Abstract
Mice are attracted to the tastes of sugar and maltodextrin solutions. Sugar taste is mediated by the T1R2/T1R3 sweet taste receptor, while maltodextrin taste is dependent upon a different as yet unidentified receptor. In a prior study sweet-sensitive C57BL/6J (B6) mice displayed similar preferences for sucrose and maltodextrin solutions in 24-h saccharide vs. water choice tests that exceeded those of sweet-subsensitive 129P3/J (129) mice. In a subsequent experiment reported here, sucrose and maltodextrin (Polycose) preference and acceptance were compared in the two strains in saccharide vs. saccharide choice tests with isocaloric concentrations (0.5 – 32%). The 129 mice displayed significantly greater maltodextrin preferences than B6 mice at mid-range concentrations (2–8%), while the mice displayed an opposite preference profile at the highest concentration (32%). As in prior studies, 129 mice consumed less total saccharide than B6 mice at lower concentrations. These findings show that the conclusions reached from tastant vs. water tests may differ from those pitting one tastant against another. The increased sucrose preference and intake of B6 mice, relative to 129 mice, is consistent with their sweet-sensitive phenotype.
Keywords: Sweet taste, Polysaccharide taste, Post-oral conditioning
1. Introduction
The taste of sugar is highly attractive to humans and many other animal species. Studies of inbred mouse strains led to the identification of the T1R2 and T1R3 receptor proteins that dimerize to form a sweet taste receptor responsive to natural sugars and artificial sweeteners [14]. Selective elimination of the T1R2 and/or T1R3 receptors in knockout (KO) mice attenuates or completely blocks the behavioral response to sweeteners [4,44]. Sweetener preferences are also blocked by deletion of taste signaling elements downstream from the T1R2/T1R3 receptor, including a-gustducin, Trpm5, Calhm1, and P2X2/P2X3 [5,33,36,39].
In addition to sugars, rodents are strongly attracted to maltodextrins derived from partial hydrolysis of starch, exemplified by the commercial maltodextrin Polycose [24,29]. Rats prefer Polycose to the disaccharides sucrose and maltose and to the monosaccharides glucose and fructose at low concentrations [16,25–28]. This and other findings indicate that the palatability of Polycose is not explained by the small amount of free sugars (~ 9% glucose and maltose) contained in the maltodextrin. Rather, rodents appear to be highly attracted to maltooligosaccharides having ~ 4–8 glucose units [6,9,20,27]. Maltodextrin and sucrose have distinctive tastes to rodents as indicated by behavioral and electrophysiological studies, and recent data from KO mice confirm this distinction. Aversions conditioned to Polycose or sucrose, for example, do not cross generalize [17,23], and various taste inhibitors selectively reduce the electrophysiological response to sucrose and maltodextrin [23,38]. The gustatory receptor that mediates maltodextrin taste does not require the T1R2 or T1R3 receptor proteins, as demonstrated by tests of KO mice missing T1R2, T1R3, or both [40–42,45]. Yet, deletion of other taste signaling elements (gustducin, Trpm5, Calhm1, P2X2/P2X3) attenuates maltodextrin preferences just as it attenuates sugar preferences in mice [5,33,36,39]. These findings confirm that maltodextrin preference is mediated by the taste system but the identity of the taste receptor remains unknown.
Comparisons of inbred strains have revealed substantial differences in responsiveness to a variety of sweet tastants [3,8,10,13]. The mouse strains have been characterized as sweet “sensitive” and “subsensitive” based on their differential preferences for sweeteners at low concentrations. Consistent with behavioral findings, sucrose and saccharin stimulate greater neural activity in gustatory nerves of sweet sensitive than of subsensitive strains [7,11,15]. Genetic differences in the TasR1 gene coding for the T1R3 sweet receptor are implicated in the differential sweet taste sensitivity of inbred mouse strains [21]. Mouse strain differences in maltodextrin taste have been less extensively studied. Recently, Poole et al. [19] compared the avidity for maltodextrin and sucrose in eight inbred mouse strains. They reported that in brief-access tests CAST/EiJ and PWK/PhJ, unlike the other strains, licked less for maltodextrin than for sucrose. In 24-h 2-bottle saccharide vs. water tests the CAST and PWK mice drank less 4% maltodextrin than sucrose, whereas the other strains drank similar amounts of the two saccharides. Poole et al. [19] proposed that strain variations in maltodextrin preference can be exploited to reveal the gene(s) coding the maltodextrin taste receptor.
