Abstract
The evolutionary advantages to the suppression of pain during a stressful event (stress-induced analgesia (SIA)) are obvious, yet the reasoning behind sex-differences in the expression of this pain reduction are not. The different ways in which males and females integrate physiological stress responses and descending pain inhibition are unclear. A potential supraspinal modulator of stress-induced analgesia is the central nucleus of the amygdala (CeA). This limbic brain region is involved in both the processing of stress and pain; the CeA is anatomically and molecularly linked to regions of the hypothalamic pituitary adrenal (HPA) axis and descending pain network. The CeA exhibits sex-based differences in response to stress and pain that may differentially induce SIA in males and females. Here, sex-based differences in behavioral and molecular indices of SIA were examined following noxious stimulation. Acute restraint stress in male and female mice was performed prior to intraplantar injections of formalin, a noxious inflammatory agent. Spontaneous pain-like behaviors were measured for 60 min following formalin injection and mechanical hypersensitivity was evaluated 120 and 180 min post-injection. Restraint stress altered formalin-induced spontaneous behaviors in male and female mice and formalin-induced mechanical hypersensitivity in male mice. To assess molecular indices of SIA, tissue samples from the CeA and blood samples were collected at the 180 min time point. Restraint stress prevented formalin-induced increases in extracellular signal regulated kinase 2 (ERK2) phosphorylation in the male CeA, but no changes associated with pERK2 were seen with formalin or restraint in females. Sex differences were also seen in plasma corticosterone concentrations 180 min post injection. These results demonstrate sex-based differences in behavioral, molecular, and hormonal indices of acute stress in mice that extend for 180 min after stress and noxious stimulation.
Keywords: sex differences, stress-induced analgesia, acute restraint, pain, central nucleus of the amygdala
Graphical Abstract
1. Introduction
Although pain has evolved as a defensive response to noxious stimuli, the suppression of pain during stressful events is evolutionarily advantageous and known as stress-induced analgesia (SIA). SIA is generated through supraspinal integration of the physiological stress response and descending pain inhibition. The amygdala is a limbic brain region involved in both of these processes, and thus a potential modulator of SIA. Signaling molecules localized in the central nucleus of the amygdala (CeA) provide direct evidence for this specific region’s link to the underlying mechanisms of SIA; stimulus-induced expression of corticotropin-releasing factor (CRF)[1] and phosphorylated extracellular signal regulated kinase 2 (pERK2)[2] couple the CeA to the stress response and pain modulation, respectively. Additional anatomical evidence comes from CeA projections to CRF-rich regions of the hypothalamus[3, 4], linking it to the HPA axis, and heterogeneous CeA projections through the periaqueductal gray (PAG)[5] to the rostral ventromedial medulla (RVM)[6] linking it to descending pain transmission.
Interestingly, the CeA exhibits sex-based differences in response to stress and pain. For instance, basal levels of CRF in the CeA vary between the sexes[7] and psychological stress and foot shock differentially regulate expression of this hormone in male and female rats[8]. In the context of pain, men exhibit increased functional connectivity between the amygdala and PAG as compared to women[9]. Additionally, localized injections of female sex hormones in the amygdala alter pain-like responses to visceral stimulation in rats[10]. Taken together, these data suggest that sex-dependent variability in amygdaloid processing of pain and stress may differentially induce SIA in males and females.
In this paper, we evaluated sex-based differences in behavioral and molecular indices of SIA. Specifically, we performed acute restraint stress in male and female mice prior to intraplantar injections of formalin, a noxious inflammatory agent. At various time points following injection, we observed pain-like behaviors, quantified circulating stress hormones, and analyzed ERK activation in the CeA. We hypothesized that females would exhibit more robust SIA since they have higher basal levels of the stress hormone corticosterone[11], greater variability in hormone responses to stress[12], and increased pain sensitivity[13].
2. Materials and Methods
2.1 Animal care
All protocols were done in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at Duquesne University, Pittsburgh, PA (Protocol Number: 1412-16). Male and female C57Bl/6J mice aged 9–12 weeks were used for all experiments. Animals were housed on a 12 hr light/dark cycle (7am – 7pm) with ad libitum access to rodent chow and water.
2.2 Behavioral testing
Only one sex was tested at a time to avoid odorant cues influencing testing. All cohorts were tested in the same room and time of day over the course of one week. Mice were placed in 25 × 25 × 35 cm ventilated Plexiglas enclosures on a wire mesh rack and habituated for at least two hr with background white noise. Male and female experimenters who were blinded to restraint stress condition performed all additional behavioral testing. All experimenters spent at least 30 min in the testing room prior to any behavioral assay to account for experimenter effects on pain-like behavior[14].
2.2.1 Acute restraint stress
Mice were restrained to induce stress before receiving a formalin paw injection (see below). Mice were restrained for 30 min in a 50 mL plastic conical tube fitted with air holes and a stopper so animals were not able to fully turn around in the tube; non-restrained control mice remained in Plexiglas enclosures. Restraint stress has been used for decades to investigate the neurobiological, behavioral, and clinical aspects of stress on the development and expression of numerous disorders[15]; it produces a significant stress response without causing physical injury to the animal. All mice were then allowed a 15-min grooming period before further testing.
2.2.2 Spontaneous formalin behavior
As previously described[16], spontaneous behaviors following intraplantar formalin injection were measured. Animals were injected subcutaneously in the plantar surface of the right hind paw with 10 μL of 2% formalin in saline (Sigma, St. Louis, MO). Restrained and control mice were videotaped (Logitech Pro 9000) following intradermal formalin injection and analyzed for nociceptive behaviors (defined as licking, lifting, and flinching of the injected paw) in five min bins for 60 min following formalin injection. The first phase of spontaneous behavior was defined as 0–10 min after injection and the second phase of testing was defined as 10–60 min after injection. The entire period was also analyzed from 0–60 min using an area under the curve analysis to determine the presence or absence of sex differences in this assay.
