Abstract
In anautogenous mosquitoes, juvenile hormone III (JH) plays an essential role in female post-eclosion (PE) development, preparing them for subsequent blood feeding and egg growth. We re-examined the JH titer during the reproductive cycle of female Aedes aegypti mosquitoes. Using liquid chromatography coupled with triple tandem mass spectrometry (LC-MS/MS/MS), we have shown that it reaches its peak at 48–54 h PE in the female hemolymph and at 72 h PE in whole body extracts. This method represents an effective assay for determination of JH titers. The 2.1-kb 5’ promoter region of the Early Trypsin (ET) gene, which is specifically expressed in the female midgut under the control of JH during the PE phase, was utilized to genetically engineer the Ae. aegypti mosquito line with the ET-Gal4 activator. We then established the ET-GAL4>UAS-enhanced green fluorescent protein (EGFP) system in Ae. aegypti. In ET-Gal4>UAS-EGFP female mosquitoes, the intensity of the midgut-specific EGFP signal was observed to correspond to the ET gene transcript level and follow the JH titer during the PE phase. The EGFP signal and the EGFP transcript level were significantly diminished in midguts of transgenic female mosquitoes after RNA interference depletion of the JH receptor Methoprene-tolerant (Met), providing evidence of the control of ET gene expression by Met. Topical JH application caused premature enhancement of the EGFP signal and the EGFP transcript level in midguts of newly eclosed ET-Gal4>UAS-EGFP female mosquitoes, in which endogenous JH titer is still low. Hence, this novel ET-Gal4>UAS system permits JH-dependent gene overexpression in the midgut of Ae. aegypti female mosquitoes prior to a blood meal.
Keywords: Juvenile hormone, Early Trypsin, Methoprene-tolerant, Mosquito, Hormone action, Transgenesis
Graphical Abstract
1. Introduction
The yellow fever mosquito Aedes aegypti represents an important vector of human diseases, transmitting Dengue fever, Yellow fever, Chikungunya and Zika virus (Barrett and Higgs, 2007; Fauci and Morens, 2016; Tsetsarkin et al., 2016; Weaver et al., 2016). There is no specific drug or vaccine available against Zika virus or other Arboviral diseases (except for yellow fever). Thus, mosquito population control must be integrated into the long-term prevention from these mosquito-borne diseases. Investigation of mosquito biology that yields important information about mosquito-specific processes and genes could pave the way toward designing novel control approaches.
Juvenile hormones play critical roles in regulating female reproduction in insects (Raikhel et al., 2005). In most insect species, these hormones are principal actors controlling vitellogenesis and egg maturation. In female mosquitoes, juvenile hormone III (JH) is involved in preparation for these events, which are later activated by blood-meal-linked nutritional factors and 20-hydroxyecdysone (20E) (Attardo et al., 2005; Clements, 1992). During the first gonadotrophic cycle, adult female mosquitoes enter a post-eclosion (PE) phase that lasts approximately 72 h and is controlled by JH (Clements, 1992; Hagedorn et al., 1979). Shapiro et al. (1986) have reported that in Ae. aegypti females, the titer of JH III was high during the PE phase, reaching its maximum at 48–50 h, and declined rapidly after a blood meal. However, according to Hernandez-Martinez et al. (2015), the JH peak in these insects is at 12 h PE, coinciding with the peak of JH biosynthesis. We re-examined the JH titer in order using the advanced liquid chromatography-triple tandem mass spectrometry (LC-MS/MS/MS) to resolve this controversy.
JH promotes the growth of ovarian primary follicles and activates the oocytes to assemble an endocytic complex that is necessary for yolk protein uptake during oogenesis (Gwadz and Spielman, 1973; Raikhel and Lea, 1985). In the fat body, JH stimulates the accumulation of glycogen and lipid droplets and cellular remodeling that includes the buildup of biosynthetic machinery and ribosomes (Hou et al., 2015; Raikhel and Lea, 1990; Zou et al., 2013). In the gut, rough endoplasmic reticulum aggregates after eclosion and this can be blocked if the corpora allata (the source of JH) was surgically removed from newly emerged adult females (Rossignol et al., 1982). Genomic studies of the fat body using microarray and RNA-sequencing demonstrated that JH and its receptor Methoprene-tolerant (Met) differentially regulate a large cohort of genes during the PE phase (Hou et al., 2015; Zou et al., 2013).
In mosquitoes, hematophagy serves as a foundation for pathogen transmission. The gut functions as the site of blood digestion and the entry point for pathogens. Blood digestion requires specialized enzymes that are present in a posterior part of the female mosquito midgut (thereafter, gut). These digestive enzymes are predominantly represented by early- and late-phase serine proteases (SPs) (Brackney et al., 2010; Isoe et al., 2009). In Ae. aegypti, the genes encoding early SPs, early trypsin (ET) and chymotrypsin-like JHA15, are expressed before blood feeding during the PE phase (Bian et al., 2008; Noriega et al., 1996a; Noriega et al., 1996b). In contrast, late SPs—late trypsin, SPVI and SPVII—are maximally expressed at 24 h post blood meal (PBM) (Isoe et al., 2009). In the gut, JH has been implicated in the transcriptional control of early-phase SP genes (Bian et al., 2008; Li et al., 2011; Noriega et al., 1997). The translation of ET mRNA is regulated by the amino acid (AA)/target of rapamycin (TOR) pathway PBM (Brandon et al., 2008; Noriega et al., 1999), and the molecular basis of ET transcriptional control by JH is well established (Li et al., 2014; Li et al., 2011). The JH action is mediated by Met and its heteromeric partner FISC; together they bind to the 9-mer Met-binding motif in the ET upstream regulatory region and activate transcription (Li et al., 2014; Li et al., 2011).
Recently, on the basis of piggyBac transposon-mediated germ-line transformation, the binary Gal4/upstream activating sequence (UAS) system for transgene expression has been established in mosquitoes (Kokoza and Raikhel, 2011; Lynd and Lycett, 2012; Zhao et al., 2014). Utilization of the vitellogenin (Vg) gene for the fat body and the carboxypeptidase (CP) gene for the gut has permitted a female-specific expression in these tissues during the PBM phase. However, a transgenic system permitting gene overexpression during the JH-dependent PE phase was lacking. In this work, utilizing the ET gene promoter and the Gal4/UAS system, we have established the transgenic line with PE stage-, gut-, and female-specific enhanced green fluorescent protein (EGFP) expression. This novel ET-Gal4>UAS system permits JH-dependent gene overexpression in the midgut of female Ae. aegypti mosquitoes.
2. Materials and Methods
2.1. Mosquito strains
Wild type (WT) and transgenic mosquito strains were maintained under laboratory conditions as described previously (Kokoza and Raikhel, 2011). Adult mosquitoes were provided with 10% sucrose solution.
