasive, safe, and reversible way to open the BBB in targeted areas.
3. Overview of focused ultrasound
The potential for FUS as a noninvasive alternative to neurosurgery is well-established. Lynn et al. first demonstrated the utility of high-intensity focused ultrasound (HIFU) for the ablation of cortical and subcortical tissue in 1942 (Lynn et al., 1942). Work by Bakay et al. in the 1950's built on this work with the first reported evidence of increased BBB permeability in the margins of HIFU induced lesions (Bakay et al., 1956), an observation also made by others (Ballantine et al., 1960; Shealy and Crafts, 1965). This early work on HIFU-induced brain tissue ablation not only established the foundation for ongoing clinical trials (Lipsman et al., 2013; Elias et al., 2013), but was also seminal to the idea of FUS-induced BBBO. While work in the 1990's suggested that the cavitation effects of FUS could be used to increase BBB permeability without associated tissue damage, inconsistencies with the technique raised concerns with respect to safety and efficacy (Vykhodtseva et al., 1995).
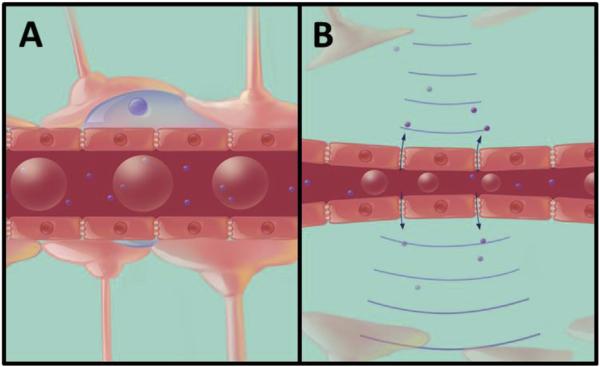
In 2001, the technique was modified by Hynynen et al. to include the intravenous administration of MBs, an ultrasound contrast agent, immediately prior to the application of FUS (Hynynen et al., 2001). As the MBs travel through the circulatory system they are dispersed and eventually pass through blood vessels within the focus of the ultrasound. Here, they begin to expand and contract in response to the cycles of rarefaction and compression of the propagating ultrasound wave (Fig. 2). The mechanical stimulation of vessel walls caused by the behavior of MBs is believed to drive the opening of the BBB (Hosseinkhah et al., 2015). Ultimately, the addition of MBs in FUS treatment enables a reduction in the acoustic power required to influence BBB permeability by lowering the cavitation threshold within the vasculature. This reduction in acoustic energy significantly reduces skull heating effects and permits safe transcranial ultrasound administration.
Fig. 2.
FUS-induced MB oscillation and BBBO. (A) Prior to sonication, MBs (red spheres in the capillary lumen) are intravenously administered and disperse in the systemic circulation. (B) When the MBs enter the focus of the ultrasound field, they expand and contract, stimulating the opening of the BBB, thereby allowing therapeutic agents (small purple spheres) to enter the brain parenchyma. Abbreviations: FUS = focused ultrasound, MB = microbubble, BBBO = blood-brain barrier opening. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Given the large number of factors that can influence FUS (discussed below), real-time feedback during treatment seems essential for safe and repeatable BBBO. The introduction of acoustic emission detection during sonication has provided a way to monitor (McDannold et al., 2006; Tung et al., 2010) and calibrate the acoustic pressure amplitude based on the in vivo behavior of MBs (Arvanitis et al., 2012) in real-time. By reducing the pressure once the sub- or ultra-harmonic emissions characteristic of stable cavitation are detected, a more consistent treatment is achieved (O'Reilly and Hynynen, 2012). The combination of optimized FUS parameters and real-time feedback of MB behavior has resulted in a technique to temporarily increase the permeability of the BBB in a targeted, safe, and reproducible manner, presenting an avenue for the delivery of therapeutic agents to the brain.
4. Targeted delivery of therapeutic agents
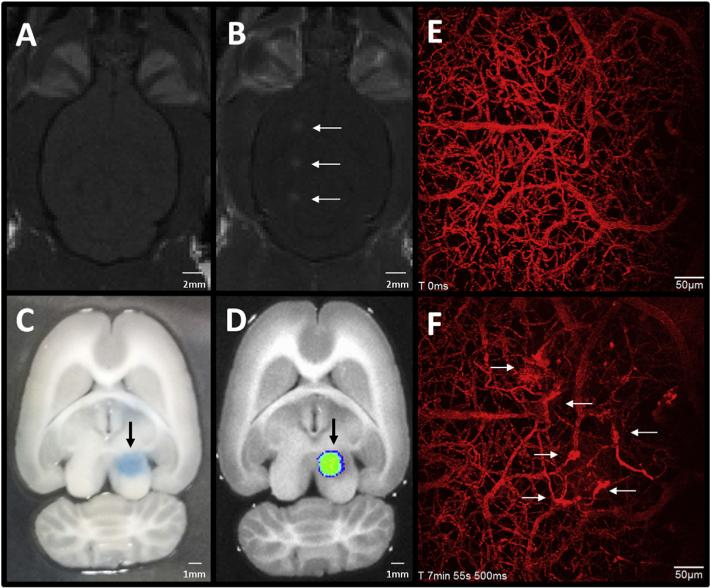
While FUS has a wide range of applications, preclinical studies involving FUS-induced BBBO have thus far largely focused on the delivery of therapeutic agents. Given the scarcity of drugs which permeate the BBB at concentrations sufficient to produce clinically relevant effects (Pardridge, 2005), work demonstrating the ability to deliver substances to the brain following FUS is in its infancy, but has shown great promise. Much of this work uses magnetic resonance imaging (MRI), which allows anatomical targeting of FUS. Target areas are chosen from MR images of each subject's brain, and BBBO is confirmed after treatment by the leakage of gadolinium contrast agent from blood vessels (Fig. 3a and b). This section will discuss some examples of MR-guided FUS studies, highlighting the range of therapeutic agents delivered to the brain to date.
Fig. 3.
Methods to visualize FUS-mediated BBBO. (A, B) T1-weighted MR images in the horizontal plane show gadolinium contrast enhancement in a rat brain (A) before and (B) 10 min after FUS-mediated BBBO. White arrows indicate the three areas of BBBO. (C, D) Evans blue dye is a commonly used indicator of BBBO. (C) A vibratome-sectioned (500 μm thick), perfused rat brain slice shows the region of Evans blue dye extravasation 2 h following FUS. (D) The same section, imaged with a Xenogen IVIS-200™ system, demonstrates the fluorescent signal emitted by Evans blue, providing a quantitative measure of BBBO. Black arrows indicate the area of BBBO. (E, F) On a microscopic scale, two-photon imaging through a cranial window provides a means of evaluating BBBO with higher temporal and spatial resolution, compared with the methods mentioned above. Here, maximum projection images of 400 μm Z-stacks are shown. (E) Prior to sonication, dextran-conjugated Texas-Red dye is intravenously injected, allowing the visualization of blood vessels, and (F) the time course of BBBO. Regions of dye leakage in the field of view can be observed following FUS (white arrows). Time stamps indicate the time from the start of imaging. Abbreviations: FUS = focused ultrasound, BBBO = blood-brain barrier opening, MR = magnetic resonance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Chemotherapeutics are one of the most useful classes of drugs in medical oncology; however, their efficacy in the treatment of brain tumors remains low due to poor penetrance (Doolittle et al., 2007). Several groups have demonstrated the ability to increase their concentration within brain tumor models following FUS, leading to decreases in tumor volume and increased survival time. Elevated concentrations of doxorubicin in the brain following FUS treatment was first demonstrated in healthy rats without tumors (Treat et al., 2007). Since then, further work has shown enhanced delivery of doxorubicin in a glioma rat model (Treat et al., 2012) and two syngenic glioblastoma mouse models (Kovacs et al., 2014) when chemotherapeutic administration is accompanied by FUS and MBs. Importantly, these treatments reduced tumor volumes and increased survival time. In addition to doxorubicin, the delivery of other chemotherapeutic drugs, such as methotrexate (Mei et al., 2009), bis-chloroethylnitrosourea (BCNU) (Liu et al., 2010a; Fan et al., 2013), and temozolomide (Wei et al., 2013), to brain tumors are also enhanced by FUS-induced BBBO.
The use of viral vectors in gene therapy represents a powerful avenue for controlling gene expression and has the potential to be a flexible tool in the treatment and prevention of a large number of pathologies. Thus far, the delivery of viral vectors to specific areas of the brain has been primarily achieved by invasive intracranial injections. FUS-induced BBBO has the potential to change this by providing a means of noninvasive targeted delivery while enabling increased transfection efficiency without raising the dose of viruses administered. This concept has been demonstrated in mice by delivering AAV9- green fluorescent protein (GFP) to cells in the striatum and hippocampus (Thévenot et al., 2012). In this study, GFP expression was found to be greatest in neurons and astrocytes of FUS-targeted regions, with minimal expression in other brain areas or peripheral organs. Several other studies have also demonstrated the ability to introduce genetic material to the brain in a targeted manner with FUS, delivering AAV2-GFP (Hsu et al., 2013), siRNA for huntingtin protein (Htt) knockdown (Burgess et al., 2012a), and rAAV1- and rAAV2-expressing eGFP under synapsin promoter (Wang et al., 2015).
While immunotherapy has shown promise in reducing amyloid beta (Aβ) in mouse models of AD (Bard et al., 2000; Wilcock et al., 2004), the low levels anti-Aβ antibodies that enter the CNS from the bloodstream may hinder the efficiency of this treatment avenue (Banks et al., 2002). Enhanced delivery of anti-Aβ antibodies to the brain following FUS-induced BBBO has been shown to be effective in reducing Aβ plaque pathology 4 d following treatment in a transgenic AD mouse model (TgCRND8 mice; Jordão et al., 2010). Although there is great debate regarding the exact contribution of Aβ plaques to cognitive deficits in AD patients (Nelson et al., 2009), the behavioral improvements measured in AD models following plaque reduction (Wilcock et al., 2004) may indicate potential for immunotherapy in the treatment of AD. In addition to anti-Aβ antibodies, delivery of dopamine receptor D4 antibodies (Kinoshita et al., 2006a) and human epidermal growth factor receptor 2 (Her2) monoclonal antibodies (Kinoshita et al., 2006b) to targeted brain areas have also been enhanced with FUS.
In addition to that described above, the enhanced delivery of natural killer cells expressing Her2 chimeric antigen receptor (Alkins et al., 2013), iron-labeled GFP-expressing neural stem cells (Burgess et al., 2011), therapeutic magnetic nanoparticles (Ting et al., 2012), and brain-derived neurotrophic factor (BDNF) (Baseri et al., 2012), have also been demonstrated. The diversity in therapeutic agents delivered to the brain with FUS demonstrates the versatility of this technology and provides an incentive for accelerated clinical testing.
5. Pharmacokinetics of focused ultrasound-mediated drug delivery
As discussed above, a wide variety of therapeutics have been shown to cross the BBB following FUS; however, the kinetics of drug delivery post-BBBO has received less attention despite the importance of this information for determining appropriate drug doses.
Studies using dynamic contrast enhanced (DCE) MRI have measured the transfer coefficient (Ktrans) for Gd-DTPA, an MRI contrast agent, to be 0.0142 ± 0.006 min−1 at 30 min post-FUS, two or more orders of magnitude higher than in non-sonicated areas (Park et al., 2012). The Ktrans for Gd-DTPA was found to decrease exponentially as a function of time with an estimated half-life of 2.22 h. This study also demonstrated that the permeability of the BBB is further increased by duplicating sonications in the same location. A duration of 10 and 120 min between FUS-mediated BBBO resulted in Ktrans values of 0.0205 and 0.0216 min−1, respectively. Importantly, a linear correlation was found between the concentration of doxorubicin measured in the targeted brain locations and the Ktrans of Gd-DTPA measured 30 min after sonication (Park et al., 2012). Additionally, work from Vlachos et al. using DCE-MRI has reported Ktrans plateaus ranging from 0.0105 ± 0.0035 to 0.0515 ± 0.0068 min−1 depending on the acoustic pressure of sonication and the size of MBs used (Vlachos et al., 2010, 2011). As acoustic pressure increased, Ktrans plateaus increased as well (0.3–0.6 MPa; 1.5 MHz sonication frequency). Similarly, MB size (1–2 μm to 7–8 μm MB diameter) was also positively correlated to Ktrans plateaus (Vlachos et al., 2011).