2.2.3 von Frey mechanosensory assessment
All behavioral testing occurred between 8 am and 3pm. von Frey filaments (North Coast Medical, San Jose, CA;[17]) were used to evaluate hind paw mechanical sensitivity. As previously described[16], mechanical testing consisted of applying von Frey filaments to the left and right hind paws until bent at approximately 30 degrees for no longer than 2 sec. If the animal removed its paw before this time, it was recorded as a withdrawal. Each filament, beginning with the smallest force filament and increasing in force thereafter, was applied five times. The mechanical threshold was determined as the smallest filament that evoked a withdrawal response in at least three of the five trials. Three to five baseline withdrawal thresholds were averaged for each hind paw. One day following baseline testing, mice were again habituated in Plexiglas enclosures. After two hr, mice were subjected to restraint stress (or control, as described above), allowed to groom for 15 min, and then injected with formalin (as described above). Mechanical sensitivity was measured 120 and 180 min following formalin in both the formalin-injected paw and the contralateral (uninjected) paw.
2.3 Blood and tissue collection
Mice were habituated as described in Plexiglas enclosures for at least two hr with background white noise; tissue and blood collection occurred between 12pm and 3pm. Restraint and formalin (or saline) injections were performed as described and then mice were returned to their Plexiglas enclosures and remained undisturbed until 180 min post-injection. At the 180 min time point, animals were transferred one at a time to another room for sacrificing. The 180 min time point was chosen for analysis because this is the time at which the CeA modulates mechanical hypersensitivity[16, 18]. Animals were sacrificed via decapitation and trunk blood was immediately collected through heparinized capillary tubes and stored on ice. Brains were isolated and cut into 1mm thick coronal sections. Using the Paxinos and Franklin brain atlas[19], 1mm punches containing the CeA were collected from the left and right hemispheres. All tissue samples were immediately stored on dry ice and then stored at −80° C until analysis. An experimenter blinded to restraint stress condition performed all molecular analyses.
2.3.1 Western blot analysis
As previously described[20], levels of ERK1/2 and pERK1/2 were analyzed in the CeA via Western analysis. All CeA samples were homogenized with ice-cold homogenization buffer (20 mM Tris, 1.5 mM EDTA, 1 mM Na4P2O7, 25 mg/mL aprotinin, 25 mg/mL leupeptin, 1X Sigma phosphatase inhibitors II and III, 100 mM PMSF), and then evaluated for total protein content using a BCA protein assay kit (Thermo Scientific, Rockford IL). 12 ug of protein from each CeA sample were separated on a 12.5% SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. Membranes were incubated in Odyssey blocking buffer for one hour and then incubated with mouse anti-pERK1/2 (Cell Signaling, 1:1,000) and rabbit anti-ERK1/2 (Cell Signaling, 1:1,000) primary antibodies for one hour. Blots were washed and rinsed with TBS with 0.1% Tween-20 (TTBS), then incubated with goat anti-mouse AlexaFluor 680 (Invitrogen, 1:20,000) and goat anti-rabbit IR 800 (Rockland, 1:20,000) secondary antibodies for one hr. Blots were rinsed with TTBS then scanned on an Odyssey Fc imaging system. Using Image Studio Lite (version 4.0) software, band densitometry was assessed for ERK1, ERK2, pERK1, and pERK2. Phosphorylated isoforms were normalized to total ERK for data analysis.
2.3.2 Corticosterone assay
Plasma corticosterone was assessed three hr after paw injection or at an equivalent time of day for naïve control mice. Trunk blood samples stored on ice were centrifuged for 15 min at 3,500 rpm at 4° C. The supernatant plasma was pipetted into 0.5 mL microcentrifuge tubes and stored at −80° C. Corticosterone blood concentrations were measured by Corticosterone Enzyme Immunoassay kit (Arbor Assay’s DetectX®) and compared to corticosterone standards.
2.4 Statistical analysis
All statistical analyses were performed with GraphPad Prism 5 software (GraphPad, La Jolla, CA). One-way or Two-way ANOVAs were used to determine main effects, while Tukey’s multiple comparison analyses or Bonferroni post hoc tests were, respectively, performed when a significant main effect was observed. All results are graphed as means +/− standard error mean. A p-value < 0.05 was considered statistically significant for all comparisons.
3. Results
3.1 Restraint stress alters formalin-induced spontaneous behavior in male and female mice
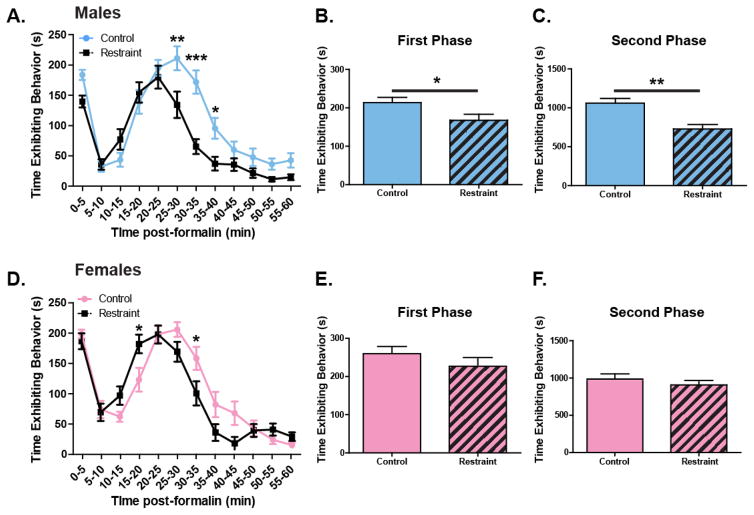
In order to examine the effects of restraint stress on formalin-induced spontaneous pain-like behaviors, male and female mice received an intraplantar injection of 2% formalin and the time spent exhibiting pain-like behaviors was evaluated over the course of the following 60 min. Both restrained (n=18) and non-restrained (n=18) males exhibited biphasic response curves (Figure 1A). Restrained mice spent significantly less time exhibiting spontaneous pain-like behaviors compared to non-restrained male animals (Figure 1A; Two-way ANOVA, effect of restraint p = 0.0003; effect of time, p<0.0001; effect of restraint x time, p<0.0001; Bonferroni post-test: 25–30 min, p<0.01; 30–35min, p<0.001; 35–40min, p<0.05). Restrained male mice specifically displayed fewer pain-like behaviors during both the first phase (Figure 1B; unpaired t-test: p=0.036) and the second phase of the test (Figure 1C; unpaired t-test: p=0.0004).