2.2. Plasmid construction
For construction of the pBac[ET-Gal4, 3×P3-EGFP afm] vector, the 2.1-kb promoter region of ET was PCR-amplified using ET promoter-specific primers from the wt DNA (Table S2). The resultant PCR product was purified, verified by sequencing, and subcloned into the pCR4-TOPO vector (ThermoFisher Scientific) to generate the pCR4-ET construct. Gal4-SV40 fragment, amplified using primers with SpeI and PmeI restriction sites, was incorporated into the pCR4-ET vector. Primers with the AscI site were used to amplify the ET-Gal4-SV40 cassette, and the resultant driver cassette was subcloned into the pBac[3×P3-EGFP afm] plasmid (Horn and Wimmer, 2000) at the AscI site to produce the ET-Gal4 driver vector pBac[ET-Gal4, 3×P3-EGFP afm].
2.3. Germ-line transformation of Ae. aegypti
A transgenic ET-Gal4 driver line was obtained by microinjection of a pBac[ET-Gal4, 3×P3-EGFP afm] transformation vector and phsp-pBac helper vector. Helper vector was used as previously described (Kokoza and Raikhel, 2011). Vectors used in injections were purified by EndoFree Plasmid Maxi Kit (QIAGEN). pBac[ET-Gal4, 3×P3-EGFP afm] (0.35 mg/ml) and helper (0.25 mg/ml) in 0.1 mM phosphate buffer (pH 6.8, containing 5 mM KCl) were microinjected into the Ae. aegypti pre-blastoderm embryos. The selection of transgenic lines and establishment of the ET-Gal4>UAS-EGFP line were performed following previously described procedures (Zhao et al., 2014).
2.4. Molecular analysis
DNA extraction, inverse polymerase chain reaction (PCR), RNA extraction, cDNA synthesis, and quantitative reverse-transcriptase PCR (qPCR) analysis were performed, as previously described (Wang et al., 2015; Zhao et al., 2014). Primers used for PCR and qPCR are listed in Table S2.
2.5. RNA interference (RNAi) knockdown
Double-stranded RNAs (dsRNAs) for Met and control luciferase were produced following the manufacturer’s protocol of MEGAscript T7 kit (Ambion). 0.3–0.5 µl dsRNA solution (4 µg/µl) was injected into 1-day-emerged female mosquitoes (PE). After injection, these mosquitoes were reared until guts were dissected for analyses. RNA interference (RNAi) depletion of luciferase (dsLuc) was used as a control.
2.6. Juvenile hormone topical application
Topical JH treatment was performed as previously described (Saha et al., 2016). A 0.3-µl aliquot of 1 µg/ml JH III (Sigma) or solvent (acetone) was applied topically to abdomens of newly eclosed female mosquitoes (3h PE). Guts were collected for photographic documentation or RNA analysis 8 h post treatment. Photography was performed using a Leica M165 FC microscope with LAS V4.0 software. For each treatment, images of ten guts were analyzed.
2.7. JH sample preparation
Sample preparation was followed as described previously (Chen et al., 2007; Hou et al., 2015; Westerlund and Hoffmann, 2004) with some modification. Briefly, whole body mosquito samples were homogenized with 400 µl 80% methanol and centrifuged for 10 min at 12000 g at 4 °C. The supernatant was transferred to a new siliconized glass tube containing 300 µl hexane; it was then vortexed vigorously and centrifuged for 10 min at 5000 g at 4 °C. The upper hexane layer was transferred to a new siliconized glass tube and dried by nitrogen. It was then re-suspended in 100 µl 50% methanol and sonicated for 5 min. The samples were centrifuged twice for 10 min at 14000 g at 4 °C, and the supernatant was stored at −80 ° C until analysis. JH III (Sigma) dilutions of 100, 50, 25, 10, 5, 1, 0.5, and 0.1 ng/ml in 50% methanol were used for calculation of the standard curve.
For hemolymph preparation, mosquito heads were removed, and bodies were placed down in QIA shredder (Qiagen) with 400 µl 80% methanol in collection tubes. They were centrifuged at 12000 g at 4 °C (Zou et al., 2010). The next steps of hexane extraction were performed as for whole body processing.
Samples of Ae. aegypti whole body homogenate and hemolymph were collected from groups of 15 or 30 female mosquitoes, respectively; four independent biological collections were used for analyses. The statistical significance between samples was defined by a p value < 0.05 using Duncan’s multiple test (prism 5.0). Bars represent ± SD.
2.8. LC-MS/MS/MS
Liquid chromatography (LC) was performed on a rapid resolution liquid chromatography system (Nexera UHPLC LC-30A, Shimadzu) equipped with two LC-30AD pumps, a SIL-30AC autosampler, a CTO-30A thermostatted column compartment and a DGU-20A5 degasser. An ACQUITY UPLC™ BEH C18 column (100 × 2.1 mm, 1.7 µm) was used for LC separation. The auto-sampler was set at 4 °C using gradient elution with 0.1% formic acid methanol as solvent A and 0.1% formic acid water as solvent B. The gradient program is as follows: 0–1 min 40% A, 1–7 min 40% A to 100 % A, 7–9 min 100% A, 9–9.1 min 100% A to 40% A, and 9.1–12 min 40% A. The flow rate was set at 0.2 ml/min, and the injection volume was 10 µl. The total run time was 12 min for each sample. The retention time of JH III was 7.07 min and the total run time - 12 min for each sample (Fig. S1).
Triple tandem mass spectrometry was performed on an AB SCIEX Triple Quad™ 4500 (Applied Biosystems) equipped with an electrospray ionization source (Turbo Ionspray). The mass spectrometry detection was operated in positive electrospray ionization mode. The [M+H] of analyst was selected as the precursor ion. The quantification parameter was set in the multiple-reaction-monitoring (MRM) approach using mass transitions (precursor ions/product ions), and ESI ion source temperature was set at 200 °C. Other mass spectrometric parameters were: curtain gas flow 10 psi; collisionally activated dissociation (CAD) gas setting medium; ion-spray voltage 5500 V; and ion gas 1 and 2 at 20 psi. Data acquisition and processing were performed using AB SCIEX Analyst 1.6 (Applied Biosystems).