Two-photon microscopy has also been used to gain insight into the kinetics of BBB leakage following FUS. The superior spatial and temporal resolution achieved with the technique, compared to that of DCE-MRI, has provided more detailed information regarding the heterogeneity of leakage between blood vessels. Nhan et al. demonstrated that the permeability of the BBB following FUS is size-dependent: larger vessels (20–70 μm) tend to be less permeable than smaller vessels (10–30 μm) (Nhan et al., 2013). This work also showed that the size of fluorescent dye used has an influence on the measured Ktrans, with permeability constants of 0.0359 min−1 and 0.0231 min−1 for 10 and 70 kDa Texas Red conjugated dextrans, respectively, when sonicating at 0.8 MPa (1.2 MHz sonication frequency) (Nhan et al., 2013).
While assessing the kinetics of BBB leakage following FUS with vascular dyes provides useful information, it may not accurately predict the extravasation of specific therapeutics. To this end, Yang et al., have investigated the pharmacokinetics of specific drugs following FUS-mediated BBBO. Using serial microdialysis sampling, they measured an increase in the maximum extracellular fluid concentration of boronophenylalanine-fructose (FUS: 6.8 ± 1.9 μg/mL; control: 3.8 ± 0.5 μg/mL), mean residence time (FUS: 503 ± 192 min; control: 313 ± 87 min), and area under the curve (FUS: 1887 ± 600 min μg/mL; control: 888 ± 225 min μg/mL) in sonicated versus non-sonicated rat gliomas (Yang et al., 2014). Similarly, they also assessed the pharmacokinetics of 111In-labeled liposomal doxorubicin following FUS in a murine glioma model using microSPECT/CT. They found that the tumor-to-contralateral brain ratios of doxorubicin after injection reached a maximum value at 48 h post-sonication, with peak values in the sonicated locations being more than twice as great as untreated regions (Yang et al., 2012a). These initial studies provide insight into the unique pharmacokinetic profile of drug delivery following FUS-mediated BBBO.
6. Physical effects of focused ultrasound and microbubbles in vasculature
The addition of ultrasound contrast agents containing preformed MBs to the FUS technique have been shown to decrease the ultrasound power necessary to cause BBBO by at least two to three orders of magnitude (Hynynen et al., 2001, 2006). MBs absorb and emit ultrasound energy in the confined space of the vasculature, thereby decreasing the acoustic energy deposited in the surrounding tissue and bone. In the vasculature, MBs exert forces against the surrounding walls by contracting and expanding through cycles of compressions and rarefactions of the acoustic wave (Qin et al., 2009).
There are four major interactions between ultrasound and MBs that are hypothesized to contribute to BBBO. First, stably oscillating MBs exert shear forces against the surrounding fluid and cells, producing microstreaming (Lewin and Bjørnø, 1982; Wu, 2002). Second, under high shear stress, ECs can detach, thereby affecting membrane integrity (VanBavel, 2007; Hosseinkhah et al., 2015). Third, the acoustic wave exerts radiation force on the MBs in the direction of the propagation of the acoustic wave (Dayton et al., 1999), and the response of MBs may be sufficient to cause BBBO (Dayton et al., 1999). Fourth, at high pressures, the surrounding fluid exerts such a large force against the MBs that they undergo a violent collapse. This causes a large local increase in temperature and a shockwave that travels at supersonic speeds, leading to microjetting that can puncture vessel walls (Apfel and Holland, 1991). Although this event, termed inertial cavitation, results in BBBO, McDannold et al. showed that it is not necessary for FUS-mediated BBBO (McDannold et al., 2006; Hynynen et al., 2006). In fact, inertial cavitation has been associated with tissue damage and thus FUS exposure levels should utilize only stable cavitation for BBBO (Hwang et al., 2006; see Section 8.4).
6.1. Ultrasound parameters that affect blood-brain barrier opening
6.1.1. Frequency
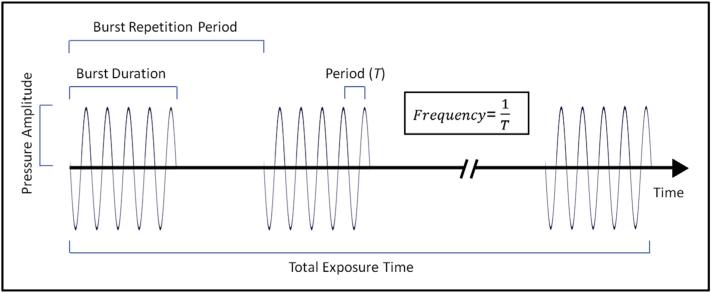
Ultrasound frequency strongly affects the response of MBs in the ultrasound field (Leighton, 1994). The pressure threshold required to cause BBBO increases with increasing frequency (Hynynen, 2008; McDannold et al., 2006, 2008a,b). BBBO has been demonstrated with acoustic frequencies ranging between 28 kHz (Liu et al., 2010b) to 8 MHz (Bing et al., 2009). However, the use of frequencies at the high end of this range for human application is unlikely due to the high pressures that would be required to overcome the high degree of attenuation caused by the skull bone (see Fig. 4 for an overview of ultrasound parameters). Conversely, use of frequencies at the low end of this range is accompanied by an increased focal size, which may also be undesirable. The optimal frequency for clinical transcranial FUS application is dependent on the desired effect, but is likely to be in the range of 0.2–1.5 MHz (Sun and Hynynen, 1999; Pajek and Hynynen, 2013).
Fig. 4.
Parameters of an ultrasound wave. The ultrasound wave is depicted as a sinusoidal wave, with areas of compression being above the x-axis and areas of rarefaction below. The relationship between burst duration, burst repetition period, period, frequency, pressure amplitude, and total exposure time are displayed.
Since the transducers used for BBBO studies differ between groups and animal models, it is often a challenge to determine the threshold for BBBO. However, when expressed as a function of the mechanical index (MI = PNP/√f, where PNP is the peak negative pressure and f is the center frequency of ultrasound wave), the threshold for BBBO (0.46) is approximately constant for several frequencies used in preclinical studies (McDannold et al., 2008b).
6.1.2. Burst duration and microbubble concentration
McDannold et al. found that the extent of BBBO increases as burst duration is increased from 0.1 to 10 ms; however, no additional effects were found beyond this point (McDannold et al., 2008a). It is postulated that longer burst lengths are not effective in increasing BBBO due to a complete destruction of MBs in the focal region during this time, necessitating a reperfusion of MBs. BBBO is also possible with short burst lengths, down to single cycle sonications, provided that the pressure amplitude is high enough to reach the threshold (O'Reilly et al., 2011).
It is intuitive to hypothesize that a higher concentration of MBs in circulation should cause more BBBO. Although some have observed increases in BBBO when MB concentration is increased (Treat et al., 2007; Yang et al., 2007), others have reported no differences in the range of 50–250 μL/kg (McDannold et al., 2008a, b).
6.1.3. Burst repetition frequency
While increasing burst repetition frequency from 0.5 Hz to 5 Hz was found to have little effect on the degree of BBBO (McDannold et al., 2008a), there is evidence to suggest that when raised from 0.1 Hz to 1 Hz, there is a significant increase in BBBO (Choi et al., 2011). However, beyond 1 Hz, there is no additional benefit. This may be attributed to the increased time between bursts allowing a more complete reperfusion of MBs into the focal region (Goertz et al., 2010).
6.1.4. Total exposure time
The duration of ultrasound exposure is also positively correlated with the degree of BBBO, as seen in contrast-enhanced MRI after treatment (Chopra et al., 2010). However, with the ultrasound parameters investigated in this study (1.08 MHz frequency; 10 ms burst length; 1 Hz burst repetition frequency; 0.38 MPa pressure amplitude), exposure times beyond 300 s caused irreversible damage (Chopra et al., 2010).
Other factors that have been shown to affect BBBO include acoustic pressure amplitude (Hynynen et al., 2005; McDannold et al., 2007, 2008a, b), MB size distribution (Choi et al., 2010; Samiotaki et al., 2012), MB type (McDannold et al., 2007), anesthetic type (McDannold et al., 2011), and MB injection method (O'Reilly et al., 2011). Overall, the above studies demonstrate that the BBBO can be induced with a wide variety of sonication parameters; however, the sonication parameters applied must be tuned for the experimental conditions to achieve BBBO without tissue damage.
7. Biological mechanisms of focused ultrasound and microbubble mediated blood-brain barrier opening
7.1. Biochemical changes
While the utility of FUS as a method of modulating BBB permeability has been demonstrated in a variety of preclinical models, a complete characterization of the biological processes that drive this opening is currently lacking. Studies have thus far provided information on the routes of entry into the brain following FUS and associated changes in protein expression. However, the knowledge required to tailor BBBO to specific therapeutic agents, pharmacologically modify the technique, or tightly control the duration of BBBO has yet to be established.
Early studies from Sheikov et al. demonstrated the occurrence of both trans- and paracellular leakage following FUS. Through the use electron microscopy, they observed an increased number of vesicles, vacuoles, fenestrations, and transcellular channels in capillary ECs, as well as dye leakage through the paracellular space, past the TJs (Sheikov et al., 2004). Further work showed that the changes in TJ integrity were mirrored at the protein level, with a significant reduction in the immunoreactivity of occluden, claudin-5, and zona occluden-1 at 1 and 2 h following FUS (Sheikov et al., 2008). Importantly, immunoreactivity of these TJ proteins returned to control levels at 4 h post-sonication, corresponding with the return of TJ integrity, as measured by the paracellular diffusion of horse-radish peroxidase (HRP) and lanthanum chloride (Sheikov et al., 2008). While it is unclear whether FUS induces a down-regulation of TJ proteins or conformational changes to these proteins which block the epitope-antibody interaction required for immuno-detection, these studies support the notion that the effects of FUS on barrier integrity are transient. Similarly, the observed increase in the number of endothelial vesicles, predominantly found in arterioles, is also supported by evidence at the protein level. An upregulation of caveolin-1 has been measured using immunohistochemistry and Western blots at 1 h following FUS (Deng et al., 2012).
More recently, two-photon microscopy has been used to observe the cerebral vasculature during FUS experiments in vivo (Fig. 3e and f). One of the major findings from this work has been the apparent existence of at least two types of leakage kinetics, mainly ‘fast’ and ‘slow’ leakage. Fast leakage describes the temporal dynamics of leakage in regions along the length of a blood vessel in which the intensity of extravasated fluorescent dye peaks within the first 10 min post-sonication (Cho et al., 2011; Nhan et al., 2013). It has been speculated that this type of leakage is caused by the opening of TJs and/or cellular sonoporation (Nhan et al., 2013). The rapid reduction in permeability in regions that display fast leakage may be aided by the recruitment of astrocytes and microglia (Burgess et al., 2012b). In contrast, slow leakage occurs more diffusely along the length of the blood vessel and begins between 5 and 15 min post-sonication (Cho et al., 2011; Nhan et al., 2013). Due to the spatial distribution and delay before slow leakage is detected, it is speculated that this type of leakage is facilitated by transcytosis. Interestingly, the diameter of blood vessel correlates strongly to the type of leakage observed, with slow leakage dominating in larger vessels (>40 μm) and fast leakage dominating in smaller vessels (<40 μm) (Nhan et al., 2013); this is in agreement with electron microscopy experiments (Sheikov et al., 2006).
Knowledge regarding the routes of entry and kinetics of leakage following FUS have advanced greatly in the past 10 years; however, the biochemical mechanisms driving this increased permeability, and the factors directing the restoration of normal barrier function are not well understood. Elevated levels of p-Akt, as well as one of its downstream signaling molecules, phosphorylated glycogen synthase kinase 3 beta (p-GSK3β), have been observed at 1.5 and 24 h post-FUS (Jalali et al., 2010). Although the Akt pathway has been implicated in increased BBB permeability following focal cerebral ischemia (Kilic et al., 2006), there have been no causal links established between increased p-Akt immunoreactivity and FUS-induced BBBO. Similarly, in vitro work has demonstrated that ultrasound-stimulated MBs induce a transient increase in intracellular calcium concentration in murine brain microvascular ECs (Park et al., 2010). While this could activate a variety of cellular processes involved in BBB permeability, including up-regulating caveolin-1 (Yang et al., 2012b) and influencing TJ reassembly (Ye et al., 1999), there is currently no in vivo experimental data supporting an influence of calcium signaling on BBB permeability following FUS.