Figure 1. Acute restraint stress alters spontaneous pain-like behaviors in male and female mice following formalin injection.
Following 30 min of restraint and a 15 min grooming period male (n=18) and female (n=17) mice received an injection of 2% formalin in the right rear paw. The amount of time animals spent exhibiting pain-like behaviors were totaled in 5 min bins over the course of 60 min. Both restrained and non-restrained males exhibited traditional biphasic response curves, however restrained animals displayed significantly fewer pain-like behaviors, particularly 25–40 min following injection (A. Two-way ANOVA: effect of restraint, p=0003; effect of time, p<0.0001; effect of restraint x time, p<0.0001; Bonferroni post-test: 25–30min, ** p<0.001; 30–35min, ***p<0.001; 35–40min, *p<0.05). When each phase was analyzed individually, there was a significant effect of acute stress on pain during both the peripherally mediated first phase (B. unpaired t-test: p=0.036) and the centrally mediated second phase (C. unpaired t-test: p=0.0004). Both restrained and non-restrained females exhibited biphasic response curves following formalin treatment. There was a significant interaction between restraint and time (D. Two-way ANOVA: effect of restraint, p>0.05; effect of time, p<0.0001; effect of restraint x time, p=0.0001; Bonferroni post-test: 15–20min, * p<0.05; 30–35′, *p<0.05). When each phase was assessed individually, restraint had no effect in neither the first phase (E. unpaired t-test: p<0.05), nor during the second phase (F. unpaired t-test: p>0.05).
Similar to male mice, restrained (n=17) and non-restrained (n=17) females exhibited biphasic response curves following formalin treatment (Figure 1D). Restraint had a subtly different role in modulating pain-like behavior in female mice; stress had no overall effect on pain-like behavior in females when analyzing all time bins (Two-way ANOVA: effect of restraint, p=0.182; Figure 1D) but there was a significant interaction between restraint and time (Two-way ANOVA: restraint x time, p<0.0001; Bonferroni post-test: 15–20min, * p<0.05; 30–35′, *p<0.05). When each phase was assessed individually, restraint failed to decrease pain-like behaviors during the first or the second phase (Figure 1E–F). Comparing male to female mice for the entire 0–60 min period (male control = 1275.4 ± 67.9 sec; male restraint = 897.5 ± 65.6 sec; female control = 1247.0 ± 67.7 sec; female restraint = 1128.6 ± 71.6 sec), there was a significant main effect of restraint but no significant effect of sex or interaction between restraint and sex (Two-way ANOVA: stress, p=0.0005; sex, p=0.14; restraint x sex, p=0.062).
3.2 Restraint stress decreases formalin-induced mechanical hypersensitivity in male but not female mice
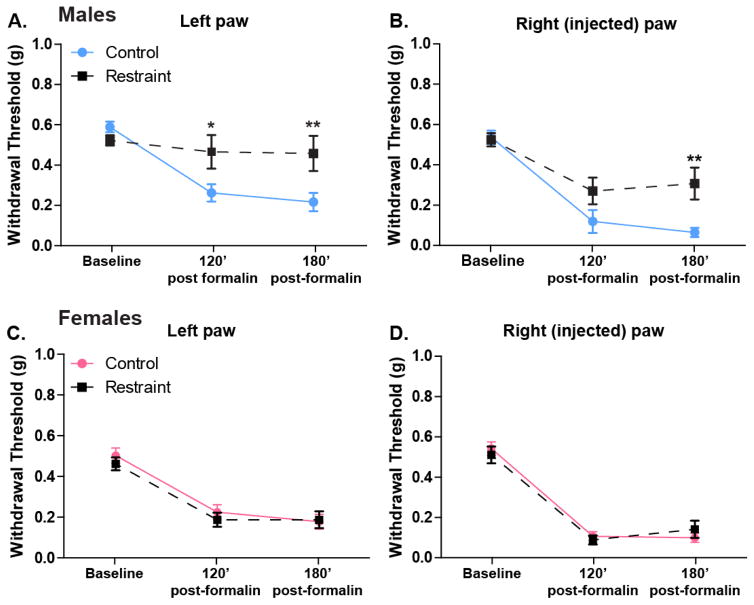
In order to examine the effects of restraint stress on formalin-induced mechanical hypersensitivity, male and female mice were restrained or were not handled prior to receiving a formalin paw injection. The non-restrained control male mice (n=18) exhibited hypersensitivity in both paws at 120 and 180 min following injection compared to baseline (Two-way ANOVA, effect of time p < 0.0001; Bonferroni post-test, 120 vs baseline p < 0.001, 180 vs baseline p < 0.001; Figure 2A–B). Consistent with the SIA seen in the spontaneous formalin test, restrained males (n=17) exhibited decreased hypersensitivity (i.e. increased withdrawal thresholds) to mechanical stimulation in the injected paw at the 180 min timepoint (Figure 2B; Two-way ANOVA, main effect of restraint p=0.026; Bonferroni post-test restraint vs. control p<0.01) and decreased hypersensitivity in the non-injected paw at both the 120 and 180 min timepoints (Figures 2A; Two-way ANOVA, p=0.063; Bonferroni post-test restraint vs. control at 120 min p<0.05 and 180 min p<0.01).
Figure 2. Acute restraint stress reduces formalin-induced paw hypersensitivity in male mice only.