3. Results
3.1. Dynamics of juvenile hormone titer during reproductive cycle
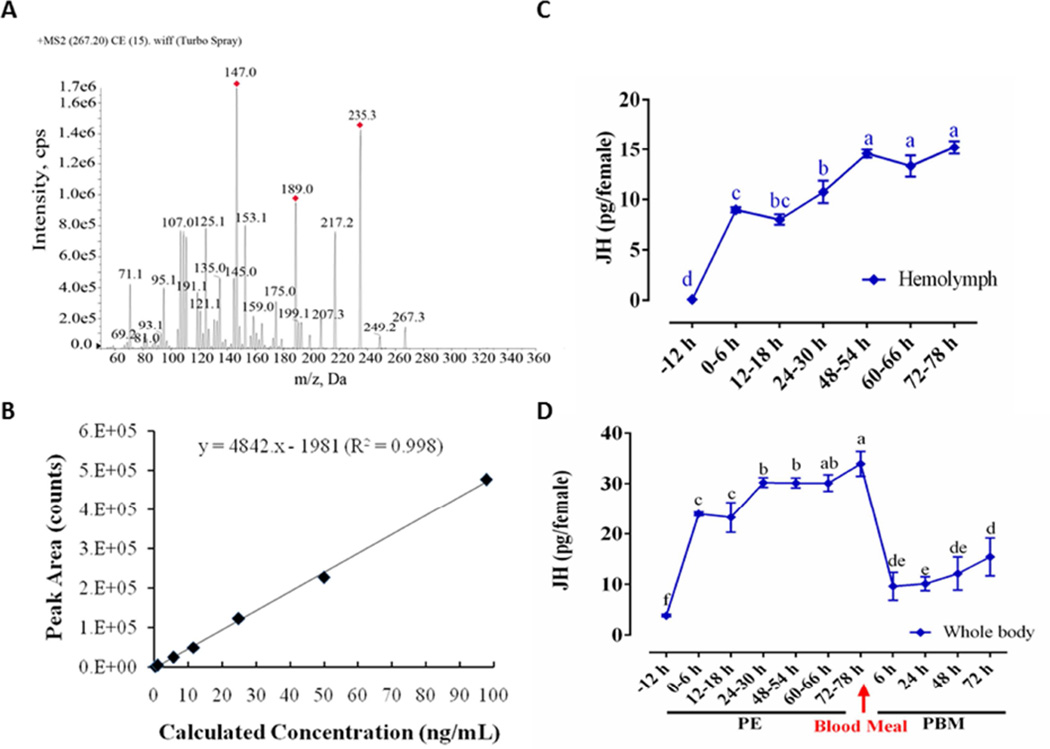
We measured JH titers in the hemolymph and whole bodies of Ae. aegypti female mosquitoes by means of liquid chromatography–coupled with triple tandem mass spectrometry (LC-MS/MS/MS). The precursor ion [M + H; m/z = 267.3] was detected by ESI-Triple-MS, which generated several product ions by collision-induced activation (Fig. 1A and B). Three major JH specific ions [m/z = 235.3, m/z = 189.0, m/z = 147.0] were found. The area of ion peaks was integrated to quantify the JH titer (Fig. 1A). According to the optimal mass condition of JHIII determination, the chromatogram of methoprene acid produced multiple peaks (Fig. S2), so we use standard curve instead of internal reference (Fig. 1B).
Figure 1.
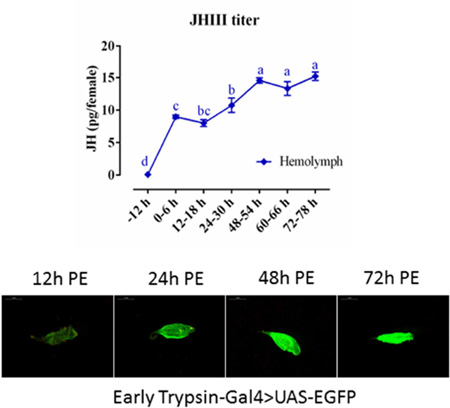
(A) The JH III mass spectra. Molecular weight of JH III is 266.2, the precursor ion plus a proton [M + H; m/z = 267.3]. (B) The standard curve of JH III includes 8 different concentration gradients, and the lowest detectable value is 0.1 ng/ml. (C) JH III titer in the hemolymph during PE stage. (D) JH III titer in whole-body homogenates during the Ae. aegypti reproduction cycle. JH III was measured in pg/female, and the letters a-f in X axis represents statistical differences at multiple time points. If the two groups have the same letters, their data are significantly different (P < 0.05). Error bars represent ± SD.
In pre-eclosion pupae, the JH titer was lower than the minimum detection line of instrument, 0.1 ng/ml. In newly eclosed mosquitoes (6 h PE), the JH titer was about 24 pg in the whole body and 9 pg in the hemolymph. The JH titer increased significantly by 24 h PE compared with that seen at 6 h and 18 h PE (Fig. 1C and 1D). Thereafter, from 24 h to 48 h PE, the JH titer in both whole bodies and hemolymph increased more slowly. In the hemolymph, the maximal JH titer was detected at 48–50 h PE and stayed at the same level until 72 h PE (Fig. 1C). In the whole bodies, JH level was increased further by 72 h PE (Fig. 1D). We also measured the JH titer in the whole bodies of female mosquitoes after blood feeding and observed it to drop sharply; it fluctuated around 10 pg/female from 6 h to 48 h PBM, and then was elevated significantly at 72 h PBM compared with PBM 24 h (Figure 1D).
3.2. Development of the Ae. aegypti ET-Gal4>UAS-EGFP transgenic line
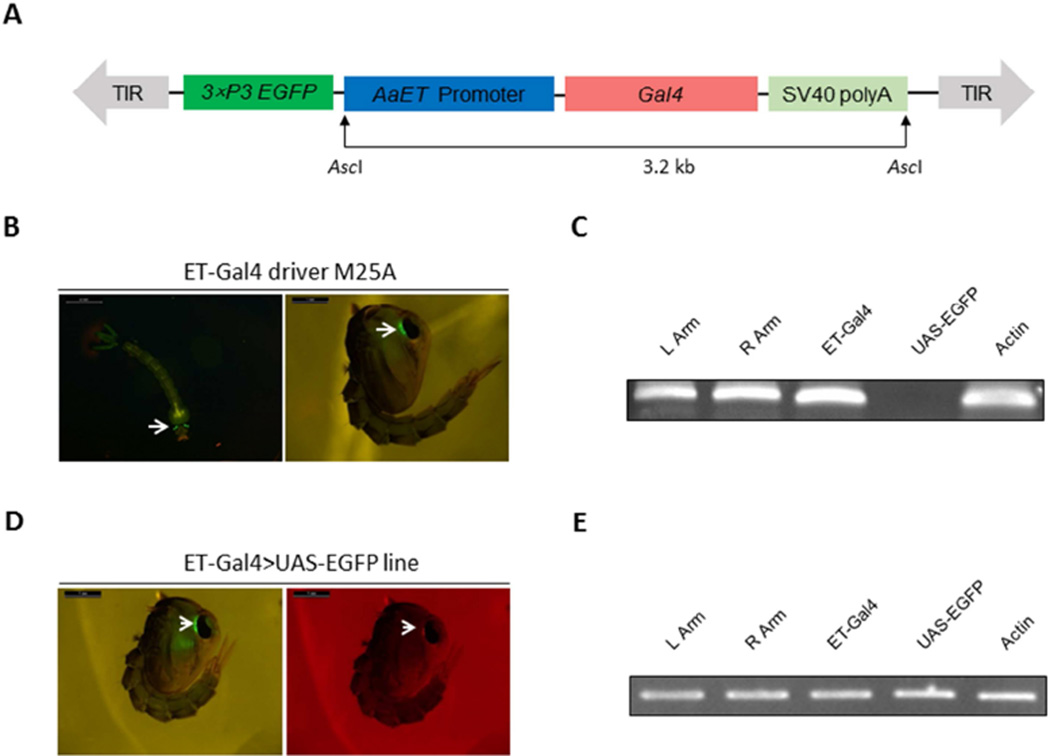
The ET-Gal4 driver vector was assembled with the ET gene 2.1-kb 5’ promoter region, the yeast transcription activator Gal4 coding sequence, and a SV40 polyadenylation signal. The ET gene sequence was cloned from the genomic Ae. aegypti DNA. The resulting ET-Gal4 driver cassette contained an EGFP selectable marker gene driven by the eye-specific 3×P3 promoter (Fig. 2A). Microinjection of the ET-Gal4 driver vector and helper vector mixture into pre-blastoderm Wt embryos generated three ET-Gal4 driver lines (M25A, M15/20 and M25B). M25B line exhibited whole body fluorescence and was excluded from further analysis. Larvae and pupae of the M25A and M15/20 lines showed a strong eye-specific marker EGFP expression (Fig. 2B). The homozygous driver line was established for the M25A line according to Kokoza and Raikhel (2011). For this driver line, we performed genomic PCR to confirm the genome integration of the ET-Gal4 vector. Our results indicate that the entire ET-Gal4 driver cassette was successfully transformed into Ae. aegypti genome (Fig. 2C) and that left and right arms of piggyBac transposon, as well as the ET-Gal4 sequence, were present in the transgene of the M25A driver line. The UAS-EGFP sequence that was used as a negative control was absent from the transgene. Actin coding sequence was employed as a positive control (Fig. 2C). To identify genome location of the ET-Gal4 driver cassette incorporation, we performed inverse PCR analysis using genomic DNA extracted from the M25A transgenic line. A single insertion was located outside of the gene coding sequence (VectorBase AaegL3.3) with canonical TTAA target sites (Table S1).