7.2. Cellular changes
Although astrocytic endfeet processes enwrap approximately 96% of the abluminal surface of brain capillaries (Jaeger and Blight, 1997), they do little to limit the paracellular diffusion of substances across the BBB (Pardridge, 2005). Astrocytes are nevertheless an important factor in regulating the extracellular milieu, and are an indispensible component of the neurovascular unit (functional unit of neurons and their associated astrocytes, smooth muscle cells, and ECs) (Abbott et al., 2006). Metabolically, astrocytes maintain ionic homeostasis in the brain (Chen and Swanson, 2003). The majority of astrocyte populations aid in maintaining the integrity of the BBB, causing tighter TJs and increased expression and polarized localization of transporters (Abbott et al., 2006). It has been shown that when astrocyte endfeet become separated from the ECs, BBB permeability increases; importantly, astrocytic endfeet can be realigned and reconnected to restored ECs (Hermann and ElAli, 2012). Hypothetically, it is possible that the mechanical vibrations associated with the MB vibrations may cause the endfeet to become disconnected and thereby contribute to the increased BBB permeability.
Microglia are the resident immune cells of the brain, and can rapidly react to changes in the physiological state of the brain (Baburamani et al., 2014). The motility and sensitivity of microglia to changes in brain parenchyma homeostasis make them an important component of the brain to consider in safety studies following manipulation of BBB integrity.
In addition to microglia, minimal entry of immune cells from the systemic circulation have been observed in the brain following FUS treatment. Specifically, helper T lymphocytes have been observed in the brain after FUS-mediated BBBO in healthy rats. This increase was not statistically significant, and the induced BBBO caused little to no red blood cell (RBC) extravasation. FUS-mediated BBBO also caused a slight increase in the tumor-infiltrating lymphocyte population in the tumor region of C-6 glioma rats. Interestingly, the authors found that administration of interleukin-12 (IL-12) with FUS-mediated BBBO significantly increased tumor-infiltrating lymphocyte populations and improved survival (Chen et al., 2015).
The relationship between glial cells and the BBB is apparent from their physical proximity and the functional dependence and cross-talk that exist between them (Abbott et al., 2006). Thus far, the majority of literature on glial cells in the FUS field has been focused on the effects of FUS-mediated BBBO in AD mouse models at ages where Aβ plaque burden is evident. In AD, both microglia and astrocytes are already in an activated state in the brain. The following section will focus on the response of microglia and astrocytes after FUS-mediated BBBO in the context of AD.
7.2.1. Microglia and astrocytes in Alzheimer's disease
The first study that observed the activation of microglia and astrocytes after FUS-mediated BBBO was observed in the TgCRND8 transgenic mouse model of AD in 2013. Analysis of astrocytes and microglia were dichotomized: the volume of cells surrounding plaques was equally divided into two categories, those proximal, and those further, or ‘distal’, to Aβ plaques. Aβ plaques are a well-known hallmark of AD. Jordão et al. showed that a single treatment of FUS-mediated BBBO targeted to the cortex in mice caused significant changes in microglia and astrocytes 4 d after treatment, compared to the untreated hemisphere. Specifically, there were statistically significant increases in the volumes and surface areas of both microglia and astrocytes distal to Aβ plaques. There was also a significantly greater Aβ plaque count and volume within microglia and astrocytes located both proximally and distally from Aβ plaques (Jordão et al., 2013). This study was a convenient segue into observing glial response to FUS-mediated BBBO due to the basal activation of microglia and astrocytes in AD. The greater response of glial cells distal to plaques suggested saturation of phagocytic activity of the glial cells that were proximal to plaques. Importantly, these relative increases in glial response were also observed in the non-transgenic controls.
Temporal differences in microglial and astrocytic responses were also observed. Although the population density of both microglia and astrocytes are typically higher proximal to Aβ plaques (Serrano-Pozo et al., 2011), most temporal differences were only statistically significant in regions that were not directly neighboring Aβ plaques. Quantitative analysis of immunoblots showed that microglial reactivity was significantly increased from 4 h until 4 d after FUS-mediated BBBO, but resolved to baseline levels by 15 d in both transgenic and non-transgenic animals. Conversely, the increase in astrocyte reactivity reached significance at 4 d but not 4 h after treatment, but only remained significantly high at 15 d in transgenic animals. The authors concluded that microglia responded faster than astrocytes to BBBO; both microglia and astrocytes were activated by 4 d which was when plaque burden was most significantly reduced, but only astrocytes remained activated by 15 d post-treatment (Jordão et al., 2013). To conclude, astrocytes and microglia were further activated by FUS-mediated BBBO and phagocytosed more Aβ than their counterparts in untreated AD animals; this is a possible mechanism contributing to the decreased plaque burden after treatment.
8. Safety of focused ultrasound and microbubble mediated blood-brain barrier opening
Due to the large surface area of the BBB (~20 m2 per 1.3 kg brain; Abbott et al., 2006) and the close proximity of neurons to capillaries (8–20 μm; Abbott et al., 2006), fluctuations in the peripheral circulation that occur throughout the day (e.g. hormones released after eating) could easily disrupt the extracellular milieu of the brain if the BBB was compromised. Thus, the temporal timeline of FUS-mediated BBBO and integrity of surrounding cells in the targeted areas are important factors to determine before clinical trials are considered.
The Food and Drug Administration (FDA) in the USA has not yet approved ultrasound therapy devices for brain treatments. The FDA safety limit for diagnostic ultrasound use is to be within an MI of 1.9 (FDA, 2008). However, the FDA has approved of HIFU for the treatment of uterine fibroids (Tempany et al., 2003), pain palliation of metastatic bone cancer (Catane et al., 2007), and most recently prostate cancer (Crouzet et al., 2014; Uchida et al., 2014). Clinical trials for some neurological diseases, such as essential tremor disorder, are currently underway (Lipsman et al., 2013). However, since these exposure levels are much higher than FUS-mediated BBBO treatments and do not include MBs, new guidelines for safety need to be established in the future.
In preclinical studies, the safety of FUS-mediated BBBO has been evaluated in three ways: (1) Analyzing the duration of increased BBB permeability in vivo using MR contrast agents (Section 8.1), (2) histologically (Section 8.2), and (3) through behavioral tests (Section 8.3). These assessments will be discussed in greater detail below, along with the effects of acute and repeated sonications. Quantitatively, parameters and thresholds for safe FUS exposure have been evaluated in two ways: (1) Pressure amplitude threshold for BBBO and tissue damage, and (2) detection and evaluation of MB cavitation activity via ultra- and subharmonics in real-time and correlating that signal to BBBO and tissue damage (Section 8.4). A summary of safety results from a selection of papers can be found in Table 1.
Table 1.
Sample of safety studies for FUS-mediated BBBO. This table summarizes the FUS parameters, experimental designs, and safety results from a selection of papers. Papers that used similar FUS parameters and experimental designs are grouped together. The asterisk (*) indicates that FUS was applied transcranially; otherwise, animals underwent a craniotomy prior to FUS treatment.
| Species | Frequency | Peak acoustic pressure | MB brand (dose) | Time of sacrifice | Evaluation method | Safety results | Reference |
|---|---|---|---|---|---|---|---|
| Rabbit | 1.63 MHz | Up to ~ 4.6 MPa | Optison (50 μL/kg) | 2 h–4 wks after sonication | Contrast-enhanced MRI Light microscopy: H&E, vanadium acid fuchsin-toluidine blue, TUNEL | 0.7 – 1 MPa: BBBO, but no neuronal damage 2–3 MPa: 25% of locations had neuronal damage >4 MPa: 70% of locations had neuronal damage Few extravasated RBCs, few apoptotic or ischemic cells around sonicated locations No delayed effects observed by MRI or histology 4 wks after treatment |
Hynynen et al., 2001 – Noninvasive MRI-guided focal opening of the BBB in rabbits McDannold et al., 2005 – MRI-guided targeted BBB disruption with FUS: Histological findings in rabbits |
| Rabbit | 0.69 MHz | Up to 3.1 MPa | Optison (50–250 μL/kg) Definity (10 μL/kg, used as well in McDannold et al., 2007) | 4–48 h after sonication | Contrast-enhanced MRI Light microscopy: H&E, TUNEL, vanadium acid fuchsin-toluidine blue Electron microscopy | MRI contrast enhancement increased with acoustic pressure, BBBO evident starting at 0.4 MPa Brain tissue necrosis at 70–80% of sonicated locations if ≥ 2.3 MPa Optison produced greater effect than Definity at same acoustic pressure amplitudes (McDannold et al., 2006) Magnitude of BBBO increased with higher burst length, but was not significantly affected by PRF or Optison dosages tested |
Hynynen et al., 2005 – Local and reversible blood-brain barrier disruption using FUS at frequencies suitable for trans-skull sonications McDannold et al., 2007 – Use of US pulses combined with Definity for targeted BBB disruption – a feasibility study McDannold et al., 2008b – Effect of acoustic parameters and ultrasound contrast agent dose on FUS induced BBB disruption |
| Rabbit | 2.04 MHz | 0.3–2.3 MPa | Optison (50 μL/kg) | 4 h after last sonication | Contrast-enhanced MRI Light microscopy: H&E | Few to no RBC extravasation at 2.04 MHz Density of RBC extravasations significantly increased at higher frequency (1.63 MHz) than at lower frequencies (representative H&E images taken from other experiments) MI threshold for BBBO = 0.46 |
McDannold et al., 2008b – BBB disruption induced by FUS and circulating preformed MBs appears to be characterized by the MI |
| Rabbit | 0.26 MHz | 0.11–0.57 MPa | Optison (50 μL/kg) | 4 h after sonication | Contrast-enhanced MRI Light microscopy: H&E | BBBO observed in contrast MRI at 0.29–0.57 MPa, associated with few to no RBC extravasation | McDannold et al., 2006 – Targeted disruption of the BBB with FUS – association with cavitation activity |
| Rat | 1 MHz | N/A | SonoVue (150–450 μL/kg) | Up to 4 h after sonication | Contrast-enhanced MRI Evans blue | Greater MB doses led to greater magnitude of BBBO | *Yang et al., 2009 – Effect of ultrasound contrast agent dose on the duration of focused-ultrasound-induced BBB disruption |
| Rat | 0.558 MHz | 0.3 MPa | Definity (20 μL/kg) | Immediately after experiment | Contrast-enhanced MRI Immunoblot Fluorescence microscopy: p-Akt, p-GSK3β, GFAP (astrocytes), NeuN (neurons), ZO-1 (TJ protein), von Willebrand Factor (endothelial damage) |
Extravasated IgG in sonicated regions Decreased interaction of occluden and ZO-1 (TJ proteins) but no loss of occluden or ZO-1 levels, increased PI3K/Akt signaling, no change in MAPK signaling, in sonicated regions Increased p-Akt and p-GSK3β levels in neurons around opened blood vessels |
*Jalali et al., 2010 – FUS-mediated BBB disruption is associated with increase in Akt activation in rats |
| Rat | 0.5515 MHz | Acoustic pressure increased incrementally until ultraharmonic emissions detected | Definity (20 μL/kg) | 2 h–8 d after sonication | Contrast-enhanced MRI Light microscopy: H&E Immunohistochemistry: NeuN (neurons) |
0.28 MPa ± 0.05 sonications resulted in BBBO Scaling pressures by 50% after ultraharmonics detected allowed BBBO without causing edema, some to no RBC extravasation, no vacuolations No neuronal loss 8 d after sonication |