Following 30 min of restraint (or control habituation) and a 15 min grooming period male (n=17–18) and female (n=18) mice received an injection of 2% formalin in the rear right paw. Similar to previous reports, non-restrained male mice developed hypersensitivity in both the non-injected (A. Two-way ANOVA: effect of time, p<0.0001) and injected (B. Two-way ANOVA: effect of time, p<0.0001) paws that was maintained at 120 (Bonferroni’s post-test: left and right paws, control baseline vs. 120′, p<0.001) and 180 min (Bonferroni’s post-test: left and right paws, control baseline vs. 180′, p<0.001) following treatment. Mice that had been restrained prior to injection demonstrated a significant decrease in mechanical hypersensitivity in both the non-injected (A. Two-way ANOVA: effect of restraint, p=0.06; Bonferroni’s post-test: 120′ control vs. restraint, *p<0.05; 180′ control vs. restraint **p<0.01) and injected (B. Two-way ANOVA: effect of restraint, p<0.05; Bonferroni’s post-test: 180′ control vs. restraint, **p<0.01) paws. Following 2% formalin injection, non-restrained female mice also demonstrated hypersensitivity in both non-injected (C. Two-way ANOVA: effect of time, p<0.0001) and injected (D. Two-way ANOVA: effect of time, p<0.0001) paws that was maintained at 2 and 3 hours following treatment (Bonferroni’s post-test: left and right paws, control baseline vs. 120′, p<0.001; control baseline vs 180′, p<0.001). However unlike their male counterparts, restrained females did not exhibit reduced increased withdrawal thresholds in either the non-injected (C. Two-way ANOVA: effect of restraint, p>0.05) or injected (D. Two-way ANOVA: effect of restraint, p>0.05) paws.
As observed in male mice, non-restrained control female mice (n=18) exhibited mechanical hypersensitivity in both the left and right paws 120 and 180 min after formalin injection compared to baseline (Two-way ANOVA, effect of time p< 0.0001; Bonferroni post-test, 120 vs baseline p<0.001, 180 vs baseline p< 0.001; Figures 2C, D). In contrast to their male counterparts however, stressed female animals (n=18) did not exhibit decreased mechanical hypersensitivity following formalin injection in either the injected (Two-way ANOVA, effect of stress p=0.935; Figure 2D) or non-injected paw (Two-way ANOVA, effect of stress p=0.590; Figure 2C) when compared to non-restrained control females.
3.3 Restraint stress in males prevents formalin-induced ERK2 phosphorylation in the CeA
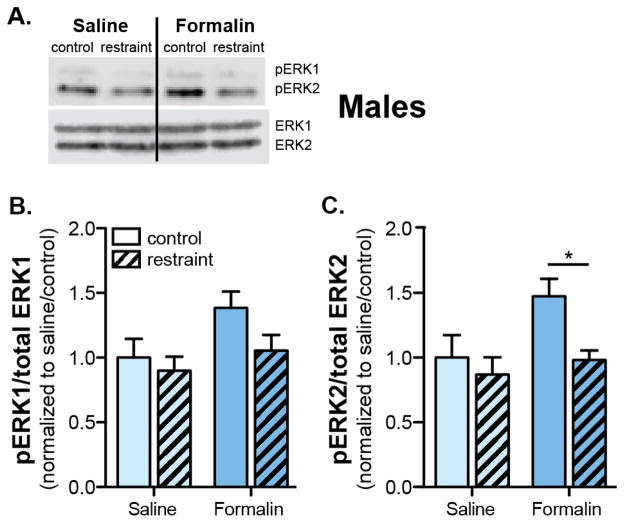
The individual and composite effects of formalin and restraint on the phosphorylation status of CeA ERK1/2 were investigated via Western blot analysis (Figure 3A). In males, both pERK1 and pERK2 expression were increased in the CeA 180 min following formalin injection (Two-way ANOVA main effect of formalin: pERK1, p=0.047; pERK2, p=0.041; Figures 3B–C). Restraint stress specifically affected ERK2, and not ERK1, phosphorylation status (Two-way ANOVA effect of restraint, p=0.031; Figure 3C); 180 min post-formalin injection, restrained, formalin-injected males expressed significantly less pERK2 compared to non-restrained, formalin-injected males (Bonferroni post-test, formalin control vs formalin restraint p<0.05; Figure 3C). These fluctuations in ERK2 phosphorylation mirror the behavioral observations in which non-restrained males demonstrated formalin-induced mechanical hypersensitivity while restrained males showed reduced mechanical hypersensitivity.
Figure 3. Acute restraint stress prevents formalin-induced increases in pERK2 in the CeA of male mice.
Following restraint (or control habituation), male mice received an injection of either 2% formalin (n=6) or sterile saline (n=6) in the rear right paw. Three hours following injection, animals were sacrificed and CeA tissue was isolated. Using Western blotting techniques, (A.) the CeA was assessed for expression of pERK1/2, well characterized pain signaling molecules, and total ERK1/2. B. Formalin treatment caused a significant increase in pERK1 (Two-way ANOVA: effect of formalin, p<0.05) three hours following injection. Pre-injection restraint stress failed to block the formalin-induced increase in pERK1 (Two-way ANOVA: effect of restraint, p>0.05) and acute restraint stress did not affect pERK1 levels in saline treated animals (Bonferroni post-test: formalin control vs. restraint, p>0.05). C. Formalin treatment caused a significant increase in pERK2 (Two-way ANOVA: effect of formalin, p<0.05) three hours following injection. Pre-injection restraint stress however, blocked the formalin-induced increase in pERK2 expression (Two-way ANOVA: effect of restraint, p<0.05; Bonferroni post-test: formalin control vs. restraint, *p<0.05). Acute restraint stress did not affect pERK2 levels in saline treated animals (Bonferroni post-test: formalin control vs. restraint, p>0.05).