Figure 2.
A–C. Schematic representation of the ET-Gal4 vector used in germ-line transformation and characterization of the Ae. aegypti ET-Gal4 driver line. (A) Schematic representation of the ET-Gal4 vector. The ET-Gal4 vector contains the promoter region of the ET gene placed upstream of the Gal4 coding sequence. The EGFP marker gene is under the control of the 3×P3 promoter. (B) Expression of the EGFP marker in the M25A ET-Gal4 driver line. (C) Genomic PCR analysis showing integration of the ET-Gal4 cassette in the genome of the Ae. aegypti M25A ET-Gal4 driver line. Primers were specific to the left arm of the piggyBac vector (L Arm), the right arm of the piggyBac vector (R Arm), the Gal4 transgene (ET-Gal4), and the EGFP transgene (UAS-EGFP). PCR analysis of Actin acted as a positive control (Actin). D–E. Characterization of the ET-Gal4>UAS-EGFP hybrid line. (D) Both eye-specific selectable markers were present in the eyes of hybrid pupae—EGFP and DsRed. (E) PCR analysis demonstrated integration of the ET-Gal4 and UAS-EGFP cassettes in the Ae. aegypti genome. Photos were obtained using a Leica M165FC fluorescent stereomicroscope equipped with GFP-B filter and LAS V4.0 software. Scale bar: 1mm.
The Ae. aegypti responder UAS-EGFP line was generated previously (Kokoza and Raikhel, 2011). The ET-Gal4>UAS-EGFP transgenic line was produced by crossing homozygous ET-Gal4 M25A driver and homozygous UAS-EGFP responder lines. The eyes of these ET-Gal4>UAS-EGFP transgenic mosquitoes exhibited the presence of both EGFP and DsRed signals (Fig. 2D). Genomic PCR analysis indicated that sequences of ET-Gal4 activator, piggyBac transposon arms and UAS-EGFP responder were simultaneously present in the ET-Gal4>UAS-EGFP transgenic line (Fig. 2E). Thus, these results clearly show the establishment of the ET-Gal4>UAS-EGFP transgenic line in Ae. aegypti.
3.3. Dynamics of the ET-Gal4>UAS-EGFP expression and EGFP accumulation in the gut of transgenic female mosquitoes during the PE phase
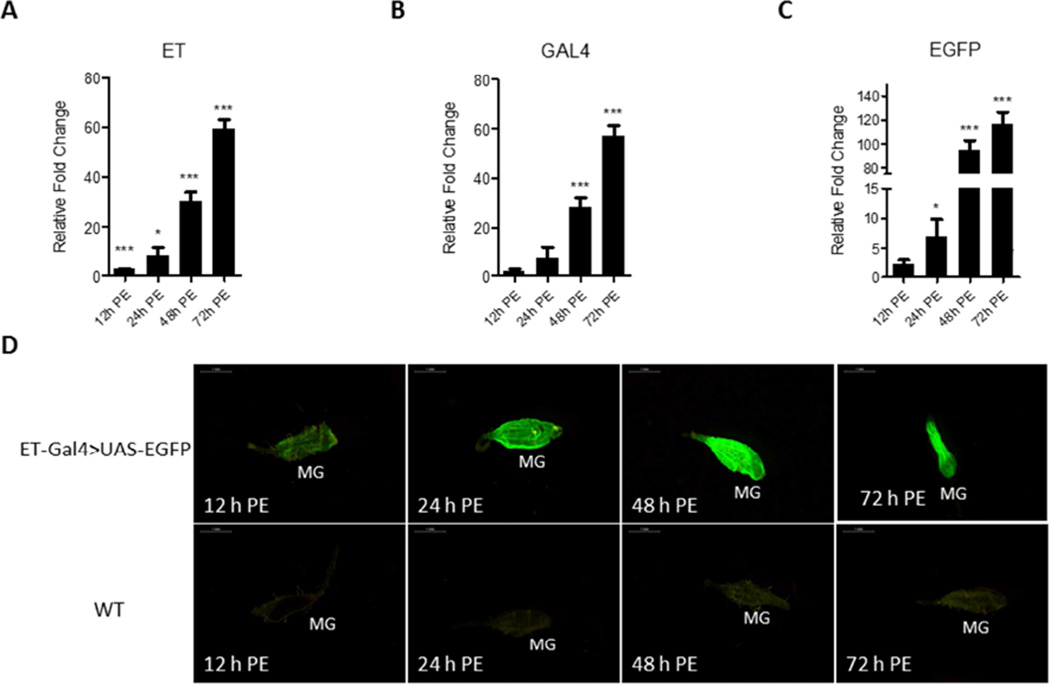
qPCR analysis of the ET-Gal4>UAS-EGFP transgene transcript revealed that its expression pattern was closely correlated with the endogenous ET expression profile (Fig. 3A–C). ET, Gal4, and EGFP transcript levels were low in the guts of newly emerged transgenic female mosquitoes (12 h PE) and started to accumulate during the PE phase. They reached the highest level at 72 h PE. To identify the EGFP signal level in the ET-Gal4>UAS-EGFP mosquitoes, we examined fluorescence in their guts using a fluorescent microscope with GFP filter at multiple time points after eclosion. This analysis has revealed that a low but detectable signal was present at 12 h PE (Fig. 3D). The EGFP signal increased dramatically by 48 h PE and reached its maximal level at 72 h PE. In contrast, only background fluorescence was observed in guts from control WT mosquitoes that were examined at the same times (Fig. 3D). These results indicate that the EGFP protein was produced in the ET-Gal4>UAS-EGFP transgenic female mosquitoes and its level corresponds to the level of its transcript and the JH titer during the PE phase.