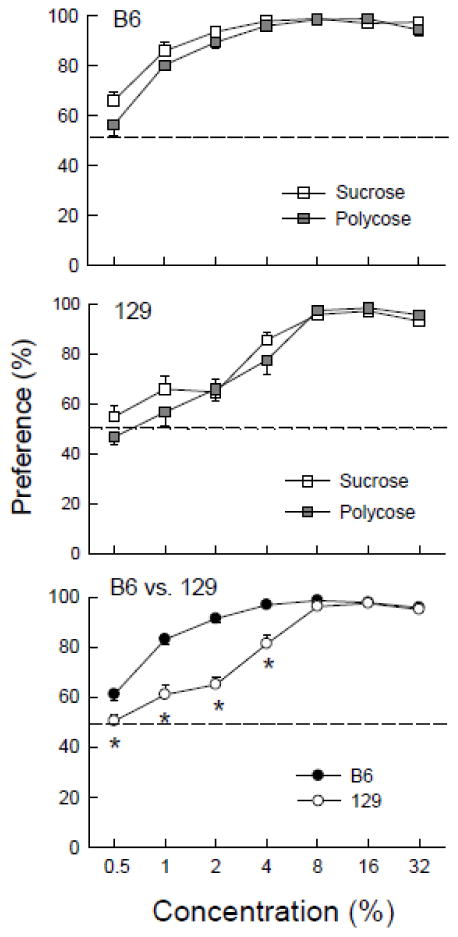
Among the strains tested by Poole et al. [19] that displayed similar preferences for 4% maltodextrin and sucrose were C57BL/6J (B6) and 129S1/SvlmJ (129) mice. However, this contrasts with early reports by Bachmanov et al. [2,3] that B6 mice have stronger preferences for dilute sucrose and maltodextrin solutions than do 129 mice (129/P3J). Sclafani [30] confirmed this finding in a subsequent study that revealed that B6 mice displayed nearly identical preferences for isocaloric sucrose and maltodextrin solutions that exceeded those of 129 mice at 0.5 to 4% concentrations (Figure 1). Based on these results, Sclafani [30] speculated that the T1R3 receptor, which is more sensitive to sweet tastants in B6 than 129 mice, may be a component of the hypothesized maltodextrin taste receptor. This idea was refuted by the discovery cited above that deletion of the T1R3 receptor greatly attenuates sweet but not maltodextrin preference in mice [40–42,45]. As reported in the present paper, the apparent similar within-strain preferences for sucrose and maltodextrin suggested by saccharide vs. water choice tests were not confirmed in two-bottle tests that gave the mice the choice between sucrose and maltodextrin. Although less common than tastant vs. water tests, direct choice tests between two tastants or nutrients provide a particularly sensitive measure of taste preferences.
Figure 1.
Mean (+/− SEM) saccharide preferences of C57BL/6J (B6) (top panel) and 129P3/J (129) (middle panel) mice in two-bottle tests with Polycose vs. water and sucrose vs. water in Sclafani [30]. Separate groups of B6 and 129 mice (n=10 each) without prior saccharide experience were given 2-day access to the 0.5 – 32% Polycose or sucrose solutions presented in an ascending order. Bottom panel: Mean (+/− SEM) saccharide preferences of the B6 vs. 129 mice. The percent saccharide preference data are the means of the B6 Polycose and sucrose groups and 129 Polycose and sucrose groups. Significant (P < 0.05) between-strain differences are indicated by an asterisk (*).
2. Methods
2.1. Subjects
Male C57BL/6J (n=10) and 129P3/J (n=10) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 7 weeks of age. The animals were housed in individual plastic tub cages in a room maintained at 22 degrees C with a 12:12 h light-dark cycle. Purina Chow (5001, PMI Nutrition International, Brentwood, MO) and, prior to carbohydrate testing, deionized water were available ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Taste Solutions and Intake Measures
Polycose (Ross Laboratories, Columbus, OH) and sucrose (Domino Foods, Inc., Yonkers, NY), solutions were prepared using deionized water. Polycose is a starch-derived maltodextrin containing 2% glucose, 7% maltose, and 91% glucose polymers, and has an average molecular weight of about 1000 [22]. Polycose and sucrose were presented at 0.5, 1, 2, 4, 8, 16 and 32% solutions by weight.