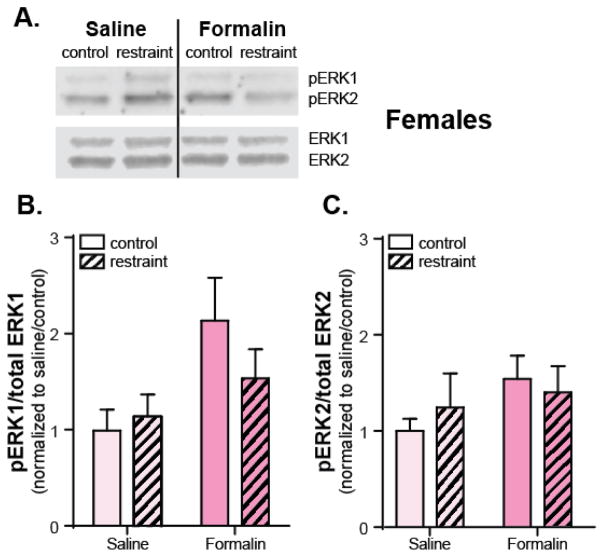
Western blot analysis was repeated in female mice to investigate if restraint stress and formalin injection had the same molecular effects (Figure 4A). Complementing the mechanical hypersensitivity observed following injection, formalin overall had a significant effect on the phosphorylation status of ERK1 in the CeA of female mice (Two-way ANOVA main effect of formalin: pERK1, p=0.019; pERK2, p=0.189; Figures 4B–C). Consistent with behavioral similarities three hours after formalin injection, there was no effect of restraint stress on either pERK1 or pERK2 expression in the CeA (Two-way ANOVA main effect of restraint: pERK1, p=0.475; pERK2, p=0.839; Figures 4B–C).
Figure 4. Formalin injection fails to increase pERK2 expression in the CeA of female mice.
Following restraint (or control habituation), female mice received an injection of either 2% formalin (n=12) or sterile saline (n=12) in the rear right paw. Three hours following injections, animals were sacrificed and CeA tissue was isolated. Using Western blotting (A.), the CeA was assessed for expression of pERK1/2 and ERK1/2. B. Similar to male mice, female mice exhibited formalin-dependent increases in pERK1 that were not blocked by acute restraint stress (Two-way ANOVA: effect of formalin, p=0.019; restraint, p>0.05). C. Unlike their male counterparts however, female mice failed to demonstrate increases in pERK2 following peripheral inflammation (Two-way ANOVA: effect of formalin and restraint, p>0.05).
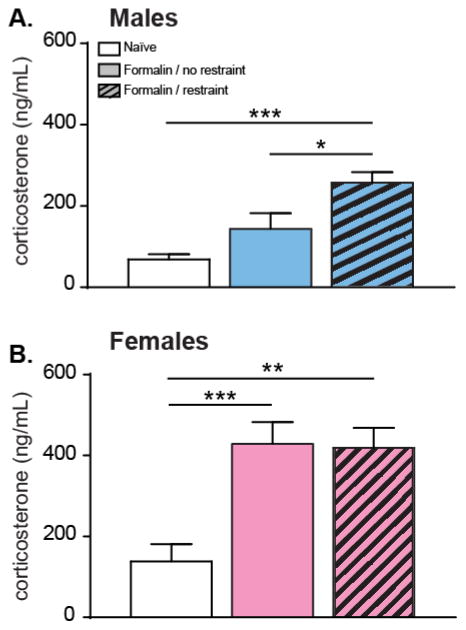
3.4 Male and female mice exhibit different levels of corticosterone 180 min post-formalin injection when restrained
In order to investigate the effects of restraint and formalin on corticosterone levels, blood samples were collected 180 min post-formalin in restrained and non-restrained groups, and from naïve mice of each sex matched for time of day. A one-way ANOVA revealed a significant overall main effect of treatment in male mice (p=0.002; Figure 5A). Additionally, multiple comparison tests revealed that male mice restrained and injected with formalin have significantly higher levels of corticosterone 180 min post injection compared to naïve and non-restrained formalin-injected males (p<0.01 and p<0.05, respectively; Figure 5A). The effects of restraint and formalin on corticosterone levels in female mice were investigated. A one-way ANOVA revealed a significant overall main effect of treatment (p < 0.001; Figure 5B), with naïve females having significantly lower levels of corticosterone compared to restrained formalin-injected and non-restrained formalin-injected females (p <0.01 and p< 0.001, respectively; Figure 5B). However, restrained formalin-injected females corticosterone concentrations 180 min post-injection were not significantly different compared to non-restrained formalin-injected females (p>0.05; Figure 5B).
Figure 5. Acute restraint stress exacerbates formalin-induced increases in corticosterone in males.
Three hours following injection, circulating levels of corticosterone were assessed in naïve male mice (n=6), male mice that had been restrained for 30 min prior to receiving a 2% formalin injection (n=8), and male mice that had not been restrained prior to injection (n=8). Restrained/injected mice had significantly more corticosterone in serum than both the non-restrained/injected mice and naïve animals that were neither restrained nor injected (A. One-way ANOVA: p<0.01; Bonferroni’s post-test: naïve vs. formalin/restraint, ***p<0.001; formalin/no restraint vs. formalin/restraint, *p<0.05). Formalin injection increased circulating corticosterone levels to the same extent in non-restrained (n=13) and restrained females (n=13) (B. One-way ANOVA: p<0.001; Bonferroni’s post-test: naïve vs. formalin/no restrain, ***p<0.001; naïve vs. formalin/restraint, **p<0.005) when compared to naive animals (n=11).
4. Discussion
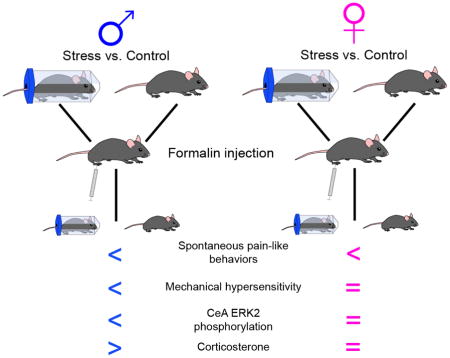
Here, we demonstrate sex-based differences in behavioral, molecular, and hormonal indices of acute SIA in mice that extend for 180 min after noxious stimulation. Behaviorally, both male and female mice exhibited fewer formalin-induced spontaneous pain-like responses following 30 min of restraint stress (Figure 1A, 1D). While the pattern of spontaneous behavior between male and female mice varied (male mice demonstrated a significant effect of restraint in the first and second phase and females having no significant effects in the first and second phase), an area under the curve analysis for the full 60 minutes of the assay did not reveal a significant sex difference between male and female mice. On the other hand, 180 minutes after formalin injection, only stressed male mice, and not their female counterparts, demonstrated decreased mechanical hypersensitivity.