Figure 3.
Expression of the ET-Gal4>UAS-EGFP transgene in the Ae. aegypti hybrid line. (A–C) The time course of transcript abundance in midguts of the ET-Gal4>UAS-EGFP hybrid transgenic female mosquitoes. RNA samples were extracted from isolated midguts of female mosquitoes at different time points: 12 h PE, 24 h PE, 48 h PE, and 72 h PE. Expression patterns of Gal4 and EGFP were highly similar to that of the endogenous ET. Relative fold changes were established by comparing transcript levels of each sample with 0 h PE mosquito sample. The abundance of 0 h is represented as 1.0, with corresponding adjustments for other time points. Values represent average ± s.e.m. from three combined biological replicates. *p < 0.05; **p < 0.01; ***p < 0.001 (t-test). (D) Images of midguts (MG) of the ET-Gal4>UAS-EGFP transgenic hybrid female mosquitoes with EGFP signal at several time points during the PE phase. Samples from wild type (WT) mosquitoes served as negative control. Images were obtained using a Leica M165FC fluorescent stereomicroscope with LAS V4.0 software. Scale bar: 1 mm.
We also evaluated the longevity of the EGFP mRNA level and the EGFP signal the ET-Gal4>UAS-EGFP transgenic female mosquitoes after a blood meal, when the JH concentration drops. The EGFP mRNA level precipitously declined after a blood meal and remained low until 72 h post-blood meal, when JH titer increased again. However, the post blood meal EGFP signal in guts of the transgenic mosquitoes was present for about 12 h longer that EGFP mRNA, eventually reducing to the 12 h PE level and increasing again at 72 h post blood meal (not shown). Thus, due to the long stability of the EGFP protein, this system has limitations for accurate indication of a post blood meal JH level. The short-lived EGFP responder should be used in the future to improve the responsiveness of the ET-Gal4>UAS-EGFP system.
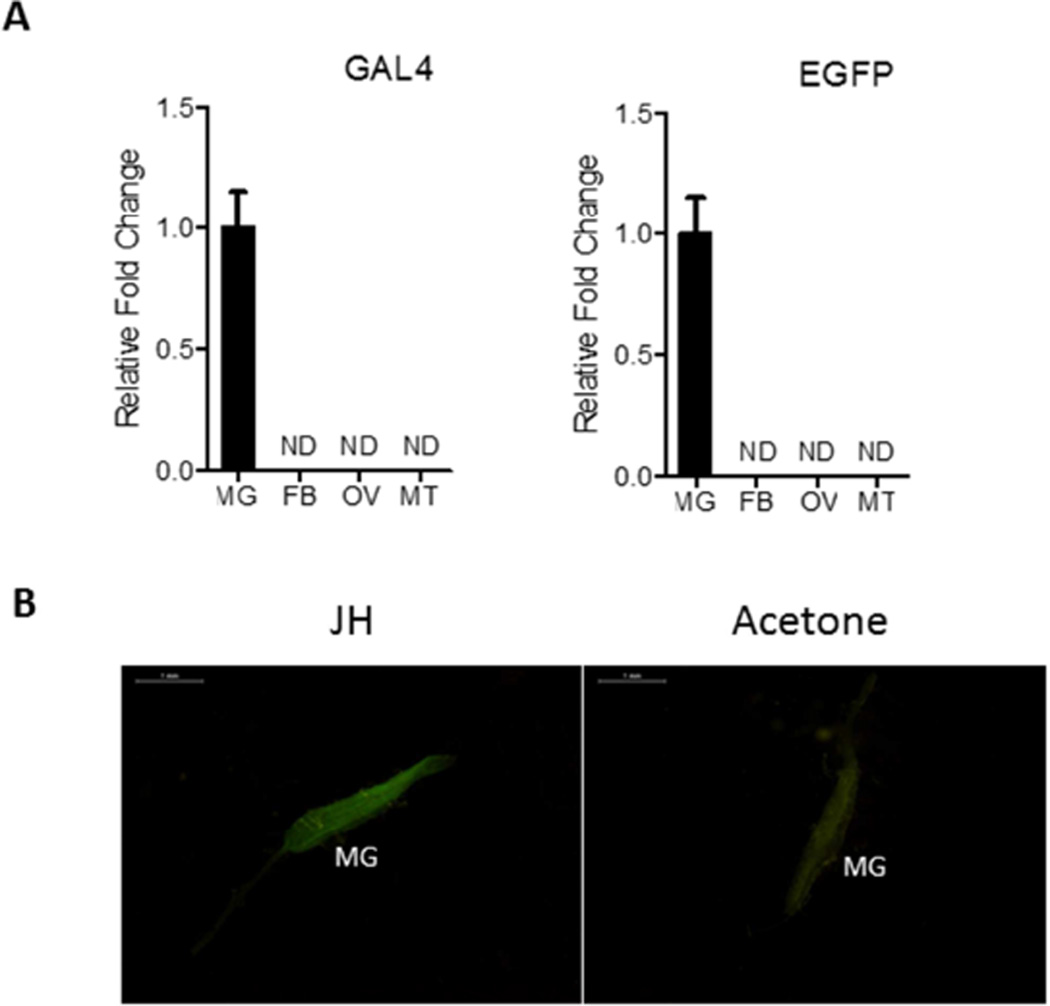
Further characterization using qPCR analysis demonstrated that ET-Gal4>UAS-EGFP transgene expression was gut specific and was not observed in other tissues (Fig. 4A). Together, these data indicate that in the ET-Gal4>UAS-EGFP transgenic mosquitoes, the transgene exhibited a tissue- and stage-specific expression pattern similar to that displayed for ET. Moreover, the ET-Gal4>UAS-EGFP transgene expression profile closely correlated with the JH titer.
Figure 4.
(A) qPCR analysis using Gal4 or EGFP primers showing the midgut-specific expression of ET-Gal4>UAS-EGFP transgene at 72 h PE. ND means non-detected. RNA samples were extracted from midgut (MG), fat bodies (FB), ovaries (OV), and Malpighian tubules (MT) at 72 h PE. Analyses were performed by means of qPCR using specific probes (Table S2). (B) Topical JH application induces premature expression of EGFP in midgut (MG) of the ET-Gal4>UAS-EGFP transgenic line (JH). Application of acetone (solvent) served as a control. Images were obtained using a Leica M165FC fluorescent stereomicroscope with LAS V4.0 software. Scale bar: 1 mm.