2.3. Procedure
The mice were adapted to the laboratory for 2 weeks. Water was available through two sipper spouts attached to 50-ml plastic tubes that were placed on top of the cage. The sipper spouts were inserted 7 mm into the cage through holes positioned 3.7 cm apart in a stainless-steel plate and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking tubes on an electronic balance interfaced to a laptop computer. Intakes were corrected for spillage estimated by recording the change in weight of two bottles placed on an empty cage. Following adaption, preference tests with Polycose vs. sucrose at 0.5 – 32% concentrations were conducted. The solutions were available 23 h/day and the bottles were weighed and refilled during the remaining 1 h. The solutions were presented in order of increasing concentration. Each concentration was presented for 2 days with the left-right position of the Polycose and sucrose alternated daily. The mice were not given water during the tests.
2.4. Statistical Analysis
Daily fluid intakes were averaged for each strain and the absolute intakes were evaluated using repeated measures analysis of variance (Strain x Concentration x Solution). Saccharide preferences were expressed as percent intakes (saccharide intake/total intake x 100). Significant interaction effects were evaluated using simple main effects tests according to Winer [43]. The significance of the solution preference at each concentration was evaluated for each strain using paired t-tests corrected for multiple comparisons using the Bonferroni procedure.
3. Results
Prior to testing, the mean body weights of the B6 and 129 mice were similar at 23.6 and 23.5 g, although the B6 consumed more water than did the 129 mice [6.0 vs. 5.0 g/day, t(18) = 3.05, p < 0.01]. Overall, the mice consumed more sucrose than Polycose [Solution F(1,18) = 22.36, P < 0.001], but the relative intakes of the two solutions varied in the B6 and 129 mice as a function of concentration [Strain x Solution x Concentration, F(6,108) = 13.23, P < 0.0001] (Figure 2).
Figure 2.
Mean (+/− SEM) saccharide intakes of C57BL/6J (B6) (top left) and 129P3/J (129) (bottom left) mice in the two-bottle test with isocaloric Polycose vs. sucrose solutions. Total solution intakes of the strains are shown in the top right panel. Polycose preference (bottom right) is expressed as the percentage of total intake for each strain. Significant (P < 0.05) differences between saccharides or strains are indicated by an asterisk (*).
The strains were compared in separate analyses of solution intakes. For Polycose, there were no main effects of strain or concentration, but a significant Strain x Concentration interaction [F(6,108) = 5.45, p < 0.0001] showed that B6 mice consumed less Polycose at 4 and 8%, and more at 32% than did 129 mice. In addition, Polycose intakes did not differ significantly by concentration in 129 mice. B6 mice consumed more sucrose than 129 mice [Strain F(1,18) = 11.0, p < 0.01]. Sucrose intake varied across the test [Concentration F(6,108) = 32.27, p < 0.0001]. Intakes were greatest at 8 and 16%, followed by 4 and 32%; the mice drank less of more dilute solutions, and intakes did not differ within the 0.5–2% concentration range. B6 mice consumed more sucrose at 2, 4 and 8%, and less at 32% than did 129 mice [Strain x Concentration F(6,108) = 14.0, p < 0.0001].
Solution intakes were also compared within strains (Figure 2, left panels). B6 mice consumed more sucrose than Polycose [F(1,9) = 45.0, p < 0.0001] and intake varied with concentration [F(6,54) = 72.3, p < 0.0001] with sucrose intake exceeding that of Polycose at 2–16% [Solution x Concentration, F(6,54) = 22.0, p < 0.0001]. For 129 mice, the main effect of Solution was not significant, but intake varied with Concentration [F(6,54) = 11.66, p < 0.0001] and the mice consumed more sucrose than Polycose at 16 and 32% [Solution x Concentration F(6,54) = 7.69, p < 0.0001].