Since SIA is largely a supraspinally-mediated phenomenon, we investigated sex-based molecular changes in the CeA, a region of the brain that mediates formalin-induced behavioral changes through GPCR and ERK2 signaling[21]. 180 min following formalin injection, non-restrained control male mice exhibited increased expression of pERK2 in the CeA (Figure 3C) but formalin failed to have a significant effect on pERK2 in female mice (Figure 4E). Complementing the behavioral data, restraint stress blocked pain-induced increases of ERK2 phosphorylation in males (Figure 3C). In addition to pain, the CeA is also linked to HPA-axis stress mediation via glucocorticoid signaling[3]. We evaluated circulating corticosterone levels and discovered that combined acute restraint stress and formalin injection significantly elevated corticosterone in male mice, while female mice showed equivalent corticosterone increases after formalin with or without restraint.
In these experiments, acute restraint stress altered pain-like behaviors in male and female mice during the entire 0–60 minute assay of the formalin test. The formalin test is a reliable rodent model of inflammatory nociception[22]. Nocifensive responses generated during the first phase are thought to be dependent on peripheral mechanisms while the second phase of testing are thought to be dependent upon central sensitization of dorsal horn neurons in the spinal cord[22]. Our data suggest that acute restraint stress may be altering nociceptive-related activity in both the periphery and in the spinal cord. This central SIA effect has previously been observed following footshock, another acute stressor. Acute footshock altered spontaneous activity and pain-evoked responses of dorsal horn neurons in intact preparations, but had no effect in spinalized preparations, suggesting a supraspinal mechanism of action[23]. It is likely that following either restraint or footshock, stress-induced activation of the hypothalamus or CeA alters activity in RVM neurons that project to these pain-mediating cells in the spinal cord[24].
In addition to measuring spontaneous formalin-induced behaviors, we also assessed mechanical hypersensitivity in both the injected and non-injected paws using von Frey filaments. Non-restrained control male and female mice demonstrated formalin-induced mechanical hypersensitivity in both paws following formalin treatment. Animals exhibited pronounced hypersensitivity in the injured paw and reduced but significant hypersensitivity in the contralateral paw. This contralateral hypersensitivity has been previously reported and may be a result of spinal cord sensitization or changes higher brain centers and the descending pain system[2, 17]. Although mechanical hypersensitivity after formalin has been observed in female rats[25], to our knowledge, our data are the first demonstration of this pain-like response in female mice at 180 min.
Restraint stress decreased mechanical hypersensitivity (i.e. increased withdrawal thresholds) in both the injected and non-injected paws of male mice. We again predict that these behavioral changes are a result of activated supraspinal analgesic systems that ultimately depress primary afferent transmission at the level of the spinal cord. The extended duration of SIA in our report is noteworthy. In previous experiments using uninjured animals, forced cold water swims induced analgesia that lasted 60 min when assessed via tail-pinch and 120 min via thermal plantar assay. In the current experiments, the combined effects of ongoing formalin-induced pain and acute stress may be engaging nociceptive systems and sustaining this activity which, in the presence of only one stimulus, would gradually diminish over time. While we did not find SIA induced changes in mechanical sensitivity in females at 120 or 180 min after formalin, it is possible that reduced hypersensitivity might be seen at earlier time points since stress did alter early spontaneous behavior in females.
We next examined pERK expression levels in the CeA to determine if sex- and stress-specific changes in this signaling cascade matched observed behavioral differences. We found that formalin injection increased phosphorylation of both ERK1 and ERK2 in the male CeA; acute restraint stress prevented formalin-induced phosphorylation of only ERK2. Although the trend observed for a reversal of formalin-induced ERK1 activation prevents us from making strong conclusions related to the two ERK isoforms, these data do suggest differences of ERK1 and ERK2 in male SIA. These isoform-specific differences are further illustrated by two findings from female mice. First, similar to male mice, formalin treatment had a significant effect on ERK1 phosphorylation in females with a trend for a reversal of the formalin effect with restraint. Second, in contrast to male mice, no effect of formalin or restraint was seen on ERK2 in female mice. Notably, other pain modalities have also failed to increase pERK1/2 expression in the female CeA; noxious bladder distension in this sex failed to induce CeA ERK1 or ERK2 phosphorylation [28]. Thus, the activation of the two pERK isoforms is sex- and pain model-dependent, suggesting a dynamic and complex role for the two isoforms of pERK in the context of pain and stress.
These data sets in male and female mice present a complicated picture that will require future studies to fully understand. Nonetheless, we reason that the sex differences observed here in ERK2 activation in the amygdala after formalin are primarily responsible for behavioral differences observed in mechanical hypersensitivity after restraint. This is not the first report of functional differences between the ERK isoforms; recent studies using global and promoter-driven knockouts of ERK2 have uncovered some of the independent and redundant functions of ERK1 and ERK2 in nociceptive signaling. Global knockouts reveal that in the CNS, ERK2 is the dominant isoform and is required for inflammation-induced behavior sensitization[26]. Deletion of ERK2 in Nav1.8-expressing sensory neurons (i.e. nociceptors) reveals that this isoform is also required for inflammation-induced behavioral sensitization in the periphery[27]. Previous data have shown that ERK2 (but not ERK1) is activated in males 180 minutes following intraplantar formalin injection and that pharmacologic activation of ERK in the CeA induces mechanical hypersensitivity[2]. Furthermore, inhibition of ERK phosphorylation in the CeA of male mice reverses formalin-induced mechanical hypersensitivity; these results relied on U0126, which prevents phosphorylation of both ERK isoforms[2]. We found that restraint only partially reversed formalin-induced hypersensitivity in male mice leaving open the possibility that ERK1 activation maintains some level of hypersensitivity in males and contributes to formalin-induced hypersensitivity in females. Overall, signaling differences in the ERK2 phosphorylation pathway in the CeA may play different roles between the sexes in regulating formalin-induced mechanical hypersensitivity but technical limitations in the ability to functionally target ERK1 versus ERK2 activation in the CeA leave open the possibility of ERK1 playing a partial role in CeA-dependent mechanical sensitivity changes in both male and female mice.