3.4. The JH signaling pathway is involved in activation of ET-Gal4>UAS-EGFP transgene expression
To identify the JH involvement in activation of ET-Gal4>UAS-EGFP transgene, we first performed an assay in which JH or acetone (control) was applied topically onto mosquito abdomens at 3 h PE, a time when JH titer is relatively low compared with that of late PE stage. Guts were dissected 8 h later and for the EGFP protein level using a fluorescence microscope equipped with GFP-B filter. A substantial EGFP signal existed only in guts of transgenic mosquitoes after exposure to JH but not to acetone (Fig. 4B). Thus, these hormone treatment experiments have suggested that JH is required for activation of ET-Gal4-driven EGFP in the gut of transgenic mosquitoes.
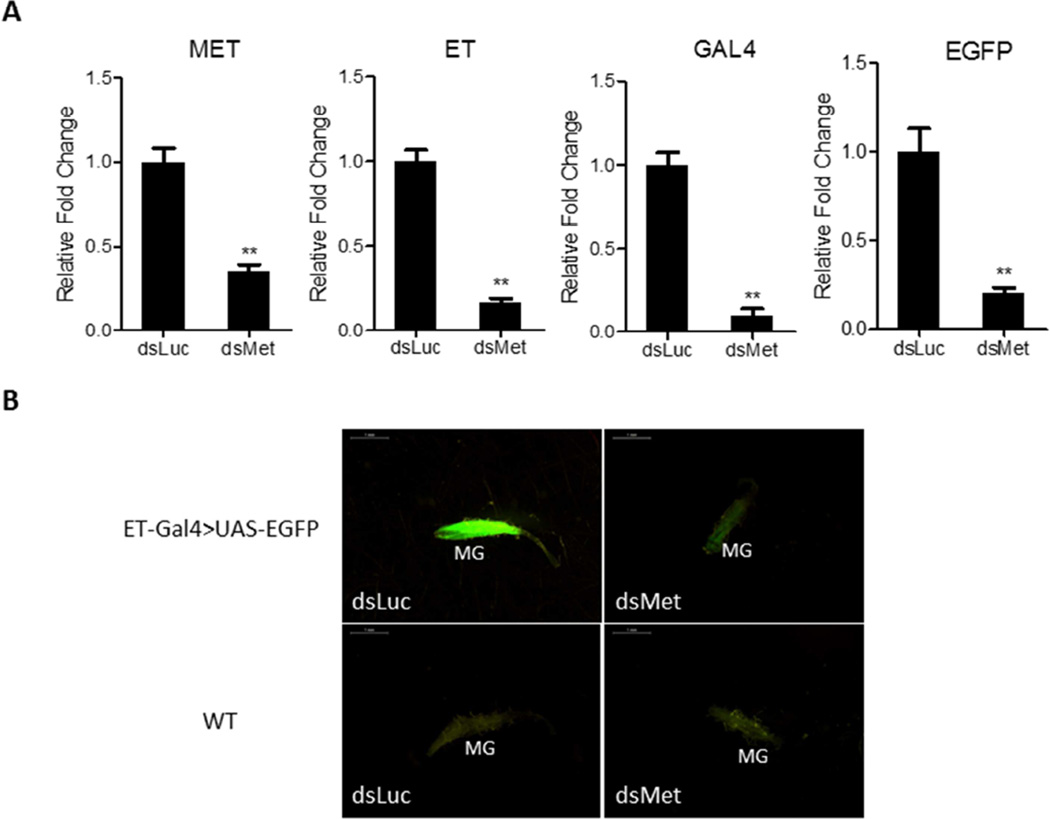
Next, we utilized Met RNAi silencing to substantiate results of the JH application assay and to test the regulatory role of JH signaling pathway in transgene expression. Met dsRNA (dsMet) or luciferase dsRNA used as a control (dsLuc) was injected into ET-Gal4>UAS-EGFP female mosquitoes at 6–12 h PE, and guts were dissected 48 h later. Met RNAi depletion had a strong inhibitory effect on the Met transcript level demonstrating the down-regulating efficiency of dsMet (Fig. 5A). The Met RNAi depletion effect was also evaluated by means of qPCR for the endogenous ET gene, and the transgenes Gal4 and EGFP (Fig. 5A). RNAi down-regulation of Met transcript resulted in a significant negative effect of the transcript levels of endogenous ET and ET-Gal4>UAS-EGFP transgenes (Fig. 5A).
Figure 5.
Depletion of Met by RNAi results in inhibitory effects on the expression of endogenous ET and ET-Gal4>UAS-EGFP transgene. (A) Knockdown of Met by RNAi results in reduced transcript levels of endogenous ET and ET-Gal4>UAS-EGFP transgenes. The abundance of dsRNA-injected samples is represented as 1.0, with corresponding adjustments for other time points. Values represent average ± s.e.m. from three combined biological replicates. *p < 0.05; **p < 0.01; ***p < 0.001 (t-test) (B) The EGFP signal was significantly reduced in midguts (MG) of dsMet-treated female mosquitoes compared with dsLuc-treated female mosquitoes. WT mosquitoes treated with dsLuc or dsMet served as negative controls. Images were obtained using a Leica M165FC fluorescent stereomicroscope with LAS V4.0 software. Scale bar: 1 mm.
We also examined the effect of Met RNAi on the EGFP signal in the gut of the transgenic mosquitoes. The signal was dramatically diminished after Met RNAi; in contrast, no negative effect was observed in dsLuc-treated transgenic mosquitoes with bright fluorescence (Fig. 5B). No fluorescence signal was observed in guts of WT mosquitoes with either dsMet or dsLuc treatments (Fig. 5B).
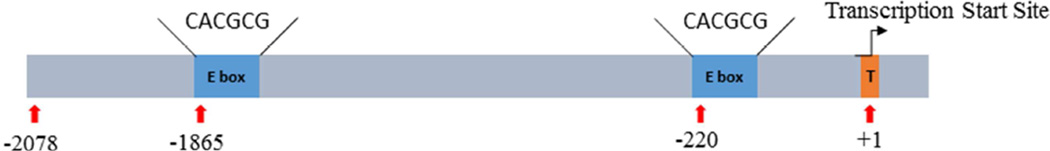
The involvement of Met in the activation of the ET-Gal4>UAS-EGFP transgene was further substantiated by the presence of the putative Met binding sites in the 5’ untranslated region of the ET gene, which was used for constructing the transgene. Analysis has revealed the presence of two E box-like motives ‘CACGCG’ for Met binding, at positions −1865 and −220 from the transcription start site (TSS) (Fig. 6 and S3). Two experimentally verified Met-binding sequences, CCACACGCGAAG and CACGCGGTG, have been identified in the promoters of Ae. aegypti ET and kruppel homolog 1 genes, respectively. These Met-binding sequences share the short motif CACGCG, which has one nucleotide difference to the palindromic canonical E-box motif, CACGTG (Li et al., 2011; Shin et al., 2012; Zou et al., 2013).