*O'Reilly and Hynynen 2012 – BBB real-time feedback-controlled FUS disruption by using an acoustic emissions based controller |
| Rat | 1.5 MHz | 0.45 MPa | Sonovue (1.5 × 108 MB/mL, 200 μL) | N/A | Contrast-enhanced MRI | BBB closed at progressively slower rate, hypothesized to be due to transcellular passage between ECs | *Marty et al., 2012 – Dynamic study of BBB closure after its disruption using ultrasound: a quantitative analysis |
| Rat | 0.4 MHz | 0.2–0.5 MPa | SonoVue (100 μL/kg) | 1 h–1 wk after sonication | Somatosensory evoked potentials (SSEPs) Functional MRI for blood-oxygen-level dependent (BOLD) measurements Light microscopy: H&E Immunohistochemistry: NeuN (neurons), TuJ1 (anti-neuron specific class III ß-tubulin) Evans blue |
No BBBO at 0.2 MPa, but BBBO at 0.35 MPa and 0.5 MPa sonication RBC extravasation highest in 0.5 MPa treated animals FUS at 0.5 MPa suppressed SSEP amplitude and prolonged latency for as long as 1 wk; at 0.35 MPa, SSEP suppression was observed for < 1h |
*Chu et al., 2015 – Neuromodulation accompanying FUS-induced BBB opening |
| Rat | 0.69 MHz | 0.66–0.80 MPa | Definity (10 μL/kg) | 1–36 h after last sonication | Contrast-enhanced MRI Light microscopy: H&E, Von Kossa (mineralization), GFAP (astrocytes) |
Contrast-enhanced T2-weighted images, but not T1-weighted images, corresponded well with histology results 0.73 MPa: Few to no microhemorrhages observed after six sonications 0.80 MPa: Microhemorrhages and some acute ischemic and necrotic damage observed after six sonications Micro-scars consisting of reactive astrocytes observed in some animals |
*Kobus et al., 2016 – Safety validation of repeated BBB disruption using FUS |
| Mouse | 1.525 MHz | 0.15–0.98 MPa | Definity (50 μL/kg) | Within 5 h of sonication | Contrast-enhanced MRI Fluorescence microscopy: Tracer injection (3 kDa Texas Red dextran) Light microscopy: H&E |
Fluorescence microscopy and MRI of tracers: BBBO threshold between 0.15 and 0.30 MPa Safest BBBO between 0.3 and 0.46 MPa 0.3 MPa: few RBC extravasation, few small microvacuolated areas 0.46 MPa: more RBC extravasations, presence of dark neurons and microvacuolations Damage increased with increasing acoustic pressure |
*Baseri et al., 2010 – Multi-modality safety assessment of BBBO using FUS and Definity MBs: A short-term study |
| Mouse | 1.525 MHz | 0.30–0.60 MPa | In-house MBs: 1–2, 4–5, or 6–7 μm(1 μL/g × 107 MB/mL) | 7 d after sonication | Contrast-enhanced MRI Light microscopy: H&E |
Magnitude of BBBO increased with sonication pressure and MB diameter BBB closure time proportional to magnitude of BBBO (24 h −5 d after sonication) Cell loss correlated with hypointensity in MRI |
*Samiotaki et al., 2012 – A quantitative pressure and MB size dependence study of FUS-induced BBBO reversibility in vivo using MRI |
| Mouse | 1 MHz | 0.7 MPa | In-house MBs (1–5×107 MBs/mL, injected 1 μL/g retroorbitally) | 30 min–1 h after sonication | Light microscopy: H&E, Nissl, vanadium acid fuchsin Evans blue Fluorescence microscopy: GFAP (astrocytes), IBA1 (microglia) |
No degenerating neurons, edema, or RBC extravasation No ischemic damage Microglial activation, but no astrogliosis, at one an 24 h after sonication |
*Leinenga and Gotz 2015 – Scanning ultrasound removes Aß and restores memory in an AD mouse model |
| Rhesus macaque | 0.5 MHz | 0.3–0.6 MPa | Definity (1.2 × 1010 MBs/mL) and in-house MBs (5 × 109 MB/mL) 500 μL MBs injected per animal |
N/A | Contrast-enhanced MRI | 0.3 MPa sufficient to cause BBBO | *Marquet et al., 2011 – Noninvasive, transient and selective BBBO in non-human primates in vivo |
| Rhesus macaque | ExAblate 4000 (InSightec, 2015) phased array 0.22 MHz | N/A | Definity (10–20 μL/kg) | 2 h–2 wks after last sonication | Contrast-enhanced MRI Functional tests Light microscopy: Nissl, H&E, Luxol Fast Blue, Bielschowsky's silver stain, Prussian blue, TUNEL Trypan blue |
BBBO varied depending on area of brain targeted No functional impairments after single or repeated BBBO in visual areas over several weeks BBBO probability 50% at 149 kPa Tissue damage probability 50% at 300 kPa |
*McDannold et al., 2012 – Temporary disruption of the BBB by use of ultrasound and MBs: Safety and efficacy evaluation in rhesus monkeys |
transcranial US.
8.1. Magnetic resonance imaging evaluation: duration of blood-brain barrier opening
Much of the concern regarding the safety of FUS-induced BBBO comes from exposing the brain to potentially harmful molecules in the systemic circulation. While this is certainly an important consideration for any method of opening the BBB, the targeted and transient nature of this FUS-induced BBBO greatly minimizes the risk of adverse exposure, especially compared to more invasive and less targeted approaches (e.g. intracranial injection (Muir et al., 2011) and mannitol-induced BBBO (Nagy et al., 1979)). Additionally, the ability to open the BBB in a targeted manner restricts potentially negative exposures to the affected regions of the brain. The duration for which BBB permeability is increased following FUS is dependent on several technical and biological parameters; thus, there is much variation in the literature regarding the timing of BBB closure. Additionally, as BBBO is not binary (open vs. closed), the method of assessing BBB integrity is highly relevant. Marty et al. investigated the half closure time (t1/2) for three different sizes of MRI contrast agents following FUS. They found a strong inverse relationship between hydrodynamic diameter and t1/2, measuring t1/2 of ~5.5 h for a 1 nm particle, ~1.5 h for a 4 nm particle, and ~30 min for a 7 nm particle (Marty et al., 2012). In general, studies commonly show the gradual reversal of BBBO, with complete closure between 1 and 10 h (Hynynen et al., 2001; Yang et al., 2009; Sheikov et al., 2008; Zhang et al., 2009), provided optimized FUS parameters are employed. However, this is contrasted by studies which have observed drastically delayed closure times, up to 5 d following FUS (with accompanying cell loss and tissue damage), when using sub-optimal exposure parameters (1.5 MHz transducer; 6–8 μm diameter MBs; 0.6 MPa PNP; 10 Hz pulse repetition frequency; total duration of 60 s) (Samiotaki et al., 2012).
8.2. Histological evaluation
The safety profile of FUS has been evaluated by assessing the brain tissue of mice, rats, rabbits, and non-human primates, using various histological staining techniques: H&E to evaluate RBC extravasation, microvacuolation, dark neurons, and blood vessel integrity (Hynynen et al., 2006; McDannold et al., 2005; Baseri et al., 2010); TUNEL staining to evaluate DNA fragmentation and apoptotic cells (Hynynen et al., 2005, 2006; McDannold et al., 2005; McDannold et al., 2012); vanadium acid fuchsin (VAF)-toluidine blue staining to evaluate ischemic neurons and RBC extravasation (McDannold et al., 2005; Hynynen et al., 2005, 2006); Nissl staining to analyze neuronal integrity (McDannold et al., 2012); Luxol fast blue to evaluate myelin integrity (McDannold et al., 2012); Bielschowsky's silver stain for axons (McDannold et al., 2012); Prussian blue for the presence of hemosiderin (McDannold et al., 2012); and HRP staining for transmission electron microscopy analysis of vessel opening (Hynynen et al., 2005, 2006). T2-and T2*- weighted MR images are also evaluated for edema and petechaie, respectively (McDannold et al., 2012).
In a short-term histological study of murine brains after FUS-mediated BBBO, dark neurons, microvacuolated sites, and erythrocyte extravasation were observed 30 min after sonication (Baseri et al., 2010). Another study showed that tissue necrosis, apoptosis, and ischemia are minimal or not observed in rabbits after sonication at the threshold for BBBO (Hynynen et al., 2006).
Histological examinations have also been conducted on the brain tissue of macaques after as many as 13 FUS sessions over 26 weeks targeted to the thalamus, putamen, cingulate cortex, visual cortex, hippocampus, and white matter structures. This paradigm of repeated FUS-mediated BBBO resulted in no significant changes in T2*-weighted MR images after sonication, no evidence of demyelination, few ischemic neurons and extravasated erythrocytes, no apoptotic cells, healthy neurons, few hemosiderin deposits, and normal axonal morphology. Importantly, daily living tasks (e.g. eating, drinking, aggressive behavior) were unaffected after FUS-mediated BBBO. At the highest ultrasound exposure levels (444–700 kPa at 220 kHz), more severe vascular and parenchymal damage were observed, but these effects were contained in the focal region (McDannold et al., 2012).
The threshold for BBBO has been described as the pressure at which the probability of causing BBBO is 50% (McDannold et al., 2008b). When expressed in the form of PNP, the threshold for BBBO increases with increasing ultrasound frequency. However, when expressed in the form of MI (see Overview for equation), BBBO threshold was found to be approximately constant at 0.46 (McDannold et al., 2008b) across many experiments (Hynynen et al., 2001, 2005; McDannold et al., 2005, 2006; McDannold et al., 2006; McDannold et al., 2007; Treat et al., 2007).
8.3. Behavioral evaluation
In addition to histological and MRI analysis, behavioral tests have also been conducted to evaluate the safety of FUS-mediated BBBO. After five FUS-mediated BBBO treatments to the lateral geniculate nucleus and primary and secondary visual areas of rhesus macaques over the course of 5–9 weeks, no changes were observed in visual acuity or in performance on a visual task learned prior to FUS treatment (McDannold et al., 2012). Another study confirmed that up to 12 FUS-mediated BBBO sessions targeted to the caudate or putamen regions of the basal ganglia in macaques had no long-term effects on visual, cognitive, motivational, and motor functions (Downs et al., 2015).
8.4. Real-time controller using microbubble cavitation activity
Various groups have established FUS parameters that are reliably believed to cause BBBO without damage, and vary according to the ultrasound frequency, acoustic pressure, and burst sequence used. Thus far, the most reliable method of producing BBBO without causing damage to the brain parenchyma is to scale back ultrasound pressures to 50% of the value at which sub- or ultra-harmonic emissions are detected using a passive cavitation detector (O'Reilly and Hynynen, 2012). These acoustic signals, emitted from MBs, are reliable indicators of stable cavitation (Neppiras, 1980) and thus provide a basis for evaluating FUS-mediated BBBO in real-time (O'Reilly and Hynynen, 2012). In addition, this method of adjusting ultrasound pressure based on MB behavior has demonstrated high fidelity in a variety of animal models (O'Reilly and Hynynen, 2012; Burgess et al., 2014), as it is intrinsically tailored to each animal's unique anatomy.
To conclude, the safety of FUS-mediated BBBO has been studied by several groups in a wide range of animal models. Novel technology, such as the real-time acoustic emissions controller, have been designed to account for the anatomical differences between subjects. The temporal and spatial resolution of FUS-mediated BBBO has been thoroughly investigated. While there are areas that remain to be investigated, the safety profile of FUS-mediated BBBO thus far appears to be promising.
9. Applications independent of therapeutic agent delivery
9.1. Alzheimer's disease
Similar to most therapeutic agents, only ~0.11% of systemically injected anti-amyloid antibodies bypass the BBB (Banks et al., 2002). Conversely, delivery of anti-Aβ antibodies via FUS-mediated BBBO result in a significant decrease in Aβ plaque load 4 d after treatment (Jordão et al., 2010).
The same group found that FUS treatment, but without delivery of therapeutics, was sufficient to significantly decrease mean Aβ plaque size and total Aβ plaque surface area in the treated hemisphere. In addition, levels of endogenous antibodies (IgG and IgM) were significantly increased after FUS treatment. Morphological analysis of microglia and astrocytes demonstrated significant increases in activation and a greater level of Aβ internalization compared to the untreated hemisphere (Jordão et al., 2013).