In addition to changes in descending analgesic systems, we also predicted that restraint stress would affect HPA axis activity. To assess this, we analyzed circulating corticosterone levels 180 min following formalin injection in animals that had been restrained and animals that had not been restrained, or in naïve animals which were neither restrained nor injected. Restrained/formalin-injected males had significantly more circulating corticosterone than both non-restrained/injected males and naïve males. Previous experiments have demonstrated that formalin injection and restraint stress independently increase corticosterone levels, but these increases gradually return to baseline approximately 90 min later in rats[29]. Our observation of increased corticosterone 180 min post-injection suggest an augmented HPA axis response caused by the synergistic effects of stress and pain. Consistent with higher basal HPA axis drive in females, naïve female mice had demonstrably more corticosterone than males[29]. Formalin injection increased corticosterone levels compared to naïve female levels. However, no differences were observed in the increased corticosterone between non-restrained/injected and restrained/injected female mice. The lack of a CeA effect of corticosterone in females may be due to consistently high levels of corticosterone under naïve conditions (compared to male naïve levels), which may saturate CeA GRs and subsequently dampen any corticosterone effect on ERK2 phosphorylation or actually prevent ERK2 phosphorylation after formalin in the first place. This suggests that restraint stress does not increase corticosterone levels to a greater extent than formalin alone in female mice.
Increased HPA axis activity in restrained/injected males relative to naïve and non-restrained/injected males could be directly or indirectly mediating SIA. Direct removal of the pituitary gland or adrenal cortex in male rats blocks SIA in the cold-water swim model[30] and formalin test[31], respectively. In a similar fashion, direct administration of corticosterone in adrenalectomized male rats restores SIA[31]. These data support the SIA-accompanying increases of corticosterone observed in this report. Indirect HPA axis modulation of SIA may be a result of glucocorticoid signaling in the CeA. Corticosterone, which is synthesized in the adrenal cortex, binds to glucocorticoid receptors (GR), which are expressed in many regions throughout the brain including the CeA[32]. Corticosterone-GR binding induces expression of CRF in the CeA[33, 34]. CRF can then bind to CRF receptor 1 (CRF1) or 2 (CRF2), which, if expressed in the anterior pituitary, drive HPA axis activity, or, if expressed in the CeA, modulate nociceptive synaptic plasticity. These two receptors have opposite nociceptive functions at the level of the CeA; CRF1 is pro-nociceptive, while CRF2 is antinociceptive[35]. CRF2 and CRF1 binding may be responsible for SIA and stress-induced hyperalgesia (SIH), a phenomenon often experienced by chronic pain sufferers following acute stress, respectively. Although the exact mechanisms of SIA and SIH are unknown, it is possible that CRF2 and CRF1 regulation of ERK signaling may be involved[35].
In this report, the data support an indirect/CeA mechanism of HPA axis SIA in mechanical sensitivity where enhanced corticosterone binding in the CeA during restraint blunts ERK2 activation leading to reduced bilateral mechanical hypersensitivity. A direct peripheral anti-inflammatory effect of corticosterone would have likely reduced mechanical sensitivity in both male and female mice and would have had a pain suppressing effect only in the inflamed right hind paw. Instead, we only found persistent SIA in male mice in both the formalin-injected and central-sensitized non-injected left paw. Together, the aforementioned male data combined with corticosterone failing to have an effect in the female CeA or on behavior support an indirect/CeA mechanism of HPA axis SIA in mechanical sensitivity in male mice.
While our data demonstrate interesting differences between SIA in male and female mice, the ultimate mechanisms of this sex difference are unknown. One might expect estrous cycling to play a role in these sex-based differences. There is mounting evidence however, that estrous cycle is not a factor in pain research[36–38]. Comprehensive analyses of large data sets have determined that there is no overall effect of cycle on pain sensitivity and further suggest that when randomly cycling animals are used, they will counteract any cycle confounds[37, 38]. Estrous cycling also has no effect on basal corticosterone levels in female rats, however it does affect stress-induced release of corticosterone; 20 minutes of restraint stress augments corticosterone release when rats are in proestrous[39]. However, cycle-dependent increases in corticosterone last only for 10 minutes following restraint; therefore we do not anticipate that our corticosterone measurements were affected by cycle differences.
5.1 Conclusions
Here, we report sex-based differences in SIA generated by acute restraint stress. Male mice exhibit robust SIA in mechanical hypersensitivity following intraplantar formalin injections that is sustained for several hours. We attribute these sex-based differences to two different supraspinal mechanisms: relative increased activity of the male HPA axis coupled with decreased nociceptive signaling of ERK2 within the male CeA. Overall, these results suggest that sex-based differences in behavior may be attributed to the interplay of hormonal and molecular factors that span both nociceptive and stress-related regions of the brain.
Highlights (optional; uploaded separately).
Restraint stress modulates spontaneous formalin behavior in both male and female mice
Restraint stress decreases formalin-induced mechanical hypersensitivity only in male mice
Restraint in males prevents formalin-induced ERK2 phosphorylation in the CeA
Corticosterone levels differ in male and female mice 180 min post-formalin injection
Acknowledgments
We would like to thank Dr. Robert Gereau IV (Washington University in St. Louis) for experimental design support. We would like to acknowledge funding for this research from the International Association for the Study of Pain Scan|Design Foundation (BJK), the National Institutes of Health (NIDDK F31DK104538 (KES); NICCIH R15AT008060 (BJK), R25DA032519 (PI Dr. Jeffrey Madura; supported CCL)), Swarthmore College (CCL), and the Duquesne University Pain Undergraduate Research Experience fellowship (CCL).