Figure 6.
A schematic diagram of the 2.1-kb ET gene promoter region used in the ET-Gal4 driver vector. This sequence harbors two E box-like motives ‘CACGCG’ (in red). Transcription start site (+1) is indicated by purple color.
4. Discussion
A characteristic feature of the female mosquito gonadotrophic cycle is a PE phase that lasts approximately 72 h (Clements, 1992; Hagedorn et al., 1979). This phase is crucial for females to attain competence for host seeking, blood feeding, and egg maturation. It is controlled by JH and determination of its titer is critical for the correct interpretation of developmental events during PE.
Measurements using coupled gas chromatography-mass spectrometry (GC-MS) have reported that in the whole bodies of Ae. aegypti females, the titer of JH III was elevated by 48–50 h PE, declined slightly by 72 h PE, and then dropped to the background level after a blood meal (Shapiro et al., 1986). Recently, however, Hernandez-Martinez et al. (2015) used high performance liquid chromatography coupled to a fluorescent detector (HPLC-FD) (Rivera-Perez et al., 2013) to evaluate the JH titer in the mosquito hemolymph and showed its peak to be at 12 h PE, after which it dramatically declined and by 48 h PE reached the same low level as at 0 h PE. To resolve these conflicting results, we used an advanced LC-MS/MS/MS technique and determined JH III levels in both the hemolymph and whole bodies of female Ae. aegypti mosquitoes. According to our results, JH III levels initially rises at 6 h PE, then exhibits a steady increase, reaching its peak at 48–50 h PE in the hemolymph and 72 h PE in the whole bodies. No decline of JH titer during evaluated 78 h PE was observed in our study. A possible explanation of this significant discrepancy between studies is a nutritional status of mosquitoes used for JH measurements. Hernandez-Martinez et al. (2015) reported that JH titer during the PE phase was dramatically affected by nutrients. In their evaluation, mosquitoes fed on 3% sucrose solution had the JH level 6-fold lower than those maintained on 20% sucrose solution at 72 h PE. These authors measured JH kinetics using female Ae. aegypti mosquitoes maintained on 3% sucrose solution, while in our experiments we used female mosquitoes fed on 10% sucrose solution. These experimental conditions could, at least in part, explain the difference between Hernandez-Martinez et al. (2015) and our studies. Further detailed investigations of the nutrition effect on JH titer kinetics are required. Interestingly, all three studies (Shapiro eet al., 1986; Hernandez-Martinez et al., 2015 and ours) are in agreement showing that the JH III level drops immediately after a blood meal, is low during the vitellogenic period and rises again at about 48 h PBM, after termination of vitellogenesis.
A large body of experimental data strongly supports that JH peaks at the end of the PE phase in female Ae. aegypti mosquitoes. The growth of ovarian primary follicles is JH dependent and this feature has been used as a bioassay for evaluation of JH levels. Removal of the corpora allata, a paired gland secreted JH, resulted in arrest of ovarian growth and development of the oocyte endocytic complex, both of which are necessary for yolk protein uptake during oogenesis (Gwadz and Spielman, 1973; Raikhel and Lea, 1985). The fat body, a tissue producing yolk protein precursors for developing oocytes under 20E control, becomes responsive to this hormone after exposure to JH, exhibiting maximal sensitivity at 60–72 h PE (Zhu et al., 2003). In the gut, transcripts of ET and JHA15 genes are elevated significantly at 36 h PE, maintaining a high level until a blood meal (Bian et al., 2008; Noriega et al., 1996a; Noriega et al., 1996b). Several molecular studies have demonstrated that large clusters of JH-activated genes exhibit their peaks at 60–72 h PE, while JH-repressed genes are at their lowest at that time (Zou et al., 2013; Hou et al., 2015; Saha et al., 2016).
In this study, we genetically engineered the Ae. aegypti ET-Gal4>UAS-EGFP line utilizing the ET gene, which is expressed in the female mosquito gut exclusively during the PE phase under the JH control. Our analysis has shown that the 2.1-kb 5’ promoter fragment of the ET gene is sufficient to drive the EGFP gene with a spatial and temporal expression pattern similar to that of the endogenous ET gene. The transgene expression was restricted to the gut, and no miss-expression was observed. In the gut, the transgene was expressed during the PE phase, with increasing transcript level corresponding to the JH titer. Interestingly, unlike the endogenous ET, which is reportedly not translated during the PE phase, the ET-driven EGFP was translated in unison with the level of the EGFP transcript abundance, providing a convenient marker for evaluating the JH titer in the mosquito for the PE phase. Indeed, topical JH application to newly eclosed Ae. aegypti ET-Gal4>UAS-EGFP female mosquitoes, in which this hormone’s titer is low, caused a significant enhancement of the EGFP signal, indicating a premature JH increase in these mosquitoes. Met RNAi silencing caused a dramatic decrease in the EGFP transgene transcript level and a diminished EGFP signal in ET-Gal4>UAS-EGFP female mosquitoes, further demonstrating that this transgene is appropriately controlled by Met via regulation of the ET promoter.
In this work, we report for the first time creation of the Gal4/UAS system specific for female gut and driving expression during the JH-dependent PE phase. We established a transgenic line with the ET-Gal4 activator using the 5’ untranslated region (UTR) of the female-, stage-, and tissue-specific ET gene. We show that in the ET-Gal4>UAS-EGFP mosquitoes, JH and its receptor Met stimulate the expression of the ET-Gal4>UAS-EGFP transgene and direct translation of the marker protein EGFP in a manner corresponding to the JH titer during the PE phase.
The development of the PE stage-, gut-, and female-specific ET-Gal4/UAS system provides a unique opportunity for the overexpression of genes and studying their biological significance in the mosquito gut during preparation for blood feeding. Establishment of the early-phase, gut-specific system is of particular importance because it would permit expression of anti-pathogen molecules prior to the gut entry by pathogens themselves. Thus, such a system might prove to be efficient for repressing pathogen development in mosquitoes.
Supplementary Material
Highlights.
The JH titer reached its peak at 48–54 h post eclosion in Aedes aegypti female mosquitoes.
The Aedes early trypsin (ET)-Gal4>UAS-EGFP system sensitive to JH activation was established.
The intensity of the gut-specific EGFP signal was observed to correspond to the level of ET mRNA and follow the JH titer.
RNA interference silencing of the JH receptor Methoprene-tolerant specifically depleted the EGFP signal in the gut of transgenic mosquitoes.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI036959, Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB11030600) and the National Science Foundation of China (No. 31472008, L1524009).