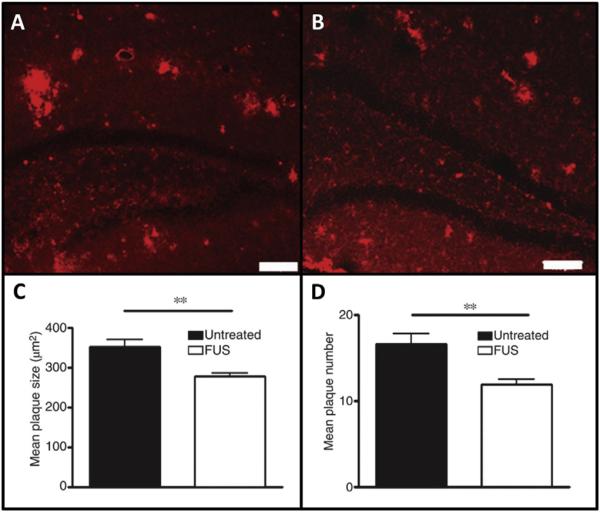
Since AD is a progressive neurodegenerative disease, it is beneficial to evaluate the effects of repeated FUS treatments to the diseased brain. In 2014, Burgess et al. produced the first study to show that three weekly treatments of FUS-mediated BBBO alone improved AD pathology on a cellular and behavioral level (Fig. 5). Specifically, they validated that FUS-mediated BBBO, bilaterally targeted to the hippocampi, significantly decreased plaque load and increased neurogenesis. This treatment also restored hippocampal-dependent spatial memory performance in the transgenic AD mice to the levels of their non-transgenic, age-matched counterparts (Burgess et al., 2014).
Fig. 5.
Reduction in Aβ plaque pathology following FUS-mediated BBBO in AD mouse model. The BBB in the hippocampi of TgCRND8 mice was opened once per week for three consecutive weeks with FUS, and the effects on AD pathology were investigated. Aβ plaques were fluorescently labeled with anti-amyloid antibody 6F3D in (A) control and (B) FUS treated hippocampi, demonstrating a significant reduction in (C) mean plaque size and (D) mean plaque number. Importantly, this study also showed a significant improvement in the performance of FUS-treated mice in hippocampal-dependent tasks compared to untreated controls (data not shown). Error bars represent standard errors of the mean. N = 6–8 per group. ** = p < 0.01 (Figure modified from Burgess et al., 2014). Abbreviations: Aβ = amyloid-beta, FUS = focused ultrasound, BBBO = blood-brain barrier opening, AD = Alzheimer's disease.
Another group, using slightly different ultrasound parameters to induce whole brain BBBO, confirmed these cellular and behavioral results in a different AD mouse model. They observed a decrease in plaque burden, improvements in different hippocampal-dependent behavioral tasks, activation of microglia, and an increase of Aβ in the lysosomal compartments of microglia, following multiple treatments (Leinenga and Götz, 2015). They did not observe a difference in inflammation in ultrasound treated animals compared to their untreated counterparts.
9.2. Neurogenesis: dentate gyrus of hippocampus
Several studies have shown that BDNF encourages neurogenesis (Henry et al., 2007; Scharfman et al., 2005), and that mice lacking endogenous levels of BDNF have lower rates of neurogenesis (Rossi et al., 2006). Based on a previous study that showed that FUS without MBs can increase BDNF (Tufail et al., 2010), Scarcelli et al. executed a series of experiments to investigate the effects of FUS-mediated BBBO on neurogenesis. They showed that FUS-mediated BBBO, unilaterally targeted to the hippocamus led to a statistically significant increase in the number of cells that were double-positive for BrdU (bromodeoxyuridine; a marker for newborn cells) and NeuN (a marker for neurons), compared to the contralateral hemisphere. This finding is supported by another study which showed a significant increase in the number of DCX (doublecortin; a marker for immature neurons) positive cells, total dendrite length, and dendritic branching in the dentate gyrus of both TgCRND8 mice and their non-transgenic littermates following repeated FUS treatment (Burgess et al., 2014). No significant differences have been observed in astrogenesis following FUS (Scarcelli et al., 2014). It is still unclear how FUS encourages neurogenesis. Although the mechanism behind this phenomenon requires further investigation, it may have a beneficial effect in diseases that result in the degeneration of neurons, such as AD.
9.3. Neuromodulation
FUS has also been shown to have an effect on neural activity, both in the presence and absence of MBs. This effect is currently being investigated as a more targeted, less invasive alternative to techniques such as deep brain stimulation, vagus nerve stimulation, implanted electrocortical stimulation, or epidural cortical stimulation (Bystritsky et al., 2011), and as an experimental tool to interrogate specific neural networks. As early as the 1950s, it was established that ultrasound had the potential to alter neural activity in the CNS of mammals (Fry, 1958). More recently, Tyler et al. showed that the application of FUS (without MBs) to hippocampal slices and ex vivo mouse brains results in the activation of sodium and voltage-gated calcium channels, triggers endocytosis, and induces an increase in synaptic transmission (Tyler et al., 2008). These results build on previous research from the 1990s on the neuromodulatory effects of FUS applied to brain slices (Rinaldi et al., 1991). Similarly, in vivo studies have demonstrated that ultrasound alone (in the absence of MBs or BBBO) can stimulate motor function when targeted to the motor cortex (Tufail et al., 2010), increase dopamine and serotinin concentrations in the thalamic areas of rats (Min et al., 2011), and modulate the performance of awake macaque rhesus monkeys in an antisaccade task (Deffieux et al., 2013).
Recent work also suggests a neuromodulatory effect of FUS-mediated BBBO. Chu et al. reported suppressed somatosensory evoked potential (SSEP) amplitudes and blood-oxygen level dependent (BOLD) responses for up to one week following FUS-mediated BBBO when sonicating at a frequency of 400 MHz and PNP of 0.5 MPa (MI of 0.8). Conversely, when the pressure was reduced to 0.35 MPa (MI of 0.55), this resulted in short-term suppression of SSEP amplitudes and BOLD responses for less than 60-min (Chu et al., 2015). Interestingly, the authors did not find significant tissue damage at MIs of 0.55 or 0.80, which are higher than the previously established threshold of 0.46 (McDannold et al., 2012).
Alternatively, FUS can also be used to deliver neuroactive substances to select regions of the brain, altering neural activity in those areas. Following BBBO in the rat somatosensory cortex, the intravenous administration of γ-aminobutyric acid (GABA) results in a dose-dependent suppression of SSEPs in response to electrical stimulation of the sciatic nerve. No suppression is observed 1–5 d afterwards, or in control animals where the BBB is not disrupted (McDannold et al., 2015). While characterization of the neuromodulatory effects of FUS-mediated BBBO in the absence of drug delivery is important from a safety and a basic science perspective, the therapeutic value of the technique remains to be determined.
10. Conclusions and future outlook
The single largest impedance to the delivery of drugs to the brain is the BBB (Pardridge, 2005). Despite grand investments and significant advancements in our understanding of various neuropathologies, effective treatments have by and large remained elusive. While assessing all of the factors that have contributed to this slow progress and determining why clinical trials for promising drugs have failed is beyond the scope of this review, it is clear that there is a need for new strategies. In providing an avenue for the noninvasive, targeted delivery of therapeutic agents to the brain, FUS-induced BBBO presents the opportunity to rethink our approach to treating neuropathologies.
Currently there are several methods of bypassing the BBB for the purpose of increasing drug delivery to the brain being investigated, each with their own benefits and drawbacks. It is our opinion that FUS-mediated BBBO is the most versatile method that is both noninvasive and targeted. The body of research conducted in pre-clinical models, including non-human primates (Marquet et al., 2011; McDannold et al., 2012; Downs et al., 2015), by several independent groups suggests that the clinical translation of FUS is both feasible and near.
As it stands, the biggest factor restricting the expediency of clinical translation is concern regarding the safety of opening the BBB with FUS. While a large body of literature supports the idea that a properly functioning BBB is crucial for ensuring proper brain homeostasis and preventing infection, several studies have demonstrated that if proper ultrasound parameters are used, temporary opening of the BBB using FUS is not accompanied by any long-term adverse side-effects. Normal barrier function is restored within 1–10 h of treatment with no evidence of apoptosis, necrosis, or infection. In addition, the targeted nature of the technique ensures that the volume of tissue exposed to any potentially harmful substances in circulation is greatly minimized, being confined to areas of pathology. However, it is important to note that the safety of this technique hinges on the use of safe but adequate ultrasound exposures. While much work has been done to determine how changes in various parameters affect the degree and duration of FUS-mediated BBBO, the most significant advancement in this regard will be in the continued development of real-time acoustic feedback control systems (O'Reilly and Hynynen, 2012) and transcranial bubble imaging (O'Reilly et al., 2014) that can provide consistent ultrasound exposures in human patients.
Although there is still work to be done to gain a more thorough understanding of the cellular and biochemical mechanisms driving BBBO following FUS, work in preclinical models demonstrate its efficacy and safety profile. As such, it may be time to cautiously move towards clinical feasibility testing that would determine if the promising animal results can be translated to the clinic. The first clinical trial of MR-guided FUS-mediated BBBO is currently underway (ClinicalTrials.gov identifier: NCT02343991). The purpose of this trial is to determine the safety of FUS-mediated BBBO in human patients, and to test the feasibility of using this technique to increase the delivery and accumulation of the chemotherapeutic doxorubicin to brain tumors. The first patient was treated in November 2015; successful BBBO was confirmed on MR images. If these treatments reach the benchmarks set for effectiveness and if long-term follow-ups confirm patient health, this will drive the advancement of FUS into more advanced trials and clinical implementation. If clinical translation is successful, FUS could change the way that many neuropathologies are treated.
Acknowledgments
We gratefully acknowledge our sources of support: Canadian Institutes of Health Research (MOP 119312) and the National Institutes of Health (R01 EB003268). The authors would also like to thank Ryan Jones for his expertise and suggestions, Hangyu Lin for his illustrations (Figs. 1 and 2), and Marcelline Ramcharan for her administrative help.