Abbreviations
- CeA
central nucleus of the amygdala
- CORT
corticosterone
- CRF
corticotropin releasing factor
- CRF1 receptor
corticotropin releasing factor receptor 1
- CRF2 receptor
corticotropin releasing factor receptor 2
- ERK
extracellular signal-regulated kinase
- GR
glucocorticoid receptor
- HPA axis
hypothalamic pituitary adrenal axis
- PAG
periaqueductal grey
- SIA
tress-induced analgesia
- RVM
rostral ventromedial medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–80. doi: 10.1016/s0006-3223(99)00164-x. Epub 1999/11/24. [DOI] [PubMed] [Google Scholar]
- 2.Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. Journal of Neuroscience. 2007;27(7):1543–51. doi: 10.1523/jneurosci.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(33):12004–9. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prewitt CMF, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. Journal of Chemical Neuroanatomy. 1998;15(3):173–85. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 5.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303(1):121–31. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140(2):376–86. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49(2):197–205. doi: 10.1016/j.yhbeh.2005.06.005. Epub 2005/08/09. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Linnman C, Beucke J-C, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. PAIN. 2012;153(2):444–54. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers B, Schulkin J, Greenwood-Van Meerveld B. Sex Steroids Localized to the Amygdala Increase Pain Responses to Visceral Stimulation in Rats. The Journal of Pain. 2011;12(4):486–94. doi: 10.1016/j.jpain.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer C, Basler HD, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. Clinical Journal of Pain. 2003;19(4):233–9. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bulls HW, Freeman EL, Anderson AJB, Robbins MT, Ness TJ, Goodin BR. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. Journal of Pain Research. 2015;8:311–9. doi: 10.2147/jpr.s84607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature methods. 2014;11(6):629–32. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 15.Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neuroscience and Biobehavioral Reviews. 2009;33(7):1089–98. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, et al. Activation of Metabotropic Glutamate Receptor 5 in the Amygdala Modulates Pain-Like Behavior. Journal of Neuroscience. 2010;30(24):8203–13. doi: 10.1523/JNEUROSCI.1216-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. Journal of Pain. 2001;2(1):2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- 18.Carrasquillo Y, Gereau RWt. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27(7):1543–51. doi: 10.1523/JNEUROSCI.3536-06.2007. Epub 2007/02/16. 27/7/1543 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Franklin KBJ. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. 4. Amsterdam: Boston: Elsevier/Academic Press; 2013. [Google Scholar]
- 20.Sadler KE, Stratton JM, DeBerry JJ, Kolber BJ. Optimization of a Pain Model: Effects of Body Temperature and Anesthesia on Bladder Nociception in Mice. Plos One. 2013;8(11) doi: 10.1371/journal.pone.0079617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, et al. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci. 2010;30(24):8203–13. doi: 10.1523/jneurosci.1216-10.2010. Epub 2010/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 23.Robbins MT, DeBerry J, Randich A, Ness TJ. Footshock stress differentially affects responses of two subpopulations of spinal dorsal horn neurons to urinary bladder distension in rats. Brain research. 2011;1386:118–26. doi: 10.1016/j.brainres.2011.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palazzo E, Marabese I, Soukupova M, Luongo L, Boccella S, Giordano C, et al. Metabotropic glutamate receptor subtype 8 in the amygdala modulates thermal threshold, neurotransmitter release, and rostral ventromedial medulla cell activity in inflammatory pain. J Neurosci. 2011;31(12):4687–97. doi: 10.1523/jneurosci.2938-10.2011. Epub 2011/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godinez-Chaparro B, Lopez-Santillan FJ, Orduna P, Granados-Soto V. Secondary mechanical allodynia and hyperalgesia depend on descending facilitation mediated by spinal 5-HT(4), 5-HT(6) and 5-HT(7) receptors. Neuroscience. 2012;222:379–91. doi: 10.1016/j.neuroscience.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RWt. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci. 2010;30(34):11537–47. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien DE, Alter BJ, Satomoto M, Morgan CD, Davidson S, Vogt SK, et al. ERK2 Alone Drives Inflammatory Pain But Cooperates with ERK1 in Sensory Neuron Survival. The Journal of Neuroscience. 2015;35(25):9491–507. doi: 10.1523/jneurosci.4404-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crock LW, Kolber BJ, Morgan CD, Sadler KE, Vogt SK, Bruchas MR, et al. Central Amygdala Metabotropic Glutamate Receptor 5 in the Modulation of Visceral Pain. Journal of Neuroscience. 2012;32(41):14217–26. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloisi AM, Ceccarelli M, Lupo C. Behavioural and hormonal effects of restraint stress on the formalin test in male and female rats. European Journal of Neuroscience. 1998;10:81. doi: 10.1016/s0361-9230(98)00063-x. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar RJ, Kelly DD, Glusman M. Stress-induced analgesia - time course of pain reflex alterations following cold water swims. Bulletin of the Psychonomic Society. 1978;11(6):333–6. [Google Scholar]
- 31.Fukuda T, Nishimoto C, Miyabe M, Toyooka H. Unilateral adrenalectomy attenuates hemorrhagic shock-induced analgesia in rats. Journal of Anesthesia. 2007;21(3):348–53. doi: 10.1007/s00540-007-0521-2. [DOI] [PubMed] [Google Scholar]
- 32.Geuze E, van Wingen GA, van Zuiden M, Rademaker AR, Vermetten E, Kavelaars A, et al. Glucocorticoid receptor number predicts increase in amygdala activity after severe stress. Psychoneuroendocrinology. 2012;37(11):1837–44. doi: 10.1016/j.psyneuen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Tran L, Greenwood-Van Meerveld B. Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behavioural Brain Research. 2012;234(2):380–5. doi: 10.1016/j.bbr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone messenger-rna in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Research. 1994;640(1–2):105–12. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 35.Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. Journal of Neurophysiology. 2008;99(3):1201–12. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- 36.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience. 2015;18(8):1081-+. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117(1–2):1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous-cycle in the rat. Endocrinology. 1991;129(5):2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]