Percent Polycose intake (Figure 2, lower right panel) was greater in 129 than B6 mice [F(1,18) = 10.49, p < 0.01] and varied with concentration [F(6,108) = 11.0, p < 0.0001]. The strains differed at intermediate and high concentrations: percent Polycose intake was greater in 129 mice at 2, 4, and 8% and less than that of B6 mice at 32% [Strain x Concentration F(6,108) = 19.2, p < 0.0001].
Analysis of total fluid intakes (Polycose + sucrose) showed that B6 mice consumed more than 129 mice [Strain F(1,18) = 10.14, p < 0.01] and totals varied with concentration [F(6,108) = 42.13, p < 0.0001] (Figure 2, top right panel). Total solution intakes were greatest at 8 and 16%, followed by 32%; the mice drank less of more dilute solutions, and intakes did not differ within the 0.5–4% concentration range. B6 total intakes exceeded those of 129 mice at the 4% and 8% concentrations [Strain x Concentration, F(6,108) = 6.46, p < 0.0001].
4. Discussion
The present findings provide a new perspective on the maltodextrin and sucrose preferences of B6 and 129 mice that has implications for the study of other mouse strains and tastants. Whereas prior studies using saccharide vs. water choice tests indicated that mice within each strain are equally attracted to the two saccharides, the present findings based on maltodextrin vs. sucrose choice tests revealed quite different strain preference profiles. In particular, the 129 mice displayed stronger maltodextrin preferences than B6 mice at intermediate isocaloric concentrations (2–8%). At higher concentrations, the two strains showed a reverse preference profile, which may be due in part to post-oral factors (see below). In contrast, the total saccharide intake data are consistent with previously established differences between these strains; B6 mice generally consume more at low and intermediate concentrations of saccharides than do 129 mice [2,3,13,30].
Responses to sucrose appeared dominant in both strains as concentration increased, though the thresholds for stimulation of sucrose intake were quite different; B6 mice began to increase sucrose intake at 2%, whereas 129 mice did not consume significantly more sucrose than Polycose until 16%. The sucrose intake pattern for both strains resembled those observed previously when the same range of concentrations was offered vs. water [30]. B6 mice consumed very little Polycose and more sucrose than 129 mice at the intermediate 4% and 8% concentrations. The preference profile of the two strains reversed, however at the highest concentration when the 129 mice consumed more sucrose than Polycose and the B6 mice consumed similar amounts of the two saccharides. One interpretation of these data is that the intake of B6 mice is more strongly driven by sweet taste than 129 mice at the lower concentrations, and they may be more sensitive than 129 mice to the differential post-oral actions of sucrose and Polycose at high concentrations, thus choosing more Polycose.
In addition to taste, sucrose and Polycose differ in two important respects. Polycose has a lower osmolarity than sucrose and the greater osmolarity of the concentrated sucrose solutions may have contributed to rapid decline in sugar intake by the B6 mice. Second, Polycose, being a glucose polymer, contains twice as much glucose as sucrose, a glucose-fructose disaccharide. The post-oral reinforcing actions of glucose are much more potent than those of fructose in B6 mice. In fact, unlike glucose, intragastric fructose infusions do not stimulate the intake of or preference for a flavored solution [35,46]. The post-oral reinforcing actions of fructose have not been evaluated in 129 mice, and it is possible that fructose has some post-oral reinforcing effect in 129 mice as in some other mouse strains (e.g., FVB, SWR) [12,37]. Consistent with this idea, orally-consumed fructose is more effective in conditioning a flavor preference in 129 mice than in B6 mice [18].
The Polycose vs. sucrose preference profile of the 129 mice is generally similar to that observed in Sprague-Dawley rats, which prefer Polycose to sucrose at low isocaloric concentrations, but sucrose to Polycose at high concentrations [25]. Yet the switch in preference observed with B6 mice from sucrose towards Polycose at the 32% concentration is consistent with the preference reversal displayed by rats with extended two-bottle testing with 32% solutions [1]. The rats’ switch in preference was attributed to the more potent post-oral glucose reinforcing effects of Polycose rather than its lower osmolarity because a similar switch in preferences was observed in testing 32% sucrose and 32% maltose (a glucose+glucose disaccharide) [1]. With extended two-bottle testing, B6 and 129 mice, like rats, also developed a significant preference for 32% Polycose over 32% sucrose (unpublished findings).