Abbreviations
- AAV
adeno-associated virus
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- BBB
blood-brain barrier
- BBBO
blood-brain barrier opening
- BCNU
bis-chloroethylnitrosourea
- BDNF
brain-derived neurotrophic factor
- BOLD
blood-oxygen-level dependent
- BrdU
bromodeoxyuridine
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DCE
dynamic contrast enhanced
- DCX
doublecortin
- DMSO
dimethyl sulfoxide
- EC
endothelial cell
- f
frequency
- FDA
Food and Drug Administration
- FUS
focused ultrasound
- GABA
γ-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- GSK3β
glycogen synthase kinase 3 beta
- Her2
human epidermal growth factor receptor 2
- HIFU
high-intensity focused ultrasound
- HRP
horseradish peroxidase
- Htt
huntingtin protein
- IBA1
ionized calcium-binding adapter molecule 1
- IL-12
interleukin-12
- Ktrans
transfer coefficient
- MB
microbubble
- MI
mechanical index
- microSPECT
micro-Single Photon Emission Computed Tomography
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- NeuN
neuronal specific nuclear protein
- PD
Parkinson's disease
- PNP
peak negative pressure
- RBC
red blood cell
- SDS
sodium dodecyl sulfate
- SSEP
suppressed somatosensory evoked potential
- t1/2
half closure time
- TJ
tight junction
- VAF
vanadium acid fuchsin
- VEGF
vascular endothelial growth factor
- ZO
zonula occludens
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability [Internet] Cell Mol. Neurobiol. Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. Available from: http://dx.doi.org/10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyteeendothelial interactions at the bloodebrain barrier [Internet] Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. Available from: http://dx.doi.org/10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, et al. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes [Internet] Elsevier B.V. J. Control Release. 2008;132(2):84–90. doi: 10.1016/j.jconrel.2008.08.017. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18805446. [DOI] [PubMed] [Google Scholar]
- Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors [Internet] Cancer Res. 2013;73(6):1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. Available from: http://dx.doi.org/10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound [Internet] Ultrasound Med. Biol. 1991;17(2):179–185. doi: 10.1016/0301-5629(91)90125-g. Available from: http://dx.doi.org/10.1016/0301-5629(91)90125-G. [DOI] [PubMed] [Google Scholar]
- Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS One. 2012;7(9):1–16. doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburamani AA, Supramaniam VG, Hagberg H, Mallard C. Microglia toxicity in preterm brain injury [Internet]. Elsevier Inc. Reprod. Toxicol. 2014;48:106–112. doi: 10.1016/j.reprotox.2014.04.002. Available from: http://dx.doi.org/10.1016/j.reprotox.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay L, Hueter TF, Ballantine HT, Sosa D. Ultrasounically produced changes in the BBB [Internet] Arch. Neurol. Psychiatry. 1956;76(5):457–467. doi: 10.1001/archneurpsyc.1956.02330290001001. Available from: http://dx.doi.org/10.1001/archneurpsyc.1956.02330290001001. [DOI] [PubMed] [Google Scholar]
- Ballantine HT, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound [Internet] J. Neurosurg. 1960;17:858–876. doi: 10.3171/jns.1960.17.5.0858. Available from: http://dx.doi.org/10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer's disease [Internet] Peptides. 2002;23(12):2223–2226. doi: 10.1016/s0196-9781(02)00261-9. Available from: http://dx.doi.org/10.1016/S0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease [Internet] Nat. Med. 2000;6(8):916–919. doi: 10.1038/78682. Available from: http://dx.doi.org/10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Deffieux T, Samiotaki M, Tung Y-S, Olumolade O, et al. Activation of signaling pathways following localized delivery of systemically-administered neurotrophic factors across the blood-brain barrier used focused ultrasound and microbbubles [Internet] Phys. Med. Biol. 2012;57(7):N65–N81. doi: 10.1088/0031-9155/57/7/N65. Available from: http://dx.doi.org/10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Tung YS, Konofagou EE. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and Definity microbubbles: a short-term study [Internet] Ultrasound Med. Biol. 2010;36(9):1445–1459. doi: 10.1016/j.ultrasmedbio.2010.06.005. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers [Internet]. Elsevier B.V. J. Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. Available from: http://dx.doi.org/10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A [Internet] Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. Available from: http://dx.doi.org/10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V, et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging [Internet] Part Fibre Toxicol. 2010;7(3):1–25. doi: 10.1186/1743-8977-7-3. Available from: http://dx.doi.org/10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing KF, Howles GP, Qi Y, Palmeri ML, Nightingale KR. Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity® in mice [Internet] Ultrasound Med. Biol. 2009;35(8):1298–1308. doi: 10.1016/j.ultrasmedbio.2009.03.012. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell RD, Salcman M, Kaplan RS. Morphologic effect of dimethyl sulfoxide on the blood-brain barrier [Internet] Sci. (80-) 1982;217(4555):164–166. doi: 10.1126/science.7089551. Available from: http://dx.doi.org/10.1126/science.7089551. [DOI] [PubMed] [Google Scholar]
- Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultra-sound for targeted delivery of siRNA and efficient knockdown of Htt expression [Internet] J. Control Release. 2012a;163(2):125–129. doi: 10.1016/j.jconrel.2012.08.012. Available from: http://dx.doi.org/10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier [Internet] PLoS One. 2011 Jan.6(11):e27877. doi: 10.1371/journal.pone.0027877. Available from: http://dx.doi.org/10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Cho EE, Shaffaf L, Nhan T, Poon C, Hynynen K. In: Multiphoton Microscopy in the Biomedical Sciences XII. König K, editor. Proc. SPIE; San Francisco (USA): Feb 9, 2012b. p. 8226. http://dx.doi.org/10.1117/12.908826. [Google Scholar]
- Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior [Internet] Radiology. 2014;273(3):736–745. doi: 10.1148/radiol.14140245. Available from: http://dx.doi.org/10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, et al. A review of low-intensity focused ultrasound pulsation [Internet] Brain Stimul. 2011 Jul.4(3):125–136. doi: 10.1016/j.brs.2011.03.007. Available from: http://dx.doi.org/10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases e preliminary clinical experience. Ann. Oncol. 2007;18(1):163–167. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas [Internet]. Elsevier B.V. Eur. J. Pharm. Sci. 2011;44:164–173. doi: 10.1016/j.ejps.2011.07.007. Available from: http://dx.doi.org/10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Chen P-Y, Wei K-C, Liu H-L. Neural immune modulation and immunotherapy assisted by focused ultrasound induced blood-brain barrier opening [Internet] Hum. Vaccin Immunother. 2015;11(11):2682–2687. doi: 10.1080/21645515.2015.1071749. Available from: http://dx.doi.org/10.1080/21645515.2015.1071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier [Internet]. Elsevier B.V. Adv. Drug Deliv. Rev. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. Available from: http://dx.doi.org/10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury [Internet] J. Cereb. Blood Flow. Metab. 2003;23(2):137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. Available from: http://dx.doi.org/10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced bloodebrain barrier opening [Internet]. Nature Publishing Group J. Cereb. Blood Flow. Metab. 2011;31(9):1852–1862. doi: 10.1038/jcbfm.2011.59. Available from: http://dx.doi.org/10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Feshitan JA, Baseri B, Wang S, Tung Y-S, Borden MA, et al. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo [Internet] IEEE Trans. Biomed. Eng. 2010;57(1):145–154. doi: 10.1109/TBME.2009.2034533. Available from: http://dx.doi.org/10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies [Internet]. Nature Publishing Group J. Cereb. Blood Flow. Metab. 2011;31(2):725–737. doi: 10.1038/jcbfm.2010.155. Available from: http://dx.doi.org/10.1038/jcbfm.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultra-sound exposures [Internet] ACS Chem. Neurosci. 2010;1(5):391–398. doi: 10.1021/cn9000445. Available from: http://dx.doi.org/10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P-C, Liu H-L, Lai H-Y, Lin C-Y, Tsai H-C, Pei Y-C. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening [Internet]. Nature Publishing Group Sci. Rep. 2015;5:15477. doi: 10.1038/srep15477. Available from: http://dx.doi.org/10.1038/srep15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet S, Chapelon JY, Rouviere O, Mege-Lechevallier F, Colombel M, Tonoli-Catez H, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur. Urol. 2014;65(5):907–914. doi: 10.1016/j.eururo.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles [Internet] Ultrasound Med. Biol. 1999:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. Available from: http://dx.doi.org/10.1016/S0301-5629(99)00062-9. [DOI] [PubMed]
- De Bock M, Wang N, Decrock E, Bol M, Gadicherla AK, Culot M, et al. Endothelial calcium dynamics, connexin channels and blood-brain barrier function [Internet] Prog. Neurobiol. 2013 Sep.108:1–20. doi: 10.1016/j.pneurobio.2013.06.001. Available from: http://dx.doi.org/10.1016/j.pneurobio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry J-F. Low-intensity focused ultrasound modulates monkey visuomotor behavior [Internet] Curr. Biol. 2013;23:2430–2433. doi: 10.1016/j.cub.2013.10.029. Available from: http://dx.doi.org/10.1016/j.cub.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles [Internet] J. Mol. Neurosci. 2012;46(3):677–687. doi: 10.1007/s12031-011-9629-9. Available from: http://dx.doi.org/10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, Peereboom DM, Christoforidis GA, Hall WA, Palmieri D, Brock PR, et al. Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the eleventh annual blood-brain barrier consortium meeting [Internet] J. Neurooncology. 2007;81(1):81–91. doi: 10.1007/s11060-006-9209-y. Available from: http://dx.doi.org/10.1007/s11060-006-9209-y. [DOI] [PubMed] [Google Scholar]
- Dorovini-Zis K, Bowman PD, Betz AL, Goldstein GW. Hyperosmotic arabinose solutions open the tight junctions between brain capillary endothelial cells in tissue culture [Internet] Brain Res. 1984;302:383–386. doi: 10.1016/0006-8993(84)90254-3. Available from: http://dx.doi.org/10.1016/0006-8993(84)90254-3. [DOI] [PubMed] [Google Scholar]
- Downs ME, Buch A, Sierra C, Karakatsani ME, Chen S, Konofagou EE, et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task [Internet] PLoS One. 2015;10(5):e0125911. doi: 10.1371/journal.pone.0125911. Available from: http://dx.doi.org/10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor [Internet] N. Engl. J. Med. 2013;369:640–648. doi: 10.1056/NEJMoa1300962. Available from: http://dx.doi.org/10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- Fan C-H, Ting C-Y, Lin H-J, Wang C-H, Liu H-L, Yen T-C, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery [Internet]. Elsevier Ltd Biomaterials. 2013 May.34(14):3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. Available from: http://dx.doi.org/10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- FDA, U.S. Department of Health and Human Services . Center for Devices and Radiological Health; 2008. Information for Manufacturers Seeking Marketingclearance of Diagnostic Ultrasound Systems and Transducers [Internet].. Available from: http://www.fda.gov/downloads/UCM070911.pdf. [Google Scholar]
- Fornaguera C, Dols-Perez A, Calderó G, García-Celma MJ, Camarasa J, Solans C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier [Internet] J. Control Release. 2015 Aug 10;211:134–143. doi: 10.1016/j.jconrel.2015.06.002. Available from: http://dx.doi.org/10.1016/j.jconrel.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK, et al. Intravascular AAV9 preferentially targets neonatal-neurons and adult-astrocytes in CNS [Internet] Nat. Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. Available from: http://dx.doi.org/10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisella W, O'Connor LH, Vogler CA, Roberts M, Walkley S, Levy B, et al. Intracranial injection of recombinant adeno-associated virus improves cognitive function in a murine model of mucopolysaccharidosis type VII [Internet] Mol. Ther. 2001;3(3):351–358. doi: 10.1006/mthe.2001.0274. Available from: http://dx.doi.org/10.1006/mthe.2001.0274. [DOI] [PubMed] [Google Scholar]
- Fry WJ. Use of intense ultrasound in neurological research. Am. J. Phys. Med. 1958;37(3):143–147. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13545380. [PubMed] [Google Scholar]
- Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance [Internet] Mol. Psychiatry. 2011;16(9):903–907. doi: 10.1038/mp.2011.52. Available from: http://dx.doi.org/10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertz DE, Wright C, Hynynen K. Contrast agent kinetics in the rabbit brain during exposure to therapeutic ultrasound [Internet] Ultrasound Med. Biol. 2010;36(6):916–924. doi: 10.1016/j.ultrasmedbio.2010.03.005. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanig JP, Morrison JM, Krop S. Ethanol enhancement of blood-brain barrier permeability to catecholamines in chicks [Internet] Eur. J. Pharmacol. 1972;18(1):79–82. doi: 10.1016/0014-2999(72)90134-3. Available from: http://dx.doi.org/10.1016/0014-2999(72)90134-3. [DOI] [PubMed] [Google Scholar]
- Hawkins Bt, Davis TP. The blood-brain barrier/neurovascular unit in health and disease [Internet] Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. Available from: http://dx.doi.org/10.1124/pr.57.2.4.173. [DOI] [PubMed] [Google Scholar]
- Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain [Internet] Eur. J. Neurosci. 2007;25(12):3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. Available from: http://dx.doi.org/10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Hermann DM, ElAli A. The abluminal endothelial membrane in neurovascular remodeling in health and disease [Internet] Sci. Signal. 2012;5(236):re4. doi: 10.1126/scisignal.2002886. Available from: http://dx.doi.org/10.1126/scisignal.2002886. [DOI] [PubMed] [Google Scholar]
- Hosseinkhah N, Goertz DE, Hynynen K. Microbubbles and blood-brain barrier opening: a numerical study on acoustic emissions and wall stress predictions [Internet] IEEE Trans. Biomed. Eng. 2015;62(5):1293–1304. doi: 10.1109/TBME.2014.2385651. Available from: http://dx.doi.org/10.1109/TBME.2014.2385651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound [Internet] PLoS One. 2013;8(2):e57682. doi: 10.1371/journal.pone.0057682. Available from: http://dx.doi.org/10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation between inertial cavitation dose and endothelial cell damage in vivo [Internet] Ultrasound Med. Biol. 2006;32(10):1611–1619. doi: 10.1016/j.ultrasmedbio.2006.07.016. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Hynynen K. Ultrasound for drug and gene delivery to the brain [Internet] Adv. Drug Deliv. Rev. 2008;60(10):1209–1217. doi: 10.1016/j.addr.2008.03.010. Available from: http://dx.doi.org/10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications [Internet] Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. Available from: http://dx.doi.org/10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits [Internet] Radiology. 2001;220(4):640–646. doi: 10.1148/radiol.2202001804. Available from: http://dx.doi.org/10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery [Internet] J. Neurosurg. 2006;105(3):445–454. doi: 10.3171/jns.2006.105.3.445. Available from: http://dx.doi.org/10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- InSightec . ClinicalTrials.gov [Internet]. National Library of Medicine (US); Bethesda (MD): 2015. Blood-brain barrier disruption using transcranial MRI-guided focused ultrasound. Available from: https://clinicaltrials.gov/ct2/show/NCT02343991. NLM Identifier: NCT02343991. [Google Scholar]
- Jaeger CB, Blight AR. Spinal cord compression injury in guinea pigs: structural changes of endothelium and its perivascular cell associations after blood-brain barrier breakdown and repair [Internet] Exp. Neurol. 1997;144(2):381–399. doi: 10.1006/exnr.1996.6405. Available from: http://dx.doi.org/10.1006/exnr.1996.6405. [DOI] [PubMed] [Google Scholar]
- Jain S, Mishra V, Singh P, Dubey PK, Saraf DK, Vyas SP. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting [Internet] Int. J. Pharm. 2003;261:43–55. doi: 10.1016/s0378-5173(03)00269-2. Available from: http://dx.doi.org/10.1016/S0378-5173(03)00269-2. [DOI] [PubMed] [Google Scholar]
- Jalali S, Huang Y, Dumont DJ, Hynynen K. Focused ultrasound-mediated bbb disruption is associated with an increase in activation of AKT: experimental study in rats [Internet] BMC Neurol. 2010;10(114):1–10. doi: 10.1186/1471-2377-10-114. Available from: http://dx.doi.org/10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation [Internet] Pharm. Res. 2007;24(9):1759–1771. doi: 10.1007/s11095-007-9379-0. Available from: http://dx.doi.org/10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound [Internet]. Elsevier Inc. Exp. Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. Available from: http://dx.doi.org/10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, et al. May. Antibodies targeted to the brain with image-guided focused ultra-sound reduced amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease [Internet] PLoS One. 2010;5(5):e10549. doi: 10.1371/journal.pone.0010549. Available from: http://dx.doi.org/10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karyekar CS, Fasano A, Raje S, Lu R, Dowling TC, Eddington ND. Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells [Internet] J. Pharm. Sci. 2003;92(2):414–423. doi: 10.1002/jps.10310. Available from: http://dx.doi.org/10.1002/jps.10310. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia [Internet] FASEB J. 2006;20(8):1185–1187. doi: 10.1096/fj.05-4829fje. Available from: http://dx.doi.org/10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the bloodebrain barrier by MRI-guided focused ultrasound [Internet] Biochem. Biophys. Res. Commun. 2006b;340(4):1085–1090. doi: 10.1016/j.bbrc.2005.12.112. Available from: http://dx.doi.org/10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption [Internet] Proc. Natl. Acad. Sci. U. S. A. 2006a;103(31):11719–11723. doi: 10.1073/pnas.0604318103. Available from: http://dx.doi.org/10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Permeability of the BBB depends on brain temperature [Internet] Neuroscience. 2009;161(3):926–939. doi: 10.1016/j.neuroscience.2009.04.004. Available from: http://dx.doi.org/10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety validation of repeated blood-brain barrier disruption using focused ultrasound [Internet] Ultrasound Med. Biol. 2016;42(2):481–492. doi: 10.1016/j.ultrasmedbio.2015.10.009. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models [Internet] J. Control Release. 2014;187:74–82. doi: 10.1016/j.jconrel.2014.05.033. Available from: http://dx.doi.org/10.1016/j.jconrel.2014.05.033. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Olsson Y. Uptake of exogenous proteins in mouse olfactory cells [Internet] Acta Neuropathol. 1971;19(2):145–154. doi: 10.1007/BF00688493. Available from: http://dx.doi.org/10.1007/BF00688493. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Lackay SN, Zhao L, Fu ZF. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection [Internet] Virus Res. 2009;144:18–26. doi: 10.1016/j.virusres.2009.03.014. Available from: http://dx.doi.org/10.1016/j.virusres.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange DG, Sedmak J. Japanese encephalitis virus (JEV): potentiation of lethality in mice by microwave radiation [Internet] Bioelectromagnetics. 1991;12(6):335–348. doi: 10.1002/bem.2250120603. Available from: http://dx.doi.org/10.1002/bem.2250120603. [DOI] [PubMed] [Google Scholar]
- Lee JK, Jin K, Endo S, Schuchman EH, Carter JE, Bae J-S. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-neta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses [Internet] Stem Cells. 2010;28:329–343. doi: 10.1002/stem.277. Available from: http://dx.doi.org/10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- Leighton TG. The Acoustic Bubble. Academic Press; San Diego: 1994. [Google Scholar]
- Leinenga G, Götz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model [Internet] Sci. Transl. Med. 2015;7(278):278ra33. doi: 10.1126/scitranslmed.aaa2512. Available from: http://dx.doi.org/10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- Lewin PA, Bjørnø L. Acoustically induced shear stresses in the vicinity of microbubbles in tissue [Internet] J. Acoust. Soc. Am. 1982;71(3):728. Available from: http://dx.doi.org/10.1121/1.387549. [Google Scholar]
- Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study [Internet]. Elsevier Ltd Lancet Neurol. 2013 May;12(5):462–468. doi: 10.1016/S1474-4422(13)70048-6. Available from: http://dx.doi.org/10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W, et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment [Internet] Radiology. 2010a;255(2):415–425. doi: 10.1148/radiol.10090699. Available from: http://dx.doi.org/10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- Liu H-L, Pan C-H, Ting C-Y, Hsiao M-J. Opening of the blood-brain barrier by low-rrequency (28-kHz) ultrasound: a novel pinhole-assisted mechanical scanning device [Internet] Ultrasound Med. Biol. 2010b;36(2):325–335. doi: 10.1016/j.ultrasmedbio.2009.10.004. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system [Internet]. Elsevier B.V. Adv. Drug Deliv. Rev. 2012;64(7):614–628. doi: 10.1016/j.addr.2011.11.002. Available from: http://dx.doi.org/10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology [Internet] J. Gen. Physiol. 1942;26(2):179–193. doi: 10.1085/jgp.26.2.179. Available from: http://dx.doi.org/10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison S. Intraperitoneal and intracranial cholecystokinin depress operant responding for food [Internet] Physiol. Behav. 1977;19(6):819–824. doi: 10.1016/0031-9384(77)90322-5. Available from: http://dx.doi.org/10.1016/0031-9384(77)90322-5. [DOI] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale [Internet] Brain Pathol. 2007;17(2):243–250. doi: 10.1111/j.1750-3639.2007.00067.x. Available from: http://dx.doi.org/10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet F, Tung Y-S, Teichert T, Ferrera VP, Konofagou EE. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo [Internet] PLoS One. 2011 Jan.6(7):e22598. doi: 10.1371/journal.pone.0022598. Available from: http://dx.doi.org/10.1371/journal.pone.0022598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M, et al. Dynamic study of bloodebrain barrier closure after its disruption using ultrasound: a quantitative analysis [Internet] J. Cereb. Blood Flow. Metab. 2012;32(10):1948–1958. doi: 10.1038/jcbfm.2012.100. Available from: http://dx.doi.org/10.1038/jcbfm.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with inertial cavitation [Internet] Phys. Med. Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. Available from: http://dx.doi.org/10.1109/ULTSYM.2005.1603078. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption [Internet] Ultrasound Med. Biol. 2008a;34(6):930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. Available from: http://dx.doi.org/10.1038/nature13314.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Zhang Y, Vykhodtseva N. Blood-brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol [Internet] Ultrasound Med. Biol. 2011;37(8):1259–1270. doi: 10.1016/j.ultrasmedbio.2011.04.019. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques [Internet] Cancer Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with definity for targeted blood-brain barrier disruption: a feasibility study [Internet] Ultrasound Med. Biol. 2007;33:584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound circulating preformed microbubbles appears to be characterized by the mechanical index [Internet] Ultrasound Med. Biol. 2008b;34(5):834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits [Internet] Ultrasound Med. Biol. 2005;31(11):1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- McDannold N, Zhang Y-Z, Power C, Arvanitis C, Vykhodtseva N, Livingstone M. Targeted delivery of GABA via ultrasound-induced blood-brain barrier disruption blocks somatosensory-evoked potentials [Internet]. Biomed. Cent. Ltd. J. Ther. Ultrasound. 2015;3(Suppl. 1):P28. Available from: http://dx.doi.org/10.1186/2050-5736-3-S1-P28. [Google Scholar]
- Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound [Internet] J. Ultrasound Med. 2009;28(7):871–880. doi: 10.7863/jum.2009.28.7.871. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19546329. [DOI] [PubMed] [Google Scholar]
- Min B-K, Yang PS, Bohlke M, Park S, R.Vago D, Maher TJ, et al. Focused ultrasound modulates the level of cortical neurotransmitters: potential as a new functional brain mapping technique [Internet] Int. J. Imaging Syst. Technol. 2011 Jun;21(2):232–240. Available from: http://dx.doi.org/10.1002/ima.20284. [Google Scholar]
- Moriyama E, Salcman M, Broadwell RD. Blood-brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: I. Temperature and power measurements [Internet] Surg. Neurol. 1991;35(3):177–182. doi: 10.1016/0090-3019(91)90068-k. Available from: http://dx.doi.org/10.1016/0090-3019(91)90068-K. [DOI] [PubMed] [Google Scholar]
- Muir KW, Sinden J, Miljan E, Dunn L. Intracranial delivery of Stem cells [Internet] Transl. Stroke Res. 2011;2:266–271. doi: 10.1007/s12975-011-0095-z. Available from: http://dx.doi.org/10.1007/s12975-011-0095-z. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Pappius HM, Mathieson G, Huttner I. Opening of tight junctions in cerebral endothelium [Internet] J. Comp. Neurol. 1979;185:569–578. doi: 10.1002/cne.901850307. Available from: http://dx.doi.org/10.1002/cne.901850307/abstract. [DOI] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, et al. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells [Internet] J. Neurovirol. 2008;14(3):186–195. doi: 10.1080/13550280801993630. Available from: http://dx.doi.org/10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship [Internet] J. Neuropathol. Exp. Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. Available from: http://dx.doi.org/10.1097/NEN.0-013e3181919-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neppiras EA. Acoustic cavitation [Internet] Phys. Rep. 1980;61(3):159–251. Available from: http://dx.doi.org/10.1016/0370-1573(80)90115-5. [Google Scholar]
- Nhan T, Burgess A, Cho EE, Stefanovic B, Lilge L, Hynynen K. Drug delivery to the brain by focused ultrasound induced blood-brain barrier disruption: quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy [Internet] J. Control Release. 2013 Nov.172(1):274–280. doi: 10.1016/j.jconrel.2013.08.029. Available from: http://dx.doi.org/10.1016/j.jconrel.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- On NH, Savant S, Toews M, Miller DW. Rapid and reversible enhancement of blood-brain barrier permeability using lysophosphatidic acid [Internet]. Nature Publishing Group J. Cereb. Blood Flow. Metab. 2013;33:1944–1954. doi: 10.1038/jcbfm.2013.154. Available from: http://dx.doi.org/10.1038/jcbfm.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MA, Hynynen K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller [Internet] Radiology. 2012;263(1):96–106. doi: 10.1148/radiol.11111417. Available from: http://dx.doi.org/10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MA, Jones RM, Hynynen K. Three-dimensional transcranial ultrasound imaging of microbubble clouds using a sparse hemispherical array. IEEE Trans. Biomed. Eng. 2014;61(4):1285–1294. doi: 10.1109/TBME.2014.2300838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused-ultrasound disruption of the blood-brain barrier using closely-timed short pulses: influence of sonication parameters and injection rate [Internet] Ultrasound Med. Biol. 2011;37(4):587–594. doi: 10.1016/j.ultrasmedbio.2011.01.008. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajek D, Hynynen K. The application of sparse arrays in high frequency transcranial focused ultrasound therapy: a simulation study [Internet] Med. Phys. 2013;40(12):122901:1–122901:10. doi: 10.1118/1.4829510. Available from: http://dx.doi.org/10.1118/1.4829510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development [Internet] NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. Available from: http://dx.doi.org/10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development [Internet] J. Clin. Pathol. 2002;55(2):158. Available from: http://dx.doi. org/10.1124/mi.3.2.90. [Google Scholar]