Abbreviations
- 20E
20-hydroxyecdysone
- AA
amino acid
- bHLH-PAS
basic helix–loop–helix-Per-Arnt-Sim
- CP
carboxypeptidase
- dsLuc
double-stranded RNA of Luciferase
- dsMet
double-stranded RNA of Methoprene-tolerant
- dsRNA
double-stranded RNA
- EGFP
enhanced green fluorescent protein
- ET
early trypsin
- FB
fat body
- GC-MS
gas chromatography-mass spectrometry
- HPLC-FD
liquid chromatography coupled to a fluorescent detector
- JH
juvenile hormone
- LC-MS/MS/MS
liquid chromatography-triple tandem mass spectrometry
- Met
Methoprene-tolerant
- MG
midgut
- MT
Malpighian tubules
- MS
mass spectrometry
- OV
ovaries
- PBM
post blood meal
- PCR
polymerase chain reactions
- PE
post eclosion
- qPCR
quantitative reverse transcriptase PCR
- RNAi
RNA interference
- SP
serine protease
- TOR
target of rapamycin
- TSS
transcription start site
- UAS
upstream activating sequence
- UTR
untranslated region
- Vg
vitellogenin
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect biochemistry and molecular biology. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annual review of entomology. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- Bian G, Raikhel AS, Zhu J. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect biochemistry and molecular biology. 2008;38:190–200. doi: 10.1016/j.ibmb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Isoe J, th WCB, Zamora J, Foy BD, Miesfeld RL, Olson KE. Expression profiling and comparative analyses of seven midgut serine proteases from the yellow fever mosquito, Aedes aegypti. Journal of insect physiology. 2010;56:736–744. doi: 10.1016/j.jinsphys.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MC, Pennington JE, Isoe J, Zamora J, Schillinger AS, Miesfeld RL. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect biochemistry and molecular biology. 2008;38:916–922. doi: 10.1016/j.ibmb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Linse KD, Taub-Montemayor TE, Rankin MA. Comparison of radioimmunoassay and liquid chromatography tandem mass spectrometry for determination of juvenile hormone titers. Insect biochemistry and molecular biology. 2007;37:799–807. doi: 10.1016/j.ibmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Development, Nutrition, and Reproduction. 1st. London: Chapman and Hall; 1992. [Google Scholar]
- Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. The New England journal of medicine. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. Journal of insect physiology. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH, Shapiro JP, Hanaoka K. Ovarian ecdysone secretion is controlled by a brain hormone in an adult mosquito. Nature. 1979;282:92–94. doi: 10.1038/282092a0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Rivera-Perez C, Nouzova M, Noriega FG. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. Journal of insect physiology. 2015;72:22–27. doi: 10.1016/j.jinsphys.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Development genes and evolution. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wang XL, Saha TT, Roy S, Zhao B, Raikhel AS, Zou Z. Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction. PLoS genetics. 2015;11:e1005309. doi: 10.1371/journal.pgen.1005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe J, Rascon AA, Jr, Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect biochemistry and molecular biology. 2009;39:903–912. doi: 10.1016/j.ibmb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza VA, Raikhel AS. Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system. Insect biochemistry and molecular biology. 2011;41:637–644. doi: 10.1016/j.ibmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu P, Wiley JD, Ojani R, Bevan DR, Li J, Zhu J. A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Molecular and cellular endocrinology. 2014;394:47–58. doi: 10.1016/j.mce.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A, Lycett GJ. Development of the bi-partite Gal4-UAS system in the African malaria mosquito, Anopheles gambiae. PloS one. 2012;7:e31552. doi: 10.1371/journal.pone.0031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, Colonna AE, Wells MA. Increase in the size of the amino acid pool is sufficient to activate translation of early trypsin mRNA in Aedes aegypti midgut. Insect biochemistry and molecular biology. 1999;29:243–247. doi: 10.1016/s0965-1748(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Pennington JE, Barillas-Mury C, Wang XY, Wells MA. Aedes aegypti midgut early trypsin is post-transcriptionally regulated by blood feeding. Insect molecular biology. 1996a;5:25–29. doi: 10.1111/j.1365-2583.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Shah DK, Wells MA. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect molecular biology. 1997;6:63–66. doi: 10.1046/j.1365-2583.1997.00154.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wang XY, Pennington JE, Barillas-Mury CV, Wells MA. Early trypsin, a female-specific midgut protease in Aedes aegypti: isolation, aminoterminal sequence determination, and cloning and sequencing of the gene. Insect biochemistry and molecular biology. 1996b;26:119–126. doi: 10.1016/0965-1748(95)00068-2. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Boston: Elsevier; 2005. pp. 433–491. [Google Scholar]
- Raikhel AS, Lea AO. Hormone-mediated formation of the endocytic complex in mosquito oocytes. General and comparative endocrinology. 1985;57:422–433. doi: 10.1016/0016-6480(85)90224-2. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. General and comparative endocrinology. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Clifton ME, Garcia EM, LeBlanc E, Noriega FG. Aldehyde dehydrogenase 3 converts farnesal into farnesoic acid in the corpora allata of mosquitoes. Insect biochemistry and molecular biology. 2013;43:675–682. doi: 10.1016/j.ibmb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol PA, Spielman A, Jacobs MS. Rough Endoplasmic-Reticulum in Midgut Cells of Mosquitos (Diptera, Culicidae) - Aggregation Stimulated by Juvenile-Hormone. Journal of medical entomology. 1982;19:719–721. [Google Scholar]
- Roy S, Saha TT, Johnson L, Zhao B, Ha J, White KP, Girke T, Zou Z, Raikhel AS. Regulation of Gene Expression Patterns in Mosquito Reproduction. PLoS genetics. 2015;11:e1005450. doi: 10.1371/journal.pgen.1005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TT, Shin SW, Dou W, Roy S, Zhao B, Hou Y, Wang XL, Zou Z, Girke T, Raikhel AS. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E735–E743. doi: 10.1073/pnas.1523838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile-Hormone and Juvenile-Hormone Esterase in Adult Females of the Mosquito Aedes-Aegypti. Journal of insect physiology. 1986;32:867–877. [Google Scholar]
- Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Chen R, Weaver SC. Interspecies transmission and chikungunya virus emergence. Current opinion in virology. 2016;16:143–150. doi: 10.1016/j.coviro.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Hu Y, Xing LS, Jiang H, Hu SN, Raikhel AS, Zou Z. A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes. PLoS pathogens. 2015;11:e1004931. doi: 10.1371/journal.ppat.1004931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral research. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund SA, Hoffmann KH. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography-mass spectrometry (LC-MS) Analytical and bioanalytical chemistry. 2004;379:540–543. doi: 10.1007/s00216-004-2598-x. [DOI] [PubMed] [Google Scholar]
- Zhao B, Kokoza VA, Saha TT, Wang S, Roy S, Raikhel AS. Regulation of the gut-specific carboxypeptidase: a study using the binary Gal4/UAS system in the mosquito Aedes aegypti. Insect biochemistry and molecular biology. 2014;54:1–10. doi: 10.1016/j.ibmb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Saha TT, Roy S, Shin SW, Backman TW, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhel AS. Distinct melanization pathways in the mosquito Aedes aegypti. Immunity. 2010;32:41–53. doi: 10.1016/j.immuni.2009.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.