The lack of preference of the B6 and 129 mice for Polycose vs. sucrose at 0.5% and 1% could be taken as an inability to detect the saccharides at those concentrations, but other data do not support this idea. Measured thresholds for responding in ascending series tests vs. water have varied, but nonetheless show a clear difference between the strains in the lowest concentration that elicits greater intake than water. B6 mice preferred Polycose at 0.3–1%, whereas 129 mice did not show a preference until 2–3% [2,30]. The strains also differ in sucrose preference thresholds, with B6 again showing preferences at lower concentrations within studies (B6 0.5–2%, 129 1–8%) [3,13,30]. However, after developing strong preferences for concentrated sucrose and Polycose vs. water in ascending test series with 0.5 – 32% saccharide solutions, both 129 and B6 mice display strong preferences (>80%) when retested with 0.5% – 4% saccharide solutions [30,32]; this demonstrates that both strains can detect the saccharides at low concentrations. These findings, along with the preference reversal observed with 32% sucrose and Polycose solutions, document how post-oral experience can modify saccharide taste preferences. Note that the similar 4% sucrose and maltodextrin preferences reported by Poole et al. [19] for B6 and 129 mice are consistent with the strong preferences displayed by experienced B6 and 129 mice in earlier studies [32]. The mice in the Poole study were exposed to concentrated saccharide solutions in the brief-access lick tests, which can alter subsequent preferences [40]. The Poole study also used a different maltodextrin than that used in the present and prior studies (Maltrin M040 vs. Polycose) but this is not likely an important factor. Note that Polycose is no longer sold by Ross Laboratories, but a similar maltodextrin product, SolCarb (Solace Nutrition, Pawcatuck, CT) is available. B6 mice equally preferred SolCarb to Polycose in 2-day, 2-bottle tests at 2–32% concentrations (Sclafani, unpublished data).
Even when 129 mice show strong preferences for dilute saccharide solutions, they persist in consuming less than B6 mice [19,30]. In addition to sucrose and Polycose, experienced 129 mice show reduced intakes of dilute soybean oil emulsions compared to B6 mice [32]. Like Polycose, soybean preference and acceptance does not depend upon the T1R3 sweet receptor, suggesting that other genetic differences between 129 and B6 mice mediate these effects [34]. The weaker acceptance of palatable nutrients by 129 mice may represent a general reduced avidity for such tastants. A possible explanation for the lack of difference between 129 and B6 mice in their intake of concentrated nutrient solutions is that post-oral satiety effects limit intake in both strains. This interpretation is not supported, however, by results obtained with a progressive ratio lick task which minimizes post-oral satiation while testing motivation to consume. After prior experience with sucrose solutions, 129 mice licked as much for 4% sucrose and more for 16% sucrose than did B6 mice in the PR task [31]. It will be of interest to compare PR responding to maltodextrin in 129 and B6 mice. It may be that 129 mice are as motivated or more so to consume maltodextrin than are B6 mice even though they consume less solution in bottle tests.
A primary implication of the present results and those of our prior PR licking study is that conclusions about a strain’s attraction to a tastant depend on the testing conditions. It is not unexpected that one saccharide would be preferred to another when their taste receptors are different, or that strains with different sensitivity to sweet taste would differ in these tests. The greater relative preference for dilute Polycose solutions displayed by 129 mice in the sucrose vs. maltodextrin tests may reflect their reduced sensitivity to sweet taste. Why naive 129 mice display reduced preferences for maltodextrin and soybean oil solutions in nutrient vs. water tests, however, remains to be determined.
The taste receptor(s) that mediates the behavioral response to maltodextrins and other maltooligosaccharide preparations remains to be identified. The identification by Poole et al. [19] of inbred strains, such as CAST and PWK mice, that have particularly low maltodextrin lick rates in brief access tests and preferences in 24-h saccharide vs. water tests should prove useful in identifying the genes responsible for the maltodextrin receptor. The present findings suggest that saccharide vs. saccharide preference tests may be useful in further characterizing strain differences in maltodextrin taste.