- Park J, Fan Z, Kumon RE, El-Sayed MEH, Deng CX. Modulation of intracellular Ca2þ concentration in brain microvascular endothelial cells in vitro by acoustic cavitation [Internet] Ultrasound Med. Biol. 2010;36(7):1176–1187. doi: 10.1016/j.ultrasmedbio.2010.04.006. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound [Internet]. Elsevier B.V. J. Control Release. 2012;162(1):134–142. doi: 10.1016/j.jconrel.2012.06.012. Available from: http://dx.doi.org/10.1016/j.jconrel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering [Internet] Phys. Med. Biol. 2009;54(6):R27–R57. doi: 10.1088/0031-9155/54/6/R01. Available from: http://dx.doi.org/10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L-B, Ding G-R, Li K-C, Wang X-W, Zhou Y, Zhou Y-C, et al. The role of protein kinase C in the opening of blood-brain barrier induced by electromagnetic pulse [Internet]. Elsevier Ireland Ltd Toxicology. 2010;273:29–34. doi: 10.1016/j.tox.2010.04.013. Available from: http://dx.doi.org/10.1016/j.tox.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Degos JD, Dreyfus PA. Peripheral injections of Freund's adjuvant in mice provoke leakage of serum proteins through the blood-brain barrier without inducing reactive gliosis [Internet] Brain Res. 1999;832:84–96. doi: 10.1016/s0006-8993(99)01479-1. Available from: http://dx.doi.org/10.1016/S0006-8993(99)01479-1. [DOI] [PubMed] [Google Scholar]
- Rinaldi PC, Jones JP, Reines F, Price LR. Modification by focused ultrasound pulses of electrically evoked responses from an in vitro hippocampal preparation [Internet] Brain Res. 1991 Aug.558(1):36–42. doi: 10.1016/0006-8993(91)90711-4. Available from: http://dx.doi. org/10.1016/0006-8993(91)90711-4. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor [Internet] J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7673356. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment [Internet] Eur. J. Neurosci. 2006;24(7):1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. Available from: http://dx.doi.org/10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Saija A, Princi P, Trombetta D, Lanza M, De Pasquale A. Changes in the permeability of the blood-brain barrier following sodium dodecyl sulphate administration in the rat [Internet] Exp. Brain Res. 1997;115(3):546–551. doi: 10.1007/pl00005725. Available from: http://dx.doi.org/10.1007/PL00005725. [DOI] [PubMed] [Google Scholar]
- Salahuddin TS, Johansson BB, Kalimo H, Olsson Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: an electron microscopic study [Internet] Acta Neuropathol. 1988;77(1):5–13. doi: 10.1007/BF00688236. Available from: http://dx.doi.org/10.1007/BF00688236. [DOI] [PubMed] [Google Scholar]
- Samiotaki G, Vlachos F, Tung Y-S, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn. Reson Med. 2012;67(3):769–777. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanovich E, Bartus RT, Friden PM, Dean RL, Le HQ, Brightman MW. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7 [Internet] Brain Res. 1995;705:125–135. doi: 10.1016/0006-8993(95)01143-9. Available from: http://dx.doi.org/10.1016/0006-8993(95)01143-9. [DOI] [PubMed] [Google Scholar]
- Scarcelli T, Jordão JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice [Internet]. Elsevier Inc. Brain Stimul. 2014;7(2):304–307. doi: 10.1016/j.brs.2013.12.012. Available from: http://dx.doi.org/10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats [Internet] Exp. Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. Available from: http://dx.doi.org/10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schilling L, Wahl M. Opening of the blood-brain barrier during cortical superfusion with histamine [Internet] Brain Res. 1994;653:289–296. doi: 10.1016/0006-8993(94)90403-0. Available from: http://dx.doi.org/10.1016/0006-8993(94)90403-0. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer's disease [Internet]. Elsevier Inc. Am. J. Pathol. 2011;179(3):1373–1384. doi: 10.1016/j.ajpath.2011.05.047. Available from: http://dx.doi.org/10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy CN, Crafts D. Selective alteration of the blood-brain barrier [Internet] J. Neurosurg. 1965;23(5):484–487. doi: 10.3171/jns.1965.23.5.0484. Available from: http://dx.doi.org/10.3171/jns.1965.23.5.0484. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier [Internet] Ultrasound Med. Biol. 2006;32(9):1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium [Internet] Ultrasound Med. Biol. 2008;34(7):1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles [Internet] Ultrasound Med. Biol. 2004 Jul.30(7):979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Sun J, Hynynen K. The potential of transskull ultrasound therapy and surgery using the maximum Available skull surface area [Internet] J. Acoust. Soc. Am. 1999;105(4):2519. doi: 10.1121/1.426863. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=765236/nhttp://scitation.aip.org/content/asa/journal/jasa/105/4/10.1121/1.426863. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hiroyuki K, Sakurada T, Tadano T, Kisara K. Intracranial injection of thyrotropin releasing hormone (TRH) suppresses starvation-induced feeding and drinking in rats [Internet] Pharmacol. Biochem. Behav. 1982;17:249–253. doi: 10.1016/0091-3057(82)90078-8. Available from: http://dx.doi.org/10.1016/0091-3057(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Sztriha L, Betz AL. Oleic acid reversibly opens the blood-brain barrier [Internet] Brain Res. 1991;550(2):257–262. doi: 10.1016/0006-8993(91)91326-v. Available from: http://dx.doi.org/10.1016/0006-8993(91)91326-V. [DOI] [PubMed] [Google Scholar]
- Tabatabaei SN, Girouard H, Carret A-S, Martel S. Remote control of the permeability of the blood-brain barrier by magnetic heating of nanoparticles: a proof of concept for brain drug delivery [Internet] J. Control Release. 2015 Feb 24;206:49–57. doi: 10.1016/j.jconrel.2015.02.027. Available from: http://dx.doi.org/10.1016/j.jconrel.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Tempany CMC, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226:897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- Thévenot E, Jordão JF, O'Reilly MA, Markham K, Weng Y-Q, Foust KD, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound [Internet] Hum. Gene Ther. 2012;23(11):1144–1155. doi: 10.1089/hum.2012.013. Available from: http://dx.doi.org/10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration [Internet] Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. Available from: http://dx.doi.org/10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Ting C-Y, Fan C-H, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, et al. Concurrent bloodebrain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment [Internet]. Elsevier Ltd Biomaterials. 2012;33(2):704–712. doi: 10.1016/j.biomaterials.2011.09.096. Available from: http://dx. doi.org/10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012;38(10):1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound [Internet] Int. J. Cancer. 2007 Aug 15;121(4):901–907. doi: 10.1002/ijc.22732. Available from: http://dx.doi.org/10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimualtes intact brain circuits [Internet] Neuron. 2010 Jun.66(5):681–694. doi: 10.1016/j.neuron.2010.05.008. Available from: http://dx.doi.org/10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Tuladhar A, Morshead CM, Shoichet MS. Circumventing the blood–-brain barrier: local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain [Internet] J. Control Release. 2015 Oct.215:1–11. doi: 10.1016/j.jconrel.2015.07.023. Available from: http://dx.doi.org/10.1016/j.jconrel.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Tung YS, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation detection during ultrasound-induced blood- brain barrier opening [Internet] Phys. Med. Biol. 2010;55:6141–6155. doi: 10.1088/0031-9155/55/20/007. Available from: http://dx.doi.org/10.1109/ULTSYM.2010.5935953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound [Internet] PLoS One. 2008 Oct.3(10):e3511. doi: 10.1371/journal.pone.0003511. Available from: http://dx.doi.org/10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Tomonaga T, Kim H, Nakano M, Shoji S, Nagata Y, et al. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer [Internet]. Elsevier Ltd J. Urol. 2014;193(1):103–110. doi: 10.1016/j.juro.2014.07.096. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25079940. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo [Internet] J. Cereb. Blood Flow. Metab. 1984;4(4):574–585. doi: 10.1038/jcbfm.1984.82. Available from: http://dx.doi.org/10.1038/jcbfm.1984.82. [DOI] [PubMed] [Google Scholar]
- VanBavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications [Internet] Prog. Biophys. Mol. Biol. 2007;93(1–3):374–383. doi: 10.1016/j.pbiomolbio.2006.07.017. Available from: http://dx.doi.org/10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood-brain barrier [Internet]. Elsevier Inc. Virology. 2009;385(2):425–433. doi: 10.1016/j.virol.2008.11.047. Available from: http://dx.doi.org/10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos F, Tung Y-S, Konofagou EE. Permeability assessment of the focused ultrasound-induced blood-brain barrier opening using dynamic contrast-enhanced MRI [Internet] Phys. Med. Biol. 2010;55(18):5451–5466. doi: 10.1088/0031-9155/55/18/012. Available from: http://dx.doi.org/10.1088/0031-9155/55/18/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos F, Tung YS, Konofagou E. Permeability dependence study of the focused ultrasound-induced blood-brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI [Internet] Magn. Reson Med. 2011;66(3):821–830. doi: 10.1002/mrm.22848. Available from: http://dx.doi.org/10.1002/mrm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo [Internet] Ultrasound Med. Biol. 1995;21(7):969–979. doi: 10.1016/0301-5629(95)00038-s. Available from: http://dx.doi.org/10.1016/0301-5629(95)00038-S. [DOI] [PubMed] [Google Scholar]
- Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus [Internet]. Nature Publishing Group Gene Ther. 2015;22(1):104–110. doi: 10.1038/gt.2014.91. Available from: http://dx.doi.org/10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei KC, Chu PC, Wang HYJ, Huang CY, Chen PY, Tsai HC, et al. Focused ultrasound-induced blood-brain barrier opening to enhance Temozolomide delivery for glioblastoma treatment: a preclinical study [Internet] PLoS One. 2013;8(3):e58995. doi: 10.1371/journal.pone.0058995. Available from: http://dx.doi.org/10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Ab in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage [Internet] J. Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. Available from: http://dx.doi.org/10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Theoretical study on shear stress generated by microstreaming surrounding contrast agents attached to living cells [Internet] Ultrasound Med. Biol. 2002;28(1):125–129. doi: 10.1016/s0301-5629(01)00497-5. Available from: http://dx.doi.org/10.1016/S0301-5629(01)00497-5. [DOI] [PubMed] [Google Scholar]
- Yang FY, Lin YL, Chou FI, Lin YC, Liu YWH, Chang LW, et al. Pharmacokinetics of BPA in gliomas with ultrasound induced blood-brain barrier disruption as measured by microdialysis [Internet] PLoS One. 2014;9(6):e100104. doi: 10.1371/journal.pone.0100104. Available from: http://dx.doi.org/10.1371/journal.pone.0100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FY, Wang HE, Liu RS, Teng MC, Li JJ, Lu M, et al. Pharmacokinetic analysis of 111In-labeled liposomal Doxorubicin in murine glioblastoma after blood-brain barrier disruption by focused ultrasound [Internet] PLoS One. 2012a;7(9):e45468. doi: 10.1371/journal.pone.0045468. Available from: http://dx.doi.org/10.1371/journal.pone.0045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FY, Fu W-M, Yang R-S, Liou H-C, Kang K-H, Lin W-L. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption [Internet] Ultrasound Med. Biol. 2007;33(9):1421–1427. doi: 10.1016/j.ultrasmedbio.2007.04.006. Available from: http://dx.doi.org/10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Yang FY, Liu S-H, Ho F-M, Chang C-H. Effect of ultrasound contrast agent dose on the duration of focused-ultrasound-induced blood-brain barrier disruption [Internet] J. Acoust. Soc. Am. 2009;126(6):3344–3349. doi: 10.1121/1.3242376. Available from: http://dx.doi.org/10.1121/1.3242376. [DOI] [PubMed] [Google Scholar]
- Yang XY, Huang CC, Kan QM, Li Y, Liu D, Zhang XC, et al. Calcium regulates caveolin-1 expression at the transcriptional level [Internet]. Elsevier Inc. Biochem. Biophys. Res. Commun. 2012b;426(3):334–341. doi: 10.1016/j.bbrc.2012.08.079. Available from: http://dx.doi.org/10.1016/j.bbrc.2012.08.079. [DOI] [PubMed] [Google Scholar]
- Ye J, Tsukamoto T, Sun A, Nigam SK. A role for intracellular calcium in tight junction reassembly after ATP depletion-repletion [Internet] Am. J. Physiol. 1999;277:F524–F532. doi: 10.1152/ajprenal.1999.277.4.F524. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10516276. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xia C, Xue Y, Liu Y. Synergistic effect of low-frequency ultrasound and low-dose bradykinin on increasing permeability of the blood-tumor barrier by opening tight junction [Internet] J. Neurosci. Res. 2009;87(10):2282–2289. doi: 10.1002/jnr.22061. Available from: http://dx.doi.org/10.1002/jnr.22061. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders [Internet] Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. Available from: http://dx.doi.org/10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]