Acknowledgments
This research was supported by grant DK31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Martin Zartarian for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Sucrose to Polycose preference shifts in rats: The role of taste, osmolality, and the fructose moiety. Physiol Behav. 1991;49:1049–1060. doi: 10.1016/0031-9384(91)90330-q. [DOI] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72:603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 5.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 6.Davis JD, Breslin PA. A behavioral analysis of the ingestion of glucose, maltose and maltooligosaccharide by rats. Physiol Behav. 2000;69:477–485. doi: 10.1016/s0031-9384(99)00265-6. [DOI] [PubMed] [Google Scholar]
- 7.Frank ME, Blizard DA. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol Behav. 1999;67:287–297. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 8.Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- 9.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, et al. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 10.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chem Senses. 2005;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft TT, Huang D, Lolier M, Warshaw D, LaMagna S, Natanova E, et al. BALB/c and SWR inbred mice differ in post-oral fructose appetition as revealed by sugar versus non-nutritive sweetener tests. Physiol Behav. 2016;153:64–69. doi: 10.1016/j.physbeh.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol. 2002;12:366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984;322:83–92. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- 16.Nissenbaum JW, Sclafani A. Sham-feeding response of rats to Polycose and sucrose. Neurosci Biobehav Rev. 1987;11:215–222. doi: 10.1016/s0149-7634(87)80029-5. [DOI] [PubMed] [Google Scholar]
- 17.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci Biobehav Rev. 1987;11:187–196. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- 18.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, et al. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2012;105:451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole RL, Aleman TR, Ellis HT, Tordoff MG. Maltodextrin acceptance and preference in eight mouse strains. Chem Senses. 2016;41:45–52. doi: 10.1093/chemse/bjv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez I. Glucose polymer taste is not unitary for rats. Physiol Behav. 1994;55:355–360. doi: 10.1016/0031-9384(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 21.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, et al. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross Laboratories. Polycose. Columbus, OH: Ross Laboratories; 2005. [Google Scholar]
- 23.Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 1994;56:741–745. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A. Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev. 1987;11:131–153. [PubMed] [Google Scholar]
- 25.Sclafani A, Clyne AE. Hedonic response of rats to polysaccharide and sugar solutions. Neurosci Biobehav Rev. 1987;11:173–180. doi: 10.1016/s0149-7634(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A, Hertwig H, Vigorito M, Feigin MB. Sex differences in polysaccharide and sugar preferences in rats. Neurosci Biobehav Rev. 1987;11:241–251. doi: 10.1016/s0149-7634(87)80032-5. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Hertwig H, Vigorito M, Sloan H, Kerzner B. Influence of saccharide length on polysaccharide appetite in the rat. Neurosci Biobehav Rev. 1987;11:197–200. doi: 10.1016/s0149-7634(87)80026-x. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Nissenbaum JW. Taste preference thresholds for Polycose, maltose, and sucrose in rats. Neurosci Biobehav Rev. 1987;11:181–185. doi: 10.1016/s0149-7634(87)80024-6. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A. The sixth taste? Appetite. 2004;43:1–3. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice strains after experience with these saccharides. Physiol Behav. 2006;87:745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 2006;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav. 2007;90:602–611. doi: 10.1016/j.physbeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of {alpha}-gustducin and Trpm5 taste signaling proteins. Am J Physiol. 2007;293:R1504–1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sclafani A, Glass D, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sclafani A, Marambaud P, Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 2014;126:25–29. doi: 10.1016/j.physbeh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: strain differences in post-oral sugar reward. Am J Physiol. 2014;307:R1448–R1457. doi: 10.1152/ajpregu.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somenarain L, Jakinovich JW. Antagonism of the gerbil’s sweetener and Polycose gustatory responses by copper chloride. Brain Res. 1990;522:83–89. doi: 10.1016/0006-8993(90)91580-a. [DOI] [PubMed] [Google Scholar]
- 39.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol. 2009;296:R855–865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treesukosol Y, Smith KR, Spector A. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci. 2011;31:13527–13534. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 2012;303:R218–235. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winer BJ. Statistical principles in experimental design. New York: McGraw Hill; 1962. [Google Scholar]
- 44.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 45.Zukerman S, Glendinning JI, Margolskee RM, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]